Abstract
Rabbit anti-thymocyte globulin (ATG) is used as prophylaxis against GVHD following allogeneic hematopoietic cell transplantation (HCT). At our institution, ATG is exclusively used in the conditioning of matched unrelated donor (URD) transplant recipients. A total of 50 URD HCT recipients who received ATG (ATG group) were retrospectively compared with 48 matched related donor (MRD) HCT recipients who did not receive ATG (no ATG group). There were no significant differences between the groups in rates of day 100 mortality, acute GVHD or relapse. Chronic GVHD incidence was significantly lower in the ATG group (P = 0.007). At a median follow-up of 36 months in the entire cohort, 50% patients are alive in the ATG group and 63% of the patients are alive in the no ATG group (P = 0.13). We conclude that the administration of ATG to patients undergoing URD HCT preserves the anti-leukemia benefit of the transplant, while reducing the risk of developing GVHD, resulting in OS rates that are comparable to MRD HCT recipients.
Keywords: ATG, allo-SCT, unrelated donor
Introduction
Donor T-cell-mediated graft versus leukemia effect following allo-SCT has made allografting the standard of care in the management of high-risk AML, ALL and myelodysplastic syndrome, particularly when an HLA-matched related donor (MRD) is available.1–5 However, despite improvements in HLA matching, unrelated donor (URD) transplantation is still not widely applied in first remission due to several lines of evidence, suggesting a high risk of GVHD and TRM.6–8 Even in patients receiving 8/8 HLA-matched URD grafts, the risk of GVHD and subsequent TRM is higher, without an accompanying decline in relapse risk in acute and chronic leukemia patients.9–11 This increased risk of GVHD among other factors is related to histo-incompatibility between HLA-matched donors and recipients in SCT from URDs, both at the level of the MHC locus,12,13 and also in other minor histocompatibility Ags.14,15 There are few prospective trials studying comparative outcomes between MRD and URD allografts. Furthermore, where URD SCT may yield comparable outcomes in high-risk AML16 it is largely unexplored in intermediate-risk AML and ALL.
The risk of GVHD stemming from donor recipient allo-reactivity may be reduced by in vivo T-cell depletion using polyclonal anti T-cell Ab preparations such as anti-thymocyte globulin (ATG). ATG was first introduced in solid organ transplant protocols where it served a tolerance-inducing function,17 helping reduce the risk of graft rejection. ATG may be of equine or rabbit origin, and because of its long half life in the circulation,18,19 both native recipient and infused donor T cells are affected owing to recognition and binding of T-cell surface Ags and depletion of CD3+ lymphocytes after administration. This has led to its use in SCT protocols, which promote tolerance induction to develop a platform for adoptive immunotherapy.20–23 Notably, patients transplanted using T-cell depletion are at a higher risk of opportunistic infection and possibly relapse.24–26 Because of the high probability of developing acute GVHD with URD SCT, ATG is often administered as a part of the conditioning regimens for these transplants to reduce this risk. This study compares clinical outcomes between URD-SCT recipients who received ATG before transplant and MRD-SCT recipients who did not receive it. We hypothesize that patients who receive ATG would have a lower risk of developing GVHD and thus, despite the use of an URD and the implications of in vivo T-cell depletion for infection risk, we would observe superior or equivalent clinical outcomes to MRD recipients.
Patients and Methods
Patients
After obtaining permission from the Virginia Commonwealth University Institutional Review Board, and in accordance with the declaration of Helsinki, a retrospective review of the medical records for allogeneic SCT recipients with AML, myeloproliferative disorders, ALL or advanced myelodysplastic syndrome transplanted between 2004 and 2009 was conducted. Recipient-donor pairs were matched at the HLA-A, B, C and DRB1 loci, using high resolution typing for recipients of URD transplantation and intermediate resolution for recipients of MRD transplants. HLA typing was performed by PCR using sequence specific oligonucleotide probes. All patients received myeloablative conditioning (Table 1).
Table 1. Patient characteristics.
| No ATG | ATG | P value | |
|---|---|---|---|
| N | 48 | 50 | |
| Number of males | 20 (42) | 32 (64) | 0.026 |
| Median age at transplant (range) | 47 (25, 63) | 48 (19, 61) | 0.57 |
| Donor type | |||
| Match related | 48 | 0 | — |
| Match unrelated | 0 | 50 | |
| Conditioning regimen | |||
| CY/TBI, BU/CY, VP16/TBI | 41 (85) | 39 (78) | 0.34 |
| BU/Flu | 7 (15) | 11 (22) | |
| GVHD prophylaxis | |||
| Tacrolimus | 14 (29) | 25 (50) | 0.07 |
| CYA | 12 (25) | 13 (26) | |
| CYA→Tacrolimus | 20 (42) | 12 (24) | |
| Sirolimus | 2 (4) | 0 | |
| ATG dose | |||
| 10 mg/kg adjusted ideal body weight | N/A | 26 | — |
| 7.5 mg/kg adjusted ideal body weight | N/A | 24 | |
| Stem cell source | |||
| PBSC | 46 (96) | 28 (56) | <0.001 |
| Mean cell dose ± s.d. | |||
| CD3 (108 cells/kg) | 3.69 ± 2.06 | 2.57 ± 2.27 | 0.012 |
| CD34 (106 cells/kg) | 5.09 ± 1.76 | 5.10 ± 2.68 | 0.98 |
| Remission status | |||
| 1st CR | 29 (60) | 30 (60) | 0.97 |
| 2nd CR | 14 (29) | 14 (28) | |
| ≥ 3rd CR | 5 (10) | 6 (12) | |
| Diagnosis at time of transplant | |||
| ALL | 7 (14) | 9 (18) | 0.19 |
| AML | 32 (67) | 30 (60) | |
| MDS | 6 (13) | 11 (22) | |
| MF | 3 (6) | 0 (0) | |
Abbreviations: Flu = fludarabine; MF = myelofibrosis; MDS = myelodysplastic syndrome. Regimens used: CY/TBI-TBI, six fractions of two Gray (Gy) each, day − 6 to − 4, and CY 60 mg/kg i.v., day − 3 and − 2; BU/CY-BU 0.8 mg/kg i.v. every 6 h, day − 7 to − 4 and CY 60 mg/kg i.v., day − 3 and − 2; BU/Flu-BU 130 mg/m2 i.v. daily, day − 5 to − 2, and Flu 40 mg/m2 i.v., day − 5 to − 2; VP16/TBI- TBI 7 fractions two Gy each and etoposide (VP16) 60 mg/kg i.v., one dose. GVHD prophylaxis: Tacrolimus target level 5–12 ng/mL; CYA, target level 250–350 ng/mL, both starting day − 2, generally tapered following day 100. CYA→Tacrolimus, i.v. CYA transitioned to oral tacrolimus following engraftment. Calcinuerin inhibitors administered with MTX 15 mg/m2 i.v. on day 1, and 10 mg/m2 on days 3, 6 and 11 and leucovorin rescue. In patients who could not tolerate MTX, mycophenolate mofetil 15 mg/kg PO/i.v. twice daily was given from day 0 to 30. Sirolimus target level 5–10 ng/mL, day − 2 to day 100. Percentages in parentheses.
Rabbit ATG
Recipients of MRD SCT did not receive ATG, whereas URD SCT recipients were routinely given rabbit ATG (Thymoglobulin, Genzyme Inc.; Cambridge, MA, USA) at a dose of 3.3 mg/kg/day (adjusted ideal body weight) for three doses (total ATG dose 10 mg/kg), from February 2004 to July 2007, after which the dose was reduced to 2.5 mg/kg/day for three doses (total ATG dose 7.5 mg/kg), administered on days − 3 to − 1 before SCT. ATG was infused over 6–12 h as tolerated after premedication with corticosteroids, diphenhydramine and acetaminophen. ATG dose reduction was necessary due to increased number of opportunistic infections observed in the earlier cohort. GVHD prophylaxis used is given in Table 1. Antimicrobial prophylaxis was routinely administered. CMV and EBV monitoring was performed using quantitative reverse transcriptase PCR on blood samples every 2 weeks.
Statistical methods
Clinical outcomes of interest were compared between the MRD recipient (no ATG) and URD recipient (ATG) cohorts. Patient characteristics between the two groups were tested for relationships using χ2 tests. The probabilities of OS and EFS were calculated with the Kaplan–Meier estimator, and were compared using the log-rank test. The probabilities of relapse, cumulative GVHD, as well as acute and chronic GVHD, and non-relapse mortality were calculated with the cumulative incidence estimator,27 and were compared between MRD and URD groups using Gray's test28 to account for the competing event of non-relapse mortality for relapse, and mortality without GVHD for acute and chronic GVHD, and relapse for non-relapse mortality. Hazard ratios (with corresponding 95% Wald confidence intervals) were estimated using the Cox proportional hazards model and compared between the no ATG and ATG cohorts. Cox proportional hazard ratios are also used to compare the outcomes between the two cohorts while accounting for pretransplant variables. Clinical outcomes of interest were compared (without respect to timing) between ATG and non-ATG subjects using χ2 tests, and odds ratios (OR; with 95% confidence intervals (CI)) were also estimated.
Results
Patients
Ninety-eight patients underwent myeloablative allo-SCT for acute leukemia or myelodysplastic syndrome in the period examined. Patients in the no ATG and ATG cohorts were similar in their composition except for the donor type, stem cell source and CD3+ cell dose infused between the two groups (Table 1). Hematopoietic engraftment was similar in both cohorts, 12 vs 12.5 days in the no ATG vs ATG groups, respectively.
Survival
At a median follow-up of 36 months in the entire cohort, 50% patients are alive in the ATG group and 63% in the no ATG group (Figure 1a; hazards ratio (HR) 0.63, 95% CI: 0.35, 1.16; P = 0.14), indicating no survival difference between the two groups. This was true regardless of patient age, diagnosis or the conditioning regimen used (Table 2). PBSC recipients in the no ATG group (MRD) and BM recipients in the ATG group (URD) had better survival compared with the PBSC recipients in the ATG group (URD; HR = 0.44, 95% CI: 0.21, 0.86, and 0.44, 95% CI; 0.18, 0.99, respectively). EFS was also similar between the no ATG and the ATG groups (Figure 1b).
Figure 1.
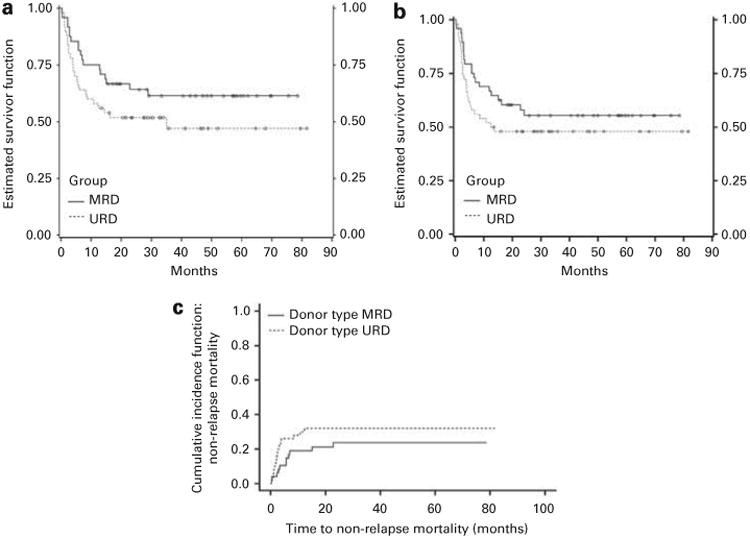
(a) K-M survival curves depicting OS in patients conditioned with or without ATG (log rank P = 0.13). (b) K-M survival curves depicting EFS in patients conditioned with or without ATG (P = 0.25). (c) Cumulative incidence curves depicting non-relapse mortality in patients conditioned with or without ATG (P = 0.28).
Table 2. Results from Cox proportional hazards model univariate analysis of clinical outcomes with respect to transplant variables.
| Age (P value) | Diagnosis (P value) | Conditioning (P value) | Stem cell source (P value) | |
|---|---|---|---|---|
| Survival | 0.10 | 0.49 | 0.06 | 0.01 |
| Relapse | 0.32 | 0.63 | 0.14 | 0.03 |
| Cumulative GVHD | 0.90 | 0.59 | 0.24 | 0.23 |
There was no significant difference (P-value = 0.21) in day 100 mortality between the no ATG group (6/48, 13%) and the ATG group (11/50, 22%; OR 0.51, 95% CI: 0.17, 1.50, P = 0.21), nor in the cumulative incidence of non-relapse mortality, accounting for the competing risk of relapse (Figure 1c). Causes of death are listed in Table 3, with infection and relapse contributing notably to the mortality observed in the ATG group, particularly in the recipients of 10 mg/kg ATG.
Table 3. Table listing causes of death in patients in each group.
| Cause of death | No ATG | ATG total | ATG 10 mg/kg cohort |
|---|---|---|---|
| Number deceased | 18 | 25 | 18 |
| Relapse | 6 | 10 | 5 |
| aGVHD | 7 | 4 | 3 |
| cGVHD | 2 | 0 | 0 |
| Infection | 1a | 7a | 6 |
| Other | 2 | 4 | 4 |
Other: no ATG—lung cancer; pulmonary edema. ATG—post-transplant lymphoproliferative disorder/pulmonary edema; alveolar hemorrhage2; thrombotic thrombocytopenic purpura.
Infection: no ATG—vancomycin-resistant Enterococcal (VRE) sepsis. ATG—VRE sepsis; disseminated toxoplasmosis (brain, heart lung); pulmonary Rhizopus and Zygomycetes; Mycobacterium avium bacteremia; Influenza A: pulmonary Aspergillosis: Pseudomonas aeruginosa encephalitis wound infection.
Relapse
There was no significant difference between the relapse rates in the no ATG group (9/48, 19%) and the ATG group (11/50, 22%; HR 0.70, 95% CI: 0.29, 1.68; P = 0.41). The cumulative incidence for relapse, accounting for the competing risk of non-relapse mortality, was similar between patient groups (Figure 2). In addition, this was the case regardless of age, diagnosis and the conditioning regimen used (Table 2); however, patients in the no ATG cohort (MRD) receiving PBSC had a lower relapse rate than patients in the ATG cohort (URD) undergoing PBSC transplant (HR = 0.51, 95% CI: 0.27, 0.96). There was, however, no difference in the BM and PBSC recipients in the ATG cohort (URD; HR = 0.51, 95% CI 0.23, 1.13).
Figure 2.
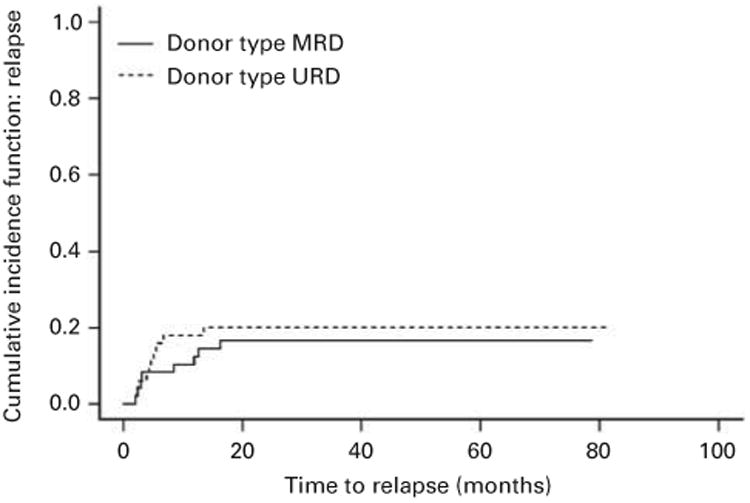
Cumulative incidence curves depicting relapse in patients conditioned with or without ATG (P = 0.64).
GVHD
There was no significant difference between the proportions of patients who developed acute or chronic GVHD occurrence in the no ATG group (28/48, 58%) and those in the ATG group (20/50, 40%; HR = 1.31, 95% CI: 0.74, 2.33, P-value = 0.36), and the cumulative incidences (P-value = 0.14) of GVHD, accounting for the competing risk of mortality without GVHD. This was true for all the patient subgroups tested (Table 2). Notably, the cumulative risk of GVHD was the same in BM and PBSC recipients in the ATG cohort (URD; HR = 0.56, 95% CI 0.28, 1.09).
Grade II-IV acute GVHD (classical and delayed onset) developed in 14 patients in the no ATG group vs 14 in the ATG group (OR 1.06, 95% CI: 0.44, 2.54; P = 0.90). Grade III-IV acute GVHD developed in seven patients in the no ATG group vs three in the ATG group (OR 2.67; 95% CI: 0.65, 11.02; P-value = 0.16). There was no difference in the cumulative incidence of acute GVHD between the MRD and URD recipients after accounting for non-GVHD mortality (Figure 3a). However, chronic GVHD was diagnosed less frequently in the ATG group vs no ATG group (5 vs 20 patients; OR 0.16, 95% CI: 0.05, 0.46; P-value = 0.003) in patients undergoing MUD SCT. MRD patients thus had a higher cumulative incidence of chronic GVHD than did URD patients after accounting for patient mortality without developing GVHD (Figure 3b).
Figure 3.
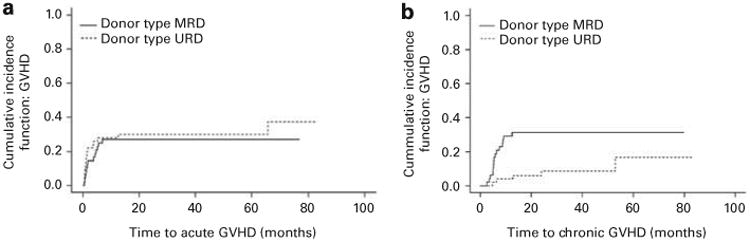
(a) Cumulative incidence curves depicting cumulative acute GVHD in patients conditioned with or without ATG (P = 0.57) after adjusting for patient mortality without GVHD. (b) Cumulative incidence curves depicting cumulative chronic GVHD in patients conditioned with or without ATG (P = 0.007) after adjusting for patient mortality without GVHD.
ATG dose effect
A subset analysis of the URD SCT recipients, exploring survival differences between the two ATG dose groups, yielded a HR of 3.23 (95% CI: 1.34, 7.77; P-value = 0.009) consistent with the lower survival rate observed in patients who received a higher dose of ATG (Figure 4). Correspondingly, there was a significantly higher (P-value = 0.005) mortality rate in the 10 mg/kg ATG dose recipients (18/26, 69%) than in the 7.5 mg/kg dose recipients (7/17, 29%), with an OR of 5.46 (95% CI: 1.63, 18.36; Table 3). This large difference stemmed from a high day-100 mortality mostly attributable to non-relapse and non-GVHD mortality in the high-dose ATG group (10/26, 39%) than in the low-dose group (1/24, 4%; OR 14.38, 95% CI: 1.67, 23.70, P-value = 0.003; Table 3).
Figure 4.
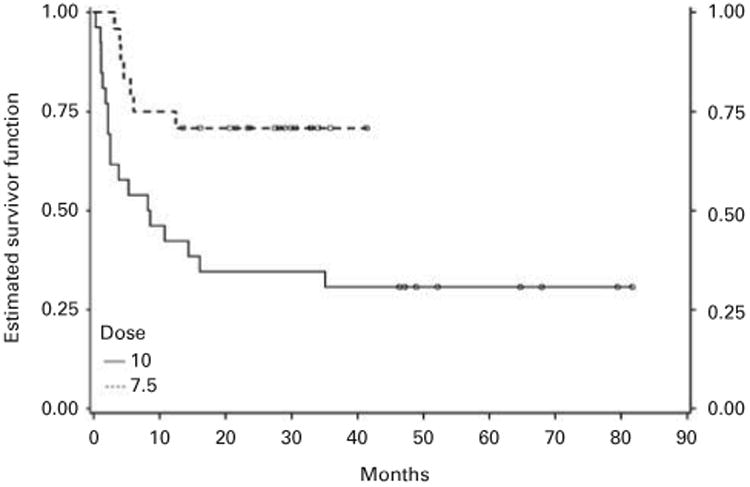
K-M survival curves depicting OS in patients conditioned with different ATG doses (P = 0.006).
Discussion
URD SCT is generally considered a high-risk intervention, and as such, is not widely established as a therapeutic modality in patients with intermediate-risk malignancies who have no HLA identical donors in the family despite the improved outcomes in recipients of MRD transplantation in recent years. This line of reasoning places this particular group of patients at risk for relapse and poor outcomes with salvage therapy and allografting beyond first remission. To address the comparability of HLA-MRDs with URDs, we examined post-transplant outcomes in cohorts of simultaneously transplanted acute leukemia and myelodysplastic syndrome patients, where URD SCT recipients received ATG as component of GVHD prophylaxis. An overall beneficial effect of ATG use was observed resulting in equivalent outcomes in MRD and URD SCT recipients.
GVHD is a manifestation of T-cell allo-reactivity directed at mismatched minor histocompatibility Ags (mHA), such as CD31, HA-1 and HA-2, disparity in which predicts higher risk of GVHD.14,15,29–31 There is a greater likelihood of mHA disparity in URD SCT recipients because of greater probability of genomic differences such as single nucleotide polymorphisms, microdeletions and copy number variations in the exons of the involved genes. Because of the HLA specificity of mHA presentation and the heterogeneity in HLA across various populations, a picture of considerable heterogeneity in outcomes has emerged in URD transplant recipients.30,32 This heterogeneity also affects the risk of GVHD observed in URD-recipient pairs matched for HLA, though this risk is lower than that recorded with one or more HLA mismatches.33,34 Whereas recipients of stringently matched URD SCT have been reported to have outcomes similar to MRD allografts in specific populations,35 other studies evaluating disparity at the MHC locus have shown that mismatching for loci such as HLA-DPB1 increases the odds for developing GVHD or reducing relapse, as does receiving a SCT from an MHC haplotype mismatched URD who is otherwise matched at allele level for the HLA-A, B, C and DRB1 loci.12,36,37 A large recent Japanese study examined highly conserved areas—termed conserved extended haplotypes—in the MHC region. It was found that patients transplanted using donors matched at all the HLA loci, but with a different haplotype from theirs have a higher rate of GVHD.13 These observations point towards a need for more intensive GVHD prophylaxis in recipients of URD SCT.
In vivo T-cell depletion with ATG has been employed for GVHD prophylaxis and other investigators have reported outcomes in both URD and MRD SCT recipients. Studies comparing cohorts of patients receiving ATG with those conditioned without ATG are summarized in Table 4.5,38–46 These data, demonstrate that, in general, patients receiving ATG, in lower doses, have similar and often better clinical outcomes when compared with those not receiving it or receiving a high dose of it, with equivalent relapse and less GVHD. Further, there have been two prospective randomized trials that have examined this question, with both assigning URD SCT recipients to standard GVHD prophylaxis with or without ATG. These studies demonstrated a reduction in the rates of both severe acute GVHD (HR = 0.5, P = 0.054) and chronic GVHD (HR 0.2, P < 0.0001) in one,47 and chronic GVHD in the other (15 vs 41% P = 0.01).38 The relapse rate as well as OS was similar in these patients regardless of ATG administration. Two other trials reported lower rates of GVHD—one acute (RR 0.3, P = 0.003),39 and one chronic (P = 0.002),40—in recipients of MRD, with an improvement in survival when ATG was used in conditioning. Similar benefit was observed in terms of chronic GVHD risk reduction in both MRD vs URD comparison, where ATG was used to condition URD SCT recipients.41 A large retrospective study, examined outcomes in URD SCT recipients conditioned with myeloablative regimens, and also demonstrated a significant reduction in chronic GVHD (4 vs 32% in the ATG vs no ATG groups: P = 0.001) with no difference in relapse, and OS when ATG was used.48
Table 4. A compilation of studies comparing outcomes between groups of patient receiving ATG versus those not receiving ATG.
| Ref. | Randomization | Dose and type of ATG | Survival | Relapse | aGVHD | cGVHD |
|---|---|---|---|---|---|---|
| Finke et al.5 MC | Y URD (202) ATG v no ATG | ATG-F 20 mg/kg | 2 Year HR 0.8 (0.5, 1.3) P = 0.47 | 2 Year HR 1.2 (0.7–2.0) P = 0.55 | III-IV HR 0.5 (0.2,1.0) P = 0.054 | Ext HR 0.2 (0.1,0.4) P < 0.0001 |
| Remberger et al.37 SC | N URD v MRD (182) ATG v no ATG | ATG - R 6 mg/kg | 5 Year 60% v 60% P = NS | 23% v 26% | III-IV 8% v 13% P = NS | Ext 24% v 51% P = <0.0001 |
| Bredeson et al.35 MC | N MRD (335) ATG v no ATG | ATG – R 4.5 mg/kg | RR = 0.6 (0.4, 0.9) P = .03 | RR = 1.9 (1.1, 3.2) P = 0.01 | II-IV RR = 0.3 (0.2,0.6) P = .0003 | Ext R = 1.3 (0.9,2.2) P = .26 |
| Russell et al.36 SC | N MRD (108) ATG v no ATG | ATG - R 4.5 mg/kg | 5 Year 66% v 50% P = 0.046 | 4 Year 43% v 22% P = 0.053 | III-IV 6% v 13% P = NS | 2 Year 55% v 96% P = 0.002 |
| Deeg et al.43 SC | N MRD/URD (113) ATG v no ATGa | ATG – R 4.5–6.0 mg/kg, | 56% v 56% | 21% v 19% | II-IV 50% v 64% | Ext 34% v 47% |
| Bacigalupo et al.34 MC | Y URD (75) ATG v no ATG | ATG - R 7.5–15 mg/kg | 6 Year 44% v 31% P = 0.8 | 19% v 18% P = 0.5 | – | Ext 15% v 41% P = 0.01 |
| Duggan et al.45 SC | N URD v MRD (114) ATG v no ATG | ATG - R 4.5–6 mg/kg | 68% v 59% 14% v 21%b | 3 Year 45% v 42% | III-IV 10% v 18% | 3 Year 44% v 51% |
| Current study SC | N URD v MRD (98) ATG v no ATG | ATG -R 10–7.5 mg/kg | 2 Year HR 0.6 (0.3–1.1) P = 0.14 | 2 Year HR 0.7 (0.3–1.7) P = 0.41 | II-IV OR 1.0 (0.4–2.5) P = 0.9 | Ext OR 0.1 (0.0–0.4) P = 0.007 |
| Bacigalupo et al.46 MC | Y URD (54/55) ATG lo/hi v no ATG | ATG - R 7.5 or 15 mg/kg | 1 Year 56% v 55% P = 0.8 43% v 43% P = 0.8 | 1 Year 12% v 10% P = 0.6 36% v 18% P = 0.8 | III-IV 41% v 36% P = 0.8 11% v 50% P = 0.00 | Ext 38% v 65% P = 0.08 41% v 59% P = 0.3 |
| Hamadani et al.44 | N URD (72) ATG hi v ATG lo | ATG-R 7.5 vs 6 mg/kg | 1 Year 64% v 84% P = 0.07 | 2 Year 25% v 31% | III-IV 23% v 11% P = 0.2 | Ext44% v 61% P = 0.09 |
| SC Ayuk et al.42 MC | N URD (83) ATG hi v ATG lo | ATG- F 30 mg/kg vs 60 mg/kg | 3 Year 56% v 72% P = 0.1 | 3 Year 16 v 15 P = 0.8 | III-IV 20% v 27% P = 0.6 | 40 v 59 P = .1 |
Abbreviations: ATG preparation: F = Fresenius; MC = multicenter trials; N = either case–control or matched pair analysis; (total number of patients reported); R = thymoglobulin; SC = single center trials; Y = randomized trial.
One of two arms included in the current table (advanced MDS).
Low-risk and high-risk disease.
What is paradoxical about these observations and the outcomes recorded in our study is the similarity of relapse rate despite lower rates of chronic GVHD, even though in recipients of T-cell replete allografts, freedom from relapse is proportional to incidence of chronic GVHD, with either myeloablative or reduced intensity-conditioning regimens.49,50 This suggests that the ATG effect observed on post-transplant outcomes derives from more than simple clonal T-cell depletion. A peripheral tolerance induction mechanism may be invoked in older recipients where the likelihood of central, thymic, tolerance developing is low. One may speculate that adoptively transferred donor T cells are rendered tolerant to ubiquitously present recipient allo-Ags disparate from the donor by peripheral tolerance induction mechanism such as co-stimulation blockade by ATG and emergence of regulatory T-cell populations.20,21,51 Chronic GVHD risk thus ameliorated; GVL is preserved by the scarcity of tumor-specific Ags at the time of SCT, which prevents induction of peripheral tolerance to tumor Ags by ATG. Eventual withdrawal of immunosuppression restores neo-Ag reactivity and in the event of leukemia progression allows GVL to develop and eradicate the malignant clone. This approach to preserving leukemia specific allo-reactivity in recipients of transplants conditioned with myeloablative regimens, based on cellular proliferation kinetics and ATG half-life, reemphasizes the role of adequate pretransplant cytoreduction.
From Table 4 it may be inferred that ATG has an overall salutary effect on SCT outcomes by reducing the risk of TRM in URD SCT recipients, preventing that variable from offsetting the benefit seen secondary to GVL effect. One may also speculate that there is relative equivalence in immune reconstitution following SCT with or without in vivo T-cell depletion with ATG. This may be the result of a lower rate of severe acute and chronic GVHD and its attendant immunosuppression, thus ATG may paradoxically help preserve GVL effect or conversely allow enough patients to survive the immediate post-transplant aftermath to develop GVL responses.
An informative consistency between the various findings depicted in Table 4 is the diminishing toxicity of ATG with lower doses, accompanied by maintenance of the GVL effect reflected by comparable relapse rates. In addition to the studies depicted, another large study examined post-transplant outcomes with thymoglobulin given at 4, 6, 8 or 10 mg/kg, and in this instance demonstrated a significantly reduced TRM (HR 0.35, P = 0.03) and better survival (HR 0.45, P = 0.027) in patients receiving the intermediate, 6 and 8 mg/kg thymoglobulin doses, as compared to patients receiving 4 and 10 mg/kg thymoglobulin, with these two groups experiencing higher rates of GVHD and infections, respectively.52 Analogous to this, we observed a high rate of infections in patients receiving 10 mg/kg of ATG and this complication has diminished considerably as reducing the dose to 7.5 mg/kg. Regardless, an increased risk of opportunistic infections, such as EBV reactivation, has been reported with the use of ATG.53 Although in this study with aggressive monitoring and preemptive therapy, the risk of development of post-transplant lympho-proliferative disease was low; lower ATG doses will likely result in fewer opportunistic infections. On the other hand, there does appear to be a threshold effect to the benefit derived from ATG, as demonstrated in a prospective trial reporting a significantly higher rate of both acute and chronic GVHD in the recipients of 2.5 mg/kg of rabbit ATG as compared with 7.5–10 mg/kg, the latter on the other hand sustained a higher rate of relapse and opportunistic infection.54 These findings clearly point to a dose response relationship between ATG and post-transplant immune reconstitution following SCT, favoring an intermediate dose range.
Within the constraints of a retrospective study, we demonstrate that the addition of ATG to the conditioning regimen of patients undergoing URD SCT reduces the risk of developing severe chronic GVHD, while preserving GVL responses, and results in OS rates equivalent to MRD SCT recipients. Our findings should be treated as a preliminary guide to designing future trials on the use of ATG in stringently HLA matched URD transplantation in first line therapy of patients with intermediate risk disease and reduce the risk of poor outcomes seen with recurrence in these individuals.
Acknowledgments
We acknowledge Ms Cheryl Jacocks-Terrell for her help in preparing the manuscript. We also acknowledge the invaluable contribution of the bone transplant program physician extenders, Mr Richard Edwards PA-C, Mr John Lamberta, Mrs Shahrzad Rabie and Mrs Veronica Buckovich, BMT coordinators Mrs Judith Davis, Angela Buskey, Dana Broadway and Katherine Candler and BMT nurses towards the care of the patients included in this study.
Footnotes
Conflict of Interest: Dr Toor, Dr McCarty and Dr Manjili have received research support from Genzyme incorporated.
References
- 1.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Witte T, Hagemeijer A, Suciu S, Belhabri A, Delforge M, Kobbe G, et al. Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European Intergroup Trial. Haematologica. 2010;95:1754–1761. doi: 10.3324/haematol.2009.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basara N, Schulze A, Wedding U, Mohren M, Gerhardt A, Junghanss C, et al. Early related or unrelated haematopoietic cell transplantation results in higher overall survival and leukaemia-free survival compared with conventional chemotherapy in high-risk acute myeloid leukaemia patients in first complete remission. Leukemia. 2009;23:635–640. doi: 10.1038/leu.2008.352. [DOI] [PubMed] [Google Scholar]
- 4.Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 6.Weisdorf DJ, Anasetti C, Antin JH, Kernan NA, Kollman C, Snyder D, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 7.Arora M, Weisdorf DJ, Spellman SR, Haagenson MD, Klein JP, Hurley CK, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27:1644–1652. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valcarcel D, Sierra J, Wang T, Kan F, Gupta V, Hale GA, et al. One-antigen mismatched related versus HLA-matched unrelated donor hematopoietic stem cell transplantation in adults with acute leukemia: Center for International Blood and Marrow Transplant Research results in the era of molecular HLA typing. Biol Blood Marrow Transplant. 2011;17:640–648. doi: 10.1016/j.bbmt.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringde´n O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisdorf DJ, Nelson G, Lee SJ, Haagenson M, Spellman S, Antin JH, et al. Chronic Leukemia Working Committee. Sibling versus unrelated donor allogeneic hematopoietic cell transplantation for chronic myelogenous leukemia: refined HLA matching reveals more graft-versus-host disease but not less relapse. Biol Blood Marrow Transplant. 2009;15:1475–1478. doi: 10.1016/j.bbmt.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw PJ, Kan F, Woo Ahn K, Spellman SR, Aljurf M, et al. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood. 2010;116:4007–4015. doi: 10.1182/blood-2010-01-261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersdorf EW, Malkki M, Gooley TA, Martin PJ, Guo Z. MHC haplotype matching for unrelated hematopoietic cell transplantation. PLoS Med. 2007;4:e8. doi: 10.1371/journal.pmed.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morishima S, Ogawa S, Matsubara A, Kawase T, Nannya Y, Kashiwase K, et al. Impact of highly conserved HLA haplotype on acute graft-versus-host disease. Blood. 2010;115:4664–4670. doi: 10.1182/blood-2009-10-251157. [DOI] [PubMed] [Google Scholar]
- 14.Kamei M, Nannya Y, Torikai H, Kawase T, Taura K, Inamoto Y, et al. HapMap scanning of novel human minor histocompatibility antigens. Blood. 2009;113:5041–5048. doi: 10.1182/blood-2008-07-171678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern M, Brand R, de Witte T, Sureda A, Rocha V, Passweg J, et al. Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am J Transplant. 2008;8:2149–2157. doi: 10.1111/j.1600-6143.2008.02374.x. [DOI] [PubMed] [Google Scholar]
- 16.Schlenk RF, Dohner K, Mack S, Stoppel M, Kiraly F, Gotze K, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010;28:4642–4648. doi: 10.1200/JCO.2010.28.6856. [DOI] [PubMed] [Google Scholar]
- 17.Gaber AO, Knight RJ, Patel S, Gaber LW. A review of the evidence for use of thymoglobulin induction in renal transplantation. Transplant Proc. 2010;42:1395–1400. doi: 10.1016/j.transproceed.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Regan JF, Lyonnais C, Campbell K, Smith LV, Buelow R US Thymoglobulin Multi-Center Study Group. Total and active thymoglobulin levels: effects of dose and sensitization on serum concentrations. Transpl Immunol. 2001;9:29–36. doi: 10.1016/s0966-3274(01)00048-x. [DOI] [PubMed] [Google Scholar]
- 19.Waller EK, Langston AA, Lonial S, Cherry J, Somani J, Allen AJ, et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2003;9:460–471. doi: 10.1016/s1083-8791(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 20.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 21.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 22.Toor A, Rodriguez T, Bauml M, Mathews H, Shanti S, Senitzer D, et al. Feasibility of conditioning with thymoglobulin and reduced intensity TBI to reduce acute GVHD in recipients of allogeneic SCT. Bone Marrow Transplant. 2008;42:723–731. doi: 10.1038/bmt.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dey BR, McAfee S, Colby C, Sackstein R, Saidman S, Tarbell N, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–329. doi: 10.1016/s1083-8791(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 24.van Burik JA, Carter SL, Freifeld AG, High KP, Godder KT, Papanicolaou GA, et al. Higher risk of cytomegalovirus and Aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 25.Wagner JE, Thompson JS, Carter SL, Kernan NA. Unrelated donor marrow transplantation trial. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell depletion trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 26.Boeckh M, Nichols W, Papanicolaou G, Rubin R, Wingard J, Zaia Y. Cytomegalo-virus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 27.Klein JP, Moeschberger ML. Survival Analysis: Techniques of Censored and Truncated Data. 2nd. Springer-Verlag; New York: 2003. [Google Scholar]
- 28.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 29.Goulmy E. Minor histocompatibility antigens: Allo target molecules for tumor-specific immunotherapy. Cancer J. 2004;10:1–7. doi: 10.1097/00130404-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 30.DeLuca DS, Eiz-Vesper B, Ladas N, Khattab BA, Blasczyk R. High-throughput minor histocompatibility antigen prediction. Bioinformatics. 2009;25:2411–2417. doi: 10.1093/bioinformatics/btp404. [DOI] [PubMed] [Google Scholar]
- 31.Matte-Martone C, Liu J, Jain D, McNiff J, Shlomchik WD. CD8+ but not CD4+ T cells require cognate interactions with target tissues to mediate GVHD across only minor H antigens, whereas both CD4+ and CD8+ T cells require direct leukemic contact to mediate GVL. Blood. 2008;111:3884–3892. doi: 10.1182/blood-2007-11-125294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spierings E, Hendriks M, Absi L, Canossi A, Chhaya S, Crowley J, et al. Phenotype frequencies of autosomal minor histocompatibility antigens display significant differences among populations. PLoS Genet. 2007;3:e103. doi: 10.1371/journal.pgen.0030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 34.Kawase T, Morishima Y, Matsuo K, Kashiwase K, Inoko H, Saji H, et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood. 2007;110:2235–2241. doi: 10.1182/blood-2007-02-072405. [DOI] [PubMed] [Google Scholar]
- 35.Ibrahim Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen– identical siblings versus human leukocyte antigen–allelic–matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French society of bone marrow transplantation and cell therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 36.Kawase T, Matsuo K, Kashiwase K, Inoko H, Saji H, Ogawa S, et al. HLA mismatch combinations associated with decreased risk of relapse: implications for the molecular mechanism. Blood. 2009;113:2851–2858. doi: 10.1182/blood-2008-08-171934. [DOI] [PubMed] [Google Scholar]
- 37.Shaw BE, Gooley TA, Malkki M, Madrigal JA, Begovich AB, Horowitz MM, et al. The importance of HLA-DPB1 in unrelated donor hematopoietic cell transplantation. Blood. 2007;110:4560–4566. doi: 10.1182/blood-2007-06-095265. [DOI] [PubMed] [Google Scholar]
- 38.Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Bredeson CN, Zhang MJ, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell JA, Turner AR, Larratt L, Chaudhry A, Morris D, Brown C, et al. Adult recipients of matched related donor blood cell transplants given myeloablative regimens including pretransplant antithymocyte globulin have lower mortality related to graft-versus-host disease: a matched pair analysis. Biol Blood Marrow Transplant. 2007;13:299–306. doi: 10.1016/j.bbmt.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Remberger M, Mattsson J, Hausenberger D, Schaffer M, Svahn BM, Ringden O. Genomic tissue typing and optimal antithymocyte globuline dose using unrelated donors results in similar survival and relapse as HLA-identical siblings in haematopoietic stem-cell transplantation for leukaemia. Eur J Haematol. 2008;80:419–428. doi: 10.1111/j.1600-0609.2008.01047.x. [DOI] [PubMed] [Google Scholar]
- 42.Ayuk F, Diyachenko G, Zabelina T, Wolschke C, Fehse B, Bacher U, et al. Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:913–919. doi: 10.1016/j.bbmt.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Deeg H, Storer B, Boeckh M, Martin P, McCune J, Myerson D, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant. 2006;12:573–584. doi: 10.1016/j.bbmt.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 44.Hamadani M, Blum W, Phillips G, Elder P, Andritsos L, Hofmeister C, et al. Improved nonrelapse mortality and infection rate with lower dose of antithymocyte globulin in patients undergoing reduced-intensity conditioning allogeneic transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2009;15:1422–1430. doi: 10.1016/j.bbmt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duggan P, Booth K, Chaudhry A, Stewart D, Ruether J, Gluck S, et al. Unrelated donor BMT recipients given pretransplant low-dose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant. 2002;30:681–686. doi: 10.1038/sj.bmt.1703674. [DOI] [PubMed] [Google Scholar]
- 46.Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 47.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial RID F-8101-2010. Lancet Oncol. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 48.Mohty M, Labopin M, Balére ML, Socié G, Milpied N, Tabrizi R, et al. Antithymocyte globulins and chronic graft-vs-host disease after myeloablative allogeneic stem cell transplantation from HLA-matched unrelated donors: a report from the Sociéte Franc¸aise de Greffe de Moelle et de Thérapie Cellulaire. Leukemia. 2010;24:1867–1874. doi: 10.1038/leu.2010.200. [DOI] [PubMed] [Google Scholar]
- 49.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 50.Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 51.Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+ CD25+ Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17:2844–2853. doi: 10.1681/ASN.2006050422. [DOI] [PubMed] [Google Scholar]
- 52.Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78:122–127. [PubMed] [Google Scholar]
- 53.Peric Z, Cahu X, Chevallier P, Brissot E, Malard F, Guillaume T, et al. Features of Epstein-Barr Virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia. 2011;25:932–938. doi: 10.1038/leu.2011.26. [DOI] [PubMed] [Google Scholar]
- 54.Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102:470–476. doi: 10.1182/blood-2002-12-3629. [DOI] [PubMed] [Google Scholar]


