Abstract
Background
Limited data are available regarding the long-term clinical outcomes of second-generation drug-eluting stents (DES) versus first-generation DES in patients with coronary chronic total occlusion (CTO) who undergo percutaneous coronary intervention (PCI). The aim of this study was to compare the clinical outcomes of second-generation DES with those of first-generation DES for the treatment of CTO.
Methods and Results
Between March 2003 and February 2012, 1,006 consecutive patients with CTO who underwent successful PCI using either first-generation DES (n = 557) or second-generation DES (n = 449) were enrolled in a multicenter, observational registry. Propensity-score matching was also performed. The primary outcome was cardiac death over a 2-year follow-up period. No significant differences were observed between the two groups regarding the incidence of cardiac death (first-generation DES versus second-generation DES; 2.5% vs 2.0%; hazard ratio [HR]: 0.86; 95% confidence interval [CI]: 0.37 to 1.98; p = 0.72) or major adverse cardiac events (MACE, 11.8% vs 11.4%; HR: 1.00; 95% CI: 0.67 to 1.50; p = 0.99). After propensity score matching, the incidences of cardiac death (HR: 0.86; 95% CI: 0.35 to 2.06; p = 0.86) and MACE (HR: 0.93; 95% CI: 0.63 to 1.37; p = 0.71) were still similar in both groups. Furthermore, no significant differences were observed between sirolimus-eluting, paclitaxel-eluting, zotarolimus-eluting, and everolimus-eluting stents regarding the incidence of cardiac death or MACE.
Conclusion
This study shows that the efficacy of second-generation DES is comparable to that of first-generation DES for treatment of CTO over 2 years of follow-up.
Introduction
Percutaneous coronary intervention (PCI) of chronic total occlusion (CTO) lesions is a challenging procedure due to the difficulty in crossing the CTO and the high restenosis rates after PCI [1–4]. However, the success rate of treating CTO lesions has improved as cardiologists have gained experience in this technique and advances have been made in PCI technology. For instance, better outcomes of PCI of CTO lesions have been achieved with bare-metal stenting (BMS) compared with balloon angioplasty alone [1, 5, 6].
Drug-eluting stents (DES) were developed for enhanced stent durability compared with BMS by inhibiting in-stent neointimal hyperplasia. Sirolimus-eluting and paclitaxel-eluting stents (SES and PES), hereafter referred to as first-generation DES, are superior to BMS with respect to the in-stent restenosis rate and target lesion revascularization after CTO PCI [7–10]. However, everolimus-eluting and zotarolimus-eluting stents (EES and ZES), hereafter referred to as second-generation DES, have been found to be superior or comparable to first-generation DES for composite outcomes in non-CTO lesions [11–15]. In the context of CTO, a few studies have compared the impacts of second-generation DES on clinical outcomes with those of first-generation DES. However, these studies had relatively small sample sizes, short follow-up periods, and yielded contradictory results [16–19]. We therefore compared the long term outcomes of patients with CTO lesions who received second-generation DES with those of patients who received first-generation DES.
Methods
Study population
This study was conducted from prospective registries at two tertiary medical centers, Samsung Medical Center and Bucheon Sejong Hospital, in South Korea. Between March 2003 and February 2012, 2,659 consecutive patients were enrolled. The inclusion criteria for the registries were: 1) at least 1 CTO detected on a diagnostic coronary angiograph; and 2) symptomatic angina and/or a positive functional ischemia study. Exclusion criteria included: 1) previous coronary bypass grafting; 2) history of cardiogenic shock or cardiopulmonary resuscitation; and 3) ST-segment elevation acute myocardial infarction (MI) during the preceding 48 hours. A CTO lesion was defined as the obstruction of a native coronary artery with a Thrombolysis In Myocardial Infarction (TIMI) flow grade 0 and an estimated duration longer than 3 months (4). Duration was estimated based on the interval from the last episode of acute coronary syndrome (ACS). For patients with no history of ACS, duration was estimated from the first episode of exertional angina consistent with the location of the occlusion or previous coronary angiogram [18, 20, 21].
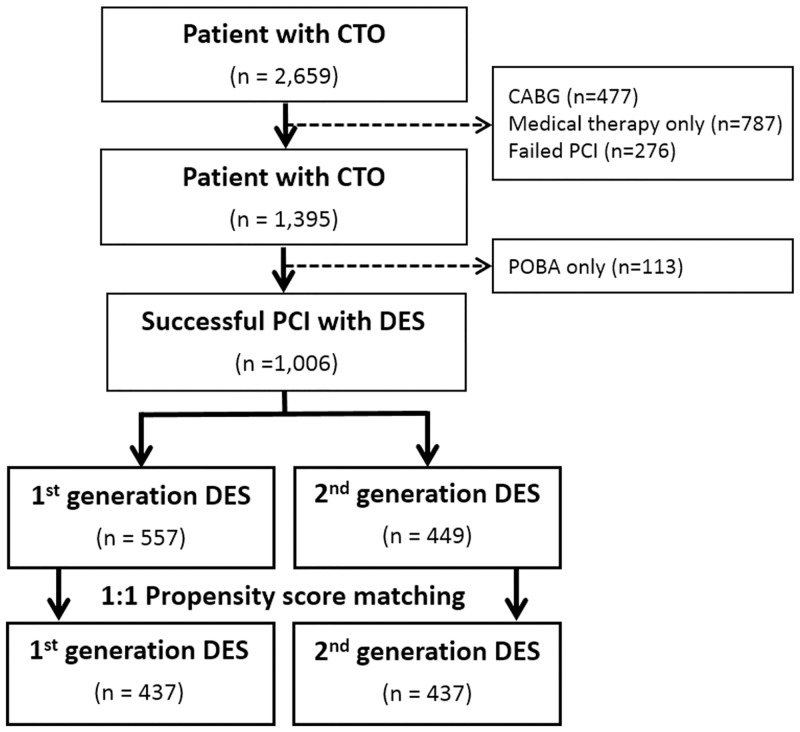
Of the 2,659 patients included in the registry, 477 patients who underwent CABG and 787 patient who treated with medical therapy only were excluded. Of the patients who performed PCI, 1,196 patients (80.2%) underwent successful revascularization. Among them, 1,006 patients who underwent PCI with DES implantation and achieved angiographic success were finally included in this analysis (Fig 1).
Fig 1. Profile of patient enrollment.
CTO = chronic total occlusion, DES = drug-eluting stents, PCI = percutaneous coronary intervention.
Data collection and follow-up
Experienced clinical research physicians and coordinators from an individual clinical research organization collected baseline clinical, angiographic and procedural characteristics from hospital charts or hospital databases according to prespecified definitions. Clinical follow-up of the registry after index coronary angiography was performed at 1, 3, 6, and 12 months, and every year thereafter. Collection of follow-up information was mainly conducted through review of inpatient and outpatient hospital charts by the clinical research coordinators, and additional follow-up information was collected through a telephone interview with patients and was confirmed with the Korean national database using a citizen registration number unique to each individual.
All baseline and procedural cine coronary angiograms were reviewed and quantitatively analyzed at the angiographic core laboratory (Cardiac and Vascular Center, Samsung Medical Center, Seoul, Korea) with an automated edge-detection system (Centricity CA 1000, GE, Waukesha, WI, USA) using standard definitions [22]. The extent of collateral flow was assessed according to the validated Rentrop classification scale [23].
Percutaneous coronary intervention
Coronary interventions were performed according to a standard technique. All procedures and treatments, including periprocedural and postprocedural medication regimens, were performed according to current practice guidelines. All patients, regardless of stent type, received a 300 mg loading dose of aspirin and a 300 to 600 mg loading dose of clopidogrel before the coronary intervention, unless they had previously received these antiplatelet medications. DES were used without restriction; the duration of dual antiplatelet therapy and the use of glycoprotein IIb/IIIa antagonists was left to the discretion of the operator.
Study outcomes and definitions
The primary outcome was cardiac death over a 2-year follow-up period. The secondary outcomes were all-cause death, MI, repeat revascularization, and major adverse cardiac events (MACEs). Repeat revascularization included target vessel revascularization (TVR) and non-TVR treated with PCI or CABG. MACEs included cardiac death, MI, and repeat revascularization. All deaths were considered to be of cardiac origin unless a definite noncardiac cause could be established. MI was defined as an elevation of the creatine kinase-MB fraction or the troponin-T/troponin-I level greater than the upper normal limit with concomitant ischemic symptoms or electrocardiographic findings indicative of ischemia [24]. Periprocedural MI was defined as an elevation of the creatine kinase-MB fraction ≥ 3 times the upper limit of normal after the index procedure [25]. Periprocedural MI was not included in this definition of MI.
Statistical analysis
Continuous variables are expressed as means ± SDs, and categorical variables are presented as absolute numbers and proportions (%). Overall comparisons between groups were performed by Student’s t-test for continuous variables and by the chi-square test or Fisher’s exact test when the Cochran rule was not met for categorical variables. The Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for clinical outcomes between two groups. Cumulative event rates were estimated by the Kaplan-Meier method, and treatment effects were assessed using stratified log-rank statistics. We also adjusted for differences in clinical and angiographic characteristics by performing propensity score matching. The “psmatching” custom dialogue was used in conjunction with SPSS version 21 (IBM, Armonk, NY, USA). The psmatching program performs all analyses in R (R foundation for Statistical Computing, Vienna, Austria) though the SPSS R-Plugin (version 2.14.2). Using the propensity score matching method, we created 557 matched pairs of patients. The adequacy of propensity matching was calculated by the overall balance test (chi-square = 4.79, df = 22.00, and p = 0.98). All tests were 2-tailed, and p values < 0.05 were considered to indicate statistical significance.
Ethical approval
The design of the study was approved by Institutional Review Board at Samsung Medical Center and Bucheon Sejong Hospital. The institutional review boards approved this study and waived the requirement for informed consent. All patients’ records/information were anonymized and de-identified prior to analysis.
Results
Patient characteristics
Of the 2,659 patients included in the registry, 1,006 patients received only first-generation (n = 557, first-generation group) or second-generation DES (n = 449, second-generation group). In the first-generation group, 59.4% of the recipients received SES (n = 331, SES group), whereas 40.6% of the recipients received PES (n = 226, PES group). In the second-generation group, 62.4% of the recipients received EES (n = 280, SES group) and 36.6% of the recipients received ZES (n = 168, ZES group).
The patients in the first-generation group were more likely to be male and less likely to have had a history of dyslipidemia or ACS compared with the patients in the second-generation group (Table 1). As shown in Table 2, patients in the first-generation group also had a significantly higher prevalence of abrupt stumps and longer total stent lengths, but lower prevalences of Rentrop grade 3 collateral flow and proximal to mid CTO lesions compared with patients in the second-generation group. After performing propensity score matching for the entire population, all 437 patients in the first-generation group were matched with patients in the second-generation group. No significant differences were observed between the first-generation DES and second-generation DES groups regarding baseline clinical, angiographic, or procedural characteristics (Tables 1 and 2).
Table 1. Baseline characteristics.
| Variables | Total population | Propensity-Matched population | |||||
|---|---|---|---|---|---|---|---|
| Total (n = 1,006) | 1st generation (n = 557) | 2nd generation (n = 449) | P value | 1st generation (n = 437) | 2nd generation (n = 437) | P value | |
| Age (yrs) | 62.5 (± 11.2) | 62.3 (± 10.9) | 62.8 (± 11.6) | 0.48 | 62.4 (± 11.0) | 62.7 (± 11.7) | 0.61 |
| Male | 784 (77.9%) | 448 (80.4%) | 336 (74.8%) | 0.03 | 339 (77.6%) | 331 (75.7%) | 0.52 |
| Diabetes | 408 (40.6%) | 231 (41.5%) | 177 (39.4%) | 0.51 | 181 (41.4%) | 173 (39.6%) | 0.58 |
| Hypertension | 626 (62.2%) | 338 (60.7%) | 288 (64.1%) | 0.26 | 281 (64.3%) | 283 (64.8%) | 0.89 |
| Dyslipidemia | 452 (44.9%) | 231 (41.5%) | 221 (49.2%) | 0.01 | 197 (45.1%) | 212 (48.5%) | 0.31 |
| Current smoker | 313 (31.1%) | 171 (30.7%) | 142 (31.6%) | 0.75 | 138 (31.6%) | 138 (31.6%) | 1.00 |
| Chronic kidney disease | 59 (5.9%) | 28 (5.0%) | 31 (6.9%) | 0.21 | 25 (5.7%) | 30 (6.9%) | 0.49 |
| ACS | 299 (29.7%) | 150 (26.9%) | 149 (33.2%) | 0.03 | 130 (29.7%) | 139 (31.8%) | 0.51 |
| CVA | 32 (7.9%) | 7 (9.0%) | 25 (7.7%) | 0.70 | 30 (6.9%) | 26 (5.9%) | 0.58 |
| Previous PCI | 210 (20.9%) | 119 (21.4%) | 91 (20.3%) | 0.67 | 93 (21.3%) | 91 (20.8%) | 0.87 |
| LVEF (%) | 57.5 (± 12.2) | 57.5 (± 11.9) | 57.4 (± 12.4) | 0.862 | 57.9 (± 11.7) | 57.6 (± 12.4) | 0.65 |
| Discharge medication | |||||||
| Statins | 773 (76.8%) | 421 (75.6%) | 352 (78.4%) | 0.29 | 335 (76.7%) | 344 (78.7%) | 0.47 |
| Beta-blocker | 599 (59.5%) | 345 (61.9%) | 254 (56.6%) | 0.09 | 262 (60.0%) | 248 (56.8%) | 0.34 |
| ACE inhibitors or ARB | 608 (60.4%) | 340 (61.0%) | 268 (59.7%) | 0.66 | 263 (60.2%) | 260 (59.5%) | 0.84 |
Values are mean ± SD or n (%). ACE = angiotensin-converting enzyme; ACS = acute coronary syndrome; ARB = angiotensin receptor blocker; CVA = cerebrovascular accident; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention.
Table 2. Angiographic characteristics.
| Angiographic parameters | Total population | Propensity-Matched population | |||||
|---|---|---|---|---|---|---|---|
| Total (n = 1,006) | 1st generation (n = 557) | 2nd generation (n = 449) | P value | 1st generation (n = 437) | 2nd generation (n = 437) | P value | |
| Abrupt stump | 360 (35.8%) | 216 (38.8%) | 144 (32.1%) | 0.03 | 148 (33.9%) | 141 (32.3%) | 0.62 |
| Bridge collaterals | 256 (25.4%) | 149 (26.8%) | 107 (23.8%) | 0.29 | 113 (25.9%) | 104 (23.8%) | 0.48 |
| Calcification | 123 (12.2%) | 70 (12.6%) | 53 (11.8%) | 0.71 | 58 (13.3%) | 49 (11.2%) | 0.35 |
| CTO location | |||||||
| Left main | 3 (0.3%) | 0 (0.0%) | 3 (0.7%) | 0.05 | 0 (0.0%) | 0 (0.0%) | - |
| LAD | 413 (41.1%) | 243 (43.6%) | 170 (37.9%) | 0.07 | 173 (39.6%) | 165 (37.8%) | 0.58 |
| LCx | 294 (29.2%) | 149 (26.8%) | 145 (32.3%) | 0.06 | 135 (30.9%) | 141 (32.3%) | 0.66 |
| RCA | 419 (41.7%) | 234 (42.0%) | 185 (41.2%) | 0.78 | 179 (41.0%) | 179 (41.0%) | 1.00 |
| Rentrop grade 3 | 302 (30.1%) | 144 (25.9%) | 158 (35.2%) | 0.001 | 138 (31.6%) | 149 (34.1%) | 0.43 |
| Mutivessel disease | 705 (70.1%) | 394 (70.7%) | 311 (69.3%) | 0.61 | 313 (71.6%) | 303 (69.3%) | 0.46 |
| Maximal stent diameter (mm) | 3.0 (± 0.4) | 3.0 (± 0.4) | 3.0 (± 0.5) | 0.46 | 3.0 (± 0.4) | 3.0 (± 0.5) | 0.94 |
| Total stent length (mm) | 33.6 (± 15.4) | 35.0 (± 15.3) | 31.9 (± 15.4.4) | 0.002 | 33.1 (± 13.5) | 31.7 (± 14.7) | 0.15 |
| Proximal to mid CTO* | 733 (72.9%) | 422 (75.8%) | 311 (69.3%) | 0.02 | 315 (72.1%) | 302 (69.1%) | 0.33 |
Values are n (%) or mean ± SD. CTO = coronary chronic total occlusion; LAD = left anterior descending artery; LCx = left circumflex artery; RCA = right coronary artery
*“CTO of the proximal to middle portions of the vessel” has been abbreviated as “Proximal to mid CTO.”
Clinical outcomes
The median follow-up duration for all surviving patients was 1,265 days [interquartile range (IQR): 764–1,968 days]. As expected, the median follow-up duration was shorter for patients treated with second-generation DES [median 881 days (IQR: 535–1,255 days)] compared with patients treated with first-generation DES [median 1,779 days (IQR: 1,186–2,330 days)]. The disparity in follow-up duration between the two groups, which was unavoidable because of the later development of second-generation DES, was accounted for in the analysis by considering only the first 24 months after PCI.
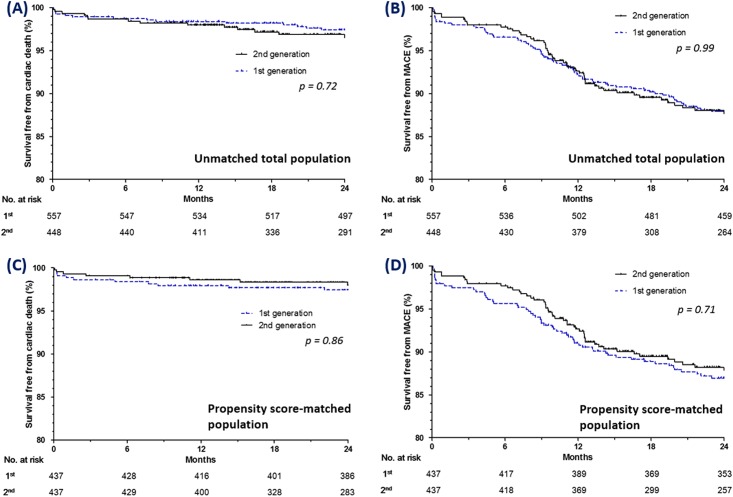
After 2 years of follow-up, 14 cardiac deaths had occurred in the first-generation group and 9 cardiac deaths had occurred in the second-generation group (2.5% versus 2.0%; HR: 0.86; 95% CI: 0.37 to 1.98; p = 0.72). The rates of all-cause death, MI, repeat revascularization, and MACE were also not different between the two groups (Table 3). Kaplan-Meier curves for survival free from cardiac death and survival free from MACE after 2 years of follow-up are shown for both groups in Fig 2A and 2B, respectively. After 1:1 propensity score matching, the clinical outcomes during follow-up were not significantly different between the two groups (Table 3, Fig 2C and 2D).
Table 3. Clinical outcomes.
| Total population | Propensity-Matched population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 1,006) | 1st generation (n = 557) | 2nd generation (n = 449) | HR (95% CI) | P value | 1st generation (n = 437) | 2nd generation (n = 437) | HR (95% CI) | P value | |
| Cardiac death | 23 (2.3%) | 14 (2.5%) | 9 (2.0%) | 0.86 (0.37–1.98) | 0.72 | 11 (2.5%) | 9 (2.1%) | 0.86 (0.35–2.06) | 0.86 |
| All-cause death | 42 (4.2%) | 27 (4.8%) | 15 (3.3%) | 0.76 (0.40–1.42) | 0.39 | 22 (5.0%) | 15 (3.4%) | 0.74 (0.38–1.42) | 0.36 |
| Myocardial infarction | 10 (1.0%) | 7 (1.3%) | 3 (0.7%) | 0.55 (0.14–2.13) | 0.39 | 6 (1.4%) | 3 (0.7%) | 0.51 (0.13–2.02) | 0.33 |
| Repeat revascularization | 98 (9.7%) | 56 (10.1%) | 42 (9.4%) | 1.01 (0.70–1.47) | 0.95 | 48 (11.0%) | 40 (9.2%) | 0.89 (0.58–1.35) | 0.89 |
| MACE* | 117 (11.6%) | 66 (11.8%) | 51 (11.4%) | 1.00 (0.67–1.50) | 0.99 | 56 (12.8%) | 49 (11.2%) | 0.93 (0.63–1.37) | 0.71 |
Values are n (%).CI = confidence interval; HR = hazard ratio; MI = myocardial infarction.
*Major adverse cardiac events (MACE) included cardiac death, MI, and repeat revascularization (included target vessel revascularization-PCI, non–target vessel revascularization-PCI, or coronary artery bypass grafting).
Fig 2. Kaplan-Meier curves for clinical outcomes in all the patients (A, B) and the propensity-matched patients (C, D).
Kaplan-Meier curves for cardiac death and MACE in the patients treated with first-generation and second generation drug-eluting stents. MACE = major adverse cardiac event.
Subgroup analysis
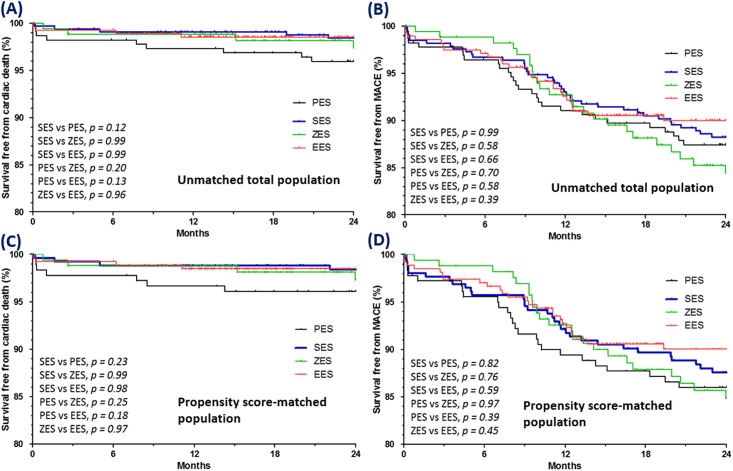
We next examined the following four subgroups for differences: SES, PES, EES, and ZES. After 2 years, no significant between-group differences were observed regarding the rates of cardiac death, MI, repeat revascularization, or MACE. Kaplan-Meier curves for survival free from cardiac death and survival free from MACE after two years of follow-up are shown for each of the four subgroups in Fig 3.
Fig 3. Kaplan-Meier curves for cardiac death and MACE according to DES subgroup for all patients (A, B) and propensity-matched patients (C, D).
MACE = major adverse cardiac event; DES = drug-eluting stent; SES = sirolimus-eluting stent; PES = paclitaxel-eluting stent; EES = everolimus-eluting stent; ZES = zotarolimus-eluting stent.
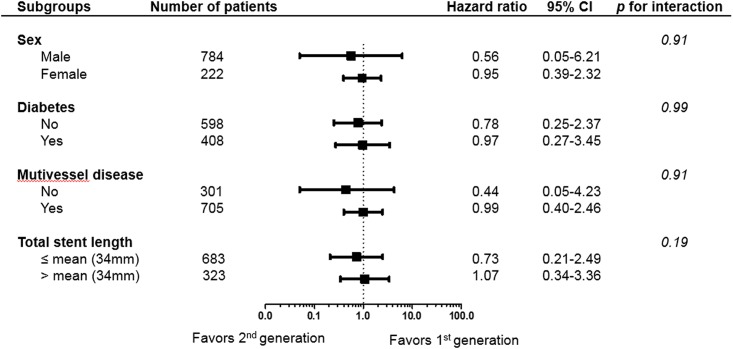
Finally, we calculated the unadjusted HR for cardiac death in various subgroups to determine whether the outcomes according to second-generation DES (vs. first-generation DES) observed in the overall population were consistent in these subgroups (Fig 4). No significant interactions were observed between the use of second-generation DES and cardiac death in any of the subgroups.
Fig 4. Comparative unadjusted hazard ratios of cardiac death for the DES subgroups out of all the patients between first-generation and second-generation drug-eluting stents.
CI = confidence interval.
Discussion
In this study, we demonstrated that first-generation and second-generation DES are similarly effective in the context of CTO lesions. No differences were found between the two groups regarding the overall incidence of cardiac death or the overall incidence of MACE, despite the mid-term (two years) period of observation. After propensity score matching of all the patients, the incidences of cardiac death and MACE in the two groups were still comparable. Subgroup analyses according to DES type (SES, PES, EES, and ZES) also revealed similar incidences of cardiac death and MACE. In fact, previous meta-analyses substantially differ from the current one. In the meta-analysis by Lanka et al. [26] only 5 studies were collected reporting 1,174 patients. Although a significant benefit on all cause death in patients assigned to second-generation DES was reported, the effects on cardiac death was not analyzed.
EES have been found to be superior to PES [11, 27–29] and comparable to SES [30, 31] with respect to cardiac death, MI, stent thrombosis, and revascularization in randomized trials and prospective cohort studies of general populations. ZES have also been found to be superior to PES [15, 32] and comparable to SES [32, 33] with respect to death rate and MI in randomized trials of general populations. Although some of these studies included patients with CTO lesions, few such analyses have been carried out.
Several randomized and nonrandomized studies have compared outcomes between first-generation and second-generation DES after PCI in CTO lesions. Valenti et al [18] examined 588 patients with CTO and found that second-generation DES (EES) were associated with reduced cardiac death and MACE (11.6% vs. 22.4%, p < 0.01) compared with first-generation DES (SES and PES). Although this study had a relatively large number of patients, the registry was from a single center and was not randomized; moreover, the follow-up duration was only 12 months. In contrast to this study, Moreno et al [16], Park et al [17], and Almalia et al [19] observed no significant differences in the rates of cardiac death and MACE between first-generation and second-generation DES. Moreno et al performed a prospective randomized study of 207 patients with CTO and found that EES was comparable to SES with respect to cardiac death (0.9% vs. 2.0%, p = 0.52) and MACE (11.3% vs. 15.8%, p = 0.34). Park et al also performed a prospective randomized study of 160 patients with CTO and found that ZES was comparable to SES with respect to cardiac death (1.3% vs. 2.5%, p = 0.56) and repeat revascularization (10% vs. 17.5%, p = 0.17). Although these two studies were prospective randomized studies, their sample sizes were relatively small and the follow-up duration was only 12 months. Therefore, we compared the impacts of second-generation and first-generation DES on clinical outcomes in patients with CTO from a large-scale, multicenter registry with a relatively long-term follow-up duration.
Second-generation DES have several advantages for PCI for CTO lesions. First, stent platforms of cobalt or platinum-chromium alloys are thinner and more deliverable than the platforms used in first-generation DES. This improved flexibility and deliverability might increase the success rate in treating CTO lesions. Moreover, second-generation DES produce a weaker inflammatory response and stimulate more rapid vessel re-endothelialization. Despite these improvements of second-generation DES, similar incidences of cardiac death, MI, and MACE have been observed in patients with bifurcated lesions [34] and ACS [35] treated with second-generation DES vs first-generation DES. Overall, the results from our CTO registry are consistent with previous 12-month follow-up prospective randomized CTO studies [16, 17] in that second-generation DES do not seem to confer any major advantages to clinical outcomes such as cardiac death, MI, or repeat revascularization.
Our study did have several limitations. First, the study design was nonrandomized due to the nature of the registry data, which means that confounding factors may have affected the results. Second, we lacked comprehensive data regarding possible alterations of medical therapies over the follow-up period. Third, we did not routinely perform angiographic follow-up examination on our patients; thus, angiographic adverse events may have been underestimated. Fourth, although the vital status of all patients, including those lost to follow-up, was confirmed with the Korean national database using a citizen registration number that is unique to each individual, we cannot exclude the possibility of under-reporting of clinical outcomes other than death such as nonfatal MI and stent thrombosis. Fifth, our attempt to mitigate the unavoidable disparity in follow-up duration due to the time lag between the development of first-generation and second-generation DES limited our outcome analysis to 2 years. Furthermore, newer specialized revascularization devices for the treatment of CTO lesions may have been used more often in the second-generation DES group, which may have led to overestimation or underestimation of MACEs. Finally, the present analysis included patients who were treated over a long period of time. During this time, changes in PCI strategies may have impacted the clinical outcomes, irrespective of the stent type used.
Conclusion
Our results suggest that the efficacy of second-generation DES is similar to that of first-generation DES for patients with CTO who undergo PCI, at least over a 2 year follow-up period.
Supporting Information
It includes data of CTO patients with file format of Microsoft Excel.
(XLSX)
Data Availability
The minimal relevant dataset is available in the Supporting Information files. Requests for additional data should be sent to the corresponding authors, SH Choi.
Funding Statement
The authors have no support or funding to report.
References
- 1.Agostoni P, Valgimigli M, Biondi-Zoccai GG, Abbate A, Garcia Garcia HM, Anselmi M, et al. Clinical effectiveness of bare-metal stenting compared with balloon angioplasty in total coronary occlusions: insights from a systematic overview of randomized trials in light of the drug-eluting stent era. American heart journal. 2006;151(3):682–9. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita I, Katoh O, Nariyama J, Otsuji S, Tateyama H, Kobayashi T, et al. Coronary angioplasty of chronic total occlusions with bridging collateral vessels: immediate and follow-up outcome from a large single-center experience. Journal of the American College of Cardiology. 1995;26(2):409–15. [DOI] [PubMed] [Google Scholar]
- 3.Olivari Z, Rubartelli P, Piscione F, Ettori F, Fontanelli A, Salemme L, et al. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). Journal of the American College of Cardiology. 2003;41(10):1672–8. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Kandzari DE, Mehran R, Colombo A, Schwartz RS, Bailey S, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation. 2005;112(15):2364–72. [DOI] [PubMed] [Google Scholar]
- 5.Buller CE, Dzavik V, Carere RG, Mancini GB, Barbeau G, Lazzam C, et al. Primary stenting versus balloon angioplasty in occluded coronary arteries: the Total Occlusion Study of Canada (TOSCA). Circulation. 1999;100(3):236–42. [DOI] [PubMed] [Google Scholar]
- 6.Stone GW, Reifart NJ, Moussa I, Hoye A, Cox DA, Colombo A, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation. 2005;112(16):2530–7. [DOI] [PubMed] [Google Scholar]
- 7.Suttorp MJ, Laarman GJ, Rahel BM, Kelder JC, Bosschaert MA, Kiemeneij F, et al. Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II): a randomized comparison of bare metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions. Circulation. 2006;114(9):921–8. [DOI] [PubMed] [Google Scholar]
- 8.Ge L, Iakovou I, Cosgrave J, Chieffo A, Montorfano M, Michev I, et al. Immediate and mid-term outcomes of sirolimus-eluting stent implantation for chronic total occlusions. European heart journal. 2005;26(11):1056–62. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura S, Muthusamy TS, Bae JH, Cahyadi YH, Udayachalerm W, Tresukosol D. Impact of sirolimus-eluting stent on the outcome of patients with chronic total occlusions. The American journal of cardiology. 2005;95(2):161–6. [DOI] [PubMed] [Google Scholar]
- 10.Werner GS, Krack A, Schwarz G, Prochnau D, Betge S, Figulla HR. Prevention of lesion recurrence in chronic total coronary occlusions by paclitaxel-eluting stents. Journal of the American College of Cardiology. 2004;44(12):2301–6. [DOI] [PubMed] [Google Scholar]
- 11.Alazzoni A, Al-Saleh A, Jolly SS. Everolimus-Eluting versus Paclitaxel-Eluting Stents in Percutaneous Coronary Intervention: Meta-Analysis of Randomized Trials. Thrombosis. 2012;2012:126369 10.1155/2012/126369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125(9):1110–21. 10.1161/CIRCULATIONAHA.111.058560 [DOI] [PubMed] [Google Scholar]
- 13.Kimura T, Morimoto T, Natsuaki M, Shiomi H, Igarashi K, Kadota K, et al. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-eluting Versus Everolimus-eluting stent Trial (RESET). Circulation. 2012;126(10):1225–36. [DOI] [PubMed] [Google Scholar]
- 14.Eisenstein EL, Leon MB, Kandzari DE, Mauri L, Edwards R, Kong DF, et al. Long-term clinical and economic analysis of the Endeavor zotarolimus-eluting stent versus the cypher sirolimus-eluting stent: 3-year results from the ENDEAVOR III trial (Randomized Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Cypher Sirolimus-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions). JACC Cardiovascular interventions. 2009;2(12):1199–207. 10.1016/j.jcin.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 15.Kirtane AJ, Leon MB, Ball MW, Bajwa HS, Sketch MH Jr., Coleman PS, et al. The "final" 5-year follow-up from the ENDEAVOR IV trial comparing a zotarolimus-eluting stent with a paclitaxel-eluting stent. JACC Cardiovascular interventions. 2013;6(4):325–33. 10.1016/j.jcin.2012.12.123 [DOI] [PubMed] [Google Scholar]
- 16.Moreno R, Garcia E, Teles R, Rumoroso JR, Cyrne Carvalho H, Goicolea FJ, et al. Randomized comparison of sirolimus-eluting and everolimus-eluting coronary stents in the treatment of total coronary occlusions: results from the chronic coronary occlusion treated by everolimus-eluting stent randomized trial. Circulation Cardiovascular interventions. 2013;6(1):21–8. 10.1161/CIRCINTERVENTIONS.112.000076 [DOI] [PubMed] [Google Scholar]
- 17.Park HJ, Kim HY, Lee JM, Choi YS, Park CS, Kim DB, et al. Randomized comparison of the efficacy and safety of zotarolimus-eluting stents vs. sirolimus-eluting stents for percutaneous coronary intervention in chronic total occlusion—CAtholic Total Occlusion Study (CATOS) trial. Circulation journal: official journal of the Japanese Circulation Society. 2012;76(4):868–75. [DOI] [PubMed] [Google Scholar]
- 18.Valenti R, Vergara R, Migliorini A, Parodi G, Carrabba N, Cerisano G, et al. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. Journal of the American College of Cardiology. 2013;61(5):545–50. 10.1016/j.jacc.2012.10.036 [DOI] [PubMed] [Google Scholar]
- 19.Almalla M, Hennings V, Marx N, Hoffmann R. Long-term clinical and angiographic outcome after treatment of chronic total occlusion with everolimus or sirolimus eluting stents. International journal of cardiology. 2012;157(3):451–2. 10.1016/j.ijcard.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 20.Godino C, Bassanelli G, Economou FI, Takagi K, Ancona M, Galaverna S, et al. Predictors of cardiac death in patients with coronary chronic total occlusion not revascularized by PCI. International journal of cardiology. 2013;168(2):1402–9. 10.1016/j.ijcard.2012.12.044 [DOI] [PubMed] [Google Scholar]
- 21.Sianos G, Werner GS, Galassi AR, Papafaklis MI, Escaned J, Hildick-Smith D, et al. Recanalisation of chronic total coronary occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2012;8(1):139–45. [DOI] [PubMed] [Google Scholar]
- 22.Lansky AJ, Dangas G, Mehran R, Desai KJ, Mintz GS, Wu H, et al. Quantitative angiographic methods for appropriate end-point analysis, edge-effect evaluation, and prediction of recurrent restenosis after coronary brachytherapy with gamma irradiation. Journal of the American College of Cardiology. 2002;39(2):274–80. [DOI] [PubMed] [Google Scholar]
- 23.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. Journal of the American College of Cardiology. 1985;5(3):587–92. [DOI] [PubMed] [Google Scholar]
- 24.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51. [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116(22):2634–53. [DOI] [PubMed] [Google Scholar]
- 26.Lanka V, Patel VG, Saeed B, Kotsia A, Christopoulos G, Rangan BV, et al. Outcomes with first- versus second-generation drug-eluting stents in coronary chronic total occlusions (CTOs): a systematic review and meta-analysis. The Journal of invasive cardiology. 2014;26(7):304–10. [PubMed] [Google Scholar]
- 27.Applegate RJ, Yaqub M, Hermiller JB, Sood P, Yu S, Doostzadeh J, et al. Long-term (three-year) safety and efficacy of everolimus-eluting stents compared to paclitaxel-eluting stents (from the SPIRIT III Trial). The American journal of cardiology. 2011;107(6):833–40. 10.1016/j.amjcard.2010.10.069 [DOI] [PubMed] [Google Scholar]
- 28.Stone GW, Rizvi A, Sudhir K, Newman W, Applegate RJ, Cannon LA, et al. Randomized comparison of everolimus- and paclitaxel-eluting stents. 2-year follow-up from the SPIRIT (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System) IV trial. Journal of the American College of Cardiology. 2011;58(1):19–25. 10.1016/j.jacc.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 29.Smits PC, Kedhi E, Royaards KJ, Joesoef KS, Wassing J, Rademaker-Havinga TA, et al. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison of the everolimus eluting XIENCE-V stent with the paclitaxel eluting TAXUS LIBERTE stent in all-comers: a randomized open label trial). Journal of the American College of Cardiology. 2011;58(1):11–8. 10.1016/j.jacc.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 30.de Waha A, Dibra A, Byrne RA, Ndrepepa G, Mehilli J, Fusaro M, et al. Everolimus-eluting versus sirolimus-eluting stents: a meta-analysis of randomized trials. Circulation Cardiovascular interventions. 2011;4(4):371–7. 10.1161/CIRCINTERVENTIONS.111.963256 [DOI] [PubMed] [Google Scholar]
- 31.Jensen LO, Thayssen P, Hansen HS, Christiansen EH, Tilsted HH, Krusell LR, et al. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation. 2012;125(10):1246–55. 10.1161/CIRCULATIONAHA.111.063644 [DOI] [PubMed] [Google Scholar]
- 32.Park DW, Kim YH, Yun SC, Kang SJ, Lee SW, Lee CW, et al. Comparison of zotarolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for coronary revascularization: the ZEST (comparison of the efficacy and safety of zotarolimus-eluting stent with sirolimus-eluting and paclitaxel-eluting stent for coronary lesions) randomized trial. Journal of the American College of Cardiology. 2010;56(15):1187–95. 10.1016/j.jacc.2010.03.086 [DOI] [PubMed] [Google Scholar]
- 33.Maeng M, Tilsted HH, Jensen LO, Krusell LR, Kaltoft A, Kelbaek H, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383(9934):2047–56. 10.1016/S0140-6736(14)60405-0 [DOI] [PubMed] [Google Scholar]
- 34.Burzotta F, Trani C, Todaro D, Mariani L, Talarico GP, Tommasino A, et al. Prospective randomized comparison of sirolimus- or everolimus-eluting stent to treat bifurcated lesions by provisional approach. JACC Cardiovascular interventions. 2011;4(3):327–35. 10.1016/j.jcin.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 35.Machado C, Raposo L, Dores H, Leal S, Campante Teles R, de Araujo Goncalves P, et al. Second-generation versus first-generation drug-eluting stents for the treatment of patients with acute coronary syndromes and obstructive coronary artery disease. Coronary artery disease. 2014;25(3):208–14. 10.1097/MCA.0000000000000078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
It includes data of CTO patients with file format of Microsoft Excel.
(XLSX)
Data Availability Statement
The minimal relevant dataset is available in the Supporting Information files. Requests for additional data should be sent to the corresponding authors, SH Choi.