Abstract
Diverse phytophagous heteropteran insects, commonly known as stinkbugs, are associated with specific gut symbiotic bacteria, which have been found in midgut cryptic spaces. Recent studies have revealed that members of the stinkbug families Coreidae and Alydidae of the superfamily Coreoidea are consistently associated with a specific group of the betaproteobacterial genus Burkholderia, called the “stinkbug-associated beneficial and environmental (SBE)” group, and horizontally acquire specific symbionts from the environment every generation. However, the symbiotic system of another coreoid family, Stenocephalidae remains undetermined. We herein investigated four species of the stenocephalid genus Dicranocephalus. Examinations via fluorescence in situ hybridization (FISH) and transmission electron microscopy (TEM) revealed the typical arrangement and ultrastructures of midgut crypts and gut symbionts. Cloning and molecular phylogenetic analyses of bacterial genes showed that the midgut crypts of all species are colonized by Burkholderia strains, which were further assigned to different subgroups of the genus Burkholderia. In addition to the SBE-group Burkholderia, a number of stenocephalid symbionts belonged to a novel clade containing B. sordidicola and B. udeis, suggesting a specific symbiont clade for the Stenocephalidae. The symbiotic systems of stenocephalid bugs may provide a unique opportunity to study the ongoing evolution of symbiont associations in the stinkbug-Burkholderia interaction.
Keywords: insect, bacteria, gut symbiosis, molecular phylogeny, evolution
Most members of the taxon Heteroptera, which includes 42,300 described species (12), display mutualistic relationships with diverse symbiotic bacteria (6, 30). While some stinkbug species of the families Lygaeidae, Artheneidae, Blissidae, and Cimicidae harbor their endosymbionts intracellularly in specific organs, called bacteriomes or mycetomes (16, 36–38, 43, 57), most phytophagus stinkbugs, particularly members of the infraorder Pentatomomorpha, extracellularly accommodate their symbiotic bacteria either in the lumen of the swollen part of the midgut (26, 60) or in the lumen of the separated sac-like tissues of the posterior midgut, called crypts or ceca (6, 54, 68).
In plant-sucking stinkbugs of the superfamily Pentatomoidea (Heteroptera: Pentatomomorpha), species of the families Acanthosomatidae, Cydnidae, Parastrachiidae, Pentatomidae, Plataspidae, Scutelleridae, and Urostylididae harbor specific bacterial symbionts, which belong to distinct lineages in Gammaproteobacteria, indicating multiple evolutionary origins of symbiotic associations (2, 3, 13, 15, 17, 19, 23–25, 31, 34, 35, 44, 51–53). The gut symbionts of the Pentatomoidea are typically transmitted vertically by specific, postnatal transmission mechanisms, such as the bacterial contamination of the egg surface during egg deposition (1, 31, 53, 55), the excretion of a bacteria-containing mucus or jelly onto the egg mass (18, 25), and the deposition of bacteria-containing capsules together with the eggs (9, 14, 15, 47).
In contrast, most representatives of the superfamilies Lygaeoidea and Coreoidea are associated with the betaproteobacterial symbionts of a specific clade in the genus Burkholderia, called the “stinkbug-associated beneficial and environmental (SBE)” group (4, 10, 22, 28, 29, 32, 33, 50). Based on a series of comprehensive studies on the coreoid species, Riptortus pedestris (Coreoidea: Alydidae), it has been reported that Burkholderia symbionts are not transmitted vertically, but are acquired anew by nymphal insects from the environment every generation (29), whereas partial vertical transmission of the Burkholderia symbiont has been reported in chinch bugs (4, 22). Due to the transmission mechanism, the phylogeny of the Burkholderia symbiont does not mirror the phylogeny of the host insects, but symbionts form a coherent group with soil-isolated strains in an intermixed manner (28, 29, 32), indicating an alternating host–symbiont relationship. In addition, a recent study revealed that representatives of the family Largidae of the superfamily Pyrrhocoroidea, a monophyletic sister taxon of Coreoidea and Lygaeoidea (67), are also associated with Burkholderia symbionts (61). However, these largid species are, in contrast to lygaeoid/coreoid species, consistently associated with Burkholderia strains of the so-called “plant-associated beneficial and environmental (PBE)” group, which are phylogenetically distinct from the SBE strains (63). In the PBE-group, stinkbug-associated strains do not form a monophyletic cluster, but are intermixed with soil-isolated and plant-associated strains, also indicating a promiscuous host–symbiont association in pyrrhocorid stinkbugs.
The family Stenocephalidae of the superfamily Coreoidea is a small stinkbug group, all members of which live and feed on various species of the Euphorbiaceae, commonly known as spurges (58). Landsbury (39, 40) identified two genera (Dicranocephalus and Psotilnus) and 36 species, whereas Moulet (45, 46) considered only one genus (Dicranocephalus) and 16 valid species. Although the group is widely distributed, most are known from the tropics and subtropics of the Eastern Hemisphere, including Australia. This small family is of special taxonomic interest because it shows characteristics that are transitional between Coreoidea (e.g., numerous hemelytral veins and a four-lobed salivary gland) and Lygaeoidea (e.g., laciniate ovipositor and an XY chromosome) (11, 12, 58). Therefore, the phylogenetic position of Stenocephalidae was controversial for a long time (11). In addition to the taxonomical importance of this family, its gut symbiotic association has not yet been characterized.
The objective of this study was to analyze and compare the bacterial populations of midgut crypts in the stenocephalid species Dicranocephalus albipes, D. agilis, and D. medius (Fig. 1A) from Europe, and D. lateralis from Japan (Heteroptera: Stenocephalidae). The phylogenetic position of the bacterial gut symbiont was elucidated by analyses of 16S rRNA and gyrB gene sequences. The localization as well as morphological characteristics of the gut symbiont of D. medius was investigated in detail by fluorescence in situ hybridization (FISH) and transmission electron microscopy (TEM), respectively. The results obtained revealed that a novel clade of Burkholderia is associated with the stenocephalid species.
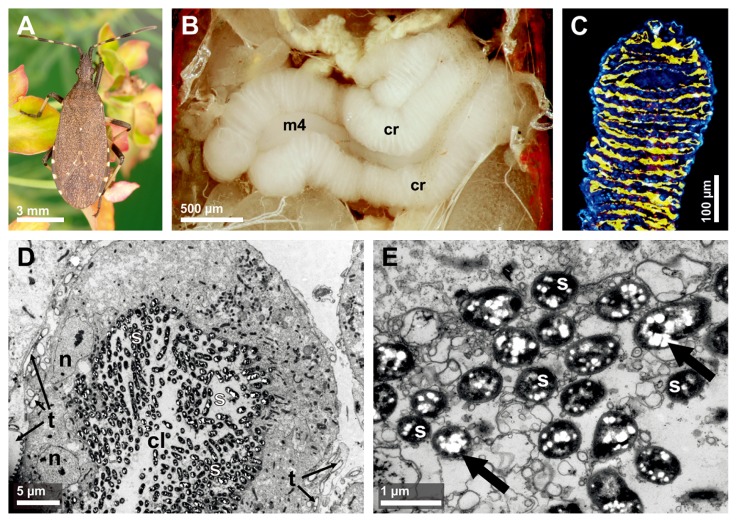
Fig. 1.
A representative of the family Stenocephalidae associated with Burkholderia symbionts in their midgut crypts. (A) An adult female of Dicranocephalus medius sitting on its Euphorbia host plant. (B) Dissected midgut of the fourth section (m4) with two rows of crypts (cr). (C) Fluorescence in situ hybridization of symbionts in midgut crypts stained with the specific Burkholderia probe (yellow) and DAPI (blue). (D) Transmission electron microscopy of thin sections through the midgut crypts, including the betaproteobacterial Burkholderia symbionts of D. medius. The crypt lumen (cl) is completely filled with symbionts (s), surrounded by a monolayer of nucleated (n) insect cells and numerous trachea (t). (E) High magnification of the Burkholderia symbionts (s). White storage granules (black arrows) are presumably cellular reserve materials.
Materials and Methods
Insects
Adults and nymphs of D. agilis, D. albipes, D. lateralis, and D. medius were collected from their host plants (Euphorbia spp.) in Europe and Japan (Table 1). The European bug species were brought alive to the laboratory and dissected in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.4]), while samples of D. lateralis preserved in acetone were dissected in the same manner. The isolated midgut tissues were subjected to DNA extraction, cloning, and sequencing. Furthermore, several individuals of D. medius were used in symbiont visualization via FISH and an ultrastructure analysis by TEM.
Table 1.
Samples of stenocephalid bugs used for cloning and/or sequencing in this study.
| Species | Insect ID | Instar | Sexa | Collection location | Collection date | Collector | Accession No. | |
|---|---|---|---|---|---|---|---|---|
| Dicranocephalus agilis | 16S rRNA | gyrB | ||||||
| Dag1 | 4th | – | Lindow (Mark), Germany | Jun 13, 2014 | C. Morkel | LT221673–LT221676 | LT221834–LT221835 | |
| Dag2 | 4th | – | “ | Jun 13, 2014 | “ | LT221677–LT221679 | LT221836–LT221843 | |
| Dicranocephalus medius | ||||||||
| Dme1 | Adult | M | Bayreuth, Germany | Apr 21, 2011 | S. Kuechler | LT221738 | LT221800 | |
| Dme2 | Adult | M | “ | May 30, 2013 | “ | LT221739–LT221741 | LT221817–LT221819 | |
| Dme3 | Adult | F | “ | May 30, 2013 | “ | LT221742–LT221743 | LT221820–LT221821 | |
| Dme4 | Adult | M | “ | Jun 04, 2014 | “ | LT221744–LT221752 | LT221822–LT221833 | |
| Dme5 | Adult | F | “ | Jun 04, 2014 | “ | LT221724–LT221730 | LT221753–LT221760 | |
| Dme6 | Adult | F | “ | Jun 16, 2014 | “ | LT221731–LT221737 | LT221761–LT221767 | |
| Dicranocephalus lateralis | ||||||||
| Dla1 | Adult | F | Ishigaki Is., Japan | Jul 28, 2002 | K. Kohno | LT221697–LT221702 | LT221768–LT221778 | |
| Dla2 | Adult | M | “ | Jul 28, 2002 | “ | LT221703–LT221712 | LT221779–LT221781 | |
| Dla3 | Adult | M | “ | Jul 28, 2002 | “ | LT221713–LT221714 | LT221782–LT221789 | |
| Dla4 | – | – | “ | Sep 10, 2009 | T. Hosokawa | LT221715–LT221723 | LT221790–LT221799 | |
| Dicranocephalus albipes | ||||||||
| Dal1 | Adult | M | Talamone, Italy | Jul 27, 2010 | S. Kuechler | LT221680–LT221693 | LT221801–LT221813 | |
| Dal2 | Adult | M | La Garde-Freinet, France | Sep 15, 2011 | S. Kehl | LT221694–LT221696 | LT221814–LT221816 | |
F, female; M, male; –, undetermined
Histology
In order to prepare tissue sections, the dissected midgut tissues of D. medius were fixed in 4% paraformaldehyde overnight, followed by a washing step in 0.5×PBS and 48% ethanol (v/v), serial dehydration in ethanol (70%, 90%, 2×100%), and a final embedding step in resin Unicryl™ (Plano GmbH, Germany). Serial sections (2 μm) were cut using a Leica Jung RM2035 rotary microtome (Leica Instruments GmbH, Wetzlar, Germany), mounted on epoxy-coated glass slides, and subjected to FISH.
FISH
Several eubacterial probes and a symbiont-specific probe (Table S1) were used to detect gut symbionts in D. medius midgut tissue. The specific probe was designed on a symbiont specific position in the 16S rRNA gene alignment and verified on probeCheck (42). In addition, a nonsense probe complementary to the bacterial probe EUB338 was used as a negative control of the experiment (Table S1). Tissue sections were incubated with a hybridization buffer (20 mM Tris-HCl [pH 8.0], 0.9 M NaCl, 0.01% sodium dodecyl sulphate [SDS], 20% formamide) containing 10 pmole mL−1 each of the fluorescent probes, kept at 46°C for 90 min, rinsed with a washing buffer (20 mM Tris-HCl [pH 8.0], 450 mM NaCl, 0.01% SDS), mounted with an anti-photobleaching solution (VectaShield Mounting Medium; Vector Laboratories, Peterborough, UK), and viewed under a fluorescent microscope (Axioplan 2 imaging, Zeiss).
TEM
The dissected tissues of D. medius were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) for 1 h, embedded in 2% agarose gel, and fixed again in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) overnight. The tissue was washed in 0.1 M cacodylate buffer for 20 min three times. Following postfixation in 2% osmium tetroxide for 2 h, the sample was washed and stained en bloc in 2% uranyl acetate for 90 min. After fixation, the tissue was dehydrated serially in ethanol (30%, 50%, 70%, 95%, and 3×100%), transferred to propylene oxide, and embedded in Epon. Ultrathin sections (70 nm) were cut using a diamond knife (Micro-Star, Huntsville, TX) on a Leica Ultracut UCT microtome (Leica Microsystems, Vienna, Austria). Ultrathin sections were mounted on pioloform-coated copper grids and stained with saturated uranyl acetate, followed by lead citrate. The sections were viewed using a Zeiss CEM 902 A transmission electron microscope (Carl Zeiss, Oberkochen, Germany) at 80 kV.
DNA extraction, cloning, and sequencing
The DNA of the dissected midgut crypts was extracted using the High Pure PCR Template Preparation Kit (Roche) following the manufacturer’s instructions. A 1.5-kb segment of the bacterial 16S rRNA gene was PCR amplified using the universal primers and a 0.65-kb segment of the bacterial gyrB (gyrase subunit B) gene was amplified for additional symbiont characterization (Table S1). In host phylogenetic analyses, several mitochondrial gene fragments were amplified: a 1.5-kb segment of cytochrome c oxidase subunit I (COI/coxI), a 0.93-kb segment of ubiquinone oxidoreductase subunit 1 (ND1/nad1), a 1.14-kb segment of cytochrome b (cytB/cob), and a 0.29-kb segment of ubiquinone oxidoreductase subunit 6 (ND6/nad6), respectively (Table S1).
All PCR reactions were performed on a Biometra thermal cycler with the following program: an initial denaturation step at 94°C for 3 min, followed by 34 cycles at 94°C for 30 s, 50°C for 2 min, and 72°C for 1 min. A final extension step at 72°C for 10 min was included. All PCR products of bacterial gene amplification were cloned using the CloneJET™ PCR Cloning Kit (Thermo Fisher). Cloned inserts offering PCR products of the correct length were examined by restriction fragment length polymorphism (RFLP). Inserts were digested by the restriction endonucleases RsaI and HhaI. Eighty clone sequences for the 16S rRNA gene and 91 clone sequences for the gyrB gene were chosen for Sanger sequencing, respectively (Table 2 and S2). Amplified mitochondrial gene segments (COI, ND1, cytB, and ND6) of the host were subjected to direct Sanger sequencing with suitable PCR primers.
Table 2.
The number of 16S rRNA gene clones in each OTU subgroup of Burkholderia symbionts.
| Species | Insect ID | Stenocephalidae-clade | Coreidae-clade | SBE clade | PBE clade | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| OTU1 | OTU2 | OTU3 | OTU4 | OTU5 | OTU6 | OTU7 | OTU8 | OTU9 | OTU10 | |||
| Dicranocephalus agilis | ||||||||||||
| Dag1 | 3 | 1 | – | – | – | – | – | – | – | – | 4 | |
| Dag2 | – | – | 3 | – | – | – | – | – | – | – | 3 | |
| Dicranocephalus medius | ||||||||||||
| Dme1 | 1 | – | – | – | – | – | – | – | – | – | 1 | |
| Dme2 | 7 | – | – | – | – | – | – | – | – | – | 7 | |
| Dme3 | 7 | – | – | – | – | – | – | – | – | – | 7 | |
| Dme4 | 3 | – | – | – | – | – | – | – | – | – | 3 | |
| Dme5 | 2 | – | – | – | – | – | – | – | – | – | 2 | |
| Dme6 | 8 | – | – | 1 | – | – | – | – | – | – | 9 | |
| Dicranocephalus lateralis | ||||||||||||
| Dla1 | – | – | – | – | – | 4 | 2 | – | – | – | 6 | |
| Dla2 | – | – | – | – | – | – | 10 | – | – | – | 10 | |
| Dla3 | – | – | – | – | – | – | 1 | 1 | – | – | 2 | |
| Dla4 | – | – | – | – | – | – | – | – | 9 | – | 9 | |
| Dicranocephalus albipes | ||||||||||||
| Dal1 | – | – | – | – | – | – | – | – | – | 14 | 14 | |
| Dal2 | 2 | – | – | – | 1 | – | – | – | – | – | 3 | |
| OTU total | 33 | 1 | 3 | 1 | 1 | 4 | 13 | 1 | 9 | 14 | 80 | |
Phylogenetic analyses
Clone sequences of gut bacteria were classified into operational taxonomic units (OTUs) using macqiime v1.9.1 (7) based on the furthest-neighbor algorithm with a >99% identity threshold for 16S rRNA gene sequences and >98% for gyrB gene sequences (Table 2 and S2). According to the OTUs calculation, high-quality sequences of the 16S rRNA and gyrB genes were aligned using the ClustalW algorithm in MEGA 6 (64) and edited manually. In the phylogenetic analysis of host insects, the mitochondrial COI, ND6, cytB, and ND1 gene sequences of allied heteropteran insects were retrieved from GenBank and aligned with the sequences of Dicranocephalus spp. by MAFFT v7.212 (G-INS-i) (27). After manually editing gap-containing sites, COI, ND6, cytB, and ND1 gene sequences were concatenated and used in subsequent analyses.
Phylogenetic trees were reconstructed under the Tamura 3-parameter (16S rRNA gene) and GTR+I+G model (gyrB and COI+ND6+cytB+ND1) of nucleotide substitution by the maximum likelihood (ML) method using MEGA 6. Additionally, the neighbor-joining (NJ) method was executed for phylogenetic analyses in MEGA 6. The bootstrap values of 1,000 replicates for all internal branches were calculated. A likelihood ratio test was also performed using MrModeltest V.2.3 (48) to find the best-fitting models for the Bayesian analysis. The Akaike criterion selected the GTR+I+G model for bacterial 16S rRNA, gyrB, and host COI+ND6+cytB+ND1 gene data. Under the evolutionary model, a Bayesian analysis with MrBayes (v.3.2.6) (21) was performed with four simultaneous Markov chains for each dataset. Regarding 16S rRNA, gyrB, and COI+ND6+cytB+ND1 gene data, 1,000,000 generations were used; 1,000 trees were obtained (samplefreq=1,000) and the first 250 of these were considered to be the ‘burn in’ and discarded.
Nucleotide sequence accession numbers
The DNA sequences of bacterial 16S rRNA and the gyrB genes and host COI, ND6, cytB, and ND1 gene sequences determined in this study were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases under the accession numbers (LT221673–LT221855), respectively.
Results
Dicranocephalus species develop midgut crypts filled with rod-shaped bacteria
Histological analyses revealed that the stenocephalid species D. agilis, D. albipes, D. medius, and D. lateralis possess a voluminous, white-colored midgut region with numerous crypts in two rows at the fourth midgut section (Fig. 1B). Typically, females had larger crypts than the smaller males. Field-collected D. medius nymphs of the 2nd instar and later instars also offered well-developed midgut crypts, while no nymph of the 1st instar was found in field collections in this study. When cross-sections of the midgut crypts were subjected to FISH with fluorescently labeled oligonucleotide probes specific to the gut symbiont, intense signals from symbionts were detected primarily in the lumen of the midgut crypts (Fig. 1C). Signals of a weak fluorescence were also observed from the lumen of the midgut main tract (data not shown). Ultrastructural examinations of the midgut crypts revealed that the lumen of the crypts was filled with rod-shaped bacteria (Fig. 1D). No intracellular bacteria were detected. Many of these rod-shaped bacteria contained multiple vesicles of weak electron density (Fig. 1E), which were presumably storage granules. A number of tracheal cells were observed between the single crypts (Fig. 1D), indicating a high rate of gas exchange and metabolic activity in the symbiotic tissue.
Diverse Burkholderia strains are associated with Dicranocephalus species
Fourteen DNA samples of midgut crypts from the four species were subjected to PCR amplification, cloning, and Sanger sequencing of a 1.5-kb 16S rRNA gene fragment and 0.65-kb gyrB gene fragment (Table 1). The top BLAST hits of our DNA datasets (80 of the 16S rRNA gene, and 91 of the gyrB clone sequences) revealed a high concordance with Burkholderia species and the clones were classified into 10 OTUs for the 16S rRNA gene (sequences showing >99% identity were designated as a single OTU) and 11 OTUs for gyrB (sequences showing >98% sequence identity were designated as a single OTU) (Table 2 and S2).
The genus Burkholderia, which currently includes more than 90 species (http://www.bacterio.net/burkholderia.html, accessed September 23, 2015), has been divided into at least three phylogenetically and ecologically distinct clades. The first clade consists of human, animal, and plant pathogens and is designated as the BCC&P (“B. cepacia complex and B. pseudomallei”) group (8, 56, 59); the second clade includes plant growth-promoting rhizobacteria and nodule-forming plant symbionts, assigned as the PBE (“plant-associated beneficial and environmental”) group (59), which also contains a recently described subclade, called the iPBE (“insect- and plant-associated beneficial and environmental”) group (63); and the third clade is described as the SBE (“stinkbug-associated beneficial and environmental”) group (22, 35), containing free-living soil Burkholderia strains, leaf-gall symbionts of Psychotria plants, and a number of gut symbionts of Coreoidea and Lygaeoidea stinkbugs.
Phylogenetic analyses based on 16S rRNA gene sequences showed that the Burkholderia OTUs detected from the midgut crypts of the stenocephalid stinkbugs were placed in three distinct groups: PBE, SBE, and another distinct cluster that contains B. sordidicola and B. udeis with a 68% support value (the ML tree is shown in Fig. 2; NJ and Bayesian trees showed basically identical topologies). The last novel cluster is designed here as the “Stenocephalidae-clade”. Depending on the species and source of collection, stenocephalid bugs were associated with different Burkholderia strains (Fig. 2, S1 and Table 2, S2). For example, Burkholderia from one individual of D. albipes collected in Italy were placed close to rhizobacteria and nodule symbionts in the PBE-group, whereas Burkholderia from another D. albipes individual collected in France clustered together with Burkholderia from D. medius and D. agilis in the “Stenocephalidae-clade”. In addition, all Burkholderia from the Japanese species D. lateralis clustered in the SBE-group, which did not correspond to any Burkholderia OTUs from European stenocephalid bugs. The phylogenetic tree based on gyrB sequences showed basically the same clusters as those estimated by 16S rRNA sequences (Fig. S1). Notably, the “Stenocephalidae-clade” was also recognized in the gyrB tree with a high support value of 100%.
Fig. 2.
Molecular phylogeny of the gut symbiotic bacteria of stenocephalid species based on 16S rRNA gene sequences. The tree displays a maximum likelihood (ML) phylogeny of ten OTUs (>99% sequence identity) of the gut symbiotic bacteria (GS) identified from Dicranocephalus albipes, D. agilis, D. lateralis, and D. medius together with selected representatives of the different Burkholderia groups. Depending on the species and their collecting site, the gut symbionts of Stenocephalidae clustered in different Burkholderia subgroups. The alignment of 1,394 nucleotide sites of the bacterial 16S rRNA gene was used. The gut symbionts of the stenocephalid species are shown in bold. The origins or sources of the isolation of Burkholderia strains/sequences are represented in parentheses. Accession numbers in the DNA database (DDBJ/EMBL/GenBank) are shown in square brackets. Burkholderia gut symbionts of other stinkbug families are marked with an asterisk. The major Burkholderia clades (BCC&P, SBE, and PBE) including the subclade “insect-associated PBE (iPBE)” and “Stenocephalidae-clade” described here are indicated on the right. Bootstrap values higher than 50% are depicted at the nodes. A phylogeny of gyrB gene sequences from stenocephalid symbionts and other Burkholderia strains/sequences is shown in Fig. S1. Bayesian (MrBayes) and neighbor-joining (NJ) analyses gave essentially the same results (data not shown).
Phylogenetic placement of the family Stenocephalidae in the infraorder Pentatomomorpha
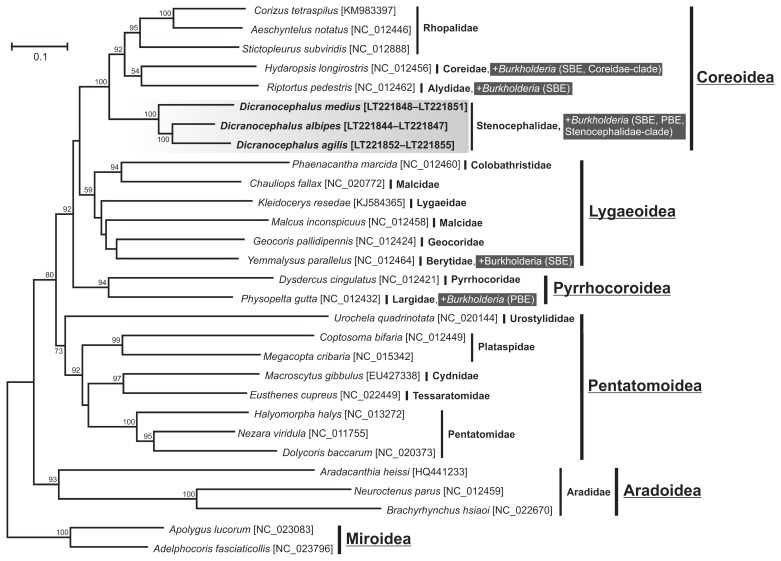
The ML tree of the members of the stinkbug infraorder Pentatomomorpha revealed that the family Stenocephalidae, represented by Dicranocephalus species used in this study, were placed into the superfamily Coreoidea (Fig. 3). Similarly, when the concatenated alignment of COI+ND6+cytB+ND1 or separate mitochondrial gene alignments were used for the analysis, all resulted in the same placement of the Stenocephalidae into the superfamily Coreoidea.
Fig. 3.
Phylogenetic position of the family Stenocephalidae within the infraorder Pentatomomorpha and their associated Burkholderia symbionts. The ML tree illustrates the position of Stenocephalidae as a basal group within the superfamily Coreoidea. In total, 3,996 sites of concatenated mitochondrial COI (1,533 bp), ND6 (417 bp), cytB (1,125 bp), and ND1 (921 bp) protein coding gene sequences of 29 species were used. The mirioid species A. lucorum and L. lineolaris were used as outgroups. Accession numbers in the DNA database (DDBJ/EMBL/GenBank) are shown in square brackets. Bootstrap values higher than 50% are depicted at the nodes. Bayesian (MrBayes) and neighbor-joining (NJ) analyses gave essentially the same results (data not shown).
Discussion
This study demonstrated that the stenocephalid species D. agilis, D. albipes, D. medius, and D. lateralis possess a voluminous, white-colored midgut region with numerous crypts in two rows at the fourth midgut section (Fig. 1), as reported in the coreoid families Coreidae and Alydidae (32). It has repeatedly been reported in diverse stinkbug species that the elimination of symbiotic bacteria results in retarded growth, reduced body size and fecundity, high mortality, and/or abnormal body coloration of the host insects (1, 3, 4, 9, 15, 19, 25, 31, 35, 53, 62, 65), indicating that symbiotic bacteria play a pivotal metabolic role in stinkbug hosts. Although the biological function of the symbionts in stenocephalid bugs remains unclear, the well-developed symbiotic organ (Fig. 1B) and high symbiont density in the midgut crypts (Fig. 1C) strongly suggest a positive influence on host development and reproduction.
Members of the superfamilies Coreoidea and Lygaeoidea are commonly associated with the SBE-group Burkholderia in the midgut crypts (4, 22, 32, 50). In the superfamily Coreoidea, members of the family Coreidae are also associated with a specific group of Burkholderia, called the “Coreidae-clade,” which is closely related to the SBE-group (32) (also see Fig. 2). Although our results for the Japanese species D. lateralis are consistent with these findings, symbionts of European stenocephalid stinkbugs reveal a loosening of this pattern. In addition to the SBE-group and Coreidae-clade Burkholderia, we demonstrated that European stenocephalid stinkbugs are consistently associated with a novel clade of the genus Burkholderia (Table 2 and S2); this clade consists of the insect symbionts and environmental species, B. sordidicola and B. udeis (41, 66), and are here designated as “Stenocephalidae-clade” Burkholderia (Fig. 2). These Stenocephalidae-clade Burkholderia may be highly specialized in the stenocephalid species and play a major role in the hosts. Although PBE-group Burkholderia was only detected once from D. albipes, it emphasizes the possibility that PBE-group Burkholderia are also a pivotal entity in the gut symbiosis of stenocephalid bugs, as demonstrated in the lygaeoid family Blissidae (4, 22) and the family Largidae of the superfamily Pyrrhocoroidea (63).
Recent studies have demonstrated that the Burkholderia symbionts of the bean bug Riptortus pedestris (Coreoidea: Alydidae) and allied-coreoid and -lygaeoid species were orally acquired by nymphal insects from the environment every generation (4, 10, 22, 29, 33), most likely from the rhizosphere of their food plants. This is a flexible system that allows for the horizontal acquisition of environmental bacteria, in contrast to the specific vertical transmission mechanism (e.g., egg smearing and capsule transmission) described in most representatives of the stinkbug superfamily Pentatomoidea (14, 31, 53) and other insects (e.g., reviewed in 5). Our results lead to the hypothesis that stenocephalid bugs also horizontally acquire Burkholderia symbionts from ambient environments every generation based on the following evidence: (1) the symbionts did not form a monophyletic group, but phylogenetically diverse Burkholderia were associated with the species (Fig. 2 and S1); (2) multiple infections of different Burkholderia strains was frequently detected (Table 2 and S2); (3) the symbionts formed a cluster with culturable, free-living Burkholderia species/strains (Fig. 2 and S1).
The phylogenetic placement of the family Stenocephalidae is a long-standing question in the taxonomy of the stinkbug infraorder Pentatomomorpha (11). Our phylogenetic analysis of stinkbugs clearly demonstrated that the family Stenocephalidae is a part of the superfamily Coreoidea with robust support values (Fig. 3). The phylogenetic tree is basically agreeable with a more extensive analysis based on complete mitochondrial genomes (67), with minor exceptions in the placement of the superfamily Pyrrhocoroidea and the different clustering of two Malcidae species within the superfamily Lygaeoidea. Nevertheless, given this phylogenetic position of the Stenocephalidae, it is assumed that the stenocephalid species are not only associated with SBE-group Burkholderia, similar to other members of the superfamily Coreoidea, but have also established a symbiotic relationship with “Stenocephalidae-clade” Burkholderia during the evolution of the insect lineage.
The discrepancy in Burkholderia symbionts harbored in different Dicranocephalus species, e.g., the specific associations between Japanese D. lateralis and SBE-group Burkholderia and between European Dicranocephalus species and “Stenocephalidae-clade” Burkholderia, may be explained by ectogenous and/or endogenous factors. If the environmental soil inhabited by Dicranocephalus bugs is dominated by only one specific group of Burkholderia, the host-symbiont association pattern may be in a region-dependent manner. Alternatively, all Burkholderia groups are ubiquitous, but the insects may select the symbiont species or strains internally. A recent study in the bean bug R. pedestris discovered an intestinal-specific organ, called the “constricted region”, for symbiont sorting (49). This organ was also found in the Dicranocephalus species (data not shown), strongly suggesting bacterial gut sorting in this species, which may play a role in the establishment of Burkholderia specificity. These ectogenous and endogenous hypotheses may be tested using the reciprocal exchange of symbionts between the Japanese and European Dicranocephalus species.
Besides the fundamental questions about the transfer and establishment of stenocephalid-Burkholderia symbionts, further analyses in respect to different collection sites of stenocephalid bugs and several additional Coreoidea/Lygaeoidea species from Europe and other continents in the world are necessary in order to elucidate which Burkholderia strains, in principle, have the ability to establish a stable symbiotic relationship with stinkbugs. Notably, it was recently demonstrated that the ongoing evolution of obligate symbiotic gut bacteria from environmental free-living bacteria occurred in natural pentatomid stinkbug populations (20). A worldwide screening of stenocephalid species may provide a unique opportunity to study the currently ongoing evolution of expanding symbiont associations in stinkbug-Burkholderia symbioses.
Supplementary Material
Acknowledgements
We thank S. Geimer and R. Grotjahn for their assistance in the electron microscopy analysis, as well as D. Scholz and B. Westermann for the opportunity to use the fluorescence microscope and for providing help. We also thank C. Morkel, S. Kehl, K. Kohno, and T. Hosokawa for collecting and providing insect samples, A. Kirpal for technical assistance, and the Japan Society for the Promotion of Science (JSPS) for affording the postdoctoral fellowship JSPS summer program (grant number SP13307) to S. M. K.
References
- 1.Abe Y., Mishiro K., Takanashi M. Symbiont of brown-winged green bug, Plautia stali Scott. Jpn J Appl Entomol Zool. 1995;39:109–115. [Google Scholar]
- 2.Bansal R., Michel A.P., Sabree Z.L. The crypt-dwelling primary bacterial symbiont of the polyphagous pentatomid pest Halyomorpha halys (Hemiptera: Pentatomidae) Environ Entomol. 2014;43:617–625. doi: 10.1603/EN13341. [DOI] [PubMed] [Google Scholar]
- 3.Bistolas K.S., Sakamoto R.I., Fernandes J.A., Goffredi S.K. Symbiont polyphyly, co-evolution, and necessity in pentatomid stinkbugs from. Costa Rica Front Microbiol. 2014;5:349. doi: 10.3389/fmicb.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucias D.G., Garcia-Maruniak A., Cherry R., Lu H., Maruniak J.E., Lietze V.-U. Detection and characterization of bacterial symbionts in the Heteropteran, Blissus insularis. FEMS Microbiol Ecol. 2012;82:629–641. doi: 10.1111/j.1574-6941.2012.01433.x. [DOI] [PubMed] [Google Scholar]
- 5.Bright M., Bulgheresi S. A complex journey: transmission of microbial symbionts. Nat Rev Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchner P. Endosymbiosis of animals with plant microorganisms. Interscience Publishers; New York, NY: 1965. [Google Scholar]
- 7.Caporaso J.G., Kuczynski J., Stombaugh J., et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W.-M., de Farja S.M., Chou J.H., James E.K., Elliott G.N., Sprent J.I., Bontemps C., Young J.P.W., Vandamme P. Burkholderia sabiae sp. nov., isolated from root nodules of Mimosa caesalpiniifolia. Int J Syst Evol Microbiol. 2008;58:2174–2179. doi: 10.1099/ijs.0.65816-0. [DOI] [PubMed] [Google Scholar]
- 9.Fukatsu T., Hosokawa T. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacopta punctatissima. Appl Environ Microbiol. 2002;68:389–396. doi: 10.1128/AEM.68.1.389-396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia J.R., Laughton A.M., Malik Z., Parker B.J., Trincot C., Chiang S.S.L., Chung E., Gerardo N.M. Partner associations across sympatric broad-headed bug species and their environmentally acquired bacterial symbionts. Mol Ecol. 2014;23:1333–1347. doi: 10.1111/mec.12655. [DOI] [PubMed] [Google Scholar]
- 11.Henry T.J. Phylogenetic analysis of family groups within the infraorder Pentatomorpha (Hemiptera: Heteroptera), with emphasis on the Lygaeoidea. Ann Entomol Soc Am. 1997;90:275–301. [Google Scholar]
- 12.Henry T.J. Biodiversity of Heteroptera. In: Foottit R.G., Adler P.H., editors. Insect Biodiversity. Wiley-Blackwell; Chichester: 2009. pp. 223–263. [Google Scholar]
- 13.Hirose E., Panizzi A.R., De Souza J.T., Cattelan A.J., Aldrich J.R. Bacteria in the gut of southern green stinkbug (Heteroptera: Pentatomidae) Ann Entomol Soc Am. 2006;99:91–95. [Google Scholar]
- 14.Hosokawa T., Kikuchi Y., Meng X.-Y., Fukatsu T. The making of symbiont capsule in the plataspid stinkbug Megacopta punctatissima. FEMS Microbiol Ecol. 2005;54:471–477. doi: 10.1016/j.femsec.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa T., Kikuchi Y., Nikoh N., Shimada M., Fukatsu T. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006;4:e337. doi: 10.1371/journal.pbio.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa T., Koga R., Kikuchi Y., Meng X.-Y., Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosokawa T., Kikuchi Y., Nikoh N., Meng X.-Y., Hironaka M., Fukatsu T. Phylogenetic position and peculiar genetic traits of a midgut bacterial symbiont of the stinkbug Parastrachia japonensis. Appl Environ Microbiol. 2010;76:4130–4135. doi: 10.1128/AEM.00616-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosokawa T., Hironaka M., Mukai H., Inadomi K., Suzuki N., Fukatsu T. Mothers never miss the moment: a fine-tuned mechanism for vertical symbiont transmission in a subsocial insect. Anim Behav. 2012;83:293–300. [Google Scholar]
- 19.Hosokawa T., Kikuchi Y., Nikoh N., Fukatsu T. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012;78:4758–4761. doi: 10.1128/AEM.00867-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosokawa T., Ishii Y., Nikoh N., Fujie M., Satoh N., Fukatsu T. Obligate bacteruial mutualists evolving from environmental bacteria in natural insect populations. Nat Microbiol. 2016;1:15011. doi: 10.1038/nmicrobiol.2015.11. [DOI] [PubMed] [Google Scholar]
- 21.Huelsenbeck J.P., Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 22.Itoh H., Aita M., Nagayama A., Meng X.-Y., Kamagata Y., Navarro R., Hori T., Ohgiya S., Kikuchi Y. Evidence of environmental and vertical transmission of Burkholderia symbionts in the oriental chinch bug, Cavelerius saccharivorus (Heteroptera: Blissidae) Appl Environ Microbiol. 2014;80:5974–5983. doi: 10.1128/AEM.01087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiwa N., Hosokawa T., Kikuchi Y., Nikoh N., Meng X.-Y., Kimura N., Ito M., Fukatsu T. Primary gut symbiont and secondary, Sodalis-allied symbiont of the scutellerid stinkbug Cantao ocellatus. Appl Environ Microbiol. 2010;76:3486–3494. doi: 10.1128/AEM.00421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiwa N., Hosokawa T., Kikuchi Y., Nikoh N., Meng X.-Y., Kimura N., Ito M., Fukatsu T. Bacterial symbionts of the giant jewel stinkbug Eucorysses grandis (Hemiptera: Scutelleridae) Zoolog Sci. 2011;28:169–174. doi: 10.2108/zsj.28.169. [DOI] [PubMed] [Google Scholar]
- 25.Kaiwa N., Hosokawa T., Nikoh N., et al. Symbiont-supplemented maternal investment underpinning host’s ecological adaptation. Curr Biol. 2014;24:2465–2470. doi: 10.1016/j.cub.2014.08.065. [DOI] [PubMed] [Google Scholar]
- 26.Kaltenpoth M., Winter S.A., Kleinhammer A. Localization and transmission route of Coriobacterium glomerans, the endosymbiont of pyrrhocorid bugs. FEMS Microbiol Ecol. 2009;69:373–383. doi: 10.1111/j.1574-6941.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 27.Katoh K., Standley D. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi Y., Meng X.-Y., Fukatsu T. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae) Appl Environ Microbiol. 2005;71:4035–4043. doi: 10.1128/AEM.71.7.4035-4043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikuchi Y., Hosokawa T., Fukatsu T. Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol. 2007;73:4308–4316. doi: 10.1128/AEM.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi Y., Hosokawa T., Fukatsu T. Diversity of bacterial symbiosis in stinkbugs. In: van Dijk T., editor. Microbial Ecology Research Trends. Nova Science Publishers, Inc.; New York: 2008. pp. 39–63. [Google Scholar]
- 31.Kikuchi Y., Hosokawa T., Nikoh N., Meng X.-Y., Kamagata Y., Fukatsu T. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009;7:2. doi: 10.1186/1741-7007-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi Y., Hosokawa T., Fukatsu T. An ancient but promiscuous host–symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 2011;5:446–460. doi: 10.1038/ismej.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuchi Y., Hosokawa T., Fukatsu T. Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl Environ Microbiol. 2011;77:4075–4081. doi: 10.1128/AEM.00358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kikuchi Y., Hosokawa T., Nikoh N., Fukatsu T. Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae) Appl Entomol Zool. 2011;47:1–8. [Google Scholar]
- 35.Kikuchi Y., Hayatsu M., Hosokawa T., Nagayama A., Tago K., Fukatsu T. Symbiont-mediated insecticide resistance. Proc Natl Acad Sci USA. 2012;109:8618–8622. doi: 10.1073/pnas.1200231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuechler S.M., Dettner K., Kehl S. Molecular characterization and localization of the obligate endosymbiotic bacterium in the birch catkin bug Kleidocerys resedae (Heteroptera: Lygaeidae, Ischnorhynchinae) FEMS Microbiol Ecol. 2010;73:408–418. doi: 10.1111/j.1574-6941.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 37.Kuechler S.M., Dettner K., Kehl S. Characterization of an obligate intracellular bacterium in the midgut epithelium of the bulrush bug Chilacis typhae (Heteroptera, Lygaeidae, Artheneinae) Appl Environ Microbiol. 2011;77:2869–2876. doi: 10.1128/AEM.02983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuechler S.M., Renz P., Dettner K., Kehl S. Diversity of symbiotic organs and bacterial endosymbionts of lygaeoid bugs of the families Blissidae and Lygaeidae (Hemiptera: Heteroptera: Lygaeoidea) Appl Environ Microbiol. 2012;78:2648–2659. doi: 10.1128/AEM.07191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landsbury I. A revision of the Stenocephalidae Dallas, 1852 (Hemiptera, Heteroptera) Entomol Monthly Mag. 1965;101:52–92. [Google Scholar]
- 40.Landsbury I. A revision of the Stenocephalidae Dallas, 1852 (Hemiptera, Heteroptera) Entomol Monthly Mag. 1966;101:145–150. [Google Scholar]
- 41.Lim Y.W., Baik K.S., Han S.K., Kim S.B., Bae K.S. Burkholderia sordidicola sp. nov., isolated from the white-rot fungus Phanerochaete sordida. Int J Syst Evol Microbiol. 2003;53:1631–1636. doi: 10.1099/ijs.0.02456-0. [DOI] [PubMed] [Google Scholar]
- 42.Loy A., Arnold R., Tischler P., Rattei T., Wagner M., Horn M. probeCheck—a central resource for evaluating oligonucleotide probe coverage and specificity. Environ Microbiol. 2008;10:2894–2896. doi: 10.1111/j.1462-2920.2008.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuura Y., Kikuchi Y., Hosokawa T., Koga R., Meng X.-Y., Kamagata Y., Nikoh N., Fukatsu T. Evolution of symbiotic organs and endosymbionts in lygaeid stinkbugs. ISME J. 2012 doi: 10.1038/ismej.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuura Y., Hosokawa T., Serracin M., Tulgetske G.M., Miller T.A., Fukatsu T. Bacterial symbionts of a devastating coffee plant pest, the stinkbug Antestiopsis thunbergii (Hemiptera: Pentatomidae) Appl Environ Microbiol. 2014;80:3769–3775. doi: 10.1128/AEM.00554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moulet P. Faune de France. Paris: 1995. Hémiptères Coreoidea (Coreidae, Rhopalidae, Alydidae), Pyrrhocoridae, Stenocephalidae, Euro-Méditerraneéns. (France et régions limitrophes 81). [Google Scholar]
- 46.Moulet P. Synonymies nouvelles dans la famille des Stenocephalidae Latrille, 1825 (Heteroptera, Stenocephalidae) Nouv Rev Entomol. 1995;11:353–364. [Google Scholar]
- 47.Müller H.J. Experimentelle Studien an der Symbiose von Coptosoma scutellatum Geoffr. (Hem. Heteropt.) Z Morphol Ökol Tiere. 1956;44:459–482. [Google Scholar]
- 48.Nylander J.A.A. MrModeltest, version 2. Evolutionary Biology Centre, Uppsala University; Uppsala, Sweden: 2004. [Google Scholar]
- 49.Ohbayashi T., Takeshita K., Kitagawa W., et al. Insect’s intestinal organ for symbiont sorting. Proc Natl Acad Sci USA. 2015;112:5179–5188. doi: 10.1073/pnas.1511454112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olivier-Espejel S., Sabree Z.L., Noge K., Becerra J.X. Gut microbiota in nymph and adults of the giant mesquite bug (Thasus neocalifornicus) (Heteroptera: Coreidae) is dominated by Burkholderia acquired de novo every generation. Environ Entomol. 2011;40:1102–1110. doi: 10.1603/EN10309. [DOI] [PubMed] [Google Scholar]
- 51.Prado S.S., Rubinoff D., Almeida R.P.P. Vertical transmission of a pentatomid caeca-associated symbiont. Ann Entomol Soc Am. 2006;99:577–585. [Google Scholar]
- 52.Prado S.S., Golden M., Follett P.A., Daugherty M.P., Almeida R.P.P. Demography of gut symbiotic and aposymbiotic Nezara viridula L. (Hemiptera: Pentatomidae) Environ Entomol. 2009;38:103–109. doi: 10.1603/022.038.0112. [DOI] [PubMed] [Google Scholar]
- 53.Prado S.S., Almeida R.P.P. Phylogenetic placement of pentatomid stinkbug gut symbionts. Curr Microbiol. 2009;58:64–69. doi: 10.1007/s00284-008-9267-9. [DOI] [PubMed] [Google Scholar]
- 54.Prado S.S., Zucchi T.D. Host-symbiont interactions for potentially managing heteropteran pests. Psyche. 2012 doi: 10.1155/2012/269473. [DOI] [Google Scholar]
- 55.Rosenkranz W. Die Symbiose der Pentatomiden. Z Morphol Ökol Tiere. 1939;36:279–309. [Google Scholar]
- 56.Sawana A., Adeolu M., Gupta R.S. Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front Genet. 2014;5:1–22. doi: 10.3389/fgene.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider G. Beiträge zur Kenntnis der symbiontischen Einrichtungen der Heteropteren. Z Morphol Ökol Tiere. 1940;36:565–644. [Google Scholar]
- 58.Schuh R.T., Slater J.A. True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History. Cornell University Press; Ithaca, New York: 1995. [Google Scholar]
- 59.Suárez-Moreno Z.R., Caballero-Mellado J., Coutinho B.G., Mendonça-Previato L., James E.K., Venturi V. Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb Ecol. 2012;63:249–266. doi: 10.1007/s00248-011-9929-1. [DOI] [PubMed] [Google Scholar]
- 60.Sudakaran S., Salem H., Kost C., Kaltenpoth M. Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae) Mol Ecol. 2012;21:6134–6151. doi: 10.1111/mec.12027. [DOI] [PubMed] [Google Scholar]
- 61.Sudakaran S., Retz F., Kikuchi Y., Kost C., Kaltenpoth M. Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. ISME J. 2015;9:2587–2604. doi: 10.1038/ismej.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tada A., Kikuchi Y., Hosokawa T., Musolin D.L., Fujisaki K., Fukatsu T. Obligate association with gut bacterial symbiont in Japanese populations of southern green stinkbug Nezara viridula (Heteroptera: Pentatomidae) Appl Entomol Zool. 2011;46:483–488. [Google Scholar]
- 63.Takeshita K., Matsuura Y., Itoh H., Navarro R., Hori T., Sone T., Kamagata Y., Mergaert P., Kikuchi Y. Burkholderia of plant-beneficial group are symbiotically associated with bordered plant bugs (Heteroptera: Pyrrhocoroidea: Largidae) Microbes Environ. 2015;30:321–329. doi: 10.1264/jsme2.ME15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor C.M., Coffey P.L., DeLay B.D., Dively G.P. The importance of gut symbionts in the development of the brown marmorated stink bug, Halyomorpha halys (Stål) PLoS ONE. 2014;9:e90312. doi: 10.1371/journal.pone.0090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vandamme P., De Brandt E., Houf K., Salles J.F., Dirk van Elsas J., Spilker T., Lipuma J.J. Burkholderia humi sp. nov., Burkholderia choica sp. nov., Burkholderia telluris sp. nov., Burkholderia terrestris sp. nov. and Burkholderia udeis sp. nov.: Burkholderia glathei-like bacteria from soil and rhizosphere soil. Int J Syst Evol Microbiol. 2013;63:4707–4718. doi: 10.1099/ijs.0.048900-0. [DOI] [PubMed] [Google Scholar]
- 67.Yuan M.-L., Zhang Q.-L., Guo Z.-L., Wang J., Shen Y.-Y. The complete mitochondrial genome of Corizus tetraspilus (Hemiptera: Rhopalidae) and phylogenetic analysis of Pentatomomorpha. PLoS ONE. 2015;106:e0129003. doi: 10.1371/journal.pone.0129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zucchi T.D., Prado S.S., Cônsoli F.L. The gastric caeca as a house for actinomycetes—Do they work as an antibiotic factory for stinkbugs? BMC Microbiol. 2012;12:101. doi: 10.1186/1471-2180-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.