Abstract
Generalized CD8+ T-cell impairment in chronic hepatitis C virus (HCV) infection and the contribution of liver-infiltrating CD8+ T-cells to the immunopathogenesis of this infection remain poorly understood. It is hypothesized that this impairment is partially due to reduced CD8+ T-cell activity in response to cytokines such as IL-7, particularly within the liver. To investigate this, the phenotype and cytokine responsiveness of blood- and liver-derived CD8+ T-cells from healthy controls and individuals with HCV infection were compared. In blood, IL-7 receptor α (CD127) expression on bulk CD8+ T-cells in HCV infection was no different than controls yet was lower on central memory T-cells, and there were fewer naïve cells. IL-7-induced signalling through phosphorylated STAT5 was lower in HCV infection than in controls, and differed between CD8+ T-cell subsets. Production of Bcl-2 following IL-7 stimulation was also lower in HCV infection and inversely related to the degree of liver fibrosis. In liver-derived CD8+ T-cells, STAT5 activation could not be increased with cytokine stimulation and basal Bcl-2 levels of liver-derived CD8+ T-cells were lower than blood-derived counterparts in HCV infection. Therefore, generalized CD8+ T-cell impairment in HCV infection is characterized, in part, by impaired IL-7-mediated signalling and survival, independent of CD127 expression. This impairment is more pronounced in the liver and may be associated with an increased potential for apoptosis. This generalized CD8+ T-cell impairment represents an important immune dysfunction in chronic HCV infection that may alter patient health.
Introduction
Acute infection of hepatitis C virus (HCV) is spontaneously cleared in a minority of those infected, and relies on effective virus-specific CD8+ T-cell mediated responses [1–4]. Failure to clear the virus is associated with HCV-specific CD8+ T-cells with impaired proliferation and cytokine production [5, 6]; a common characteristic of chronic viral infections such as hepatitis B virus (HBV), HIV [7, 8], and HIV-HCV co-infection, as shown by Barrett et al. [9]. This dysfunction is reportedly more pronounced compared to CMV-, EBV-, or influenza-specific cells within the same individual [7, 10, 11]. However, impairment has been observed regardless of antigen specificity in bulk CD8+ T-cells, characterized by increased potential for inducible apoptosis and lower basal perforin expression [12, 13]. Hence, CD8+ T-cell dysfunction in HCV infection is a generalized phenomenon. While there is no specific clinical immunodeficiency in hepatic viral infections, cirrhosis-associated immune dysfunction syndrome (CAIDS) [14] and increased risk of community-acquired infections such as pneumonia [15, 16] are not uncommon. There is some evidence that progressive liver fibrosis is correlated with impairment of HCV-specific and HCV non-specific CD8+ T-cells [17]. Furthermore, bystander CD8+ T-cell dysfunction may contribute to a more rapid progression to AIDS in HIV-HCV co-infection compared to HIV mono-infection [18–20].
The mechanisms mediating CD8+ T-cell dysfunction in chronic HCV infection are not well understood. Increased IL-10 production by peripheral blood mononuclear cells (PBMC) and IL-10+ HCV-specific CD8+ T-cells may impair the response [21, 22]. Expression of the inhibitory receptors PD-1 and Tim-3, on both bulk and HCV-specific CD8+ T-cells, are associated with reduced proliferation and IFN-γ production [23–26]. Early expression of these receptors on HCV-specific CD8+ T-cells can predict progression to chronic infection while high interleukin-7 receptor α (CD127) expression foretells spontaneous clearance and protection [4, 25, 27, 28].
IL-7 is critical for T-cell development and is important for memory cell generation, homeostasis [29–31], as its signalling molecules are directly linked to CD8+ T-cell activity (i.e. proliferation, perforin accumulation, Bcl-2 production, and glucose uptake) [32]. In chronic HCV infection, low CD127 expression on HCV-specific CD8+ T-cells inversely correlates with viral load, though the expression on bulk CD8+ T-cells is similar to controls [33]. The potential role of impaired IL-7 responsiveness in CD8+ T-cell dysfunction observed in HCV infection is unknown.
In chronic HCV infection, the dysfunction of CD8+ T-cells extends to liver-infiltrating intrahepatic (IH) T-cells. Higher co-expression of PD-1 and Tim-3 on IH-bulk and IH-HCV-specific CD8+ T-cells [23, 25, 34], and lower CD127 expression on IH-HCV-specific CD8+ T-cells has been observed compared to circulating cells in the same individual [23, 35]. HCV-specific IH-CD8+ T-cells have decreased IFN-γ production in response to their cognate antigens compared to other non-HCV-specific memory CD8+ T-cells [11], although the function of bulk IH-CD8+ T-cells remains largely unknown.
Understanding generalized CD8+ T-cell dysfunction in HCV infection will provide insight into the mechanisms establishing chronic infection, progression of liver fibrosis, and other associated immunological impairments. In this report, we tested the hypothesis that bulk circulating and IH-CD8+ T-cells in HCV infection have a reduced response to IL-7, and found that CD8+ T-cells are phenotypically different with impaired responsiveness to IL-7 detectable among bulk CD8+ T-cells.
Materials and Methods
Patients
Study participants were healthy, HCV- donors or treatment naïve, chronically infected HCV+ individuals (i.e. ≥ 6 months HCV RNA+). Age, gender, and ethnicity are summarized in Table 1. Fibrosis scores were determined by fibroscan, and were grouped by fibrosis stage (F0-F2 or F3-F4, with those that are classified as F2-F3 included in the F3-F4 grouping). This study was approved by The Ottawa Health Science Network Research Ethics Board, and written informed consent was obtained from all individuals.
Table 1. Baseline Characteristics of Study Participants.
| Controls | HCV+ individuals evaluated in functional experiments | HCV+ individuals included in whole blood phenotype study | |
|---|---|---|---|
| n | 51 | 29 | 50 |
| Sex (male, female)a | M26, F25 | M21, F8 | M39, F11 |
| Mean age | 35.0 ± 10.8 | 48.9 ± 12.5 | 49.4 ± 9.9 |
| Ethnicity (%White) | 88% | 86% | 92% |
| HCV Genotype | |||
| 1 | 22/29 | 33*/49 | |
| 2 | 1/29 | 6/49 | |
| 3 | 6/29 | 8/49 | |
| 4 | 0/29 | 3*/49 | |
| Fibrosis Stageb | |||
| 0–2 | 20/28 | 35/44 | |
| 2–4 | 8/28 | 9/44 | |
| Mean HCV RNA (IU/ml) | 7.58x106 ± 1.1x107 | ||
| Mean ALTb | 90 ± 58 |
a M (male), F (female)
b measured by liver biopsy (Metavir system) or by fibroscan
*One participant with genotype 1 and 4 co-infection
Note: There is no fibrosis or genotype data for some HCV+ individuals.
Isolation and culture of lymphocytes
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient density centrifugation (Lymphoprep, Stemcell Technologies, Vancouver, Canada), and CD8+ T-cells were then isolated by magnetic bead positive selection (Stemcell Technologies, Vancouver, Canada). IH-lymphocytes were isolated from fresh liver biopsies (1mm x 1mm x 3cm) collected from HCV+ individuals as part of their routine care and processed by mechanical disruption and enzyme digestion as described previously [36, 37]. Briefly, tissue was cut into small (1mm) pieces and incubated with 500U collagenase IV and 2% FCS in HBSS (Gibco, Life Technologies, Burlington, Ontario, Canada), 50U DNase I (Sigma-Aldrich, Oakville, Ontario, Canada), and 0.6% BSA for 20 minutes at 37°C. Tissue was then manually disrupted with a syringe end and filtered through a 70μm filter (Fisher Scientific, Waltham, MA, USA) to remove undigested tissue. Cells were washed in HBSS and cultured. Both blood-derived CD8+ T-cells and IH-lymphocytes were cultured at 1x106/ml in complete RPMI (supplemented with 20% FCS, 1% L-glutamine, and 0.5% penicillin/streptomycin (Gibco, Life Technologies)) at 37°C, 5% CO2.
Flow cytometry and phenotypic analysis of CD8+ T-cells
The phenotypes of CD8+ T-cells in whole blood (using Optylyse, Beckman Coulter, Marseille, France) or isolated cells were distinguished by flow cytometry using multiple antibodies: CD127-PE (5μl, clone R34.34, AB_131301, Beckman Coulter), CD8-FITC/PeCy5 (5μl, clone HIT8a, AB_395996 and AB_395998), CD45RA-APC/PECy5 (3μl, clone HI100, AB_398468 and AB_395881, BD Pharmingen, BD Bioscience, San Jose, CA, USA) and CCR7-APCCy7 (5μl, clone G043H7, AB_10916390, Biolegend, San Diego, CA, USA). Freshly isolated cells (1x105 lymphocytes per sample) were incubated in 1% BSA-PBS (100μl) for 30 minutes on ice, followed by 2 washes with 1% BSA-PBS, protocol adapted from Nascimbeni and Rehermann [37]. Cell subsets were distinguished as follows: naïve (TN, CD45RA+CCR7+), central memory (TCM, CD45RA-CCR7+), effector memory (TEM, CD45RA-CCR7-), and terminally differentiated effector memory (TEMRA, CD45RA+CCR7-). When analyzing IH-CD8+ T-cells, the flow cytometer was calibrated using blood CD8+ T-cells to conserve the number of IH-CD8+ T-cells available for study, which revealed a higher degree of autofluorescence in IH-CD8+ T-cells compared to blood-derived cells, as previously reported by others [38]. All flow cytometry analyses excluded dead cells, on the basis of forward and side scatter profiles, and gates were set using the principle of fluorescence minus one (FMO).
Measurement of pSTAT5
Isolated blood-derived CD8+ T-cells or IH-lymphocytes (0.5x106/ml) were incubated with IL-7 (0.01–10 ng/ml) and/or IL-2 (100 ng/ml) and IL-15 (10 ng/ml, Sigma Aldrich, St. Louis, MO, USA) for 15 minutes at 37°C and phosphorylation of STAT5 (pSTAT5) was measured by flow cytometry, as described previously using the anti-pSTAT5 pY694 alexafluor 488 antibody (5μl/100μl cells, clone 47/STAT5(pY694), AB_399881, BD Phosflow, BD Bioscience) with fixation in 4% paraformaldehyde and permeabilization in 100% cold methanol [32] (average age of controls was 39 ± 13, HCV+ individuals was 43 ± 12). The expression level of pSTAT5 in CD8+ T-cell subsets was determined after cells were stained for phenotypic markers using CD45RA-APC and CCR7 antibodies (average age of controls was 35 ± 11, HCV+ individuals was 56 ± 10). To distinguish CD8+ T-cells from other IH-lymphocytes, 5μl CD8-PeCy5 was added with the pSTAT5 antibody. The autofluorescence of IH-lymphocytes is higher than blood-derived cells, and this was taken into account during data analysis [38].
Proliferation of CD8+ T-cells
Isolated CD8+ T-cells were labeled with carboxyfluoresceinsuccinimidyl ester (CFSE, 8μM, Cell Trace CFSE Cell Proliferation Kit, Molecular Probes, Life technologies) and cultured with IL-7 (10ng/ml) and a suboptimal concentration of the T-cell mitogen phytohaemagglutinin (PHA, 0.2ug/ml, Sigma Aldrich) for 5 days, as described previously [39] (average age of controls was 31 ± 11, HCV+ individuals was 52 ± 02). Colchicine (100ng/ml, Sigma Aldrich) was used a negative control. Proliferation (CFSE dilution) was determined by flow cytometry.
Measurement of Bcl-2 Expression
Expression of Bcl-2 in CD8+ T-cells was determined after overnight rest of isolated cells and after incubation with IL-7 (0.01-10ng/ml) for 48 hours. Specifically, cells were analysed by flow cytometry using an anti-Bcl-2 FITC (5μl/100μl cells, clone Bcl-2/100, AB_396382) antibody, and an IgG1-FITC isotype control (5μl/100μl cells, clone MOPC-21, BD Pharmingen) as described previously [32] with fixation in 4% paraformaldehyde and permeabilization in 1% saponin (Sigma Aldrich) (average age of controls was 32 ± 11, HCV+ individuals was 46 ± 12). To distinguish CD8+ T-cells from other IH-lymphocytes, 5μl CD8-PeCy5 was added before fixation in 1% BSA-PBS (100μl) for 20 minutes at room temperature. The autofluorescence of IH-lymphocytes relative to blood cells was taken into account during data analysis [38].
Analysis and Statistics
Flow cytometry was completed using an FC500 Beckman Coulter flow cytometer followed by analysis using FCS Express Research Edition 4.0 (De Novo Software, Los Angeles, CA, USA). Graphs and statistics were generated using GraphPad Prism 5.0 Software (San Diego, CA, USA). Where necessary, statistical analyses included two-way, unpaired Student’s t-test, one-way ANOVA with Dunnett post-test, and/or non-linear regression (p ≤ 0.05), and data are presented in text and graphical form as means ± standard deviation.
Results
Blood-derived CD8+ T-cell subsets differ between HCV- controls and HCV-infected individuals
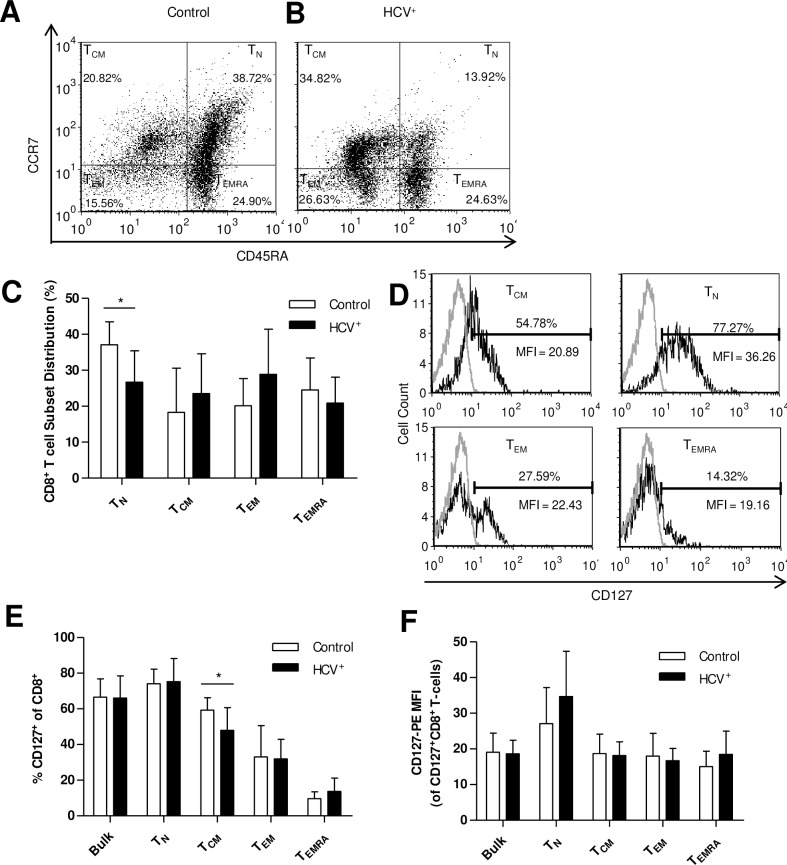
The distribution of blood-derived CD8+ T-cell subsets was evaluated to detect any inherent differences in chronic HCV infection. PBMCs were stained with anti-CD8 antibody, and subsets distinguished with anti-CD45RA and anti-CCR7 antibodies, as established by Sallusto et al [40]. Blood-derived CD8+ T-cell subset distribution in HCV- individuals was as follows (mean ± SD): TN (37.1% ± 2.0) > TEMRA (24.5% ± 2.8) > TEM (20.12% ± 2.4) > TCM (18.3% ± 3.9) (Fig 1A), and is consistent with previous reports [31, 41]. In chronic HCV infection, the ranking of subsets was subtly different: TEM (28.9% ± 3.6) > TN CD8+ T-cells (26.7% ± 2.5) > TCM (23.5% ± 3.2) > TEMRA (20.9% ± 2.1) (Fig 1B). There were no significant differences in the proportions of TEM, TCM and TEMRA cells compared to controls. However, the proportion of TN cells was significantly lower in HCV+ individuals (p = 0.006 by Student’s t-test, Fig 1C and 1D). There was no detectable association between CD8+ T-cell subset distribution and fibrosis stage or HCV genotype. However, HCV+ individuals in the older age group (≥58 years of age, ≈ 75% percentile of individuals tested) did have an increased proportion of TEMRA cells. In all other experiments, within HCV+ individuals tested, age was not associated with differences in the measured result.
Fig 1. HCV+ individuals have fewer blood-derived naïve CD8+ T-cells and a lower expression of CD127 on central memory cells than HCV- controls.
CD8+ T-cell subset distribution was determined by CD45RA and CCR7 staining in (A) controls (n = 10) and (B) HCV infection (n = 12). (C) Subset distribution data for controls and HCV-infected individuals are graphically represented as means (TN p = 0.006). (D) The expression of CD127 was measured on blood-derived bulk CD8+T-cells (control n = 30, HCV+ n = 50) and their subsets (control n = 10, HCV+ n = 12) and presented as (E) percentage (TCM p = 0.02, unpaired Student’s t-test) and (F) mean fluorescence intensity of CD127 expressing cells (error bars represent ±S.D.).
The expression of CD127 on bulk blood-derived CD8+ T-cells does not differ, though is lower on TCM cells, in HCV infection
To determine if the degree of CD127 receptor expression could contribute to IL-7 responsiveness in subsequent experiments, receptor expression was assessed. In bulk blood-derived CD8+ T-cells, there was no difference in percentage of CD127 expression between HCV- controls and HCV-infected individuals (66.7% ± 1.9 and 66.1% ± 1.8, respectively, Fig 1E), nor in the degree of CD127 expressed on these cells (MFI, Fig 1F). The proportion of CD8+ T-cells expressing CD127 varied by subset; in controls, it was as follows (mean ± SD): TN (74.1% ± 2.6) > TCM (59.3% ± 2.2) > TEM (33.1% ± 5.5) > TEMRA (9.6%± 1.2) (Fig 1D and 1E). This hierarchical pattern of CD127 expression was similar in HCV infection for 3 subsets: TN (75.3% ± 3.8) > TEM (32.0% ± 3.2) > TEMRA (13.7% ± 2.2). There were significantly fewer TCM cells expressing CD127 in HCV infection (48.0% ± 3.7) compared to controls (p = 0.02 by Student’s t-test, Fig 1E. The intensity of CD127 expression on subsets expressing CD127 was the highest in TN cells, with similar intensity in the other 3 subsets, for both experimental groups (Fig 1F). Levels of CD127 expression were not associated with age, fibrosis stage or HCV genotype among HCV+ individuals.
IL-7 signalling through STAT5 is impaired in blood-derived CD8+ T-cells in HCV infection
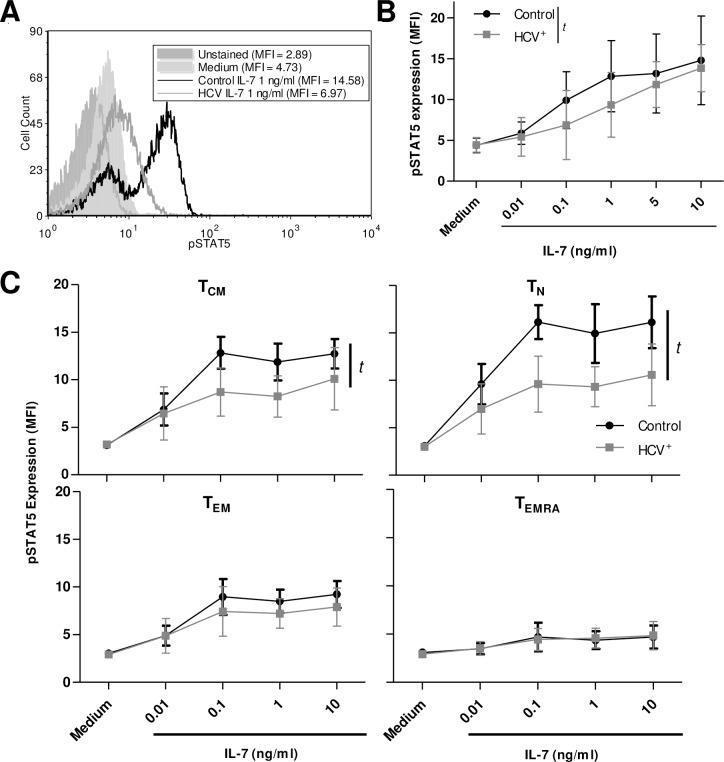
To investigate activation of the Jak/STAT pathway in response to IL-7 in health and chronic HCV infection, the phosphorylation of STAT5 (pSTAT5) was evaluated in CD8+ T-cells. Phosphorylation of STAT5 in response to IL-7 (0.01-10ng/ml) occurred in a dose dependent manner in CD8+ T-cells isolated from HCV- controls and HCV-infected individuals (one-way ANOVA p<0.0001 and p = 0.0003, respectively), as expected [32, 42] (Fig 2A and 2B). Upon IL-7 stimulation, the minimum, and physiological, concentration of IL-7 required for a significant increase in pSTAT5 was lower for controls (0.1ng/ml) than in HCV infection (1ng/ml) (p≤ 0.05, Dunnett post-test). There were no associations with pSTAT5 production and age detected. Since there were no individuals with fibrosis scores >2 among those tested in this assay, it was not possible to analyze any association between the degree of STAT5 activation and liver fibrosis. Overall, across these dose responses, there was less STAT5 activation in IL-7-stimulated CD8+ T-cells from HCV-infected individuals compared to controls (non-linear regression, p = 0.005).
Fig 2. IL-7-induced signaling of blood-derived CD8+ T-cells is impaired in HCV infection.
(A) Phosphorylation of STAT5 was measured as mean fluorescence intensity (MFI) as shown in a representative histogram. (B) The expression of pSTAT5 was significantly increased by increasing concentrations of IL-7 (0.01–10 ng/ml) in blood-derived CD8+ T-cells from controls (p < 0.001, n = 10) or chronically infected HCV+ individuals (p = 0.003, n = 9) is summarized, as assessed by ANOVA, yet responses of the latter group were significantly less pronounced than controls (t: p = 0.005, non-linear regression analysis). (C) The expression of pSTAT5 in CD8+ T-cell subsets were distinguished by CD45RA and CCR7 expression, with significance in TCM and TN subsets (t: p<0.0001 for each subset, non-linear regression, control n = 7, HCV+ n = 5). Error bars in the graphs represent ± S.D.
The degree of pSTAT5 expression by IL-7-stimulated CD8+ subsets in controls (Fig 2C) corresponded with their stratified expression of CD127 (Fig 1E). In control individuals, TN cells phosphorylated STAT5 to the greatest extent, followed by TCM and TEM cells, while TEMRA cells did not phosphorylate STAT5. In HCV infection, IL-7 stimulation activated STAT5 in TN and TCM cells but was significantly lower than in controls (p<0.0001, non-linear regression). Unlike in bulk CD8+ T-cells, the level of pSTAT5 with the highest concentration of IL-7 (10ng/ml) was lower in HCV infection than controls. Unfortunately, the relation between the degree of fibrosis and IL-7-induced pSTAT5 levels could not be examined as only 2 individuals in the study of bulk CD8+ T-cells and 1 individual in the CD8+ T-cell subset studies had fibrosis scores > F2.
Proliferation is not impaired but blood-derived CD8+ T-cells express less Bcl-2 in response to IL-7 in HCV infection
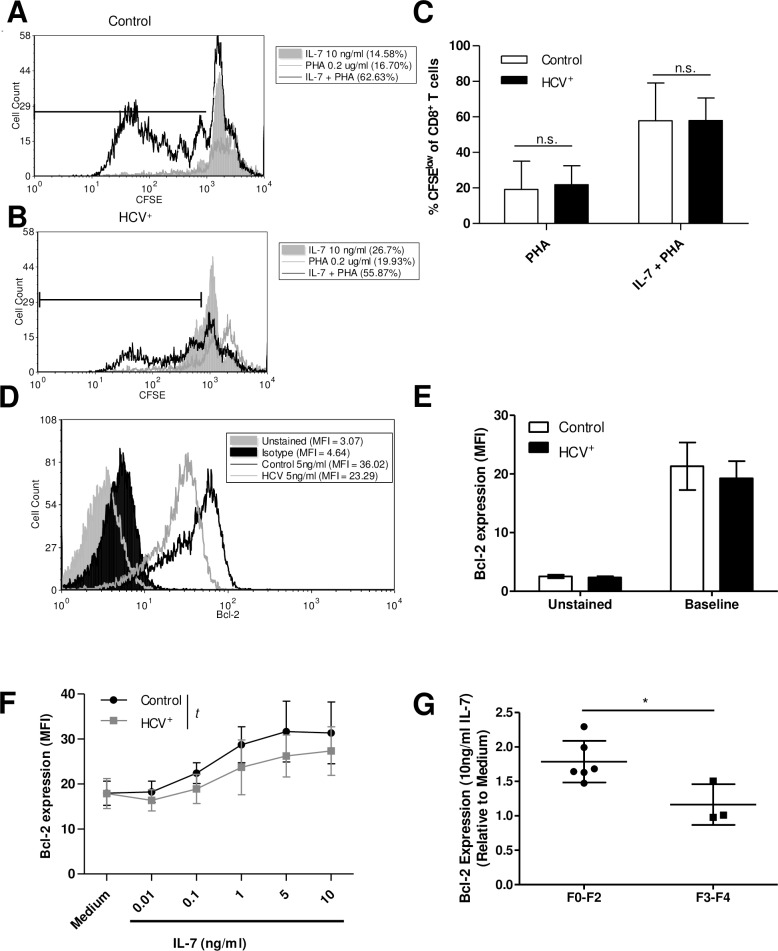
To quantify IL-7-mediated proliferation of blood-derived CD8+ T-cells, CFSE-labelled cells were stimulated with suboptimal amounts of T-cell mitogen (PHA, 0.2 ug/ml), since T-cell activation is required to enable human T-cells to proliferate in response to IL-7 [43]. Cells were cultured with a dose of IL-7 known to induce detectible cell division (10ng/ml) [42]. There was minimal proliferation of cells following culture with IL-7 or PHA alone, while IL-7 + PHA induced multiple cell divisions (%CFSElow cells) (Fig 3A and 3B). However, there was no difference between the IL-7 + PHA mediated proliferation of CD8+ T-cells isolated from HCV- controls (n = 8, 57.9% ± 7.5) and HCV+ individuals (n = 8, 58.0% ± 4.5), nor was there a difference in their proliferation in response to PHA alone (19.2% ± 5.6 and 21.9% ± 3.8, respectively, Fig 3C). No association between age and proliferation was detected among HCV+ individuals, and a correlation analysis of fibrosis score with proliferation was not possible as only 2 out of 9 individuals tested here had scores greater than F2.
Fig 3. IL-7-induced proliferation is not impaired while production of Bcl-2 is reduced in blood-derived CD8+ T-cells from HCV+ individuals.
Cell proliferation in response to IL-7 (10ng/ml) and/or PHA (0.2mg/ml) was measured as CFSE dilution of CD8+ T-cells from (A) HCV- (n = 8) and (B) HCV+ individuals (n = 8), with markers indicating the proportion (%) of CFSElow (dividing) cells. (C) Proliferation of isolated CD8+ T-cells induced by IL-7 + suboptimal PHA was significantly increased in both groups (control p = 0.0001 and HCV+ p<0.0001, unpaired Student’s t-test), yet there was no difference between these groups (n.s. = not significant). (D) Bcl-2 expression of blood-derived CD8+ T-cells was measured. (E) Bcl-2 expression (MFI) of unstimulated CD8+ T-cells is summarized (control n = 4, HCV+ n = 5) and (F) Bcl-2 production in response to IL-7 after 48 hours is summarized as MFI (t p = 0.0006, non-linear regression, control n = 8, HCV+ n = 9). (G) The IL-7-induced expression of Bcl-2 in blood-derived CD8+ T-cells from HCV+ individuals with low fibrosis (F0-F2) was compared to that of high fibrosis (F3-F4). Values are expressed relative to medium alone (* p = 0.02, unpaired Student’s t-test, error bars represent ±S.D.).
To determine the potential for cell survival, the expression of anti-apoptotic Bcl-2 was measured. The ex vivo expression of Bcl-2 of blood-derived CD8+ T-cells from controls and HCV infection were similar (Fig 3E). Culture of CD8+ T-cells with IL-7 (0.01-10ng/ml) increased Bcl-2 levels in a dose-dependent manner in cells isolated from either controls or HCV-infected individuals (Fig 3D and 3F) (one-way ANOVA p<0.0001 for each group). However, despite similar Bcl-2 basal expression across the groups after overnight culture, the magnitude of increase in Bcl-2 expression in response to increasing concentrations of IL-7 was lower in HCV infection (p = 0.0006 by non-linear regression). Similar to STAT5 activation (Fig 2B), Bcl-2 levels were not significantly increased in CD8+ T-cells of HCV-infected individuals when stimulated with less than 1ng/ml of IL-7, while cells from controls produced significantly more Bcl-2 with a log lower concentration of IL-7 (0.1ng/ml, p≤ 0.05, Dunnett post-test).
Of the individuals tested in these Bcl-2 assays, 3 had fibrosis scores of F3-F4 and 6 had scores of F0-F2. Individuals with higher scores of liver fibrosis (i.e. F3-F4 scores) produced significantly less Bcl-2 in response to IL-7 (10ng/ml) compared to those with lower degrees of fibrosis (i.e. F0-F2) (p = 0.02, unpaired Student’s t-test) (Fig 3G). Therefore, while proliferation of CD8+ T-cells in response to IL-7 was not different between controls and HCV-infected individuals, there was a significant reduction in IL-7-induced Bcl-2 production that was inversely associated with the extent of fibrosis.
Intrahepatic CD8+ T-cells have an increased proportion of TEM cells compared to blood-derived cells in HCV infection, while CD127 expression remains unchanged
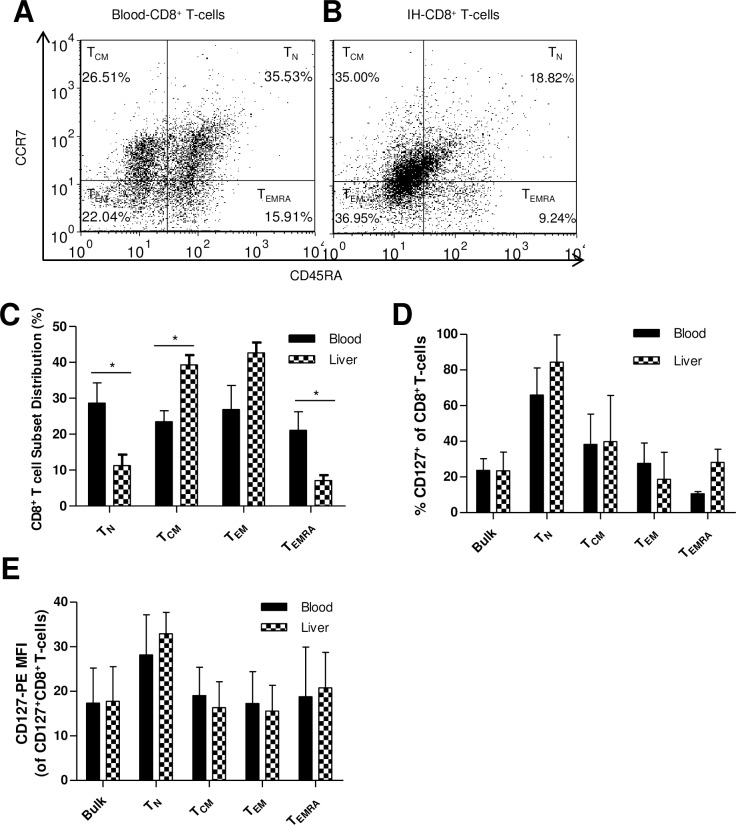
IH-lymphocytes were isolated from 4 separate liver biopsy samples obtained from HCV+ individuals and the proportions of CD8+ T-cell subsets were simultaneously compared to blood-derived CD8+ T-cells from the same individuals. The phenotypic distribution of blood CD8+ T-cells in these individuals was relatively similar to proportions reported in Fig 1 (Fig 4A and 4B). The phenotype of IH-CD8+ T-cells differed significantly compared to blood CD8+ T-cells, with a higher proportion sharing cell markers with TCM (39.3% ± 5.3 vs. 23.5% ± 3.1, p = 0.03, unpaired Student’s t-test), and a lower proportion expressing TN (11.2% ± 6.2 vs. 28.6% ± 5.6, p = 0.01) and TEMRA (7.08% ± 3.0 vs. 21.07% ± 5.2, p = 0.03) surface proteins. There was also a trend of more cells lacking CCR7 and CD45RA surface proteins, and appearing to be TEM than in blood CD8+ T-cells (42.64% ± 5.7 vs. 26.8% ± 6.7, p = 0.06) (Fig 4C). The CD8+ T-cells found in the liver appear to have a single, memory-like phenotype.
Fig 4. The proportion of CD8+ T CM cells is increased, while TN and TEMRA cells are decreased in the liver in HCV infection and CD127 expression does not differ between IH- and blood-derived CD8+ T-cells.
Representative dot plots of subset distribution are shown for (A) Blood-derived and individually matched (B) IH-CD8+ T-cell subsets, as analysed by flow cytometry which distinguished between subsets on the basis of CD45RA and CCR7 expression. (C) The means of these observations are summarized in a bar graph (* TN p = 0.03, TCM p = 0.01, TEMRA p = 0.03, unpaired Student’s t-test, n = 3, error bars represent ± S.D.). Membrane CD127 expression on bulk and CD8+ T-cells subsets did not differ between locations, as measured by (D) percentage expression nor (E) intensity of expression (MFI).
The level of CD127 expression was equivalent between blood-derived and IH-CD8+ T-cells (27.8% ± 3.8 and 23.6% ± 6.0, respectively) (Fig 4D). Similarly, CD127 expression among CD8+ T-cell subsets was the same between controls and HCV-infected individuals, and followed the same hierarchical pattern: TN (66.0% ± 8.8 and 81.3% ± 13.9) > TCM (38.2% ± 17.1 and 42.6% ± 21.8) > TEM (27.6% ± 6.6 and 22.3% ± 14.3) ≈ TEMRA (10.7% ± 0.7 and 23.5% ± 11.0) (Fig 4D). The intensity of CD127 expression was similar to percentage expression, with TN cells expressing the most CD127 in both blood and liver (Fig 4E).
Observational data from 2 individuals has indicated that IH-CD8+ T-cells expressed high basal levels of pSTAT5 compared to blood CD8+ T-cells, and IL-7 and other common γ chain (γc) cytokines (e.g. IL-2 and IL-15) did not further increase pSTAT5 (S1A Fig). The degree of basal Bcl-2 expression after overnight culture was lower in IH-CD8+ T-cells compared to blood-derived CD8+ T-cells in HCV mono-infection (S1B Fig). Further investigation of these novel findings is not possible given recent limitations in access to liver biopsies for HCV-infected patients at The Ottawa Hospital where standard of care now mandates the use of ultrasound diagnostics (i.e. Fibroscan).
Discussion
CD8+ T-cells isolated from individuals with HCV infection exhibit significantly impaired responsiveness to IL-7, independent of CD127 expression, and this was widespread, as detected in bulk blood-derived and IH-CD8+ T-cells. Impaired HCV-specific CD8+ T-cell has been well described [7, 10, 21, 22, 26, 28, 35], while bystander CD8+ T-cell dysfunction has been less well understood [12, 13]. Impaired response of bulk CD8+ T-cells to cytokine stimulation suggests broad CD8+ T-cell dysfunction in HCV infection, which this study has simultaneously observed in both circulating and liver-infiltrating CD8+ T-cells. This was a challenging study, as the relatively low number of IH-CD8+ T-cells in a liver biopsy of an HCV-infected individual limited the breadth of these investigations. In addition, the recent increased use of elastography diagnostics (i.e. fibroscan), instead of liver biopsies, has significantly reduced the availability of this tissue for this research. Despite these challenges, our small data set from this tissue provides invaluable insights into the state of IH-CD8+ T-cells in HCV infection, which complements our findings in circulating cells.
We found no role for CD127 expression in the CD8+ T-cell dysfunction observed here. In HCV infection, there were fewer TN blood-derived CD8+ T-cells compared to healthy controls (Fig 1C), similar to a previous report [44], but no CD127 expression differences were observed except in the TCM cell subset (Fig 1D and 1F). This suggests an inherent cell deficiency similar to that of dysfunctional CD8+CD127+ T-cells in HIV-infected individuals (e.g. reduced IL-7 signalling, survival and proliferation) [42, 45], contrasted in part by the marked decrease of CD127 expression on bulk CD8+ T-cells in HIV infection [46–49]. In addition, receptor expression was not associated with age in HCV infection, unlike in HIV infection [50]. It is not likely that other elements of the IL-7 receptor complex contribute to this impairment, as IL-7 does not alter CD132 expression and chronic viral infection can increase the proportion of CD132 expression [51, 52].
A previous study where increased susceptibility of CD8+ T-cells to apoptosis was noted [12] supports our finding that IL-7 did not increase Bcl-2 expression of CD8+ T-cells in HCV infection to the same level as in controls (Fig 3F). The decreased expression of Bcl-2 did not translate into decreased CD8+ T-cells isolated in HCV infection, as the percentage of CD8+ cells among PBMC for control and HCV infection were similar (17.1 ± 7.4 and 17.5 ± 6.0, respectively). This decreased Bcl-2 finding also confirms our previous report, and that of others, that Bcl-2 production is dependent on STAT5 activation [32, 53], which was also found to be lower in IL-7-stimulated cells from HCV-infected individuals compared to controls. The lower level of STAT5 activation with IL-7 stimulation observed in HCV infection was most evident in blood-derived TCM and TN cells, subsets with the highest level of STAT5 activation (Fig 2C). This was not associated with lower CD127 expression as only TCM cells had a lower CD127 (Fig 1F). The proliferation of CD8+ T-cells induced by IL-7 was similar between controls and HCV infection (Fig 3C), unlike HIV infection [42]. The activation of STAT5 is also associated with T-cell proliferation, however activation of the Akt pathway may have compensated for the inadequacies of STAT5 signaling in this instance, although our experimental efforts could not reliably assess Akt activity in these samples [54].
In viral hepatitis (HCV and HBV), IH-CD8+ T-cells frequently have an activated phenotype (i.e. decreased CD28 and increased IFN-γ expression) [55], similar to the TCM/TEM type phenotype of the IH-CD8+ T-cells observed here (Fig 4B). Our further analysis of cytokine signaling and survival potential was limited to observational results of two individuals of blood and liver matched samples (S1 Fig) due to the recent lack of access to liver biopsies from HCV-infected individuals. The high level of basal STAT5 activation observed (S1A Fig) may be due to cytokine secretion by hepatocytes, a known source of IL-7 [56]. As a tertiary lymphoid organ, liver dendritic cells, hepatocytes, and Kupffer cells expressing co-stimulatory molecules can also activate CD8+ T-cells in inflammatory conditions [57–60]. However, T-cells activated in the liver are more prone to apoptosis and produce less IFN-γ and IL-2 [58, 61, 62]. This may contribute to the reduced Bcl-2 expression observed here in IH-CD8+ T-cells in HCV infection (S1B Fig), and may partially explain the increased susceptibility of IH-CD8+ T-cells to apoptosis. The effect of such decreases in Bcl-2 expression on the life span CD8+ T-cells and their function in vivo is not known as decreases in Bcl-2 levels do not guarantee apoptosis, and would have to be assessed directly. However, if future access to liver sample permits, further analysis of blood-derived vs. IH-CD8+ T-cells would be required to confirm these findings.
Higher stages of fibrosis (F3-F4) were associated with lower IL-7-mediated Bcl-2 expression by blood-derived CD8+ T-cells (Fig 3G). No other associations were detected between CD8+ T-cell activities and fibrosis stage, although the selection of study subjects was not stratified to examine immune function associations with liver damage. Few associations between fibrosis and CD8+ T-cell function in HCV infection have been described; fibrosis has been associated with increased infiltration of CD8+ T-cells as well as increased CD8+ T-cell apoptosis in pediatric and adult livers in HCV infection [63, 64]. Whether the impairment observed is due to chronic liver disease, or HCV specifically, was not determined. The inclusion of controls with non-HCV chronic liver disease may have offered some insight in this regard. However, in alcoholic liver disease, the extent of liver damage has been implicated as a potential contributor to reduced T-cell responses, in the absence of infection [65]. Similarly, cirrhosis-associated immune dysfunction syndrome includes states of immune depression, including CD8+ T-cell exhaustion and senescence that is dependent on the severity and etiology of the liver disease [14, 66]. Nevertheless, NASH, alcoholic liver disease (ALD) and HBV/HCV infections recruit many CD8+ T-cells to the liver regardless of their antigen specificity. Activation of these cells in this organ in these diseases is associated with increased pro-inflammatory and pro-fibrogenic cytokine production [22, 67–70]. In HCV infection, increased CD8+ T-cell infiltrates in fibrosis is also associated with elevated levels of liver CD8+ T-cell apoptosis [63, 64], suggesting a potential common feature of liver disease.
Potential contributors to the suppression of T-cell activity and cytokine response include direct viral effects (plasma HCV core protein) or changes in the expression of cytokine signalling regulatory proteins (e.g. suppressors of cytokine signalling) [8, 71, 72]. In addition, the duration of the infection, host genetics, smoking or age were not evaluated here. A sizeable proportion of the CD8+ T-cell pool is comprised of CMV-specific CD8+ T-cells in healthy individuals, and numbers increase with age (>60yrs), found principally as a late differentiation phenotype (e.g. TEMRA) [73–75]. While CMV-specific T-cell responses are known to be strong, their proliferative potential in vitro is poor and their effector functions are weak following solid organ transplantation and in immunocompromised individuals [76, 77]. In this study, the HCV-infected individuals tested in the STAT5 and Bcl-2 assays were on average < 60 yrs of age (STAT5: 55.8 (±9.9); Bcl-2: 47.1 (±13.5)) and impairments were not observed in the TEMRA subset. While the average age of HCV+ individuals tested was higher than controls in most experiments, among HCV+ individuals, age was not associated with any differences. Lastly, while the CMV serostatus of our subjects was not determined, if there was active CMV infection in the HCV+ individuals, this would have been expected to reduce CD127 expression [78] and a difference in CD127 expression was not observed in most cases (Fig 1E and 1F). While CMV status and virus-specific cells do play a role in observed immune senescence and the decline of immune response in the elderly, and are numerous among circulating cells, we do not think this has significantly influenced the findings here.
In summary, CD8+ T-cells are phenotypically different and have impaired responsiveness to IL-7 in chronic HCV infection that is independent of CD127 expression and prevalent among bulk CD8+ T-cells. This dysfunction was particularly pronounced in the liver where high basal pSTAT5 levels could not be surmounted with added cytokine and basal Bcl-2 expression was significantly lower than in the blood. A consequence of progressive liver disease and generalized CD8+ T-cell dysfunction may result in insufficient responses to other concurrent infections, negatively influence vaccine immunogenicity (e.g. influenza, HBV) or contribute to the risk for hepatocellular carcinoma. Preliminary trials of T-cell mediated HCV vaccination of individuals with chronic HCV infection induced weaker T-cell responses than controls, suggesting that CD8+ cell dysfunction poses relevant challenges to the development of an effective therapeutic HCV vaccine [79]. Ongoing research may provide insights into the design of immune therapeutics for individuals whose cure rates with direct acting antiviral therapy are lower, such as those with advanced fibrosis, cirrhosis and those co-infected with HIV. Finally, HCV infection may be an important model of how liver disease affects CTL function, no matter its etiology, including nonalcoholic steatohepatitis and hepatocellular carcinoma.
Supporting Information
(A) Blood-derived CD8+ T-cells and IH-lymphocytes from the same donors (n = 2) were cultured with STAT5-activating γc cytokines (IL-7 (0.1 or 1 ng/ml), IL-2 (100 ng/ml), or IL-15 (10 ng/ml)) and pSTAT5 expression (MFI). (B) Bcl-2 expression of unstimulated CD8+ T-cells was measured after overnight rest at 37°C (n = 1).
(TIF)
(DOCX)
Acknowledgments
We acknowledge the voluntary blood and liver biopsy donations of those who participated in this study. We are thankful for the efforts of the nursing staff of The Ottawa Hospital (TOH) phlebotomy clinic, TOH and Regional Hepatitis Clinic and TOH Medical imaging in acquiring these biological samples. We are very grateful to Dr. Jonathan B. Angel for his assistance in editing this manuscript.
Data Availability
Relevant data are within the paper and its Supporting Information files.
Funding Statement
S.B.S. was the recipient of the Ontario Graduate Scholarship and the Queen Elizabeth II Graduate Scholarship in Science and Technology. A.M.C. is the recipient of a New Investigator salary award from the Canadian Institutes of Health Research, and a Junior Investigator Development Award from the Ontario HIV Treatment Network (OHTN). This research was funded by the OHTN, Canadian Foundation for AIDS Research and the J.P. Bickell Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. The Journal of experimental medicine. 2003;197(12):1645–55. 10.1084/jem.20030239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Wang X, Douglas SD, Metzger DS, Woody G, Zhang T, et al. CD8+ T cell depletion amplifies hepatitis C virus replication in peripheral blood mononuclear cells. The Journal of infectious diseases. 2005;192(6):1093–101. Epub 2005/08/19. 10.1086/432957 . [DOI] [PubMed] [Google Scholar]
- 3.Jo J, Aichele U, Kersting N, Klein R, Aichele P, Bisse E, et al. Analysis of CD8+ T-cell-mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology. 2009;136(4):1391–401. 10.1053/j.gastro.2008.12.034 . [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Hakeem MS, Bedard N, Murphy D, Bruneau J, Shoukry NH. Signatures of protective memory immune responses during hepatitis C virus reinfection. Gastroenterology. 2014;147(4):870–81 e8. 10.1053/j.gastro.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. The Journal of experimental medicine. 2001;194(10):1395–406. Epub 2001/11/21. ; PubMed Central PMCID: PMCPmc2193681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer-Olson D, Shoukry NH, Brady KW, Kim H, Olson DP, Hartman K, et al. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. The Journal of experimental medicine. 2004;200(3):307–19. 10.1084/jem.20040638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. Journal of immunology. 2002;169(6):3447–58. Epub 2002/09/10. . [DOI] [PubMed] [Google Scholar]
- 8.Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, Braciale TJ, Hahn YS. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. The Journal of clinical investigation. 2000;106(10):1239–49. Epub 2000/11/22. 10.1172/jci10323 ; PubMed Central PMCID: PMCPmc381434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett L, Gallant M, Howley C, Ian Bowmer M, Hirsch G, Peltekian K, et al. Stronger hepatitis C virus-specific CD8+ T-cell responses in HIV coinfection. Journal of viral hepatitis. 2011;18(3):170–80. 10.1111/j.1365-2893.2010.01293.x . [DOI] [PubMed] [Google Scholar]
- 10.Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. Journal of virology. 2001;75(12):5550–8. 10.1128/JVI.75.12.5550-5558.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spangenberg HC, Viazov S, Kersting N, Neumann-Haefelin C, McKinney D, Roggendorf M, et al. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42(4):828–37. 10.1002/hep.20856 . [DOI] [PubMed] [Google Scholar]
- 12.Zhao BB, Zheng SJ, Gong LL, Wang Y, Chen CF, Jin WJ, et al. T lymphocytes from chronic HCV-infected patients are primed for activation-induced apoptosis and express unique pro-apoptotic gene signature. PloS one. 2013;8(10):e77008 10.1371/journal.pone.0077008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas M, Vargas-Cuero AL, Lauer GM, Barnes E, Willberg CB, Semmo N, et al. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. Journal of immunology. 2004;172(3):1744–53. Epub 2004/01/22. . [DOI] [PubMed] [Google Scholar]
- 14.Sipeki N, Antal-Szalmas P, Lakatos PL, Papp M. Immune dysfunction in cirrhosis. World journal of gastroenterology: WJG. 2014;20(10):2564–77. 10.3748/wjg.v20.i10.2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, et al. Bacterial infection in patients with advanced cirrhosis: a multicentre prospective study. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2001;33(1):41–8. . [DOI] [PubMed] [Google Scholar]
- 16.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2011;9(9):727–38. 10.1016/j.cgh.2011.02.031 . [DOI] [PubMed] [Google Scholar]
- 17.Graham CS, Wells A, Liu T, Sherman KE, Peters M, Chung RT, et al. Antigen-specific immune responses and liver histology in HIV and hepatitis C coinfection. AIDS. 2005;19(8):767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–8. 10.1002/hep.510300409 . [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Sierra C, Arizcorreta A, Diaz F, Roldan R, Martin-Herrera L, Perez-Guzman E, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36(4):491–8. 10.1086/367643 . [DOI] [PubMed] [Google Scholar]
- 20.Mohsen AH, Easterbrook PJ, Taylor C, Portmann B, Kulasegaram R, Murad S, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52(7):1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett L, Gallant M, Howley C, Bowmer MI, Hirsch G, Peltekian K, et al. Enhanced IL-10 production in response to hepatitis C virus proteins by peripheral blood mononuclear cells from human immunodeficiency virus-monoinfected individuals. BMC immunology. 2008;9:28 10.1186/1471-2172-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel M, Sene D, Pol S, Bourliere M, Poynard T, Charlotte F, et al. Intrahepatic virus-specific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006;44(6):1607–16. 10.1002/hep.21438 . [DOI] [PubMed] [Google Scholar]
- 23.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. Journal of virology. 2007;81(17):9249–58. 10.1128/JVI.00409-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. 10.1038/nature04444 . [DOI] [PubMed] [Google Scholar]
- 25.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. The Journal of clinical investigation. 2010;120(12):4546–57. 10.1172/JCI43127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. Journal of virology. 2009;83(18):9122–30. 10.1128/JVI.00639-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golden-Mason L, Burton JR Jr., Castelblanco N, Klarquist J, Benlloch S, Wang C, et al. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44(5):1098–109. 10.1002/hep.21365 . [DOI] [PubMed] [Google Scholar]
- 28.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. Journal of virology. 2006;80(22):11398–403. 10.1128/JVI.01177-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth MJ, Norihisa Y, Gerard JR, Young HA, Ortaldo JR. IL-7 regulation of cytotoxic lymphocytes: pore-forming protein gene expression, interferon-gamma production, and cytotoxicity of human peripheral blood lymphocytes subsets. Cellular immunology. 1991;138(2):390–403. Epub 1991/12/01. . [DOI] [PubMed] [Google Scholar]
- 30.Hofmeister R, Khaled AR, Benbernou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7: physiological roles and mechanisms of action. Cytokine & growth factor reviews. 1999;10(1):41–60. Epub 1999/06/24. . [DOI] [PubMed] [Google Scholar]
- 31.O'Connor AM, Crawley AM, Angel JB. Interleukin-7 enhances memory CD8(+) T-cell recall responses in health but its activity is impaired in human immunodeficiency virus infection. Immunology. 2010;131(4):525–36. 10.1111/j.1365-2567.2010.03325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawley AM, Vranjkovic A, Faller E, McGuinty M, Busca A, Burke SC, et al. Jak/STAT and PI3K signaling pathways have both common and distinct roles in IL-7-mediated activities in human CD8+ T cells. Journal of leukocyte biology. 2014;95(1):117–27. 10.1189/jlb.0313122 . [DOI] [PubMed] [Google Scholar]
- 33.Larrubia JR, Lokhande MU, Garcia-Garzon S, Miquel J, Gonzalez-Praetorius A, Parra-Cid T, et al. Persistent hepatitis C virus (HCV) infection impairs HCV-specific cytotoxic T cell reactivity through Mcl-1/Bim imbalance due to CD127 down-regulation. Journal of viral hepatitis. 2013;20(2):85–94. 10.1111/j.1365-2893.2012.01618.x . [DOI] [PubMed] [Google Scholar]
- 34.Kroy DC, Ciuffreda D, Cooperrider JH, Tomlinson M, Hauck GD, Aneja J, et al. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology. 2014;146(2):550–61. 10.1053/j.gastro.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, von Weizsacker F, et al. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. Journal of virology. 2007;81(2):945–53. 10.1128/JVI.01354-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vali B, Yue FY, Jones RB, Sheth PM, Kaul R, Betts MR, et al. HIV-specific T-cells accumulate in the liver in HCV/HIV co-infection. PloS one. 2008;3(10):e3454 10.1371/journal.pone.0003454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nascimbeni M, Rehermann B. Determination of hepatitis B virus-specific CD8+ T-cell activity in the liver. Methods in molecular medicine. 2004;96:65–83. Epub 2004/02/06. 10.1385/1-59259-670-3:65 . [DOI] [PubMed] [Google Scholar]
- 38.Sheahan T, Rice C. Single cell analysis of HCV infected patient hepatocytes: The science is no longer science fiction. Gastroenterology. 2013;145(6): 10.1053/j.gastro.2013.10.035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawley AM, Faucher S, Angel JB. Soluble IL-7R alpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. Journal of immunology. 2010;184(9):4679–87. 10.4049/jimmunol.0903758 . [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. 10.1038/44385 . [DOI] [PubMed] [Google Scholar]
- 41.Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, et al. Substantial reduction of naive and regulatory T cells following traumatic stress. Brain, behavior, and immunity. 2009;23(8):1117–24. 10.1016/j.bbi.2009.07.003 . [DOI] [PubMed] [Google Scholar]
- 42.Vranjkovic A, Crawley AM, Angel JB. In vitro HIV Type 1 infection indirectly alters CD127 expression on CD8(+) T cells. AIDS research and human retroviruses. 2012;28(3):295–8. 10.1089/aid.2011.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura MY, Pobezinsky LA, Guinter TI, Thomas J, Adams A, Park JH, et al. IL-7 signaling must be intermittent, not continuous, during CD8(+) T cell homeostasis to promote cell survival instead of cell death. Nature immunology. 2013;14(2):143–51. 10.1038/ni.2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartling HJ, Gaardbo JC, Ronit A, Salem M, Laye M, Clausen MR, et al. Impaired thymic output in patients with chronic hepatitis C virus infection. Scandinavian journal of immunology. 2013;78(4):378–86. 10.1111/sji.12096 . [DOI] [PubMed] [Google Scholar]
- 45.Juffroy O, Bugault F, Lambotte O, Landires I, Viard JP, Niel L, et al. Dual mechanism of impairment of interleukin-7 (IL-7) responses in human immunodeficiency virus infection: decreased IL-7 binding and abnormal activation of the JAK/STAT5 pathway. Journal of virology. 2010;84(1):96–108. 10.1128/JVI.01475-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacPherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999). 2001;28(5):454–7. Epub 2001/12/18. . [DOI] [PubMed] [Google Scholar]
- 47.Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, Jacod S, et al. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients—effects of antiretroviral therapy. Journal of acquired immune deficiency syndromes (1999). 2006;42(3):277–85. 10.1097/01.qai.0000214823.11034.4e . [DOI] [PubMed] [Google Scholar]
- 48.Colle JH, Moreau JL, Fontanet A, Lambotte O, Joussemet M, Delfraissy JF, et al. CD127 expression and regulation are altered in the memory CD8 T cells of HIV-infected patients—reversal by highly active anti-retroviral therapy (HAART). Clinical and experimental immunology. 2006;143(3):398–403. 10.1111/j.1365-2249.2006.03022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paiardini M, Cervasi B, Albrecht H, Muthukumar A, Dunham R, Gordon S, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174(5):2900–9. . [DOI] [PubMed] [Google Scholar]
- 50.Bazdar DA, Kalinowska M, Sieg SF. Interleukin-7 receptor signaling is deficient in CD4+ T cells from HIV-infected persons and is inversely associated with aging. The Journal of infectious diseases. 2009;199(7):1019–28. 10.1086/597210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faller EM, McVey MJ, Kakal JA, MacPherson PA. Interleukin-7 receptor expression on CD8 T-cells is downregulated by the HIV Tat protein. Journal of acquired immune deficiency syndromes (1999). 2006;43(3):257–69. 10.1097/01.qai.0000230319.78288.f4 . [DOI] [PubMed] [Google Scholar]
- 52.Sasson SC, Zaunders JJ, Zanetti G, King EM, Merlin KM, Smith DE, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and Increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. The Journal of infectious diseases. 2006;193(4):505–14. 10.1086/499309 . [DOI] [PubMed] [Google Scholar]
- 53.Tripathi P, Kurtulus S, Wojciechowski S, Sholl A, Hoebe K, Morris SC, et al. STAT5 is critical to maintain effector CD8+ T cell responses. Journal of immunology. 2010;185(4):2116–24. 10.4049/jimmunol.1000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swainson L, Kinet S, Mongellaz C, Sourisseau M, Henriques T, Taylor N. IL-7-induced proliferation of recent thymic emigrants requires activation of the PI3K pathway. Blood. 2007;109(3):1034–42. 10.1182/blood-2006-06-027912 . [DOI] [PubMed] [Google Scholar]
- 55.Valiante NM, D'Andrea A, Crotta S, Lechner F, Klenerman P, Nuti S, et al. Life, activation and death of intrahepatic lymphocytes in chronic hepatitis C. Immunological reviews. 2000;174:77–89. Epub 2000/05/12. . [DOI] [PubMed] [Google Scholar]
- 56.Larrea E, Riezu-Boj JI, Aldabe R, Guembe L, Echeverria I, Balasiddaiah A, et al. Dysregulation of interferon regulatory factors impairs the expression of immunostimulatory molecules in hepatitis C virus genotype 1-infected hepatocytes. Gut. 2014;63(4):665–73. 10.1136/gutjnl-2012-304377 . [DOI] [PubMed] [Google Scholar]
- 57.Chen M, Tabaczewski P, Truscott SM, Van Kaer L, Stroynowski I. Hepatocytes Express Abundant Surface Class I MHC and Efficiently Use Transporter Associated with Antigen Processing, Tapasin, and Low Molecular Weight Polypeptide Proteasome Subunit Components of Antigen Processing and Presentation Pathway. The Journal of Immunology. 2005;175(2):1047–55. 10.4049/jimmunol.175.2.1047 [DOI] [PubMed] [Google Scholar]
- 58.Tokita D, Sumpter TL, Raimondi G, Zahorchak AF, Wang Z, Nakao A, et al. Poor allostimulatory function of liver plasmacytoid DC is associated with pro-apoptotic activity, dependent on regulatory T cells. Journal of hepatology. 2008;49(6):1008–18. 10.1016/j.jhep.2008.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nature immunology. 2013;14(6):574–83. 10.1038/ni.2573 . [DOI] [PubMed] [Google Scholar]
- 60.Jenne CN, Kubes P. Immune surveillance by the liver. Nature immunology. 2013;14(10):996–1006. 10.1038/ni.2691 . [DOI] [PubMed] [Google Scholar]
- 61.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the 'liver tolerance effect'. Immunology and cell biology. 2002;80(1):84–92. Epub 2002/03/01. 10.1046/j.0818-9641.2001.01048.x . [DOI] [PubMed] [Google Scholar]
- 62.Holz LE, Benseler V, Vo M, McGuffog C, Van Rooijen N, McCaughan GW, et al. Naive CD8 T cell activation by liver bone marrow-derived cells leads to a "neglected" IL-2low Bimhigh phenotype, poor CTL function and cell death. Journal of hepatology. 2012;57(4):830–6. 10.1016/j.jhep.2012.05.015 . [DOI] [PubMed] [Google Scholar]
- 63.Valva P, Gismondi MI, Casciato PC, Galoppo M, Lezama C, Galdame O, et al. Distinctive intrahepatic characteristics of paediatric and adult pathogenesis of chronic hepatitis C infection. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(12):O998–o1009. Epub 2014/06/20. 10.1111/1469-0691.12728 . [DOI] [PubMed] [Google Scholar]
- 64.Feuth T, van Baarle D, van Erpecum KJ, Siersema PD, Hoepelman AI, Arends JE. CD4/CD8 ratio is a promising candidate for non-invasive measurement of liver fibrosis in chronic HCV-monoinfected patients. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2014;33(7):1113–7. Epub 2014/01/23. 10.1007/s10096-014-2053-7 . [DOI] [PubMed] [Google Scholar]
- 65.Nouri-Aria KT, Alexander GJ, Portmann BC, Hegarty JE, Eddleston AL, Williams R. T and B cell function in alcoholic liver disease. Journal of hepatology. 1986;2(2):195–207. . [DOI] [PubMed] [Google Scholar]
- 66.Marquez M, Fernandez-Gutierrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodriguez-Ramos C, et al. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clinical and experimental immunology. 2009;158(2):219–29. 10.1111/j.1365-2249.2009.04005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. The Journal of experimental medicine. 2000;191(8):1269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferreyra Solari NE, Inzaugarat ME, Baz P, De Matteo E, Lezama C, Galoppo M, et al. The role of innate cells is coupled to a Th1-polarized immune response in pediatric nonalcoholic steatohepatitis. J Clin Immunol. 2012;32(3):611–21. 10.1007/s10875-011-9635-2 . [DOI] [PubMed] [Google Scholar]
- 69.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54–62. . [DOI] [PubMed] [Google Scholar]
- 70.Colombat M, Charlotte F, Ratziu V, Poynard T. Portal lymphocytic infiltrate in alcoholic liver disease. Human pathology. 2002;33(12):1170–4. 10.1053/hupa.2002.129414 . [DOI] [PubMed] [Google Scholar]
- 71.Collins AS, Ahmed S, Napoletano S, Schroeder M, Johnston JA, Hegarty JE, et al. Hepatitis C virus (HCV)-induced suppressor of cytokine signaling (SOCS) 3 regulates proinflammatory TNF-alpha responses. Journal of leukocyte biology. 2014;96(2):255–63. 10.1189/jlb.2A1211-608RRRR . [DOI] [PubMed] [Google Scholar]
- 72.Yao ZQ, Prayther D, Trabue C, Dong ZP, Moorman J. Differential regulation of SOCS-1 signalling in B and T lymphocytes by hepatitis C virus core protein. Immunology. 2008;125(2):197–207. 10.1111/j.1365-2567.2008.02829.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Looney RJ, Falsey A, Campbell D, Torres A, Kolassa J, Brower C, et al. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clinical immunology. 1999;90(2):213–9. 10.1006/clim.1998.4638 . [DOI] [PubMed] [Google Scholar]
- 74.Moss P, Khan N. CD8(+) T-cell immunity to cytomegalovirus. Hum Immunol. 2004;65(5):456–64. 10.1016/j.humimm.2004.02.014 . [DOI] [PubMed] [Google Scholar]
- 75.Gillespie GM, Wills MR, Appay V, O'Callaghan C, Murphy M, Smith N, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74(17):8140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin X, Demoitie MA, Donahoe SM, Ogg GS, Bonhoeffer S, Kakimoto WM, et al. High frequency of cytomegalovirus-specific cytotoxic T-effector cells in HLA-A*0201-positive subjects during multiple viral coinfections. J Infect Dis. 2000;181(1):165–75. 10.1086/315201 . [DOI] [PubMed] [Google Scholar]
- 77.Singhal S, Shaw JC, Ainsworth J, Hathaway M, Gillespie GM, Paris H, et al. Direct visualization and quantitation of cytomegalovirus-specific CD8+ cytotoxic T-lymphocytes in liver transplant patients. Transplantation. 2000;69(11):2251–9. . [DOI] [PubMed] [Google Scholar]
- 78.Boutboul F, Puthier D, Appay V, Pelle O, Ait-Mohand H, Combadiere B, et al. Modulation of interleukin-7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS. 2005;19(17):1981–6. . [DOI] [PubMed] [Google Scholar]
- 79.Houghton M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunological reviews. 2011;239(1):99–108. Epub 2011/01/05. 10.1111/j.1600-065X.2010.00977.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Blood-derived CD8+ T-cells and IH-lymphocytes from the same donors (n = 2) were cultured with STAT5-activating γc cytokines (IL-7 (0.1 or 1 ng/ml), IL-2 (100 ng/ml), or IL-15 (10 ng/ml)) and pSTAT5 expression (MFI). (B) Bcl-2 expression of unstimulated CD8+ T-cells was measured after overnight rest at 37°C (n = 1).
(TIF)
(DOCX)
Data Availability Statement
Relevant data are within the paper and its Supporting Information files.