Abstract
Freshly ejaculated human semen has the appearance of a loose gel in which the predominant structural protein components are the seminal vesicle-secreted semenogelins (Sg). The primary structure of the 439-residue SgI has previously been obtained by cDNA cloning. This cDNA cross-hybridizes to a larger transcript coding for a second secretory protein, SgII. Here we report the almost complete structure of a precursor of SgII established by lambda gt11 clones isolated from epididymal and seminal vesicular cDNA libraries. The deduced amino acid sequence of the 559-residue mature protein has a molecular weight of 62,931 but an increase in weight may be provided by asparagine-linked oligosaccharide attachment at residue 249. SgII, which has 78% overall identity with SgI, contains eight 60-residue regions that display conspicuous internal sequence similarity, whereas SgI only contains six of these regions. The SgII structure is translated from an open reading frame in a polyadenylylated 2.4-kilobase transcript. The message is abundant in the seminal vesicles but rare in the epididymis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama K., Nakamura T., Iwanaga S., Hara M. The chymotrypsin-like activity of human prostate-specific antigen, gamma-seminoprotein. FEBS Lett. 1987 Dec 10;225(1-2):168–172. doi: 10.1016/0014-5793(87)81151-1. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Pentecost B. T., McLachlan J. A., Teng C. T. The androgen-dependent mouse seminal vesicle secretory protein IV: characterization and complementary deoxyribonucleic acid cloning. Mol Endocrinol. 1987 Oct;1(10):707–716. doi: 10.1210/mend-1-10-707. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christensson A., Laurell C. B., Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990 Dec 27;194(3):755–763. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Herr J. C. Immunohistochemical localization of the MHS-5 antigen in principal cells of human seminal vesicle epithelium. Anat Rec. 1986 Apr;214(4):372-7, 390-1. doi: 10.1002/ar.1092140406. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom J. E., Harvey S., Madden B., McCormick D., Wieben E. D. Androgens affect the processing of secretory protein precursors in the guinea pig seminal vesicle. II. Identification of conserved sites for protein processing. Mol Endocrinol. 1989 Nov;3(11):1797–1806. doi: 10.1210/mend-3-11-1797. [DOI] [PubMed] [Google Scholar]
- Herr J. C., Summers T. A., McGee R. S., Sutherland W. M., Sigman M., Evans R. J. Characterization of a monoclonal antibody to a conserved epitope on human seminal vesicle-specific peptides: a novel probe/marker system for semen identification. Biol Reprod. 1986 Oct;35(3):773–784. doi: 10.1095/biolreprod35.3.773. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985 Nov;76(5):1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H., Abrahamsson P. A., Lundwall A. Semenogelin, the predominant protein in human semen. Primary structure and identification of closely related proteins in the male accessory sex glands and on the spermatozoa. J Biol Chem. 1989 Jan 25;264(3):1894–1900. [PubMed] [Google Scholar]
- Lilja H. Cell biology of semenogelin. Andrologia. 1990;22 (Suppl 1):132–141. doi: 10.1111/j.1439-0272.1990.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Lilja H., Jeppsson J. O. Amino acid sequence of the predominant basic protein in human seminal plasma. FEBS Lett. 1985 Mar 11;182(1):181–184. doi: 10.1016/0014-5793(85)81179-0. [DOI] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B., Jeppsson J. O. Characterization of the predominant basic protein in human seminal plasma, one cleavage product of the major seminal vesicle protein. Scand J Clin Lab Invest. 1984 Sep;44(5):439–446. doi: 10.3109/00365518409083835. [DOI] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B. Liquefaction of coagulated human semen. Scand J Clin Lab Invest. 1984 Sep;44(5):447–452. doi: 10.3109/00365518409083836. [DOI] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B. The predominant protein in human seminal coagulate. Scand J Clin Lab Invest. 1985 Nov;45(7):635–641. doi: 10.3109/00365518509155271. [DOI] [PubMed] [Google Scholar]
- Lilja H., Oldbring J., Rannevik G., Laurell C. B. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987 Aug;80(2):281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundwall A., Lilja H. Molecular cloning of human prostate specific antigen cDNA. FEBS Lett. 1987 Apr 20;214(2):317–322. doi: 10.1016/0014-5793(87)80078-9. [DOI] [PubMed] [Google Scholar]
- Mansson P. E., Sugino A., Harris S. E. Use of a cloned double stranded cDNA coding for a major androgen dependent protein in rat seminal vesicle secretion: the effect of testosterone in gene expression. Nucleic Acids Res. 1981 Feb 25;9(4):935–946. doi: 10.1093/nar/9.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee R. S., Herr J. C. Human seminal vesicle-specific antigen during semen liquefaction. Biol Reprod. 1987 Sep;37(2):431–439. doi: 10.1095/biolreprod37.2.431. [DOI] [PubMed] [Google Scholar]
- McGee R. S., Herr J. C. Human seminal vesicle-specific antigen is a substrate for prostate-specific antigen (or P-30). Biol Reprod. 1988 Sep;39(2):499–510. doi: 10.1095/biolreprod39.2.499. [DOI] [PubMed] [Google Scholar]
- Moore J. T., Hagstrom J., McCormick D. J., Harvey S., Madden B., Holicky E., Stanford D. R., Wieben E. D. The major clotting protein from guinea pig seminal vesicle contains eight repeats of a 24-amino acid domain. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6712–6714. doi: 10.1073/pnas.84.19.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller J., Akiyama K., Tsuda R., Hara M., Marti T., Rickli E. E. Isolation, characterization and amino-acid sequence of gamma-seminoprotein, a glycoprotein from human seminal plasma. Eur J Biochem. 1987 Dec 30;170(1-2):111–120. doi: 10.1111/j.1432-1033.1987.tb13674.x. [DOI] [PubMed] [Google Scholar]
- Schneider K., Kausler W., Tripier D., Jouvenal K., Spiteller G. Isolierung und Strukturbestimmung von zwei Peptiden aus humanem Seminalplasma. Biol Chem Hoppe Seyler. 1989 Apr;370(4):353–356. [PubMed] [Google Scholar]
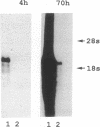
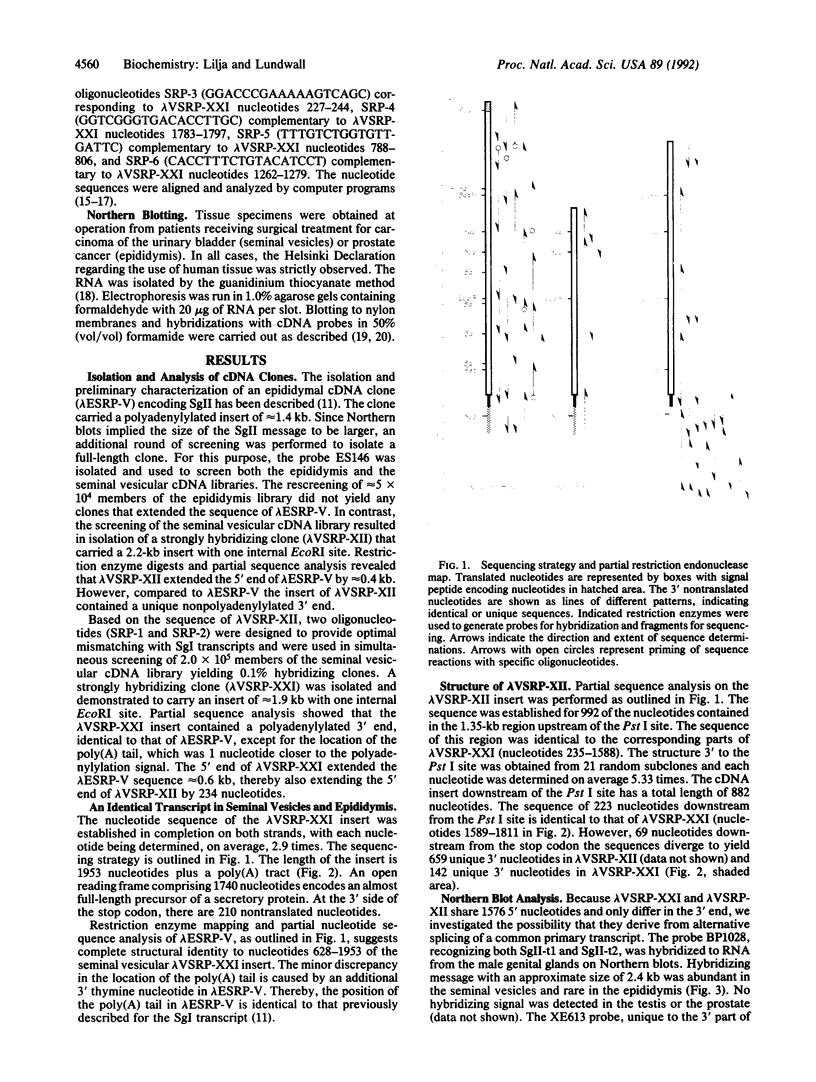
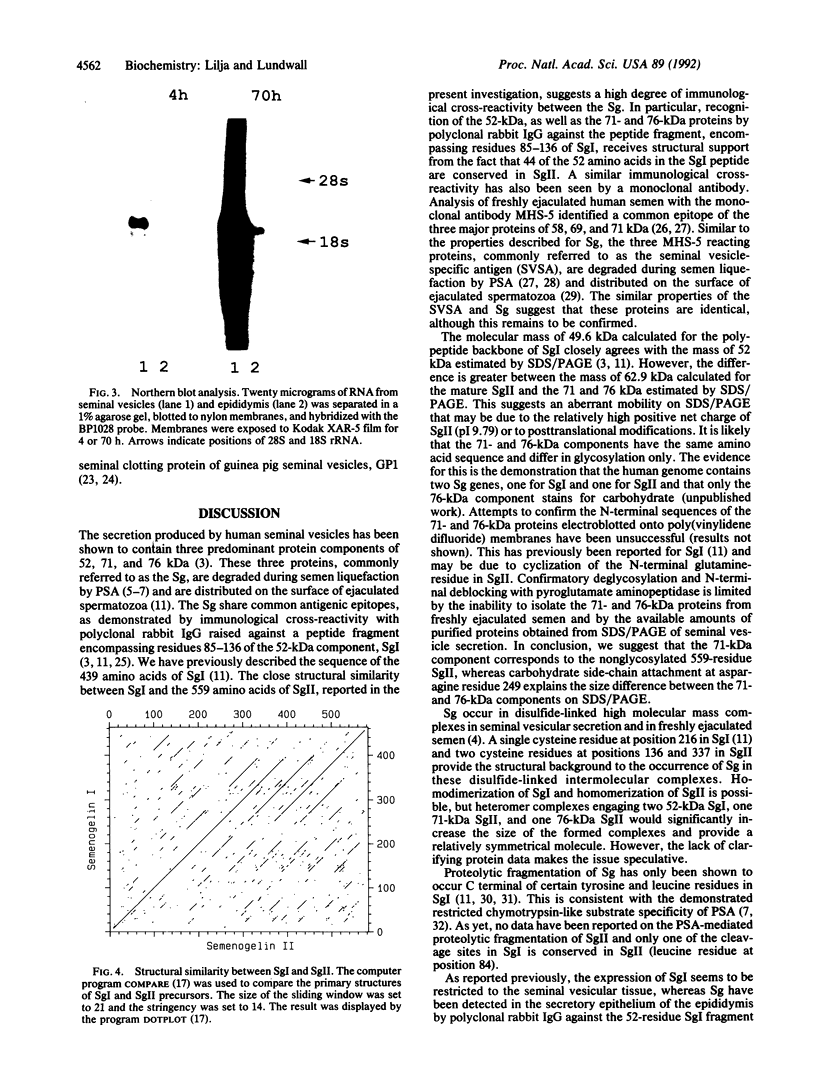
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt K. W., Lee P. J., M'Timkulu T., Chan W. P., Loor R. Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc Natl Acad Sci U S A. 1986 May;83(10):3166–3170. doi: 10.1073/pnas.83.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]