Abstract
Braak's staging concept of Lewy body disease pathogenesis is based on a spatiotemporal sequence of alpha-synuclein deposition, with autonomic nervous system involvement before synucleinopathy in substantia nigra neurons. A patient with primary chronic autonomic failure underwent biennial brain 6-[18F]DOPA and myocardial 6-[18F]dopamine scanning over 4 years. Low myocardial radioactivity indicated cardiac noradrenergic denervation that persisted. Striatal 6-[18F]DOPA-derived radioactivity initially was normal, 2 years later was decreased subtly, and by 4 years was clearly decreased, accompanied by dementia and parkinsonism. In this case, neuroimaging evidence of cardiac noradrenergic denervation and subsequent progressive striatal dopaminergic denervation fit with Braak staging.
Keywords: Parkinson, Synuclein, Braak, Sympathetic, nervous system, Norepinephrine
Introduction
Braak's staging approach for the pathogenetic sequence of Parkinson disease is based on post mortem studies of alpha-synuclein [1]. According to this concept, alpha-synuclein deposition occurs early in the olfactory bulb, autonomic nerves, and dorsal motor nucleus of the vagus nerve, later in the midbrain substantia nigra (corresponding to parkinsonism), and eventually diffusely in the cerebral cortex (corresponding to dementia).
Consistent with Braak staging, Lewy bodies found incidentally at autopsy are associated with decreased tyrosine hydroxylase immunoreactivity in epicardial nerves, suggesting cardiac sympathetic denervation in premotor Parkinson disease [3, 16]. Since one cannot know whether individuals with incidental Lewy bodies would go on to develop clinical Parkinson disease, post mortem cross-sectional studies are limited for evaluating the sequence of lesions.
Clinical laboratory assessments such as cardiac sympathetic neuroimaging and baroreflex testing can identify autonomic abnormalities preceding signs of central neurodegeneration [11], and cases have been reported of autonomic failure as an early finding in Parkinson disease [5, 13]; however, it is well recognized that by the time loss of nigrostriatal dopaminergic neurons becomes clinically evident, most of the striatal terminals are already lost. Because of the possibility of asymptomatic loss of nigrostriatal dopaminergic neurons, findings in such cases may not apply to the spatiotemporal sequence that is the key feature of Braak staging.
Recently, Tijero et al. [17] described a case of E46 K mutation of the alpha-synuclein gene who had neuroim-aging evidence of cardiac sympathetic denervation and concurrent normal striatal dopaminergic innervation. Although this combination suggests that cardiac sympathetic denervation can precede nigrostriatal neuronal loss in inherited synucleinopathy, the report does not mention whether the patient actually went on to develop a striatal dopaminergic lesion or clinical signs of central neurodegeneration.
Another recent study [15], based on surgical specimens from patients undergoing resection of abdominopelvic organs, included mention of a patient with tissue alpha-synuclein aggregates who had previously been diagnosed with REM behavior disorder and olfactory dysfunction. Myocardial 123I-metaiodobenzylguanidine scanning indicated cardiac sympathetic denervation, while striatal 123I-ioflupane-derived radioactivity was normal. At 30 months after biopsy the UPDRS-III Motor Subscale was elevated at 17; however, the baseline value when the biopsy was done was not noted, and the report did not include verification of striatal dopaminergic denervation at the time of onset of motor signs suggestive of PD.
To our knowledge no report to date has described follow-up of a patient with cardiac sympathetic denervation and initially normal striatal dopaminergic innervation to determine if striatal denervation developed subsequently, as predicted by the Braak staging concept. Here we report such a case.
Case summary
The IRB of the National Institute of Neurological Disorders and Stroke approved the protocol under which this patient was studied. The patient gave informed written consent.
The patient carried a diagnosis of pure autonomic failure, based on previously published consensus criteria and supportive data about plasma levels of catechols [8] and cardiac sympathetic neuroimaging [7]. His orthostatic hypotension was neurogenic, because of abnormal beat-to-beat blood pressure responses to the Valsalva maneuver and attenuated orthostatic increments in plasma norepinephrine levels.
The patient had a history of a few years of heat intolerance, decreased energy, orthostatic intolerance, constipation, decreased sense of smell, and presyncope; violent nightmares dating back to post-traumatic stress related to combat in the Vietnam era; and a strong family history of Parkinson disease. He noted normal gait and memory, occasional erectile failure, chronic constipation, decreased sweating, and fatigue, without urinary complaints. Physical examination showed marked orthostatic hypotension (Table 1), normal gait and muscle tone, and normal short-term memory. He had cardiac sympathetic denervation as indicated by a low myocardial concentration of 18F-DA-derived radioactivity (Fig. 1), extra-cardiac sympathetic noradrenergic denervation as indicated by low arterial and venous plasma levels of dihydroxyphenylglycol, and decreased sympathetic cholinergic function as indicated by a low rate of sweat production during the quantitative sudomotor axon reflex test. The patient had bilaterally normal putamen:occipital cortex (PUT:OCC) ratios of 6-[18F]DOPA-derived radioactivity (more than 3.0 on each side about 2 h injection of the imaging agent) and normal brain magnetic resonance imaging.
Table 1.
Clinical laboratory results values in boldface are abnormal. Arrows indicate direction of abnormality
| Parameter | Initial | 2 years | 4 years |
|---|---|---|---|
| Age | 69 | 71 | 73 |
| Supine BP (mmHg) | 155/87 | 155/95 | 225↑/89 |
| Heart rate (bpm) | 67 | 73 | 57 |
| Orthostatic ΔBPs (mmHg) | –39 ↑ | –50 ↑ | –71 ↑ |
| Valsalva ratio | 1.11 ↓ | 1.00 ↓ | 1.05 ↓ |
| Baroreflex gain (ms/mmHg) | 2.00 ↓ | 3.96 | 1.92 ↓ |
| Phase II_L Valsalva BP | Progressive fall | Progressive fall | Progressive fall |
| Phase IV overshoot | Absent | Absent | Absent |
| Pressure recovery time (s) | 12 ↑ | 8 ↑ | |
| [NEa] (pg/mL) | 123 | ||
| [NEv] (pg/mL) | 154 | 157 | 117 |
| [DHPGa] (pg/mL) | 429 ↓ | ||
| [DHPGv] (pg/mL) | 391 ↓ | 436 ↓ | 413 ↓ |
| UPSIT score | 11 ↓(Anosmia) | 12 ↓(Anosmia) | |
| QSART (μL/10 min) | 0.14 ↓ | 0.32 ↓ | |
| Mentation | Normal | Visual hallucinations | MMSE 23 ↓ |
| MRI | Normal | Cortical volume loss | |
| 18F-Dopamine (nCi-kg/cc-mCi) | 2151 ↓ | 1702 ↓ | 3231 ↓ |
| PUT:OCC (R) | 3.10 | 2.70 | 2.11 ↓ |
| PUT:OCC (L) | 3.38 | 3.23 | 2.42 ↓ |
The Valsalva maneuver consisted of straining against a resistance (30 mm Hg) for 12 s with the patient supine (head on pillow). The Valsalva ratio was calculated from the maximum interbeat interval after the maneuver divided by the minimum interbeat interval during the maneuver
BP blood pressure, ABPs change in systolic pressure, NEa arterial norepinephrine, NEv venous norepinephrine, DHPGa arterial dihydroxyphenylglycol, DHPGv venous dihydroxyphenylglycol, UPSIT University of Pennsylvania Smell Identification Test, QSART quantitative sudomotor axon reflex test (forearm), PUT:OCC putamen:occipital cortex ratio of 6-[18F]DOPA-derived radioactivity at about 120 min
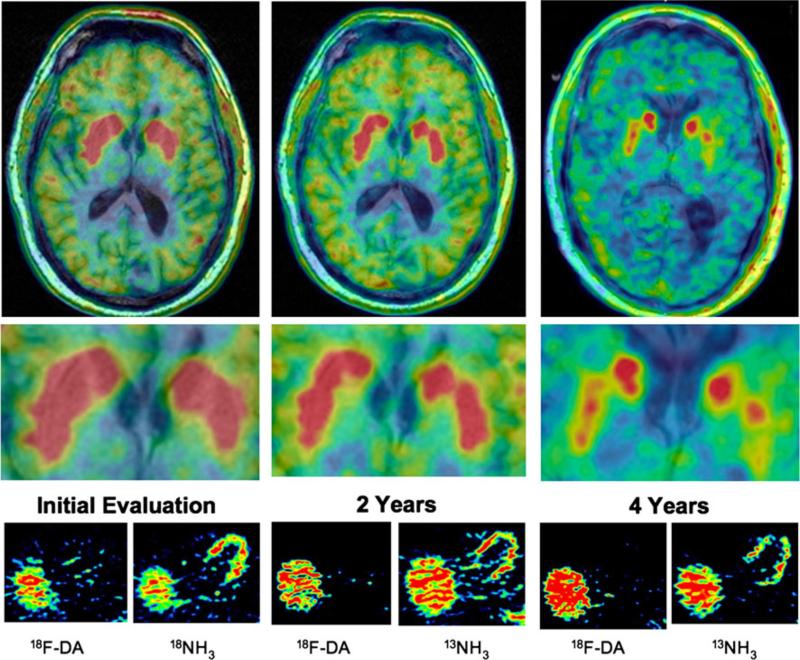
Fig. 1.
Serial striatal dopaminergic and cardiac sympathetic images. Spectral scales were used, with peak radioactivity in red and zero radioactivity in blue-black in each scan. (Top) 6-[18F]DOPA high resolution positron emission tomographic images superimposed on the patient's magnetic resonance image. (Middle) Blow-ups of the striata from the same scans. (Bottom) Thoracic transaxial images after administration of the sympathetic neuroimaging agent 6-[18F]fluorodopamine (18F-DA) and the perfusion imaging agent 13N-ammonia (13NH3). Throughout the period of study the patient had absence of myocardial 18F-DA-derived radioactivity despite approximately normal perfusion. Initially he had normal striatal 6-[18F]DOPA-derived radioactivity but subsequently had progressively decreased radioactivity, especially in the putamen. By 4 years he also had diffusely decreased 6-[18F]DOPA-derived radioactivity in the brain and had cerebroventricular enlargement from cortical atrophy
Two years later the patient reported visual hallucinations, decreased postural stability, and variably decreased memory. He recalled only 1 of 3 objects at 5 min. The PUT:OCC ratio of 6-[18F]DOPA-derived radioactivity decreased than before but was still within normal limits. 18F-DA scanning continued to show cardiac sympathetic denervation.
At 4 years the patient now reported altered gait, with a tendency to drift to the left while walking, depression for which he was treated successfully with venlafaxine, uri-nary urgency without incontinence, continued decreased sweating, and erectile failure. He noted variably decreased ability to attend to conversations. His visual hallucinations were worse, and he had weight loss without dieting. Neurological examination showed a subtle decrease in left arm swing, possibly decreased facial expression, left blepharospasm, and decreased rapid alternating movements and finger taps on the left side, but no rigidity or resting tremor. Mini-mental status examination score was subnormal at 23, and dementia rating scale (DRS-2) testing showed memory at 1% of normal, initiation/perseveration at 3–5% of normal, conceptualization 19–28%, construction 41–59%, and attention > 99%. The PUT:OCC ratio was now obviously abnormally low. 6-[18F]DOPA-derived radioactivity was also noticeably decreased diffusely in the cerebral hemispheres (Fig. 1). While in the hospital he fell during an episode of dream enactment behavior, lacerating his scalp. Computed tomography of the head showed no intracranial bleeding but demonstrated cortical atrophy. Magnetic resonance imaging showed progressive ventricular dilatation and cortical atrophy compared to when he had first been tested. From these findings we concluded that the patient had probable dementia with Lewy bodies.
Discussion
This case provides in vivo evidence that cardiac and extra-cardiac sympathetic denervation can precede striatal dopaminergic denervation, in line with the Braak staging concept of synucleinopathy.
Our patient was diagnosed initially with pure autonomic failure, since he had persistent, consistent neurogenic orthostatic hypotension, baroreflex failure, neuroimaging and neurochemical evidence of cardiac and extra-cardiac sympathetic noradrenergic denervation, and no signs initially of parkinsonism or cognitive dysfunction. The patient also had anosmia, which occurs commonly in pure autonomic failure [4, 9]. He had severe dream enactment behavior, probably representing REM behavior disorder. Although REM behavior disorder is by now well established as a non-motor manifestation of Parkinson disease and occurs commonly in dementia with Lewy bodies, whether it is typical of pure autonomic failure remains uncertain. At 2 years of follow-up the patient reported visual hallucinations, a core feature of dementia with Lewy bodies [14]. At 4 years the patient had clear evidence of dementia and subtle parkinsonism. From these findings we diagnosed probable dementia with Lewy bodies. Whether pure autonomic failure constitutes a restricted Lewy body synucleinopathy or evolves into Parkinson disease or dementia with Lewy bodies is unclear [12].
Relatively young patients might not follow the pathological progression proposed by Braak. We updated our findings about the prevalence of cardiac sympathetic denervation as a function of the age at clinical onset of the movement disorder. Across all PD patients, the group with cardiac sympathetic denervation (interventricular septal myocardial 18F-DA-derived radioactivity less than 5,000 nCi-kg/cc-mCi) was significantly older at the time of motor onset (59 ± 1 years) than the group with radioactivity within two standard deviations of the normal mean (53 ± 2 years, p = 0.006). Our patient was more than 70 years old when subtle parkinsonian signs first came to light. Future studies should consider age at motor onset as a factor influencing the prevalence of early autonomic involvement in the pathogenetic sequence.
We have studied other patients who had established Parkinson disease and yet had neuroimaging evidence of only partial loss of or even intact cardiac sympathetic innervation [10]. These findings do not fit well with Braak's staging concept of synucleinopathy. Moreover, across patients with synucleinopathies there is no relationship between the extent of putamen dopaminergic denervation by 6-[18F]DOPA scanning and myocardial sympathetic denervation by cardiac 18F-DA scanning [6].
A potential explanation for these findings is that different types of monoaminergic neurons have different susceptibilities to the same etiologic process. According to this notion, patients with relatively vulnerable noradrenergic neurons might have anosmia, REM behavior disorder, baroreflex failure, orthostatic hypotension, and dementia—as in the present case—whereas patients with more vulnerable dopaminergic neurons might mainly have parkinsonism. Studies using 11C-methylreboxetine, a recently introduced ligand for the cell membrane norepinephrine transporter [2], may help test this idea.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke. Ms. Tereza Jenkins (Clinical Neurocardiology Section) coordinated patient travel. Sandra Pechnik, RN (Clinical Neurocardiology Section) assisted with clinical procedures and scheduling.
Abbreviations
- 18F-DA
6-[18F]Fluorodopamine
- PUT:OCC
Putamen:occipital cortex
Footnotes
Conflict of interest None.
References
- 1.Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol. 2000;247(Suppl 2):II3–II10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 2.Ding YS, Singhal T, Planeta-Wilson B, Gallezot JD, Nabulsi N, Labaree D, Ropchan J, Henry S, Williams W, Carson RE, Neu-meister A, Malison RT. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S, S)-[(11)C]O-methylreboxetine and HRRT. Synapse. 2010;64:30–38. doi: 10.1002/syn.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujishiro H, Frigerio R, Burnett M, Klos KJ, Josephs KA, Delledonne A, Parisi JE, Ahlskog JE, Dickson DW. Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson's disease. Mov Disord. 2008;23:1085–1092. doi: 10.1002/mds.21989. [DOI] [PubMed] [Google Scholar]
- 4.Garland EM, Raj SR, Peltier AC, Robertson D, Biaggioni I. A cross-sectional study contrasting olfactory function in autonomic disorders. Neurology. 2011;76:456–460. doi: 10.1212/WNL.0b013e31820a0caf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein DS. Orthostatic hypotension as an early finding in Parkinson disease. Clin Auton Res. 2006;16:46–64. doi: 10.1007/s10286-006-0317-8. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DS, Holmes C, Bentho O, Sato T, Moak J, Sharabi Y, Imrich R, Conant S, Eldadah BA. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Park Rel Dis. 2008;14:600–607. doi: 10.1016/j.parkreldis.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein DS, Holmes C, Sharabi Y, Brentzel S, Eisenhofer G. Plasma levels of catechols and metanephrines in neurogenic orthostatic hypotension. Neurology. 2003;60:1327–1332. doi: 10.1212/01.wnl.0000058766.46428.f3. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein DS, Sewell L. Olfactory dysfunction in pure autonomic failure: implications for the pathogenesis of Lewy body diseases. Park Rel Dis. 2009;15:516–520. doi: 10.1016/j.parkreldis.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein DS, Sewell L, Sharabi Y. Autonomic dysfunction in PD: a window to early detection? J Neurol Sci. 2011 doi: 10.1016/j.jns.2011.04.011. in press. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS, Sharabi Y, Karp BI, Bentho O, Saleem A, Pacak K, Eisenhofer G. Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Clin Auton Res. 2007;17:118–121. doi: 10.1007/s10286-007-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann H, Goldstein DS. Pure autonomic failure: a restricted Lewy body synucleinopathy or early Parkinson disease? Neurology. 2010;74:536–537. doi: 10.1212/WNL.0b013e3181d26982. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann H, Nahm K, Purohit D, Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004;63:1093–1095. doi: 10.1212/01.wnl.0000138500.73671.dc. [DOI] [PubMed] [Google Scholar]
- 14.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 15.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M, Concha A, Munoz DG. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology. 2007;68:2012–2018. doi: 10.1212/01.wnl.0000264429.59379.d9. [DOI] [PubMed] [Google Scholar]
- 16.Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, Takahashi H. Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson's disease. Brain. 2008;131:642–650. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 17.Tijero B, Gomez-Esteban JC, Llorens V, Lezcano E, Gonzalez-Fernandez MC, de Pancorbo MM, Ruiz-Martinez J, Cembellin JC, Zarranz JJ. Cardiac sympathetic denervation precedes nigrostriatal loss in the E46 K mutation of the alpha-synuclein gene (SNCA). Clin Auton Res. 2010;20:267–269. doi: 10.1007/s10286-010-0068-4. [DOI] [PubMed] [Google Scholar]