Abstract
Liver fluke infection caused by Opisthorchis viverrini remains a major public health problem in many parts of Asia including Thailand, Lao PDR, Vietnam and Cambodia, where there is a strikingly high incidence of cholangiocarcinoma (CCA - hepatic cancer of the bile duct epithelium). Among other factors, uptake of O. viverrini excretory/secretory products (OvES) by biliary epithelial cells has been postulated to be responsible for chronic inflammation and proliferation of cholangiocytes, but the mechanisms by which cells internalize OvES are still unknown. Herein we incubated normal human cholangiocytes (H69), human cholangiocarcinoma cells (KKU-100, KKU-M156) and human colon cancer (Caco-2) cells with OvES and analysed the effects of different endocytic inhibitors to address the mechanism of cellular uptake of ES proteins. OvES was internalized preferentially by liver cell lines, and most efficiently/rapidly by H69 cells. There was no evidence for trafficking of ES proteins to cholangiocyte organelles, and most of the fluorescence was detected in the cytoplasm. Pretreatment with clathrin inhibitors significantly reduced the uptake of OvES products, particularly by H69 cells. OvES induced proliferation of liver cells (H69 and CCA lines) but not intestinal (Caco-2) cells, and proliferation was blocked using inhibitors of the classical endocytic pathways (clathrin and caveolae). OvES drove IL6 secretion by H69 cells but not Caco-2 cells, and cytokine secretion was significantly reduced by endocytosis inhibitors. This the first known study to address the endocytosis of helminth ES proteins by host epithelial cells and sheds light on the pathways by which this parasite causes one of the most devastating forms of cancer in south-eastern Asia.
Keywords: Opisthorchis viverrini, Endocytosis, Carcinogenesis, Excretory/secretory products, IL6, Cholangiocyte
Graphical Abstract
1. Introduction
Opisthorchiasis caused by infection with the carcinogenic liver fluke, Opisthorchis viverrini, remains an important health problem in Laos, Cambodia, Vietnam and Thailand, where >10 million people are infected (Sithithaworn et al., 2012). Humans acquire the infection by eating raw or undercooked cyprinoid fish, which act as the secondary intermediate host containing infective metacercariae (Kaewpitoon et al., 2008; Sripa et al., 2011). Adult worms inhabit the biliary system of the mammalian host where they can survive for many years (Kaewpitoon et al., 2008). Chronic O. viverrini infection is associated with several hepatobiliary diseases including cholangitis, biliary hyperplasia, periductal fibrosis and cholangiocarcinoma (CCA), a fatal type of bile duct cancer (Sripa et al., 2007, 2012a). Indeed, the north-eastern region of Thailand where O. viverrini is endemic has the highest worldwide incidence of CCA (Sripa and Pairojkul, 2008).
Opisthorchis viverrini-induced biliary pathology includes mechanical damage caused by the parasite’s physical attachment to and grazing on the biliary epithelium, the release of excretory/secretory products (OvES), and immunopathology (Sripa, 2003; Sripa et al., 2012a). Indeed, immune-mediated pathogenesis in response to liver fluke infection is a major driving force in the onset of biliary disease, including CCA (Sripa et al., 2007, 2012a). OvES released from the tegument and excretory openings of the fluke are highly immunogenic (Wongratanacheewin et al., 1988; Sripa and Kaewkes, 2000a, b; Choi et al., 2003). Sripa and Kaewkes (2000a) observed an intense inflammatory response in areas of the biliary epithelium where parasite antigens were present, particularly those tissues in contact with the fluke. Moreover, numerous studies have provided evidence, albeit with low-resolution microscopy, that fluke antigens are detected inside cholangiocytes lining the biliary epithelium in O. viverrini-infected hamsters (Sripa and Kaewkes, 2000a; Pinlaor et al., 2009; Smout et al., 2009). Until now, the mechanism by which liver fluke antigens are internalised by cholangiocytes remained elusive.
Endocytosis is a major pathway for cell-cell communication and internalisation of extracellular proteins in eukaryotic organisms (Doherty and McMahon, 2009). Two major pathways have been described: (i) clathrin mediated endocytosis whereby molecules are taken up via clathrin-coated vesicles, and (ii) non-clathrin mediated endocytosis (or caveolae-mediated endocytosis) which requires cholesterol/sphingolipid-rich caveolae in membrane invagination and internalization (Le Roy and Wrana, 2005). The clathrin pathway controls targeting of signaling molecules to specialized membrane compartments (Ceresa and Schmid, 2000), while the caveolae mediated pathway acts as a regulator of cell signaling (Lipkowitz, 2003). In this study we used state-of-the-art microscopy techniques to investigate the internalisation mechanisms of OvES by human cholangiocytes and its function, particularly in cell proliferation and inflammatory cytokine IL6 production. This may lead to better understanding of the pathogenesis of O. viverrini-induced hepatobiliary pathology and CCA.
2. Materials and methods
2.1. Chemicals
Bovine serum albumin and all cellular process inhibitors (protease inhibitor E64, chlorpromazine (CPZ, a cationic amphiphilic inhibitor acts on clathrin-coated pits), sucrose (hypertonic media that interferes with clathrin and adaptor protein interactions), bafilomycin A1 and filipin) were purchased from Sigma USA). ER-Tracker™Red, BODIPY TR C5-ceramide, LysoTracker, mouse anti-Rad5, Hoechst, propidium iodide, Alexa Fluor 488 and carboxylic acid succinimidyl ester were obtained from Molecular Probes (USA).
2.2. Parasites, animal infections and ethics approvals
Opisthorchis viverrini metacercariae were obtained from naturally infected cyprinoid fish captured from an endemic area in Khon Kaen province, north-eastern Thailand, as described previously (Ninlawan et al., 2010). Briefly, fish were digested with pepsin-HCl, and after several washes with normal saline, metacercariae were collected, identified under a dissecting microscope and used to infect hamsters. Adult O. viverrini worms were obtained from the liver, gallbladders and extrahepatic bile ducts of hamsters infected for 3 months. All the hamsters used for this study were maintained at the animal facility, Faculty of Medicine, Khon Kaen University, Thailand and the protocols used for animal experimentation were approved by the Animal Ethics Committee of Khon Kaen University based on the Ethics of Animal Experimentation of the National Research Council of Thailand.
2.3. Preparation of parasite ES products
OvES was prepared as previously described with minor modifications (Sripa and Kaewkes, 2000a, b). Briefly, fresh worms were cultured in RPMI-1640 containing antibiotic and the protease inhibitor E64. Worms were maintained in vitro at 37°C and supernatants containing the OvES were collected twice each day for up to 7 days and centrifuged at 2,090 g for 10 min to remove the eggs. The clarified supernatants were pooled, dialyzed in PBS, concentrated and absorbed with Triton-X114 to remove residual lipopolysaccharide (LPS) (Aida and Pabst, 1990), followed by Bio-Beads SM2 (Bio-Rad, (USA) to remove Triton-X114. Finally, OvES was filtered through a 0.2 μm membrane and then aliquoted and stored at −80°C. The LPS concentration was determined by Limulus amoebacyte assay (less than 100 ng/ml had no effect on cell proliferation or cytokine production).
2.4. Fluorescent labeling of OvES proteins
OvES proteins were labeled with Alexa Fluor488 (Invitrogen, USA) following the manufacturer’s protocol. Briefly, 0.5 ml of 2 mg/ml OvES was gently mixed with 50 μl of 1.0 mg/ml Alexa Fluor 488 in DMSO for 1 h at room temperature (RT). A G10 gel filtration column (Amersham Biosciences, UK) was used to isolate labeled proteins from unbound dye. The protein concentration was measured by Bradford assay (Bio-Rad), and labeled protein was kept at 4°C until required for cell culture.
2.5. Cell culture
Normal human cholangiocytes (H69) were maintained in DMEM/HamF-12 (Gibco, USA) supplemented with insulin, adenine, epinephrine, T3-T, epidermal growth factors (EGF) and hydrocortisone (Ninlawan et al., 2010). Human cholangiocarcinoma (KKU-100 and KKU-M156) were maintained in Ham-F12 (Gibco) and human colon cancer (Caco-2) cell lines were maintained in DMEM containing L-gutamine and non-essential amino acids (Gibco) at 37°C, 5% CO2 with 10% FCS (Gibco) containing 100 U/ml of penicillin and 100 μg/ml of streptomycin solution (Gibco).
2.6. Internalization of OvES
To determine the uptake of OvES by cells, H69, KKU-100, KKU-M156 and Caco-2 cell lines were incubated with 10 μg/ml of unlabeled OvES at 37°C. The cells were collected at different time points (0, 15, 30, 45, 60 min), washed in PBS, fixed with cold 4% paraformaldehyde for 20 min at RT and permeabilised with 0.1% Triton X-100 for 15 min. The cells were then blocked for non-specific binding with 3% BSA in PBS for 20 min and incubated with rabbit anti-ES antiserum (Sripa and Kaewkes, 2000a) diluted 1: 300 with 1% BSA in PBS for 1 h, followed by goat anti-rabbit-Alexa Fluor 488 (Invitrogen) for 1 h. Nuclei were stained with propidium iodide or Hoechst and samples were viewed under a confocal microscope (Olympus-FV1000 or Zeiss, LSM 700) and a three-dimensional (3D) structure illumination microscope (SIM) for providing orthogonal views using Zen 2009 software (©Carl Zeiss MicroImaging GmbH, Germany). The total intracellular fluorescence was quantified by manually drawing a region of interest around the cytoplasm and analyzed using FV10-ASW V.2.1 software (Olympus, Japan).
Internalization of OvES was also analyzed by flow cytometry. Briefly, OvES co-cultured cells were trypsinised, permealised and immunostained as above. After thorough washing with PBS the cells were examined using a flow cytometer (FC 500, Beckman Coulter, Inc., USA) and data was analyzed with FlowJo software (Tree Star, Inc., USA). For inhibition experiments, cells were pretreated with the following endocytosis inhibitors for 30 min prior to adding 10 μg/ml of OvES: 5 μg/ml of CPZ, 0.3 M sucrose, or 4 μg/ml of filipin. All experiments were performed in triplicate and data are expressed as mean-S.E. Statistical differences were determined using one-way ANOVA using GraphPad Prism™ version 5.3. P <0.05 was considered as significant for rejection of the null hypothesis.
2.7. IL6 production
H69 and Caco-2 cells were seeded at 2,000 cells/well in complete media as described in Section 2.5 for 24 h and starved for 12 h in media without serum prior to subsequent experiments. Cells were pretreated with endocytosis inhibitors (5 μg/ml of CPZ, 4 μg/ml of filipin and 1 nM bafilimycin A1) for 30 min, and subsequently cultured with 1.2 μg/ml of OvES for 48 h. Cells incubated with and without OvES proteins and endocytosis inhibitors were used as controls. The culture media was collected and centrifuged at 929 g for 10 min to remove cell debris. Supernatant was then collected and IL6 levels determined using a human IL6 ELISA kit (R&D Systems, USA) following the manufacturer’s recommendations.
2.8. Subcellular localization of OvES in biliary cells
To determine OvES uptake and trafficking, H69, KKU-100 and KKU-M156 cells were pretreated with the following specific organelle trackers: endoplasmic reticulum (ER) - ER-Tracker™Red; lysosomes – LysoTracker; Golgi - BODIPY TR C5-ceramide; early endosomes - anti-Rab5. Cells were stained with Hoechst dye for 30 min before incubating with Alexa Flour 488-conjugated OvES (10 μg/ml) for 2 h. After three rounds of washing, cells were fixed in cold 4% paraformaldehyde for 20 min at RT, mounted in 80% glycerol and viewed on a Nikon A1 confocal fluorescence microscope, equipped with a 60× (NA1.4 plan Apo) oil immersion objective. Z series images were collected in three channels (Ex 405 nm, Em 425–475 nm; Ex 488 nm, Em 500–550 nm; Ex 561 nm, Em 570–620 nm) with a step size of 200 nm.
2.9. Effect of OvES and endocytosis inhibitors on cell proliferation
To test the effect of OvES and various endocytosis inhibitors on cell proliferation, real-time monitoring of cell growth using an xCELLigence system (Roche, USA) was employed. Briefly, cells were seeded (2,000 cells/well) in an E plate (Roche) for 24 h. Cells were treated with the clathrin-specific inhibitor CPZ (5 μg/ml of CPZ) for 30 min and, subsequently, 1.2 μg/ml of OvES were added and incubated for 48 h. Cells treated only with OvES (and not with inhibitors) were used as controls. Real-time cell growth was measured as cell index (CI) as previously described (Xing et al., 2005).
3. Results
3.1. Internalization of OvES by cancerous and non-cancerous biliary cell lines
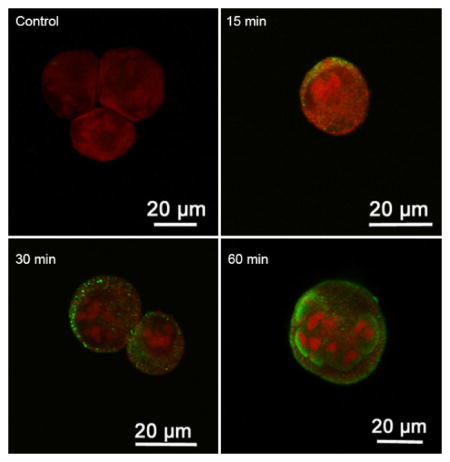
To study the internalization of OvES into biliary cells, different cell lines (H69, KKU-100, KKU-M156 and Caco-2) were cultured with OvES and visualized at different time-points using confocal microscopy (FV100). Internalization rates of OvES proteins were dependent on the cell type. For the first 15 min, OvES proteins were internalized by H69 and KKU-M156 cell lines and were attached to the surface membranes of KKU-100 cells, but, in contrast, significantly less fluorescence was detected on or inside Caco-2 cells (Fig. 1A), suggesting that OvES internalization is cell type-dependent. To validate the internalization of OvES products by H69 cholangiocytes in three dimensions, we performed a 3D image analysis, with the orthogonal view of the Z-stack 3D-SIM imaging (Figs. 1B – D). Orthogonal views confirmed that labeled OvES was attached to the cholangiocyte plasma membrane within 15 min and detected in the cell cytoplasm after 60 min.
Fig. 1.
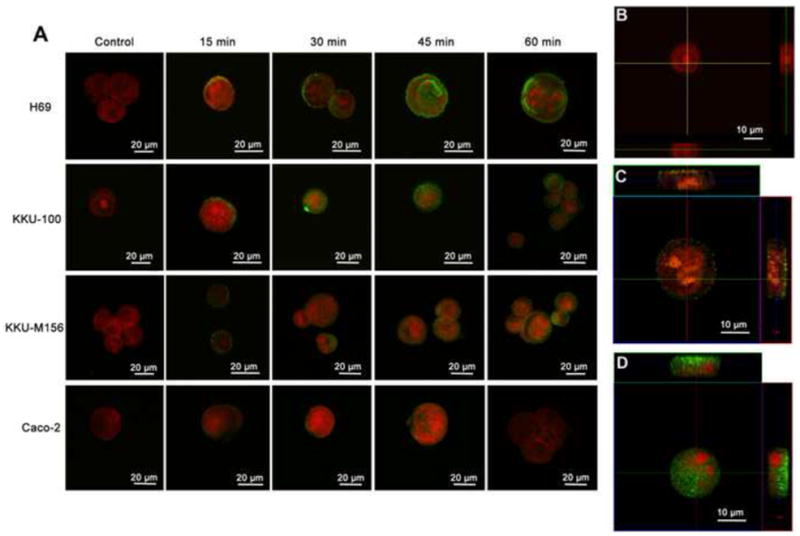
Internalization of Opisthorchis viverrini excretory/secretory products (OvES) by biliary cells (H69, KKU-100, KKU-M156) and colon cancer cells (Caco-2) at 15, 30, 45 and 60 min post-incubation. Internalization of OvES (green fluorescence) by different cell types over time (A). Orthogonal views using Z-stack immunofluorescence microscopy showing three-dimensional internalization of OvES at 0 min (B), 15 min (C) and 120 min (D) in H69 cholangiocytes. Note that OvES is more readily internalized by biliary cells than by colonic epithelial cells. Nucleus was stained with propidium iodide (red fluorescence).
Using flow cytometry, we found that significantly greater quantities of OvES were detected inside cells of hepatic origin (H69, KKU-100 and KKU-156) compared with control colon-derived Caco-2 cells (Fig. 2). The maximum fluorescence intensity was detected in H69 cholangiocytes after 2 h of co-culture (Supplementary Fig. S1).
Fig. 2.
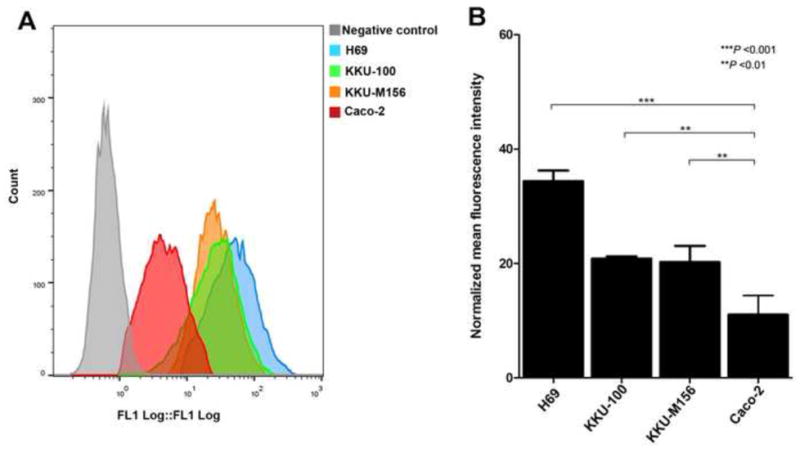
Mean intracellular fluorescence depicting the internalization of Opisthorchis viverrini excretory/secretory products (OvES) by normal cholangiocytes (H69), cholangiocarcinoma (KKU-100, KKU-M156) and colon cancer (Caco-2) cell lines over 2 h. Fluorescence intensity was measured by flow cytometry (A). Histogram is the average intensity of OvES internalization from three independent experiments ± S.E.M. (B).
3.2. OvES uptake by cholangiocytes is via clathrin-mediated endocytosis
To investigate the pathway(s) implicated in OvES internalization by host cells, different endocytosis inhibitors were employed – sucrose, filipin and CPZ. Cells were treated with filipin, sucrose or CPZ for 30 min, following incubation with OvES at 37°C for 2 h and imaged using a confocal microscope. CPZ and sucrose inhibited OvES protein internalization by cholangiocytes and cholangiocyte-derived cancer cells (H69, KKU-100 and KKU-M156) (Fig. 3). Treatment of H69 cells with CPZ, sucrose and filipin resulted in 52 – 64% decrease of OvES uptake (Fig. 4). In contrast, CPZ and sucrose were more effective at preventing uptake of OvES by KKU-100 and KKU-M156 CCA cell lines than was filipin (Fig. 4). Total fluorescence calculation also showed that CPZ and sucrose inhibited OvES internalization (Supplementary Figs. 2A – C). These results suggest that OvES proteins are internalized by cholangiocytes via clathrin- and caveolae-mediated endocytosis pathways.
Fig. 3.

Confocal fluorescence microscopy images showing internalization of Opisthorchis viverrini excretory/secretory products (OvES) in normal (H69) and cholangiocarcinoma (KKU-100 and KKU-M156) biliary cells with and without endocytosis inhibitors. Cells treated with an inhibitor of the clathrin pathway (chlorpromazine, CPZ) showed reduced internalization of OvES (green fluorescence) in all cell types tested. Similarly, sucrose-treated cells showed substantially reduced internalization of OvES in the cholangiocarcinoma lines KKU-100 and KKU-M156 and partially reduced internalization in H69 cholangiocytes.
Fig. 4.
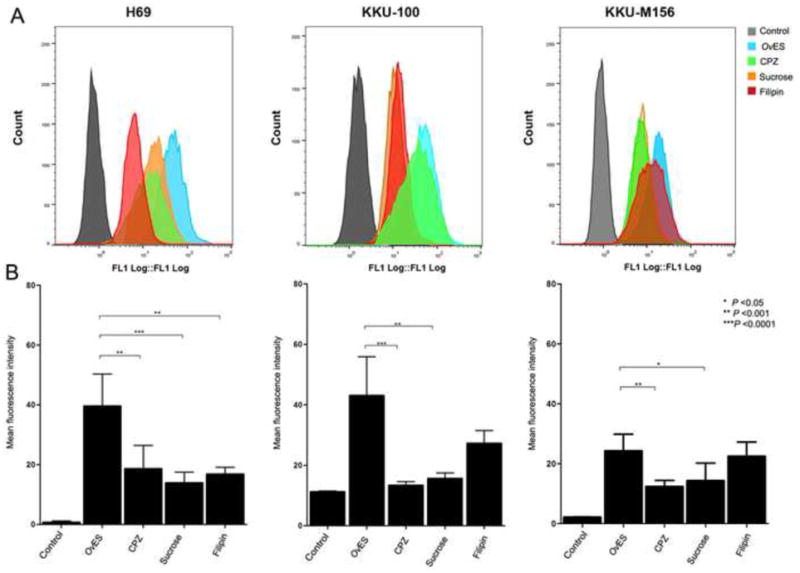
Endocytosis pathway inhibitors block the uptake of Opisthorchis viverrini excretory/secretory products (OvES) by normal (H69) and cholangiocarcinoma (KKU-100 and KKU-M156) biliary cell lines using flow cytometry. Cells were pretreated with the endocytosis inhibitors chlorpromazine (CPZ), sucrose (clathrin-mediated inhibitor) and filipin (caveolae-mediated inhibitor) before incubation with OvES for 2 h. Fluorescence intensity was measured by flow cytometry (A). The histogram depicts the average of three independent experiments ± S.E.M. (B).
3.3. Cellular translocation of OvES proteins
To investigate OvES intracellular localization within cholangiocytes, Alexa Flour 488-conjugated OvES products were cultured with H69 cholangiocytes and different organelles were stained with specific fluorescent dyes. Using confocal microscopy, there was no evidence of OvES co-localization with Golgi, early endosomes, lysosomes, ER or nuclei, suggestive of a cytoplasmic location (Fig. 5).
Fig. 5.
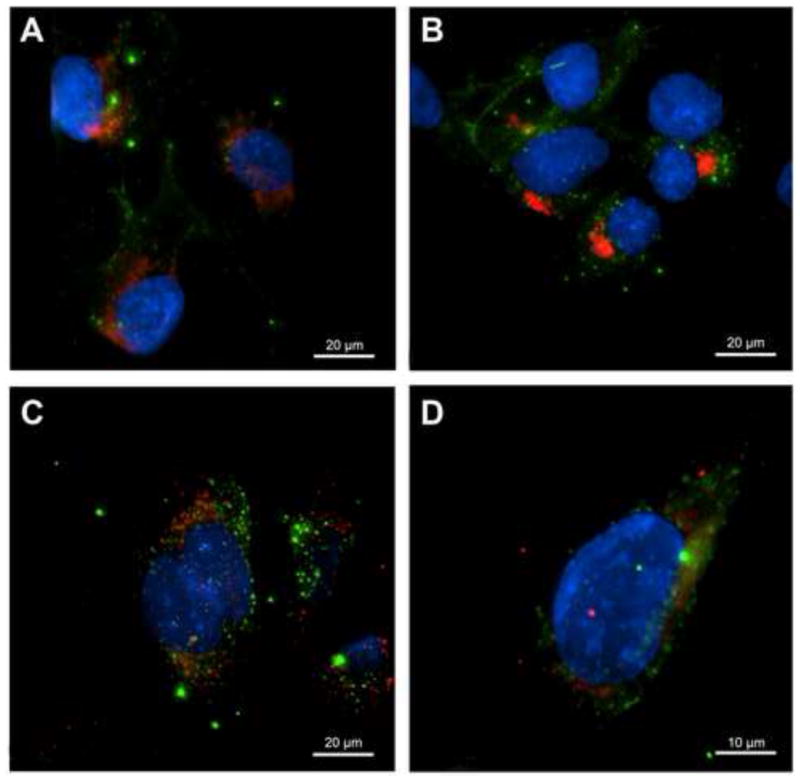
Co-staining of H69 cholangiocytes for internalized Opisthorchis viverrini excretory/secretory products (OvES) and cell organelles revelaed an absence of co-localization. OvES is observed as punctate green fluorescence. Cells were co-stained for endoplasmic reticulum (ER) with ER-Tracker-red (A), Golgi with Golgi-tracker-red (B), early endosomes with Anti-Rab5-red (C) and lysosomes with LysoTracker-red (D). Nuclei were counterstained with Hoechst dye (blue).
3.4. Endocytosis of OvES proteins modulates cell proliferation and pro-inflammatory cytokine production
To determine whether endocytosis of OvES proteins by cholangiocytes promoted cell proliferation we monitored cell proliferation in real-time using an xCelligence system (Xing et al., 2005). H69 cholangiocytes, KKU-100 and KKU-M156 CCA cell lines and the Caco-2 colon cancer cell line were incubated with 1.2 μg/ml of OvES products in the presence or absence of CPZ and monitored for 48 h. ES products promoted proliferation of all three cholangiocyte-derived cell lines (H69 and the CCA lines) but not the colon-derived Caco-2. In the presence of CPZ, OvES stimulated proliferation of the two CCA lines within the first 48 h of co-culture but thereafter significantly attenuated proliferation (Figs. 6A – C), while drug alone had no effect on cell growth (data not shown). Interestingly, OvES had no effect on proliferation of Caco-2 cells (Fig. 6D).
Fig. 6.
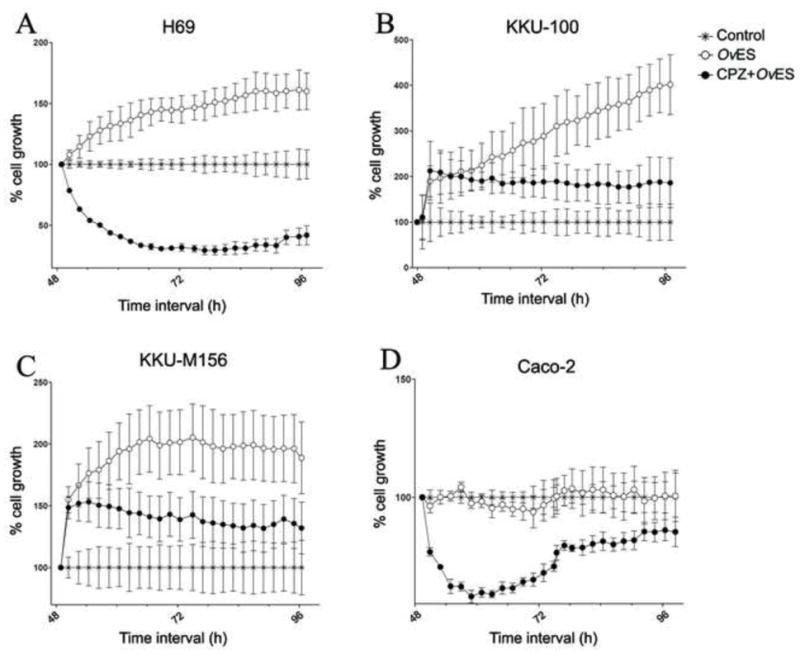
Real time cell proliferation of biliary (H69, KKU-100, KKU-M156) and colon (Caco-2) cells induced by Opisthorchis viverrini excretory/secretory products (OvES) in the presence or absence of chlorpromazine (CPZ) using an xCELLigence system. OvES stimulated proliferation of bilary cells (A – C) but not colon cells (D) compared with media control over 96 h of culture. CPZ significantly inhibited growth of all cell types. Growth of each cell type was normalized to its media control without OvES.
It has been previously described that IL6 is a pro-inflammatory cytokine associated with advanced periductal fibrosis in O. viverrini infected patients (Sripa et al., 2009, 2012b). IL6 production from both cholangiocytes and colon cancer cells incubated with and without endocytosis inhibitors (CPZ, filipin and bafilomycin A1) before addition of OvES was measured. Addition of OvES promoted IL6 production by cholangiocytes but had no effect on IL6 production by Caco-2 cells (Fig. 7A). Moreover, all endocytosis inhibitors attenuated cholangiocyte IL6 production by 69%, 55% and 53%, respectively, with the greatest reduction occurring in the presence of the clathrin-mediated endocytosis inhibitor, CPZ (P < 0.001; Fig. 7B).
Fig. 7.
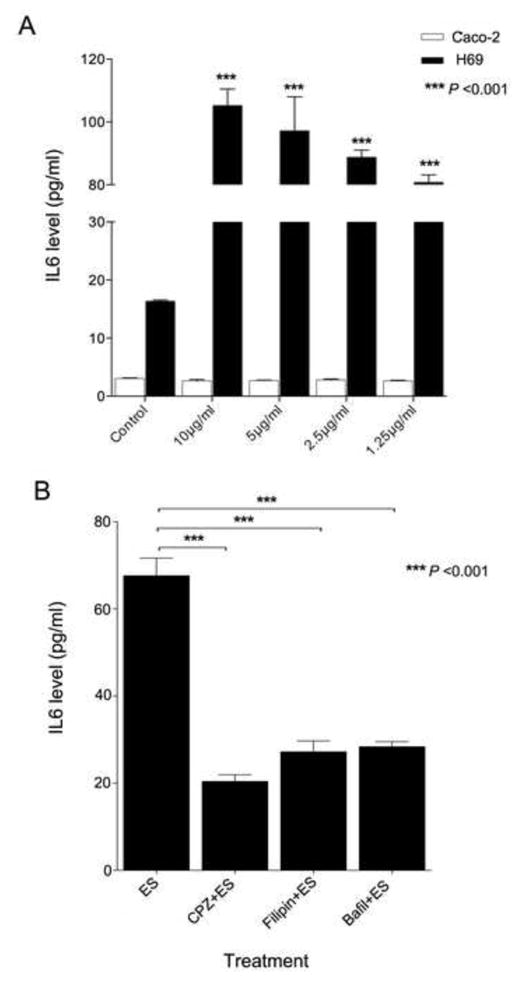
IL6 production. IL6 production in normal human cholangiocytes (H69) and human colon cancer (Caco-2) co-cultured with Opisthorchis viverrini excretory-secretory products (OvES) in several dilutions for 48 h (A). The biliary cells were strongly stimulated by OvES protein, but had no effect in colon cancer cells when compared with control cells. IL6 production from normal human cholangiocyte cell (H69) with and without endocytosis inhibitors (chlorpromazine; CPZ, Filipin and Bafilomycin A1) before OvES stimulation for 48 h using the ELISA technique (B). OvES stimulated IL6 production while all inhibitors of endocytosis significantly suppressed the levels of IL6 secreted. Histograms represent the average of three independent experiments ± S.E.M. of the absorbance at 450 nm measured by a Versamax microplate reader using SoftMax pro V.5 program (*** P < 0.001).
4. Discussion
The metabolic products secreted from the tegument and excretory openings of Opisthorchis viverrini are highly immunogenic and have diverse effects on host cells (Sripa, 2003). Perhaps the most intriguing aspect of the interactions between OvES and human host cholangiocytes is the active internalization of ES proteins, and the potential carcinogenic ramifications of this process. We have shown previously that OvES proteins can be detected inside biliary epithelial cells in the vicinity of O. viverrini in the bile ducts of infected hamsters. Moreover, ES products were detected inside epithelial cells in the upper biliary tree where adult flukes are too large to reach (Sripa and Kaewkes, 2000a). Since this initial description, we and others raised antibodies to defined recombinant OvES products and showed that individual ES products, including thioredoxin peroxidase (Laha et al., 2007), granulin (Smout et al., 2009) and cathepsin F (Pinlaor et al., 2009) were internalized by cholangiocytes. While other pathogens have been shown to produce proteins that are internalized by host cells whereupon they induce toxic and functional changes, including pre-cancerous events, this is the first known evidence of the uptake by host cells of a secreted parasite protein. The bacterium Helicobacter pylori secretes cag A, a virulence factor whose uptake by epithelial cells can result in gastric cancer (Hatakeyama, 2004). Given that OvES products induce severe inflammation by up-regulation of cholangiocyte Toll-like receptor (TLR)4 mRNA expression, thereby promoting IL6 and IL-8 production (Ninlawan et al., 2010), it is reasonable to assume that internalized OvES products also promote a tumorigenic phenotype.
We show herein that OvES was internalized by cholangiocytes but not by the Caco-2 line of intestinal epithelial cells (IEC). IEC serve as a critical barrier to luminal bacteria and food, and are active participants in the intestinal innate immune response, responding to signals in both the luminal (apical) and lamina propria (basal) compartments (Madara, 1997; Hecht, 1999). While IEC are reported to be unresponsive to LPS, Caco-2 cells have very low levels of tlr4 mRNA and weakly detectable myeloid differentiation (md)-2 expression compared with human dermal microvessel endothelial cells (Abreu et al., 2001). We suggest that the uptake of OvES by host cells might depend on the presence of a specific receptor, such as TLR4, that is highly expressed on cholangiocytes compared with IECs.
To further address the mechanism of internalization of OvES products by different cell lines we analysed the role of the endocytic pathway. This pathway is the key process involved in the internalization of molecules from the cell surface to internal membrane compartments, and two major mechanisms are involved in this receptor-mediated endocytic pathway: clathrin-mediated endocytosis and the non-classical caveolae-mediated endocytosis (Le Roy and Wrana, 2005). Clathrin-mediated endocytosis requires clathrin and adaptor proteins such as AP-2 and Esp15 to form a coat pit (Keen, 1987), whereas the caveolae-mediated pathway depends on the balance between the caveolin-1 protein and lipid raft components such as cholesterol and glycosphingolipids (Sharma et al., 2004). By using different endocytosis blocking agents such as CPZ, sucrose and filipin, we showed that CPZ and sucrose significantly blocked the internalization of OvES in all hepatic cell types studied. CPZ is known to cause a loss of clathrin-coated pits at cell surfaces and is associated with accumulation of clathrin and AP-2 in the endosomal compartment (DiPaola et al., 1984; Sofer and Futerman, 1995), while sucrose serves as a hypertonic medium and induces abnormal clathrin polymerization into empty microcages (Heuser and Anderson, 1989). Despite the requirement of clathrin-mediated endocytosis for internalization of OvES products, filipin, a sterol-binging agent that disrupts structure and function of caveolae (Schnitzer et al., 1994; Orlandi and Fishman, 1998) also blocked the uptake of OvES by normal cholangiocytes (H69), but not by CCA cell lines. These results imply that different OvES proteins use different pathways to enter host cells, although clathrin-mediated endocytosis clearly plays a major role in the overall process.
We have shown that OvES products specifically induce production of IL6 by human cholangiocytes, but not by human colon cancer epithelial cells, suggesting that cholangiocytes might be the primary source of IL6 in the biliary epithelium of infected individuals. High levels of IL6 have been associated with chronic periductal fibrosis and CCA in opisthorchiasis patients (Sripa et al., 2012b). Moreover, IL6 has also been implicated in the maintenance of chronic inflammation that could lead to tumorigenesis (Schafer and Werner, 2008). Thuwajit et al. (2004) reported that OvES products may act as growth factors by inducing proliferation of a mouse fibroblast cell line (NIH-3T3) in vitro accompanied by increased expression of the TGF-β receptor (TGFβR). Interestingly, clathrin- and caveolae-mediated endocytosis can regulate TGFβR signaling and turnover (Di Guglielmo et al., 2003). In addition, different internalization pathways are associated with distinct intercellular fates. Several signaling receptors, including TGFβR, receptor tyrosine kinases (RTKs), GPCRs, NOTCH and WNT are involved in both clathrin-mediated endocytosis and non-clathrin endocytosis and influence the final signaling output (Le Roy and Wrana, 2005). Clathrin-mediated entry is associated with long-term signaling (Sadowski et al., 2009) whereas caveolae-mediated entry associated with Smad7-Smurf2 ubiquitin ligase complex is directly involved in degradation of the receptor by lysosome (Di Guglielmo et al., 2003). OvES products stimulate cell proliferation and signal transduction via clathrin- and caveolae-mediated endocytosis in normal cholangiocytes but only the clathrin pathway in CCA cells. The endocytosis pathway regulates the balance in the receptor systems acting as an effector or attenuator of the signal transduction (Lipkowitz, 2003). Thus, the internalization of OvES via clathrin-mediated endocytosis may steer receptors away from a degradation fate, and enhance signaling from the plasma membrane, thereby increasing inflammatory cascade signaling and contributing to the immunopathogenesis of opisthorchiasis. However, more studies are required to assess this hypothesis.
We reveal here a role for the clathrin-mediated and caveolae endocytic pathways in the internalization of OvES by biliary epithelial cells, and subsequent stimulation of cell proliferation and the production of the pro-inflammatory cytokine IL6. This is a key early step in pathogenesis of opisthorchiasis and CCA development, particularly seeing as inflammatory IL6 can stimulate inflammation leading to free radical production causing oxidative DNA damage (Sripa et al., 2012a). DNA damage can be from exogenous (i.e. nitrosamine in fermented dietary foods) or endogenous nitrosation from O. viverrini infection (Sripa et al., 2007). These biliary cells that have undergone DNA damage due to OvES exposure are induced to proliferate, and fixed genetic alterations ultimately occur. Accumulated genetic alterations can ensue with uncontrolled growth and subsequent malignancy. However, OvES also displays inhibitory effects on host cells, such as anti-apoptotic activity (Sripa et al., 2012a). Further work is now required to characterize multiple pathways downstream of the process of parasite protein internalisation, as well as the individual fluke proteins involved and their cellular receptors. Recent reports have shown that parasitic helminths secrete extracellular vesicles (Marcilla et al., 2012; Buck et al., 2014), and future work should explore the possibility that O. viverrini secretes exosome-like vesicles that are internalized by host cells. A better understanding of the process of host cell-mediated internalization of liver fluke proteins will shed light on the immunopathogenesis of the infection and provide novel pathways to target in the development of vaccines against this carcinogenic infection.
Supplementary Material
The kinetics of internalization of Opisthorchis viverrini excretory-secretory products (OvES) at 0, 15, 30, 45, 60 min in normal cholangiocytes (H69), cholangiocarcinoma cell lines (KKU-100 and KKU-M156) and Caco-2 colon cancer cells. Histograms represent the average of three independent experiments ± S.E.M. of the fluorescence intensity measured by flow cytometry.
Fluorescence intensity of internalization of Opisthorchis viverrini excretory-secretory products (OvES) by H69 cholangiocytes, KKU-100 and KKU-M156 cholangiocarcinoma cell with and without the endocytosis inhibitors cholorpromazine (CPZ) and sucrose. CPZ and sucrose have significant inhibitory effects on OvES internalization in all biliary cell types (A, B, C). Histograms represent the average of three independent experiments ± S.E.M. of the fluorescence intensity measured by image analysis.
Highlights.
Opisthorchis viverrini excretory-secretory products (OvES) were internalised by biliary cells but not intestinal cells.
The internalisation was accomplished by clathrin and caveolae endocytic pathways.
Endocytosis of OvES induced biliary cell proliferation and production of IL6.
Acknowledgments
This research was supported by the National Health Security of Thailand, the Thailand Research Fund (TRF) under the TRF Senior Scholar and was partially supported by awards P50AI098639 (BS, AL), from the National Institute of Allergy and Infectious Disease (NIAID), USA, R01CA155297 (AL, BS), R01CA164719 (BS, AL) from the National Cancer Institute (NCI), National Institute of Health (NIH), USA and a project grant from the National Health and Medical Research Council of Australia (NHMRC). AL is the recipient of a principal research fellowship from NHMRC. The United States Army Medical Research and Materiel Command (USAMRMC), partially supported the work (contract number W81XWH-12-C-0267). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID, NCI or NIH. Sujittra Chaiyadet was supported by the TRF-the Royal Golden Jubilee PhD scholarship (RGJ) through Dr. Banchob Sripa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609– 1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191– 195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- Buck AH, Coakley G, Simbari F, McSorley HJ, Quintana JF, Le Bihan T, Kumar S, Abreu-Goodger C, Lear M, Harcus Y, Ceroni A, Babayan SA, Blaxter M, Ivens A, Maizels RM. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014;5:5488. doi: 10.1038/ncomms6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa BP, Schmid SL. Regulation of signal transduction by endocytosis. Curr Opin Cell Biol. 2000;12:204– 210. doi: 10.1016/s0955-0674(99)00077-0. [DOI] [PubMed] [Google Scholar]
- Choi MH, Park IC, Li S, Hong ST. Excretory-secretory antigen is better than crude antigen for the serodiagnosis of clonorchiasis by ELISA. Korean J Parasitol. 2003;41:35– 39. doi: 10.3347/kjp.2003.41.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410– 421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- DiPaola M, Keith CH, Feldman D, Tycko B, Maxfield FR. Loss of alpha 2-macroglobulin and epidermal growth factor surface binding induced by phenothiazines and naphthalene sulfonamides. J Cell Physiol. 1984;118:193– 202. doi: 10.1002/jcp.1041180212. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857– 902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688– 694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol. 1999;277:C351– C358. doi: 10.1152/ajpcell.1999.277.3.C351. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Anderson RG. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389– 400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: the carcinogenic human liver fluke. World J Gastroenterol. 2008;14:666– 674. doi: 10.3748/wjg.14.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JH. Clathrin assembly proteins: affinity purification and a model for coat assembly. J Cell Biol. 1987;105:1989– 1998. doi: 10.1083/jcb.105.5.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha T, Pinlaor P, Mulvenna J, Sripa B, Sripa M, Smout MJ, Gasser RB, Brindley PJ, Loukas A. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112– 126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Lipkowitz S. The role of the ubiquitination-proteasome pathway in breast cancer: ubiquitin mediated degradation of growth factor receptors in the pathogenesis and treatment of cancer. Breast Cancer Res. 2003;5:8– 15. doi: 10.1186/bcr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL. The chameleon within: improving antigen delivery. Science. 1997;277:910– 911. doi: 10.1126/science.277.5328.910. [DOI] [PubMed] [Google Scholar]
- Marcilla A, Trelis M, Cortes A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sanchez del Pino MM, Munoz-Antoli C, Toledo R, Bernal D. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One. 2012;7:e45974. doi: 10.1371/journal.pone.0045974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninlawan K, O’Hara SP, Splinter PL, Yongvanit P, Kaewkes S, Surapaitoon A, LaRusso NF, Sripa B. Opisthorchis viverrini excretory/secretory products induce toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol Int. 2010;59:616– 621. doi: 10.1016/j.parint.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905– 915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinlaor P, Kaewpitoon N, Laha T, Sripa B, Kaewkes S, Morales ME, Mann VH, Parriott SK, Suttiprapa S, Robinson MW, To J, Dalton JP, Loukas A, Brindley PJ. Cathepsin F cysteine protease of the human liver fluke, Opisthorchis viverrini. PLoS Negl Trop Dis. 2009;3:e398. doi: 10.1371/journal.pntd.0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Exp Cell Res. 2009;315:1601– 1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628– 638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217– 1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114– 3122. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int l. 2012;61:10– 16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout MJ, Laha T, Mulvenna J, Sripa B, Suttiprapa S, Jones A, Brindley PJ, Loukas A. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer A, Futerman AH. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the Golgi apparatus and the subsequent elevation of cyclic AMP. J Biol Chem. 1995;270:12117– 12122. doi: 10.1074/jbc.270.20.12117. [DOI] [PubMed] [Google Scholar]
- Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209– 220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120(Suppl 1):S158–168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012a;28:395– 407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000a;30:735– 740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000b;22:139– 145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, Tessana S, Loukas A, Brindley PJ, Bethony JM. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50:1273– 1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349– 356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Thinkhamrop B, Mairiang E, Laha T, Kaewkes S, Sithithaworn P, Periago MV, Bhudhisawasdi V, Yonglitthipagon P, Mulvenna J, Brindley PJ, Loukas A, Bethony JM. Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS Negl Trop Dis. 2012b;6:e1654. doi: 10.1371/journal.pntd.0001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuwajit C, Thuwajit P, Kaewkes S, Sripa B, Uchida K, Miwa M, Wongkham S. Increased cell proliferation of mouse fibroblast NIH-3T3 in vitro induced by excretory/secretory product(s) from Opisthorchis viverrini. Parasitology. 2004;129:455– 464. doi: 10.1017/s0031182004005815. [DOI] [PubMed] [Google Scholar]
- Wongratanacheewin S, Chawengkirttikul R, Bunnag D, Sirisinha S. Analysis of Opisthorchis viverrini antigens by immunoprecipitation and polyacrylamide gel electrophoresis. Parasitology. 1988;96(Pt 1):119– 128. doi: 10.1017/s0031182000081701. [DOI] [PubMed] [Google Scholar]
- Xing JZ, Zhu L, Jackson JA, Gabos S, Sun XJ, Wang XB, Xu X. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem Res Toxicol. 2005;18:154– 161. doi: 10.1021/tx049721s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The kinetics of internalization of Opisthorchis viverrini excretory-secretory products (OvES) at 0, 15, 30, 45, 60 min in normal cholangiocytes (H69), cholangiocarcinoma cell lines (KKU-100 and KKU-M156) and Caco-2 colon cancer cells. Histograms represent the average of three independent experiments ± S.E.M. of the fluorescence intensity measured by flow cytometry.
Fluorescence intensity of internalization of Opisthorchis viverrini excretory-secretory products (OvES) by H69 cholangiocytes, KKU-100 and KKU-M156 cholangiocarcinoma cell with and without the endocytosis inhibitors cholorpromazine (CPZ) and sucrose. CPZ and sucrose have significant inhibitory effects on OvES internalization in all biliary cell types (A, B, C). Histograms represent the average of three independent experiments ± S.E.M. of the fluorescence intensity measured by image analysis.


