Abstract
Objective:
To evaluate the characteristics and spontaneous recovery of tinnitus related to idiopathic sudden sensorineural hearing loss (ISSNHL).
Study Design:
Retrospective analysis from two randomized placebo-controlled clinical trials for treatment of ISSNHL within 48 hours from onset (Study A), or of tinnitus related to ISSNHL within 3 months from onset (Study B).
Setting:
Forty-eight European sites (academic tertiary referral centers, private ENT practices).
Patients:
One hundred thirteen adult patients of which 65 with hearing loss ≥30 dB (Study A) and 48 with persistent acute tinnitus (Study B) at baseline.
Interventions:
Intratympanic (i.t.) injection of placebo gel in single dose or in triple dose during 3 consecutive days.
Main Outcome Measures:
Frequency of tinnitus, subjective tinnitus loudness, rates of complete tinnitus remission, and complete hearing recovery during 3 months follow-up.
Results:
In acute ISSNHL, tinnitus loudness decreased rapidly in cases of mild-moderate hearing loss, and tinnitus had completely resolved in two-thirds of patients after 3 months. Hearing recovery preceded tinnitus resolution. When associated with severe-profound hearing loss, tinnitus improved significantly less. Complete hearing recovery and full tinnitus remission were both about three times more frequent in mild-moderate hearing loss patients than in severe-profound cases. Improvement in tinnitus loudness over time can be approximated by a negative exponential function.
Conclusions:
Prognosis for ISSNHL-related tinnitus is relatively poor in case of severe-profound hearing loss and the longer it has persisted. Alleviation or management of tinnitus should be a key therapeutic objective especially in pronounced ISSNHL cases.
Keywords: Hearing recovery, Intratympanic, ISSNHL, Spontaneous recovery, Tinnitus
In idiopathic sudden sensorineural hearing loss (ISSNHL), tinnitus is the most frequent secondary symptom; recent ISSNHL trials reported incidence rates as high as 73 to 84% (1–3). The primary symptom of acute hearing loss, which may present as a complaint of a full or blocked ear, often is of less immediate concern for patients than tinnitus, which as a new and unexpected experience may be perceived as much more frightening (4). Although the incidence of concomitant tinnitus and its severity seems to be lower in ISSNHL than, for example, in cases of acute acoustic trauma (5), and the rate of spontaneous recovery is considered to be substantial, it may still become chronic and have a serious impact on the patient's well-being and quality of life (QoL) (6,7).
To date there is sparse published information available describing the characteristics and natural history of ISSNHL-tinnitus. While the presence of tinnitus is frequently reported as part of baseline patient characteristics and potential prognostic factor in ISSNHL trials, rarely are follow-up data provided. Also, hardly any data seem to exist on spontaneous recovery rates (8), which reflects a dearth of observational or placebo-controlled ISSNHL trials. A recent Cochrane review of steroids in the treatment of ISSNHL failed to meet its secondary objective of analyzing the effect on tinnitus because of the low number of well-designed controlled studies and for lack of specific tinnitus data (9).
While not directly comparable, the small number of published ISSNHL trials that do provide follow-up data seem to suggest that tinnitus recovery rates are linked to initial ISSNHL severity and correlate with hearing recovery rates. Studies enrolling ISSNHL patients with mild to moderate hearing loss already show after 7 to 10 days not only substantial hearing recovery, but also a large decrease in tinnitus incidence from 50 to 70% to 30 to 40% (2,10). On the other extreme, studies enrolling ISSNHL patients with more severe hearing loss show only small to moderate hearing recovery and small to moderate reductions in tinnitus incidence, if at all (11,12); for example, 12 months after onset tinnitus was still present in 60% of patients with severe ISSNHL (11). Recovery in hearing in general seems to occur more rapidly than a decrease in tinnitus loudness or tinnitus remission (13).
Given the scarcity of information on ISSNHL-tinnitus, we sought to obtain more detailed data and gain further insights from two recent prospective double-blind, randomized, placebo-controlled studies that involved ISSNHL patients. One of the studies enrolled ISSNHL patients within 48 hours from onset and followed them for 3 months, with a primary focus on hearing loss (14). The other study enrolled ISSNHL-tinnitus patients within 3 months from onset and followed them for 3 months, with a primary focus on tinnitus (15). Thanks to the inclusion of placebo control groups, the two studies allow for a detailed analysis of the acute and post-acute time course and spontaneous recovery of ISSNHL-related tinnitus.
MATERIALS AND METHODS
Study Design and Participants
For the purposes of the present study we retrospectively analyzed data from a total of 113 placebo-treated ISSNHL patients participating in one of the two randomized double-blind clinical trials AM-111-CL-08-01 (“Study A”; 65 patients) or AM-101-CL-08-01 (“Study B”; 48 patients). Details of the study design and procedures have been described elsewhere (14,15).
Study A enrolled patients (18–60 years of age) suffering from unilateral ISSNHL with hearing loss of at least 30 dB at the average of the three worst affected contiguous audiometric test frequencies (pure tone average [PTA]), determined against the contralateral ear (or a pre-existing audiogram or ISO norm values in case of history of asymmetric hearing loss). They were treated within 48 hours from onset. The presence of tinnitus was not a requirement for study participation. Study B enrolled patients (18–65 years of age) with persistent uni- or bilateral tinnitus following ISSNHL within 3 months from onset. Persistent tinnitus was considered given when patients indicated that they could always hear their tinnitus when they were thinking of it in the past 2 weeks and that their tinnitus during that period occurred either all of the time, most of the time, or a good bit of the time. The presence of hearing loss was not a requirement for study participation.
Randomization and Masking
At baseline (Day 0), study participants were randomized to receive the investigational drug, AM-111 (Study A) or AM-101 (Study B), respectively, or placebo (a viscous phosphate-buffered gel formulation based on 0.7% sodium hyaluronate) at a 2:1 ratio. The study drug formulation was identical in appearance for active and placebo doses and revealed no differences during administration. Study patients and investigators remained blinded throughout the entire study.
Procedures
Participants in Study A received on Day 0 a single dose intratympanic (i.t.) injection, whereas participants in Study B received 3 i.t. injections on Days 0, 1, and 2 under local anesthesia through a small myringotomy with the patient's head placed in a position tilted 45 degrees towards the untreated ear. In Study B, only the worse affected ear was treated if bilaterally affected (<14% of patients). Patients remained in their supine position for approximately 30 minutes. Patients were followed for 90 days; interim follow-up visits were scheduled at Days 3 (only Study A), 7 and 30. Tinnitus loudness was recorded on a 0 to 10 (Study A) respectively, 0 to 100 (Study B) point numerical rating scale with a recall period of “right now” (0 = “no tinnitus” and 10 = “extremely loud” [Study A], respectively “very loud” [Study B]). For comparison with Study A, values from Study B were converted to a 0 to 10 scale. Hearing loss in Study A was classified by baseline audiogram type (16), initial frequency range (low, medium, or high), and baseline severity (mild to moderate: PTA < 60 dB, severe to profound: PTA ≥ 60 dB; [17]). Complete tinnitus remission was assumed when tinnitus loudness was rated as 0. Hearing was considered as fully recovered if PTA recovered to within 10 dB of the baseline value. In Study A, study participants with insufficient hearing recovery (defined as <10 dB from baseline to Day 7 at the average of the three worst affected contiguous audiometric test frequencies) were given the option to receive oral prednisolone 50 mg b.i.d. for 5 days as a reserve therapy.
Statistical Methods
Statistical analyses were performed with SAS (SAS Institute, Cary, NC, version 9.3). Tests and graphs were based on analysis sets that included all placebo-treated patients for whom data were available both at baseline and Day 90. Mean values of tinnitus loudness at treatment visits and change from baseline were compared using the t test, assuming equal variances for the compared subgroups. A non-linear time function of tinnitus loudness was estimated using the SAS “proc nlin” function. The frequency of complete tinnitus remission and complete hearing recovery was compared between subgroups using Fisher's exact test. For pairwise correlation of PTA and tinnitus loudness at baseline and Day 90, Spearman's correlation coefficient rs was calculated. A contingency table with complete hearing recovery and complete tinnitus remission (yes/no) was generated to evaluate their interrelations within subjects; in addition a χ2 test for independence was performed.
RESULTS
Tinnitus in the Acute ISSNHL Patient
Out of a total of 65 ISSNHL patients enrolled within 48 hours from acute hearing loss onset, 72.3% reported the concurrent onset of tinnitus. 7.7% of patients had pre-existing tinnitus and 20.0% reported no tinnitus at all. The incidence of novel tinnitus (47 patients in total) was 75.9% in cases of severe-profound hearing loss (n = 22) and 70.3% in mild-moderate cases (n = 25; Table 1). In the subgroup of patients with novel-onset tinnitus, cases with severe-profound hearing loss showed a higher proportion of predominantly high-frequency hearing loss and of audiogram types C or E (descending slope or total or subtotal anacusis) than those with mild-moderate hearing loss. Mean subjective tinnitus loudness in the 47 patients with novel-onset tinnitus was 5.7 points on the 0 to 10 point numerical rating scale; it was significantly lower in the mild-moderate subgroup (5.2 points) than in the severe-profound subgroup (6.4 points; p < 0.05).
TABLE 1.
Baseline characteristics of acute idiopathic sudden sensorineural hearing loss patients
| Hearing Loss | |||
| Mild to Moderate | Severe to Profound | Total | |
| Age, years | |||
| Mean (SD) | 39.6 (11.3) | 46.6 (10.9) | 42.8 (11.6) |
| Range | 19–60 | 19–61 | 19–61 |
| Time from onset, hours | |||
| Mean (SD) | 25.9 (11.8) | 31.9 (12.1) | 28.6 (12.3) |
| Range | 3–47 | 6–48 | 3–48 |
| Presence of tinnitus, number (%) patients | 36 (100.0) | 29 (100.0) | 65 (100.0) |
| Tinnitus present, concurrent onset | 25 (70.3) | 22 (75.9) | 47 (72.3) |
| Tinnitus pre-existing | 4 (13.5) | 1 (3.4) | 5 (7.7) |
| No tinnitus present | 7 (16.2) | 6 (20.7) | 13 (20.0) |
| Novel-onset tinnitus only: | |||
| Tinnitus loudness (0–10) | |||
| Mean (SD) | 5.2 (1.7) | 6.4 (1.9) | 5.7 (1.9) |
| Range | 2–8 | 3–10 | 2–10 |
| Initial frequency range, number (%) patients | 25 (100.0) | 22 (100.0) | 47 (100.0) |
| Low frequency hearing loss | 11 (44.0) | 5 (22.7) | 16 (34.0) |
| Medium frequency hearing loss | 5 (20.0) | 4 (18.2) | 9 (19.1) |
| High frequency hearing loss | 9 (36.0) | 13 (59.1) | 22 (46.8) |
| Audiogram type, number (%) patients | 25 (100.0) | 22 (100.0) | 47 (100.0) |
| Type A (ascending) | 4 (16.0) | 0 (0.0) | 4 (8.5) |
| Type B (flat) | 5 (20.0) | 3 (13.6) | 8 (17.0) |
| Type C (descending) | 9 (36.0) | 8 (36.4) | 17 (36.2) |
| Type D (U or V shaped) | 6 (24.0) | 1 (4.5) | 7 (14.9) |
| Type E (total or subtotal anacusis) | 0 (0.0) | 8 (36.4) | 8 (17.0) |
| Not assignable | 1 (4.0) | 2 (9.1) | 3 (6.4) |
| Hearing threshold (pure tone average), dB | |||
| Mean (SD) | 47.7 (5.7) | 85.7 (21.0) | 65.5 (24.2) |
| Range | 31.7–58.3 | 60.0–120.0 | 31.7–120.0 |
| Hearing loss, dB | |||
| Mean (SD) | 36.1 (6.2) | 72.0 (22.4) | 52.9 (24.0) |
| Range | 20.0–48.3 | 41.0–116.7 | 20.0–116.7 |
PTA indicates pure tone average (mean hearing threshold at three most affected contiguous audiometric test frequencies); SD, standard deviation. Placebo-treated patients in clinical trial AM-111-CL-08-01 (Study A; n = 65).
Seventeen out of the 47 patients with fresh-onset tinnitus received prednisolone reserve therapy starting after the Day 7 visit because of unsatisfactory hearing recovery up to that point; 14 of them suffered from severe-profound hearing loss at baseline. Since active treatment might conflict with our aim of determining spontaneous recovery after Day 7, we compared the change in mean PTA and tinnitus loudness. While those patients who went on to receive reserve therapy on Day 7 had initially improved less than those who did not receive steroids—hence the former's eligibility for it—the changes thereafter were essentially in parallel. The differences in mean changes in tinnitus loudness and PTA from Day 7 to Day 30 and from Day 7 to Day 90 were statistically not significant (p = 0.62 and p = 0.29 and p = 0.54 and p = 0.06, respectively). As the reserve therapy could not influence data points up to Day 7 and had no significant impact thereafter and to avoid selection bias, we performed all analyses in the full data set, that is, including steroid treated patients.
Overall, tinnitus loudness dropped rapidly as tinnitus in many cases resolved completely or at least became less intense. Indeed at the follow-up visit on Day 7 mean tinnitus loudness had already more than halved to 2.7 points, and 15.6% of patients experienced complete tinnitus remission. Additional substantial improvement was observed to Day 30, as mean tinnitus loudness decreased further to 2.0 points, and a cumulated 35.6% of patients had complete tinnitus remission. Between Days 30 and 90 the rate of incremental improvement decreased substantially; at the last follow-up visit, the mean tinnitus loudness was down to 1.3 points, and the complete tinnitus remission rate was 44.4%.
The level of tinnitus loudness and rate of complete tinnitus remission differed substantially between the subgroups of mild-moderate and severe-profound acute hearing loss (Fig. 1). Although the incidence of fresh-onset tinnitus was not much lower at baseline in the mild-moderate subgroup, tinnitus loudness was significantly lower not only at baseline, but even more so at all follow-up visits (p < 0.01); mean loudness decreased from 5.3 to 0.7 points at Day 90 (mean decrease 87.6%) versus a decrease from 6.4 to 2.0 points (mean decrease 69.3%) in the severe-profound subgroup. Initially, tinnitus loudness dropped much more quickly than in the severe-profound subgroup; the difference in absolute tinnitus loudness reduction was statistically significant for the change from baseline to Day 7 (p < 0.05). Complete tinnitus remission in the mild-moderate subgroup set in much earlier and benefited a significantly higher share of patients (Fig. 2; Table 2). 30.4% of patients in the mild-moderate hearing loss subgroup experienced already at Day 7 complete tinnitus remission, whereas in the severe-profound hearing loss subgroup such complete resolution was observed for the first time only by Day 30. By Day 90, the incidence of complete tinnitus remission was almost three times higher in the mild-moderate subgroup than in the severe-profound subgroup (65.2 versus 22.7%; p < 0.01).
FIG. 1.
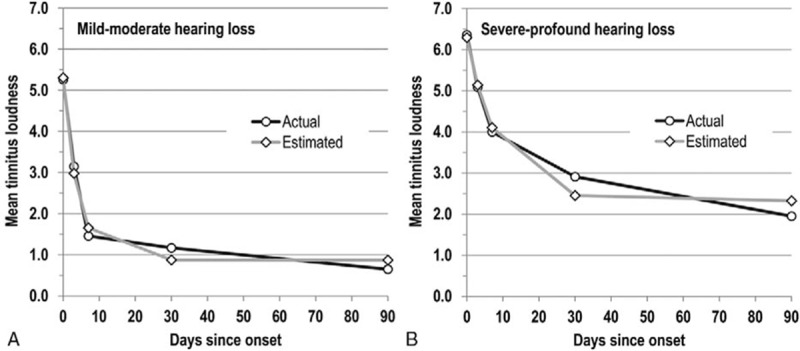
FIG. 2.
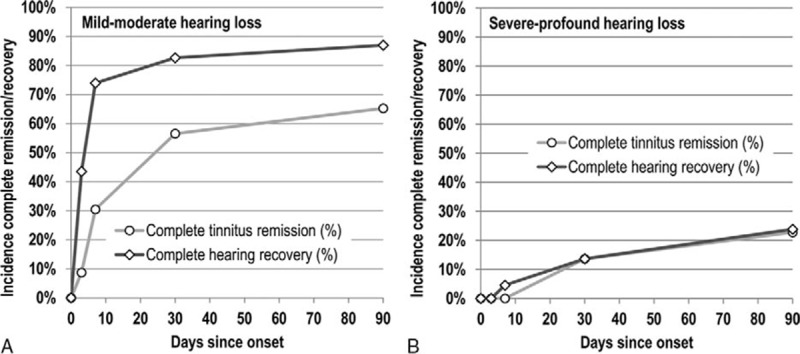
Percentage of patients suffering from idiopathic sudden sensorineural hearing loss and concurrent onset of tinnitus within the past 48 hours who experienced complete tinnitus remission and complete hearing recovery (to within 10 dB of pre-ISSNHL thresholds), respectively. A, Subgroup of patients with mild to moderate hearing loss at baseline (n = 23). B, Subgroup of patients with severe to profound hearing loss at baseline (n = 22). Placebo-treated patients in clinical trial AM-111-CL-08-01 (Study A).
TABLE 2.
Complete tinnitus remission and complete hearing recovery rates
| Complete… | Subgroup | Baseline | Day 3 | Day 7 | Day 30 | Day 90 |
| Tinnitus remission | Mild-moderate (n = 23) | 0 (0.0%) | 2 (8.7%) | 7 (30.4%) | 13 (56.5%) | 15 (65.2%) |
| Severe-profound (n = 22) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (13.6%) | 5 (22.7%) | |
| p value | 0.489 | 0.009 | 0.005 | 0.007 | ||
| Hearing recovery | Mild-moderate (n = 23) | 0 (0.0%) | 10 (43.5%) | 17 (73.9%) | 19 (82.6%) | 20 (87.0%) |
| Severe-profound (n = 22; 21 at Day 90) | 0 (0.0%) | 0 (0.0%) | 1 (4.5%) | 3 (13.6%) | 5 (23.8%) | |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
Acute idiopathic sudden sensorineural hearing loss patients reporting onset of tinnitus concurrently with onset of acute hearing loss who were treated with placebo in clinical trial AM-111-CL-08-01 and had a baseline tinnitus loudness rating (Study A; n = 45).
The time course of the mean tinnitus loudness was modeled as a negative exponential function
whereas parameters for the hearing loss severity subgroups differed substantially (Fig. 1). For both subgroups, the function appeared to provide a good fit for early time points, but underestimated the tinnitus level at Day 30 and overestimated it at Day 90. While the actual curve suggests the potential for further, albeit only small improvement beyond Day 90, the function yields no or hardly any additional reduction in tinnitus loudness beyond Day 30.
Patterns for complete hearing recovery were similar to those observed for complete tinnitus remissions in both hearing loss severity subgroups (Table 2). The difference was statistically significant for complete hearing recovery at all follow-up visits (p < 0.001) and for complete tinnitus remission beginning at Day 7 (p < 0.01). Whereas 73.9% of patients in the mild-moderate hearing loss subgroup enjoyed complete hearing recovery already at Day 7, this was the case for only 4.5% of patients in the severe-profound hearing loss subgroup. At Day 90, the incidence of complete hearing recovery was 3.7 times higher in the mild-moderate hearing loss subgroup (87.0 versus 23.8%; p < 0.001).
The level of tinnitus loudness at Day 90 was statistically significantly correlated with the level of PTA both at baseline (rs = 0.47, p = 0.001) and at Day 90 (rs = 0.48, p = 0.001); the correlation between the PTA at baseline and Day 90, however, was even stronger (rs = 0.82; p < 0.001). A χ2 test showed that occurrence of complete hearing recovery and complete tinnitus remission were not independent of each other (p < 0.01); Cramer's V was 0.427 and thus confirmed the association between these two discrete variables. 36.4% of patients experienced both complete hearing recovery and complete tinnitus remission, and 34.1% neither of the two; complete tinnitus remission without complete hearing recovery was the least frequent combination (Table 3).
TABLE 3.
Interrelation between complete tinnitus remission and complete hearing recovery
| Complete Tinnitus Remission | ||||
| N | No | Yes | Total | |
| Complete hearing recovery | No | 15 | 4 | 19 |
| % overall | 34.1% | 9.1% | 43.2% | |
| % row | 78.9% | 21.1% | 100.0% | |
| Yes | 9 | 16 | 25 | |
| % overall | 20.4% | 36.4% | 56.8% | |
| % row | 36.0% | 64.0% | 100.0% | |
| Total | 24 | 20 | 44 | |
| % overall | 54.5% | 45.5% | 100.0% | |
Acute idiopathic sudden sensorineural hearing loss patients reporting onset of tinnitus concurrently with onset of acute hearing loss who were treated with placebo in clinical trial AM-111-CL-08-01 and had a tinnitus loudness rating both at baseline and Day 90 (Study A).
Complete tinnitus remission lagged complete hearing recovery in both subgroups (Fig. 2, A and B). In the mild-moderate hearing loss subgroup the majority of the observed hearing improvement was achieved already at Day 7, whereas the majority of complete tinnitus remission occurred only between Day 7 and Day 30. In the severe-profound hearing loss subgroup, the frequency of complete tinnitus remission and hearing recovery moved essentially in parallel at relatively low levels.
Tinnitus Course after Initial Stage
For the post-acute ISSNHL stage, we focused on those tinnitus patients who had enrolled in Study B between 30 and 90 days from onset—Study A had showed only little further tinnitus reduction beyond the first month. Here, mean tinnitus loudness decreased over the following 90 days from 5.3 to 3.1 points (corresponding to approximately 5 months from tinnitus onset); 17.1% of patients experienced complete tinnitus remission (Table 4). Post-acute stage improvement was smaller than in the acute ISSNHL stage and its magnitude even below that for acute severe-profound hearing loss patients. The rate of tinnitus improvement was again time-dependent: tinnitus loudness dropped more, and spontaneous tinnitus remission occurred more frequently among the patients enrolling in the second month from onset compared with those presenting only in the third month (Fig. 3). The two subgroups showed a similar reduction in the first 7 days from baseline. After 90 days follow-up (corresponding to about 4.5 and 5.5 months from onset), tinnitus loudness dropped to 2.3 points in the earlier and 3.9 points in the later subgroup (Table 4).
TABLE 4.
Tinnitus evolution in ISSNHL patients presenting within 2 to 3 months from onset
| Days to Enrollment | |||
| Total | 30–59 | 60–90 | |
| N | 41 | 20 | 21 |
| Mean tinnitus loudness (SD) | |||
| Baseline | 5.3 (2.1) | 5.2 (2.2) | 5.4 (2.1) |
| Day 90 | 3.1 (2.6) | 2.3 (1.6) | 3.9 (3.0) |
| Mean change to Day 90 | |||
| Points (SD) | −2.2 (2.2) | −2.9 (2.1) | −1.5 (2.2) |
| % of baseline | −44.0% | −57.4% | −31.3% |
| % patients with complete tinnitus remission | 17.1% | 20.0% | 14.3% |
SD indicates standard deviation. Idiopathic sudden sensorineural hearing loss patients with tinnitus who were treated 30 to 90 days from onset with placebo in clinical trial AM-111-CL-08-01 (Study B).
FIG. 3.
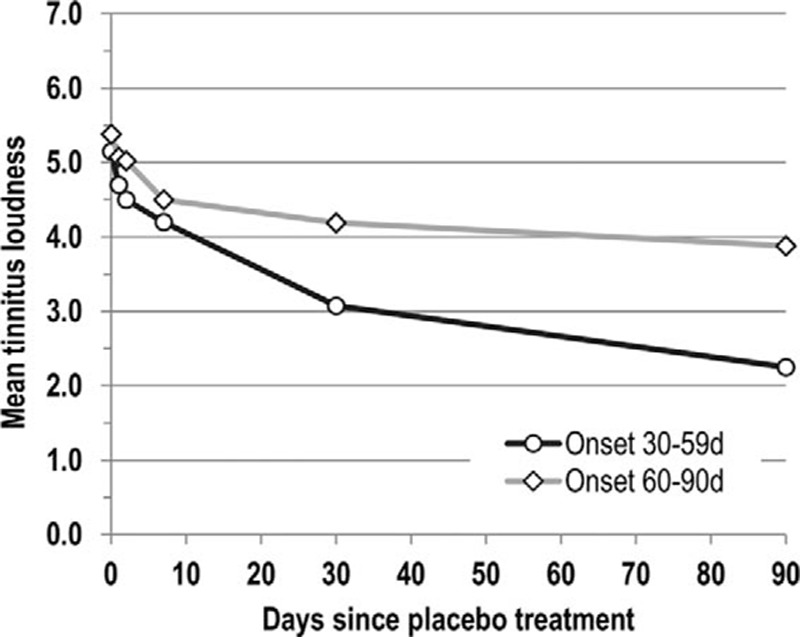
Time course of subjective tinnitus loudness from baseline to Day 90 rated on a 0 to 10 scale by patients suffering from tinnitus arising with idiopathic sudden sensorineural hearing loss who were treated three times with intratympanic placebo within 30 to 59 (n = 21) and 60 to 90 days (n = 20) from onset in clinical trial AM-101-CL-08-01 (Study B).
DISCUSSION
ISSNHL has been the object of a growing number of clinical research studies in otology, yet there is still only limited information available on the natural history. This observation relates to hearing loss and hearing recovery, and even more so to its most frequent comorbidity, tinnitus. Frequently, for hearing recovery reference is made to a “one third rule” (about one-third of patients recover completely, one-third recover partially, while one-third show a remaining hearing loss). This rule draws upon findings from some of the few studies that have assessed spontaneous recovery in non- or placebo-treated patients (18,19). However, these data have to be interpreted and compared across studies with caution given important differences in outcome measures (e.g., tested audiometric frequencies; [20]) or patient characteristics (e.g., time to diagnosis, affected hearing frequencies).
Similar reservations have to be made with regards to the scarce tinnitus data that are available from ISSNHL studies. To our knowledge, the present study represents the first attempt to assess in detail the characteristics of ISSNHL-tinnitus and its spontaneous recovery based on data from well-defined patient populations.
The outcomes from the present study confirm the high incidence of tinnitus that accompanies ISSNHL at the time of onset that has been reported previously. They further show how closely related the two symptoms hearing loss and tinnitus are in terms of severity and recovery patterns. At baseline, tinnitus was not much more frequent in patients with severe-profound acute hearing loss than in those with mild-moderate hearing loss, but its subjective loudness was significantly higher. Over the following 3 months, there was no significant difference between the subgroups in absolute reduction in tinnitus loudness; however, mild-moderate patients enjoyed a significantly higher rate of complete remission. In that, tinnitus recovery followed a similar pattern as in hearing recovery—mild-moderate acute hearing loss recovered more rapidly and to a larger extent than severe-profound hearing loss. Ninety days after onset, complete hearing recovery and complete tinnitus remission were both about three times more frequent in the mild-moderate acute hearing loss subgroup than in the severe-profound hearing loss subgroup.
In spite of important recovery in the average ISSNHL patient, 90 days following the originating incident, there were still 34.8 and 77.3% of patients in the mild-moderate and severe-profound hearing loss subgroups, respectively, who had tinnitus. Data that was available from the second trial from patients enrolling 30 to 90 days after the tinnitus triggering event showed that improvement in tinnitus is still possible beyond the first 90 days, albeit at a lower rate and with complete remission rates of <20%. Whether these patients initially had severe-profound hearing loss at the time of onset or—regardless of hearing loss severity—simply did not improve at all or only slightly is not known. The negative exponential function determined in Study A for severe-profound hearing loss cases would suggest lower tinnitus loudness values than those actually observed post-acutely in Stratum B; accordingly, it appears most likely that both patterns were represented.
With regards to the long-term impact of ISSNHL-tinnitus, a retrospective Swedish study showed that annoying tinnitus (defined as being present always or often) together with remaining vertigo was the strongest predictor of negative impact on the QoL of ISSNHL-patients (7). The authors found significant correlations with the QoL measures Hospital Anxiety and Depression Scale, EuroQoL-5D, and a problems impact rating scale; in addition, ISSNHL-patients suffering from annoying tinnitus were significantly more often on sick leave directly after onset or in the years thereafter. 22 to 24% of patients in their studied ISSNHL population, which included also cases of less than severe hearing loss, reported the long-term presence of annoying tinnitus.
The present study confirmed earlier findings (13) wherein the pace of tinnitus improvement lags hearing recovery. Active treatment with AM-111 in Study A had also showed earlier effects on hearing recovery than on complete tinnitus remission in patients with severe-profound hearing loss (14). While both hearing loss and tinnitus are symptoms of a dysfunction within the auditory system, there seems to be a difference between the capacity for recovery of afferent input for hearing and for resolution/suppression of the tinnitus perception. Presuming that tinnitus is the result of maladaptive plasticity within the auditory system (for a review see [21–24]), reversing such plasticity changes may take longer than intrinsic repair mechanisms inside the cochlea and may actually depend to some extent on them. In case of insufficient hearing recovery, auditory rehabilitation seems to be warranted to manage or alleviate the negative impact of tinnitus on the well-being and QoL of ISSNHL-tinnitus patients (6,7).
In conclusion, ISSNHL-related tinnitus overall has a good prognosis for rapid improvement up to complete remission in patients suffering from mild-moderate acute hearing loss at onset, whereas recovery is led by improvement in hearing. Watchful monitoring and waiting appear to be appropriate management options, as recommended e.g., in the United States treatment guideline (4). In severe-profound hearing loss patients, both hearing recovery and tinnitus remission are much rarer and prognosis is much poorer. In the absence of effective treatments for the acute hearing loss and/or tinnitus, patient education and auditory rehabilitation should be performed proactively. Tinnitus should be recognized as a patient burden that in case of pronounced ISSNHL may be even more important and bothersome than persisting hearing loss.
Although all patients in the present study received inactive gel injections, which may have evoked some type of placebo response, outcomes can be considered to depict the natural history of tinnitus rather closely. Importantly, in Study A the primary focus of patients and investigators was on hearing loss rather than tinnitus with both sensations being very recent, which may have mitigated any placebo effect on the tinnitus. That some patients took a course of oral corticosteroids as active treatment 7 days after enrollment does not invalidate their inclusion as the reserve therapy did not have any apparent effect on the course of tinnitus, which is in line with other studies’ outcomes (25,26). In Study B the primary focus was on tinnitus, and there was a reduction of tinnitus loudness of 16 to 18% within the first week of trial participation that seemed atypical of spontaneous recovery at this stage; however, any placebo response may have been mitigated by previous unsuccessful treatment attempts.
Given the limited size of the database for the present study, further analyses in a larger number of patients seem to be warranted. It would especially be worthwhile to compare ISSNHL cases with and without tinnitus as comorbidity; however, ISSNHL without tinnitus represents a clear minority. In addition, given the acute nature of the condition, it may be challenging or impossible to obtain pure observational or placebo data at all.
Acknowledgments
The authors wish to thank Drs. Manfred Wargenau and Frauke Friedrichs for their biostatistical contributions, Prof Marlies Knipper for her critical review of the manuscript, and the two anonymous reviewers for their thoughtful suggestions.
Footnotes
The study was supported in full by Auris Medical AG. M.S. and D.B. received honoraria from the Sponsor for work as medical experts related to discussions with a regulatory body; D.B. and G.M. received honoraria from the Sponsor as speakers in a scientific symposium; M.S. received honoraria from the Sponsor for serving as safety officer for Study B. T.M. is the Chairman, Chief Executive Officer and a major shareholder of Auris Medical Holding AG.
The other authors disclose no conflicts of interest.
REFERENCES
- 1.Nosrati-Zarenoe R, Hultcrantz E. Corticosteroid treatment of idiopathic sudden sensorineural hearing loss: randomized triple-blind placebo-controlled trial. Otol Neurotol 2012; 33:523–531. [DOI] [PubMed] [Google Scholar]
- 2.Klemm E, Bepperling F, Burschka MA, Mösges R. Hemodilution therapy with hydroxyethyl starch solution (130/0.4) in unilateral idiopathic sudden sensorineural hearing loss: a dose-finding, double-blind, placebo-controlled, international multicenter trial with 210 patients. Otol Neurotol 2007; 28:157–170. [DOI] [PubMed] [Google Scholar]
- 3.Rauch SD, Halpin CF, Antonelli PJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA 2011; 305:2071–2079. [DOI] [PubMed] [Google Scholar]
- 4.Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 2012; 146:S1–S35. [DOI] [PubMed] [Google Scholar]
- 5.Spoendlin H. Feldmann H. Inner ear pathology and tinnitus. Proceedings of the Third International Tinnitus Seminar. Karlsruhe: Harsch Verlag; 1987. 42–51. [Google Scholar]
- 6.Chiossoine-Kerdel JA, Baguley DM, Stoddart RL, Moffat DA. An investigation of the audiologic handicap associated with unilateral sudden sensorineural hearing loss. Am J Otol 2000; 21:645–651. [PubMed] [Google Scholar]
- 7.Carlsson PI, Hall M, Lind KJ, Danermark B. Quality of life, psychosocial consequences, and audiological rehabilitation after sudden sensorineural hearing loss. Int J Audiol 2010; 50:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Labus J, Breil J, Stützer H, Michel O. Meta-analysis for the effect of medical therapy vs. placebo on recovery of idiopathic sudden hearing loss. Laryngoscope 2010; 120:1863–1871. [DOI] [PubMed] [Google Scholar]
- 9.Wei BP, Stathopoulos D, O’Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Sys Rev 2013; 7:CD003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mösges R, Köberlein J, Heibges A, et al. Rheopheresis for idiopathic sudden hearing loss: results from a large prospective, multicenter, randomized, controlled clinical trial. Eur Arch Otorhinolaryngol 2009; 266:943–953. [DOI] [PubMed] [Google Scholar]
- 11.Westerlaken BO, de Kleine E, van der Laan B, Albers F. The treatment of idiopathic sudden sensorineural hearing loss using pulse therapy: a prospective, randomized, double-blind clinical trial. Laryngoscope 2007; 117:684–690. [DOI] [PubMed] [Google Scholar]
- 12.Wen YH, Chen PR, Wu HP. Prognostic factors of profound idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol 2014; 271:1423–1429. [DOI] [PubMed] [Google Scholar]
- 13.Ishida IM, Sugiura M, Teranishi M, et al. Otoacoustic emissions, ear fullness and tinnitus in the recovery course of sudden deafness. Auris Nasus Larynx 2008; 35:41–46. [DOI] [PubMed] [Google Scholar]
- 14.Suckfüll M, Lisowska G, Domka W, et al. Efficacy and safety of AM-111 in the treatment of acute sensorineural hearing loss: a double-blind, randomized, placebo-controlled phase II study. Otol Neurotol 2014; 35:1317–1326. [DOI] [PubMed] [Google Scholar]
- 15.Van de Heyning P, Mühlmeier G, Cox T, et al. Efficacy and safety of AM-101 in the treatment of acute inner ear tinnitus—a double-blind, randomised, placebo-controlled phase II study. Otol Neurotol 2014; 35:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huy PT, Sauvaget E. Idiopathic sudden sensorineural hearing loss is not an otologic emergency. Otol Neurotol 2005; 26:896–902. [DOI] [PubMed] [Google Scholar]
- 17.Jerger J, Jerger S. Paparella MM, Shumrick DA. Measurement of hearing in adults. Otolaryngology 2nd ed.Philadelphia: WB Saunders; 1980. 1225–1249. [Google Scholar]
- 18.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol 1980; 106:772–776. [DOI] [PubMed] [Google Scholar]
- 19.Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol 1977; 86:463–480. [DOI] [PubMed] [Google Scholar]
- 20.Plontke SK, Bauer M, Meisner C. Comparison of pure-tone audiometry analysis in sudden hearing loss studies: lack of agreement for different outcome measures. Otol Neurotol 2007; 28:753–763. [DOI] [PubMed] [Google Scholar]
- 21.Singer W, Zuccotti A, Jaumann M, et al. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiol 2013; 47:261–279. [DOI] [PubMed] [Google Scholar]
- 22.Guitton MJ. Tinnitus: pathology of synaptic plasticity at the cellular and system levels. Front Syst Neurosci 2012; 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggermont JJ. Tinnitus and neural plasticity (Tonndorf lecture at XIth International Tinnitus Seminar, Berlin, 2014). Hear Res 2015; 319:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Knipper M, Van Dijk P, Nunes I, et al. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Progr Neurobiol 2013; 111:17–33. [DOI] [PubMed] [Google Scholar]
- 25.Araújo MF, Oliveira CA, Bahmad FM. Intratympanic dexamethasone injections as a treatment for severe, disabling tinnitus: does it work? Arch Otolaryngol Head Neck Surg 2005; 131:113–117. [DOI] [PubMed] [Google Scholar]
- 26.Topak M, Sahin-Yilmaz A, Ozdoganoglu T, et al. Intratympanic methylprednisolone injections for subjective tinnitus. J Laryngol Otol 2009; 123:1221–1225. [DOI] [PubMed] [Google Scholar]


