Supplemental Digital Content is available in the text
Abstract
Combined use of heparin and aspirin is frequently prescribed for treatment of recurrent miscarriage (RM) in patients with antiphospholipid syndrome (APS), or in those without apparent cause of RM other than thrombophilia; however, this strategy is largely based on expert opinion and has not been well studied. The option for the use of different antithrombotic therapies to improve live birth remains unclear. In this network meta-analysis, we incorporated direct and indirect evidence to evaluate effects of different antithrombotic treatments on prevention of pregnancy losses.
We searched PubMed and Embase for randomized clinical trials comparing effects of at least 2 antithrombotic treatments on live birth in RM patients published from 1965 through the early of May 2015. Potential risk bias of eligible trials was evaluated according to the Cochrane Collaboration guidelines. Bayesian network meta-analysis was used to estimate relative effects on live birth.
A total of 19 trials involving 2391 RM patients with or without thrombophilia and 543 with APS were included. No beneficial effect of antithrombotic treatment was observed either in RM patients with or without thrombophilia or in patients with APS; however, for patients with or without thrombophilia, low molecular weight heparin therapy had the greatest probability (61.48%) of being the best option in terms of live birth; for patients with APS, unfractionated heparin plus aspirin was the superior treatment for RM with the highest possibility (75.15%) of being top 2 places for reducing pregnancy losses. Aspirin was inferior in both groups.
Our results do not support the use of combined low molecular weight heparin and aspirin for RM treatment, and suggested aspirin may have negative effects for lowering the risk of pregnancy loss.
INTRODUCTION
Recurrent miscarriage (RM) is a major health issue and is devastating for women and their families. Up to 5% of women experience 2 or more miscarriages and approximately 1% of women suffer from ≥3.1 A common risk factor for RM is exaggerated hemostatic response, a condition often seen in antiphospholipid syndrome (APS) and thrombophilia, leading to placental thrombosis and infarction,2–5 which is also responsible for unexplained RM that accounts for roughly 60% of total RM cases.6
Antithrombotic therapies or combinations, including aspirin, heparin (unfractionated heparin [UFH] or low molecular weight heparin [LMWH], are typically prescribed as they have antiplatelet or anticoagulant activity to combat the thrombotic causes of RM. Combined use of low-dose aspirin and heparin has been recommended in several guidelines for women diagnosed with APS and with a history of RM7,8; however, this recommendation is mainly based on expert opinion, rather than substantial evidence. Results on the benefits of combination therapy reported from several randomized clinical trials (RCTs) have been inconsistent.9–12 Some antithrombotic treatments (such as LMWH plus aspirin vs LMWH alone) have never been compared directly in clinical trials. Thus, no clear consensus has been reached on the choice of antithrombotic regimen for women with RM and APS. Additionally, for women with RM and thrombophilia or with unexplained RM, the benefits of antithrombotic therapy remain inconclusive,13–15 although anticoagulants are frequently prescribed in practice.
Although some meta-analyses16–18 have studied the effect of aspirin or heparin on live birth, they only focused on the relative effects between 2 of antithrombotic treatments or did not rank the different antithrombotic therapies. Clinicians will still be confused about which one should be provided in practice without an overall picture. In addition, additional studies19–21 have been published since these studies. Therefore, in this network meta-analysis and systematic review, we updated the evidence and evaluated effects of different antithrombotic treatments on the prevention of pregnancy loss in RM patients with APS and patients without apparent cause of RM other than thrombophilia, combining both direct and indirect evidence including those that had never been previously directly compared. Further, we provided a clear ranking of the efficacy conferred by different antithrombotic treatments to gain an evidence-based understanding of each choice of antithrombotic therapy in women with RM.
METHODS
Data Sources and Search Strategy
A systematic search of literature from 1965 to the early of May 2015 in the electronic databases PubMed and Embase was initially conducted using Medical Subject Headings (MeSH) and the following free keywords: ‘miscarriage”; “abortion”; “pregnancy loss”; “stillbirth”; “fetal loss”; “antithrombotic”; “anticoagulants”; “anticoagulant agent”; “heparin”; “low-molecular-weight heparin”; “unfractionated heparin”; and “aspirin”. RCTs investigating any antithrombotic treatment for patients with a history of at least 2 pregnancy losses were included in our meta-analysis. Additionally, all references cited in all relevant original and review articles were searched manually to prevent relevant studies from being excluded. Ethical approval was not necessary as this study was based on published data and had no direct contact with patient.
Study selection
Studies were included if they met the following criteria: randomized controlled clinical trial comparing the effects of one thrombotic therapy with another or with placebo including intensive pregnancy surveillance; enrolled women with a history of at least 2 miscarriages and APS or without apparent causes of RM other than thrombophilia; reported live birth as the main outcome measure. For each trial, the most recent and complete data was used in our analysis.
Data Extraction and Quality Assessment
Data extraction and quality assessment were independently performed by 2 investigators (TY.Z. and TT.Z). The following information was extracted and entered into a database: author, year of publication, study design, sample size, patients’ characteristics, therapies, and outcomes. Potential risk bias in eligible trials was evaluated according to the Cochrane Collaboration guidelines (random sequence generation; allocation concealment; blinding of participants; blinding of outcome assessment investigator; incomplete outcome data; selective reporting; and other bias).22 Any disagreement between the 2 authors was resolved by discussion. If consensus could not be reached, the principal investigator (J.H.) made the final judgment.
Statistical Analysis
Firstly, we did traditional pair-wise meta-analysis for direct comparisons between 2 treatment arms using a random-effects model. The pooled estimates of odds ratios (OR) and 95% confidence intervals (95% CIs) was calculated for each study population (women with APS and women without apparent cause other than thrombophilia). If an article reported different populations (patients with thrombophilia, without thrombophilia, or patients with APS), we considered each as a different study for calculation. Heterogeneity across studies was assessed using the χ2 test and I2 statistic and P values of < 0.10 was considered as indicative of significant heterogeneity.23 The probability of publication bias was evaluated with the Egger regression test.24 If publication bias existed, the effect of publication bias was evaluated by the trim and fill method.25
Next, we used network meta-analysis methods to compare different antithrombotic therapies (aspirin alone, LMWH, LMWH plus aspirin, UFH plus aspirin, as well as placebo, or intensive pregnancy surveillance) relative to each other, incorporating evidence on both direct and indirect comparisons. The Bayesian hierarchical random effects model26 was adopted to take multiarm trials and differences among trials into account. The pooled estimates were calculated using the Markov Chains Monte Carlo method. The OR was estimated using the median of the posterior distribution, and 95% credibility intervals (CrI) were obtained based on the 2.5th and 97.5th percentiles of the posterior distribution, which can be interpreted in the same way as conventional 95% CIs. The goodness of model fit was measured by residual deviance, which is similar to the number of data points when the model provides an adequate fit.
Inconsistency of the model was evaluated using the node splitting method that separated evidence on a particular comparison into direct and indirect evidence.27,28 The Bayesian P value was reported to measure the agreement between the direct and indirect evidence for each split node. In addition, sensitivity analysis was carried out using the same computations with the fixed effect model and by excluding trials that may bias the pooled effects.
Finally, the treatment was ranked in each Markov Chain Monte Carlo cycle according to the effect size. Rank probabilities were calculated on the basis of the proportion of the cycles in which the given treatment ranked first (the most effective therapy), second (the second best) and so on, which is presented in the form of rankograms and cumulative rankograms.29–34 The surface under the cumulative ranking curve (SUCRA) was also estimated to obtain a treatment hierarchy. SUCRA would be 1 if a treatment is certain to be the best and 0 if a treatment is certain to be the worst.33
Analyses were conducted with WinBUGS1.4.3 (MRC Biostatistics Unit, Cambridge, UK), R 3.0.3 and Stata 12.0 (StataCorp LP, College Station, TX). Figures of risk of bias were generated using Review Manager Version 5.1. Statistical tests were two sided and P < 0.05 was considered to be of statistical significance.
RESULTS
Characteristics of Selected Studies
We identified 213 relevant articles from the initial database search. After removing duplicates, the total number of potential articles was 210. Of these, 169 records were excluded on the basis of their titles and abstracts. The full texts of the remaining articles were further evaluated. Finally, a total of 2934 patients from 19 trials9–15,19–21,35–43 met our inclusion criteria. Figure 1 outlines the selection process in detail and Table 1 summarizes general characteristics of each study. Of the included trials, 12 were conducted in a group of women with or without thrombophilia,13–15,20,21,35–39,42,43 6 in patients with APS,9–11,19,40,41 and 1 in both.12Figure 2 shows the network of direct comparisons for different populations. For the included studies, the risk of bias was mainly from the fact that participants and personnel were not blinded because heparin was administered subcutaneously, and therefore, blinding participants was virtually impossible (Table S1, http://links.lww.com/MD/A489, Figure S1, http://links.lww.com/MD/A489, and Figure S2, http://links.lww.com/MD/A489).
FIGURE 1.
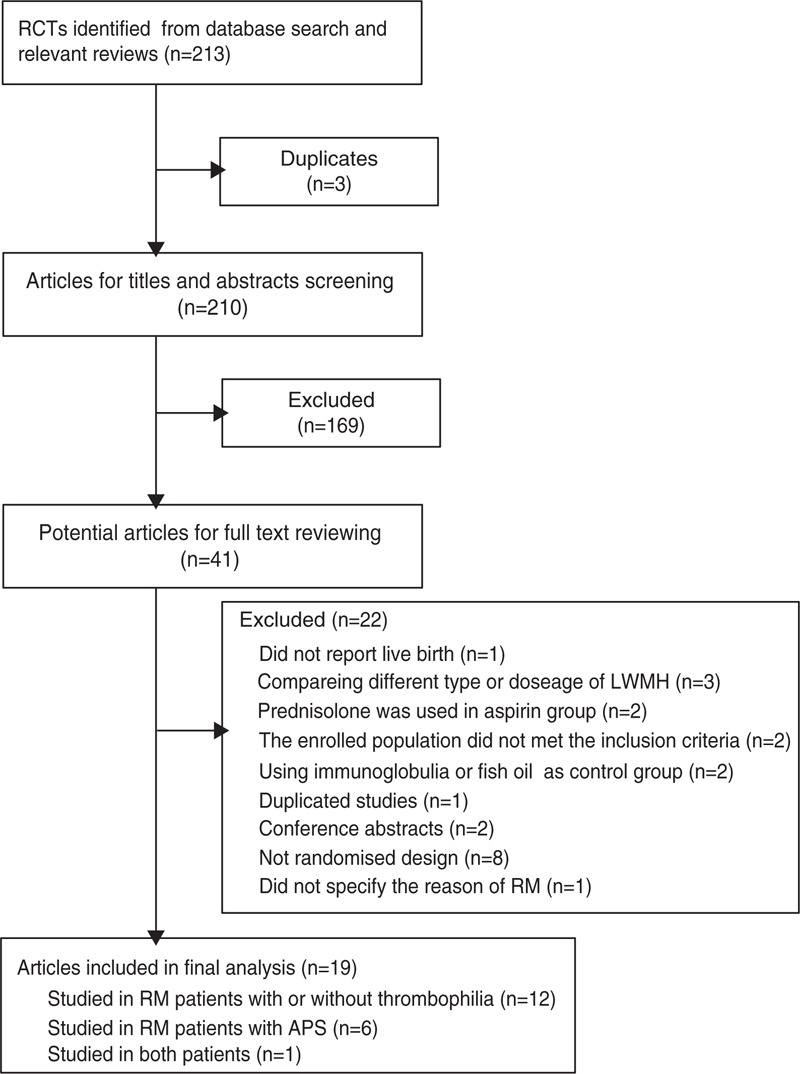
Flow diagram of the database search and trial selection process.
TABLE 1.
Characteristics of Included Randomized Clinical Trials
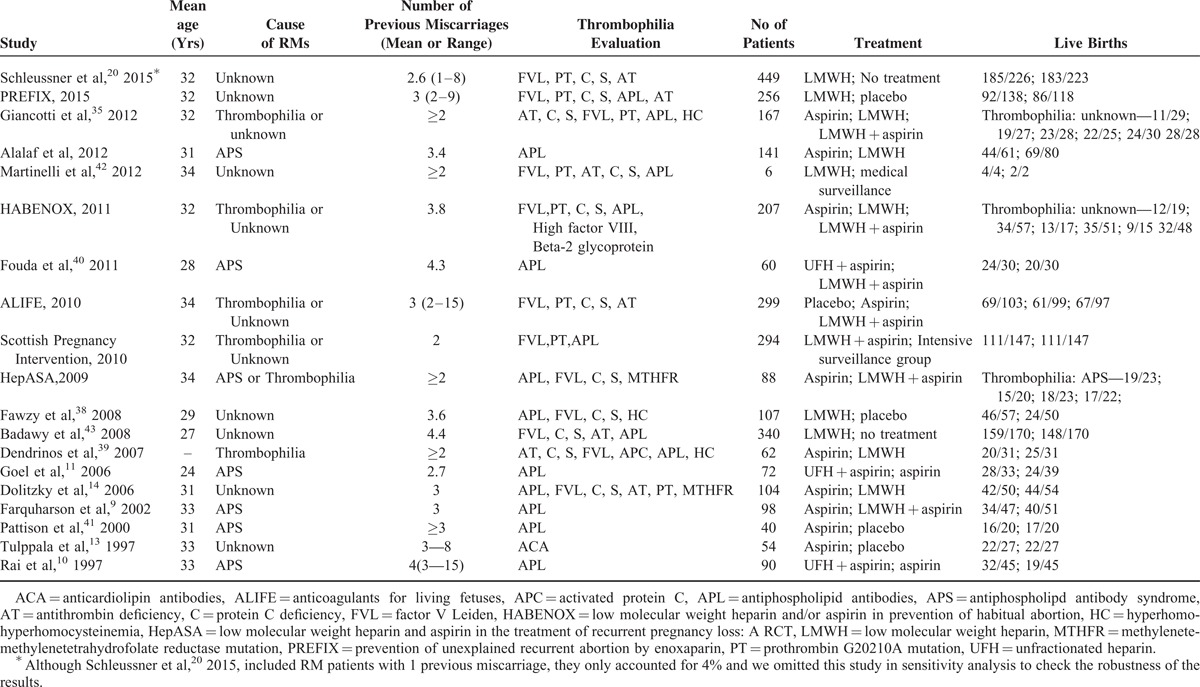
FIGURE 2.
Network among eligible treatments in patients with or without thrombophilia and patients with APS. The node size indicates the sample size in the treatment group that the node stands for; the thickness of the link represents the sample size of the direct comparisons. APS = antiphospholipid syndrome, LMWH = low molecular weight heparin, UFH = unfractionated heparin.
Effects of Antithrombotic Treatments on Live Birth in Patients With or Without Thrombophilia
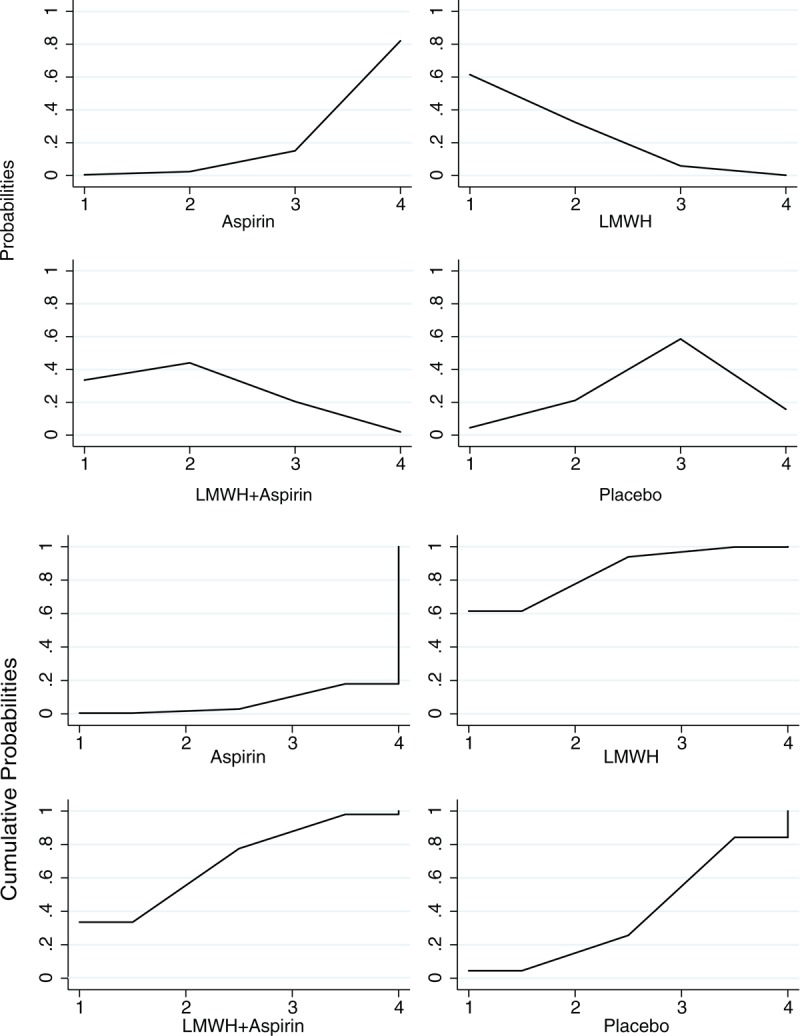
A total of 2391 patients were included in this analysis, with 362 patients in the aspirin group, 801 in the LMWH group, 388 in the combination of LMWH and aspirin group, and 840 in the placebo or intensive surveillance group. Table 2 and Figure 3 present the pooled effect estimates for the results of Bayesian network and traditional pair-wise meta-analyses on the outcome of live birth in RM patients with or without thrombophilia. Figure 4 and Table S3, http://links.lww.com/MD/A489 show the distribution of probabilities of each treatment strategy being ranked at different positions based on the protective effects on live birth in RM patients with or without thrombophilia. Compared with placebo, none of antithrombotic treatments showed a significant effect of improving live birth. The only significant difference was observed between LMWH and aspirin (LMWH vs aspirin: OR 2.02, 95% CrI 1.13–3.95); however, LMWH had the highest SUCRA (85.10%) and showed the greatest probability (61.48%) of being ranked at the first place to improve live birth for RM patients with or without thrombophilia. Whereas aspirin had the lowest SUCRA (7.00%) and showed the greatest probability of being least beneficial (82.04%). The residual deviance (38.67) was closed to data points (35), meaning goodness fit for the model was adequate. Using traditional pair-wise meta-analysis or excluding the trial20 that enrolled a small proportion of patients with 1 miscarriage (4%), the results were consistent. In the sensitivity analysis based on the fixed effects model, the beneficial effect of LMWH plus aspirin on live births also reached the level of significance compared with aspirin (OR 1.64, 95% CrI 1.16–2.32). The order of ranking of treatment strategies was not changed. Heterogeneity was shown in the comparison between LMWH plus aspirin versus aspirin alone (I2 = 51.2%, P = 0.04) and comparison between LMWH and placebo (I2 = 73.5%, P < 0.01). No publication bias or inconsistency was identified (Table S4, http://links.lww.com/MD/A489 and Table S5, http://links.lww.com/MD/A489).
TABLE 2.
Results of Bayesian Network, Traditional Pair-Wise and Sensitivity Analysis
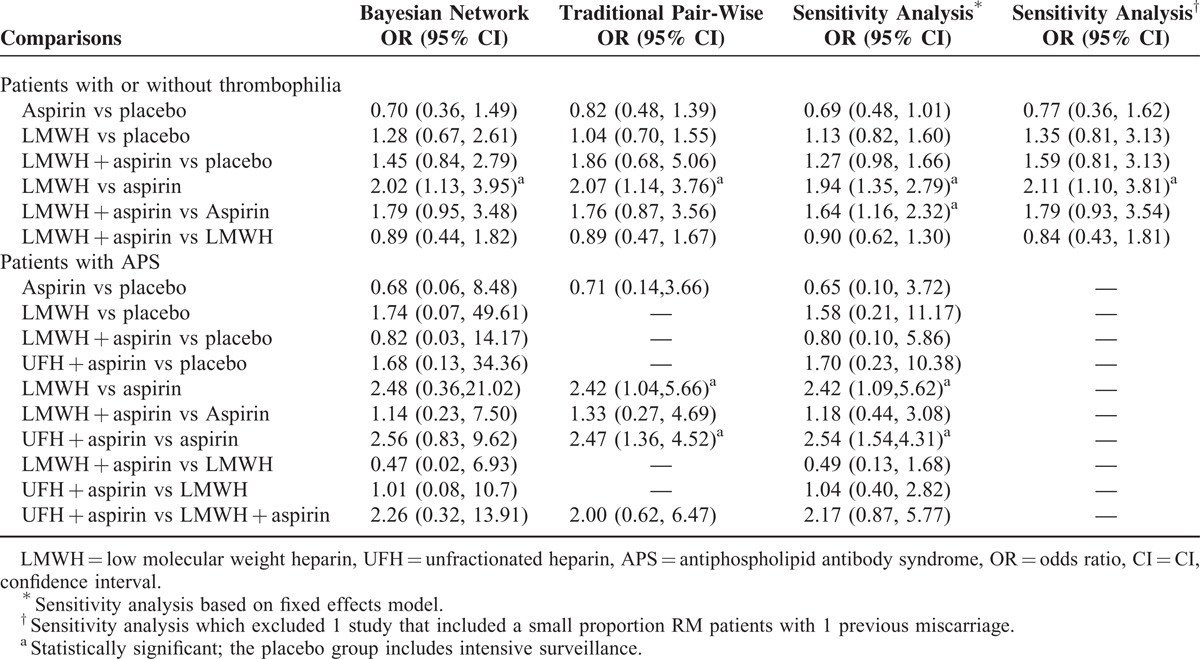
FIGURE 3.
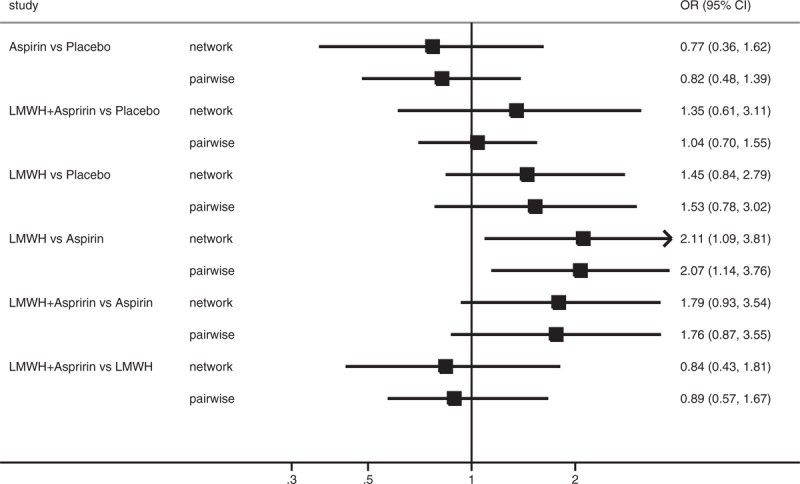
Forest plot for OR of live birth based on Bayesian network and traditional pair-wise meta-analyses in patients with or without thrombophilia. The black squares represent the pooled effect estimates, which mean the OR of live birth between the corresponding pair of drugs, whereas the horizontal lines depict the 95% credible intervals in Bayesian network meta-analysis and 95% confidence intervals in traditional pair-wise meta-analysis. LMWH = low molecular weight heparin, OR = odds ratio, UFH = unfractionated heparin.
FIGURE 4.
Rank and cumulative probabilities of different antithrombotic treatments in patients with or without thrombophilia based on the protective effects on live birth. The horizontal axis represents the positions that the corresponding drug may rank at based on the protective effects on the outcome of live birth, whereas the vertical axis means the probabilities or the cumulative probabilities of the drug being ranked at the corresponding positions on the horizontal axis. For example, as the figure shows, the probabilities of aspirin being at first, second, third, and the last place for the protective effects on live birth are 0.5%, 2.4%, 15.07%, 82.03%, respectively, and the cumulative probabilities are 0.5%, 2.9%, 17.97%, 100%, respectively. LMWH = low molecular weight heparin.
Effects of Antithrombotic Treatments on Live Birth in Patients With APS
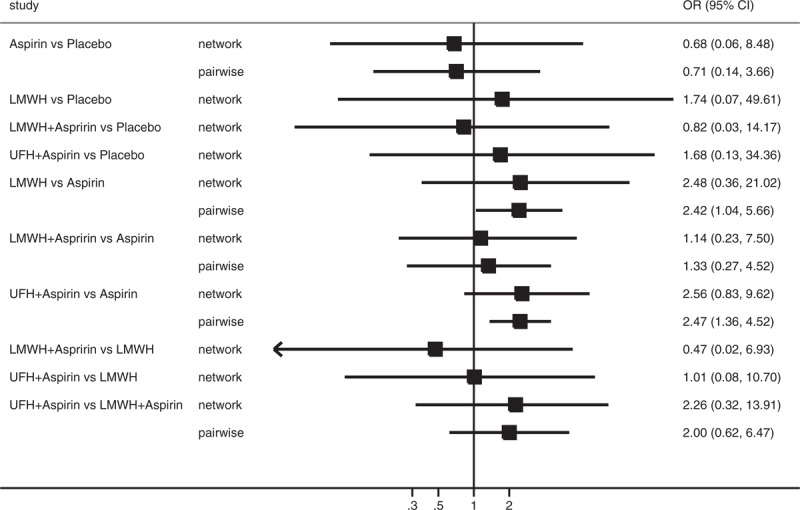
A total of 543 patients with APS were included in this analysis, with 232 patients in the aspirin group, 80 in the LMWH group, 103 in the combination of LMWH and aspirin group, 108 in the combination of UFH and aspirin group, and 20 in the placebo group. Table 2 and Figure 5 present the pooled effect estimates for the results of Bayesian network and traditional pair-wise meta-analyses on the outcome of live birth in RM patients with APS. Figure 6 and Table S3, http://links.lww.com/MD/A489 show the distribution of probabilities of each treatment strategy being ranked at different positions based on the protective effects on live birth in RM patients APS. None of antithrombotic treatments showed a significant beneficial effect on improving live birth compared with placebo; however, the combination of UFH and aspirin had the highest SUCRA (75.50%) and showed the greatest probability (75.15%) of being at the top 2 positions in the effect of reducing pregnancy loss, followed by LMWH (SUCRA, 71.00%; being in the top 2 places with probability of 65.87%). Whereas aspirin had the lowest SUCRA (23.00%) and showed the highest probability (79.14%) of being at last 2 places. The residual deviance (13.27) was similar to data points (35), which meant goodness fit for the model was satisfactory. In the traditional pair-wise meta-analysis and sensitivity analysis based on the fixed effects model, UFH plus aspirin (pair-wise analysis: OR 2.47, 95% CrI 1.36–4.52; sensitivity analysis: OR 2.54, 95% CrI 1.54–4.31) and LMWH alone (pair-wise analysis: OR 2.42, 95% CrI 1.04–5.66; sensitivity analysis: OR 2.42, 95% CrI 1.09–5.62) significantly improved live births compared with aspirin. The ranking order was not changed. There was no evidence of heterogeneity, publication bias, or inconsistency (Table S4, http://links.lww.com/MD/A489 and Table S5, http://links.lww.com/MD/A489).
FIGURE 5.
Forest plot for OR of live birth based on Bayesian network and traditional pair-wise meta-analyses in patients with APS. The black squares represent the pooled effect estimates, which mean the OR of live birth between the corresponding pair of drugs, whereas the horizontal lines depict the 95% credible intervals in Bayesian network meta-analysis and 95% confidence intervals in traditional pair-wise meta-analysis. APS = antiphospholipid syndrome, LMWH = low molecular weight heparin, OR = odds ratio, UFH = unfractionated heparin.
FIGURE 6.
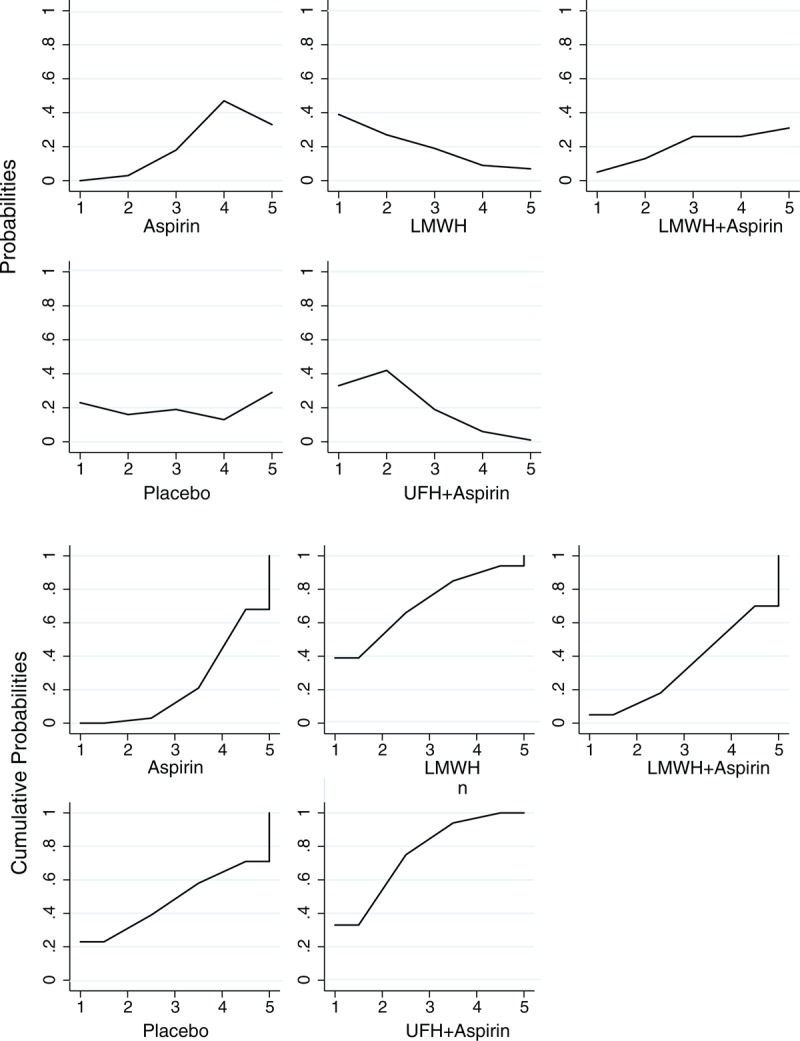
Rank and cumulative probabilities of different antithrombotic treatments in patients with antiphospholipid syndrome based on the protective effects on live birth. The horizontal axis represents the positions that the corresponding drug may rank at based on the protective effects on the outcome of live birth, whereas the vertical axis means the probabilities or the cumulative probabilities of the drug being ranked at the corresponding positions on the horizontal axis. LMWH = low molecular weight heparin, UFH = unfractionated heparin.
Safety Profile
Because of differences in reporting adverse events (AEs) among studies and insufficient data for AEs, we did not carry out network analysis for the safety of treatments; however, we summarized the AEs reported in each study in Table 3 . The most common AE was bleeding.
TABLE 3.
Adverse Events Reported in Each Clinical Trial
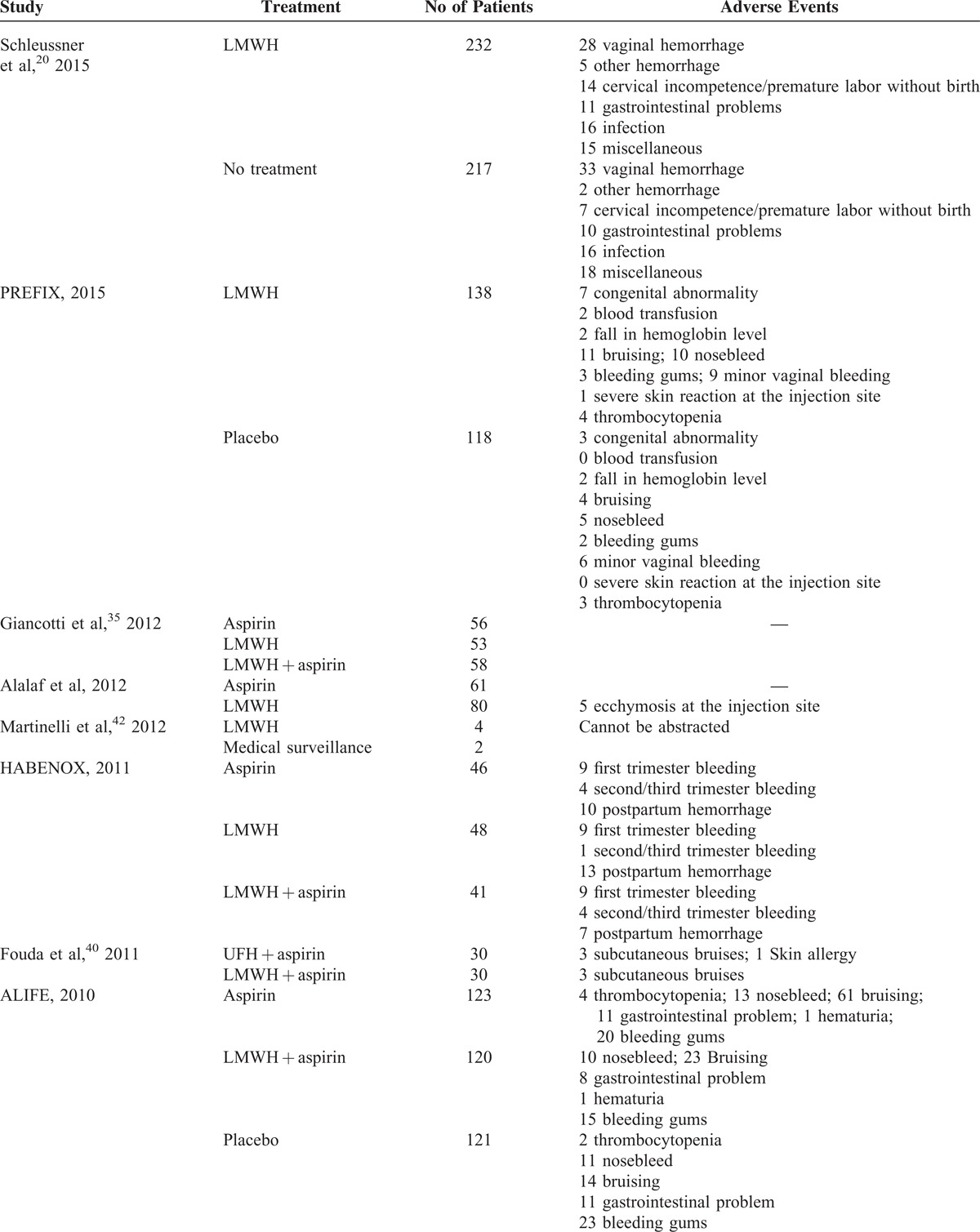
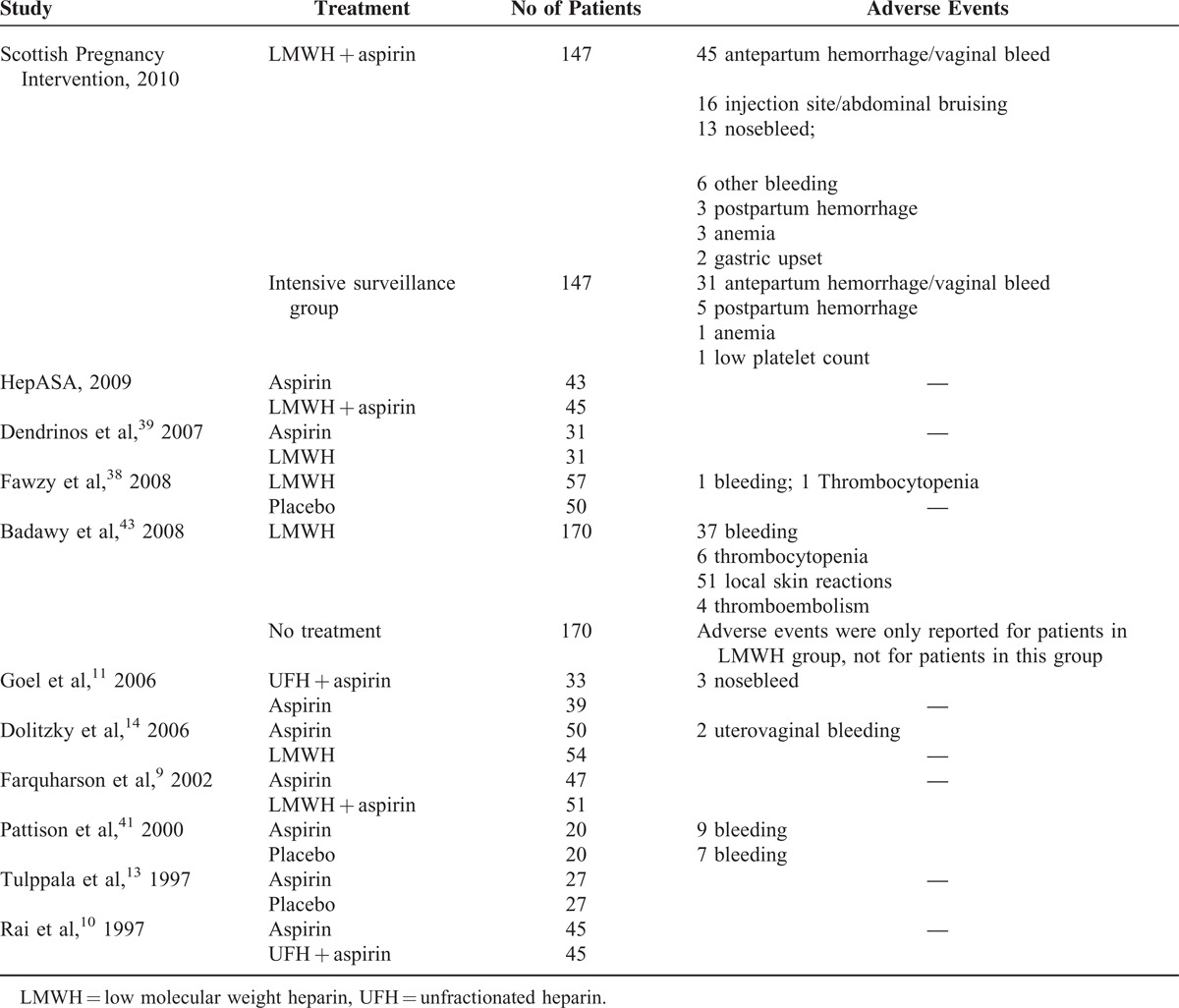
TABLE 3 (Continued).
Adverse Events Reported in Each Clinical Trial
DISCUSSION
Principal Findings
In this network meta-analysis, we evaluated the effects of different antithrombotic treatment strategies on live births for RM patients with thrombophilia or without apparent cause and patients with RM and APS, separately. There was no beneficial effect conferred by antithrombotic treatment either in RM patients with or without thrombophilia or in patients with APS; however, for patients with RM, with or without thrombophilia, LMWH therapy had the greatest probability of being the best option in terms of live births; for patients with RM and APS, this meta-analysis indicated that the combination of UFH and aspirin is the superior treatment for RM with the highest possibility of being best option for reducing pregnancy loss. Aspirin, by contrast, seemed inferior among antithrombotic treatments in both groups of patients.
Results in Relation to Other Studies
Our findings are consistent with those of previous pair-wise meta-analyses, but go beyond in that our study mines more information on the effects of different antithrombotic therapies on live births in women with RM. The meta-analysis conducted by Mak et al,16 which included nonrandomized trials, showed that the combination of heparin and aspirin was significantly superior to aspirin alone in reducing the risk of miscarriage in RM patients with APS. Ziakas et al17 observed a significant benefit in live births conferred by the combination of UFH and aspirin, but not by LMWH plus aspirin; however, both analyses only focused on the relative effects of the combination therapy versus aspirin alone in women with RM and APS. Several comparisons among other antithrombotic treatments, such as LMWH plus aspirin versus placebo in patients with RM and APS, have not been reported in previous meta-analyses due to the lack of studies of direct comparisons. The most comprehensive meta-analysis carried out by de Jong et al,18 which compared effect of anticoagulant treatment on live birth each other in RM patients with or without inherited thrombophilia, also found no beneficial effect of anticoagulants compared with placebo; however, it only based on direct evidence and did not rank the different therapies. In this Bayesian network analysis, we updated evidence and summarized all antithrombotic therapies used in clinical practice, incorporating both direct and indirect comparisons including those that had never been previously directly compared (such as LMWH plus aspirin vs placebo), providing more comprehensive evidence to guide clinical decisions.
Perhaps one of the most important findings of this study was that the common practice, the combination of LMWH and aspirin, seemed not to be the best strategy for RM either in patients without apparent cause other than thrombophilia or in patients with APS. Based on the ranking of antithrombotic treatments, the combination of LMWH plus aspirin followed behind LMWH and aspirin ranked behind placebo in both groups of patients. It is reasonable to suspect from this that aspirin had a potentially deleterious effect on live births. This has been mentioned in previous systematic reviews by Mantha et al44 because of the poor outcomes in the aspirin-only arm. In fact, the association between aspirin and the risk of miscarriage has been observed in a cohort study carried out by Li et al.45 The mechanism may be that aspirin can suppress the biosynthesis of prostaglandin and prostaglandin plays an important role in embryo implantation into the uterus.46–49 Small sample size may have contributed to the fact that the difference between aspirin and placebo did not reach statistical significance; however, these results demonstrate that there is no benefit against pregnancy loss conferred by aspirin, as shown in the study conducted by Schisterman et al.50 Therefore, our findings do not support the use of aspirin in patients with RM.
Furthermore, in our study, no beneficial effect of antithrombotic treatment was found either in RM patients with or without thrombophilia or in patients with APS; however, LMWH and UFH plus aspirin showed the greatest probability of ranking the first option for RM patients with or without thrombophilia and patients with APS, respectively. Whether the benefit of antithrombotic therapy does not exist or still not be observed due to limited sample size is urgently needed to be studied, which highlighted the need for large-scale RCTs.
LIMITATIONS
Our study has several limitations. First, moderate heterogeneity was seen in the comparison between LMWH plus aspirin and aspirin alone. This may be due to variances in drug doses, laboratory standardization (such as variety of thrombophilia evaluated, differences in cutoffs), and the inclusion criteria of subjects (early or late pregnancy loss); however, further stratification would not be feasible due to the limited sample size, which might lead to insufficient statistical power. Nonetheless, the comparison between the combination of LMWH plus aspirin and aspirin alone was stable in traditional meta-analysis and sensitivity analysis. Second, several comparisons showed small differences in sensitivity analyses (such as LMWH plus aspirin vs aspirin in patients with or without thrombophilia, UFH plus aspirin vs aspirin in APS patients). Small sample size and the conservative nature of the Bayesian hierarchical random-effects model may be responsible; however, credible intervals generally overlapped and there was no significant inconsistency within the networks. Finally, we did not carry out network analysis for the safety of treatments, because of insufficient data and differences in reporting AEs among studies; however, we summarized the AEs reported in each study and parented in the form of table. Despite these limitations, our analysis can guide clinical use of antithrombotic therapies in the treatment of RM until large RCTs are reported. Moreover, our study offers advice for investigators to perform further research.
CONCLUSIONS
In conclusion, our analysis do not support combined use of LMWH and aspirin in treating RM. Aspirin may have negative effects in lowering the risk of pregnancy loss. Additionally, we do not find benefit of antithrombotic treatments; however, definite conclusions cannot be made due to limited sample size and favorable trend toward heparin. Further studies are urgently needed to evaluate effects and safety of antithrombotic therapy for RM.
Acknowledgements
The authors thank the grants from the leading talents of science in Shanghai 2010(022), the key discipline construction of evidence-based public health in Shanghai (12GWZX0602), the National Natural Science Foundation of China (No. 81202285, No. 81373105), the Project supported by Foundation for Young Scholars of SMMU (2014QN09) and the Natural Science Foundation of Shanghai (12ZR1453700) that supported our study. In addition, the authors would like to thank Editage [http://www.editage.cn/] for English language editing.
Footnotes
Abbreviations: APS = antiphospholipid syndrome, CI = confidence interval, CrI = credibility intervals, LMWH = low molecular weight heparin, OR = odds ratio, RCTs = randomized clinical trials, RM = recurrent miscarriage, SUCRA = surface under the cumulative ranking curve, UFH = unfractionated heparin.
Drs Tianyi Zhang, Xiaofei Ye, and Xiang Xiao, Tiantian Zhu contributed equally to the writing of this article.
TYZ, XFY, TTZ, XX and JH discussed and developed the question for this article. TYZ and TTZ carried out the searches, assessed the eligibility of the studies for inclusion, and extracted data. TYZ and XFY contributed to the study design and data analysis. All authors were involved in interpreted and discussed results. TYZ wrote the first draft of this article and it was reviewed by XFY and JH. All authors agreed on the final draft of this study. JH is the guarantor.
The authors have no funding and conflicts interests to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Rai R, Regan L. Recurrent miscarriage. Lancet 2006; 368:601–611. [DOI] [PubMed] [Google Scholar]
- 2.Rushton DI. Placental pathology in spontaneous miscarriage. Early Pregnancy Loss. 1988; London:Springer, 149–157. [Google Scholar]
- 3.Rai RS, Regan L, Chitolie A, et al. Placental thrombosis and second trimester miscarriage in association with activated protein C resistance. Br J Obstet Gynaecol 1996; 103:842–844. [DOI] [PubMed] [Google Scholar]
- 4.Rai RS, Regan L, Clifford K, et al. Antiphospholipid antibodies and beta 2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod 1995; 10:2001–2005. [DOI] [PubMed] [Google Scholar]
- 5.Rey E, Kahn SR, David M, et al. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet 2003; 361:901–908. [DOI] [PubMed] [Google Scholar]
- 6.Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril 2010; 93:1234–1243. [DOI] [PubMed] [Google Scholar]
- 7.Derksen RH, Khamashta MA, Branch DW. Management of the obstetric antiphospholipid syndrome. Arthritis Rheum 2004; 50:1028–1039. [DOI] [PubMed] [Google Scholar]
- 8.Erkan D, Patel S, Nuzzo M, et al. Management of the controversial aspects of the antiphospholipid syndrome pregnancies: a guide for clinicians and researchers. Rheumatology (Oxford) 2008; 47 suppl 3:iii23–iii27. [DOI] [PubMed] [Google Scholar]
- 9.Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol 2002; 100:408–413. [DOI] [PubMed] [Google Scholar]
- 10.Rai R, Cohen H, Dave M, et al. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997; 314:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel N, Tuli A, Choudhry R. The role of aspirin versus aspirin and heparin in cases of recurrent abortions with raised anticardiolipin antibodies. Med Sci Monit 2006; 12:CR132–CR136. [PubMed] [Google Scholar]
- 12.Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol 2009; 36:279–287. [DOI] [PubMed] [Google Scholar]
- 13.Tulppala M, Marttunen M, Soderstrom-Anttila V, et al. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect on prostacyclin and thromboxane A2 production. Hum Reprod 1997; 12:1567–1572. [DOI] [PubMed] [Google Scholar]
- 14.Dolitzky M, Inbal A, Segal Y, et al. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil Steril 2006; 86:362–366. [DOI] [PubMed] [Google Scholar]
- 15.Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med 2010; 362:1586–1596. [DOI] [PubMed] [Google Scholar]
- 16.Mak A, Cheung MW, Cheak AA, et al. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: a meta-analysis of randomized controlled trials and meta-regression. Rheumatology (Oxford) 2010; 49:281–288. [DOI] [PubMed] [Google Scholar]
- 17.Ziakas PD, Pavlou M, Voulgarelis M. Heparin treatment in antiphospholipid syndrome with recurrent pregnancy loss: a systematic review and meta-analysis. Obstet Gynecol 2010; 115:1256–1262. [DOI] [PubMed] [Google Scholar]
- 18.de Jong PG, Kaandorp S, Di Nisio M, et al. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev 2014; 7:CD004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alalaf S. Bemiparin versus low dose aspirin for management of recurrent early pregnancy losses due to antiphospholipd antibody syndrome. Arch Gynecol Obstet 2012; 285:641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schleussner E, Kamin G, Seliger G, et al. Low-molecular-weight heparin for women with unexplained recurrent pregnancy loss. Ann Intern Med 2015; 162:485. [DOI] [PubMed] [Google Scholar]
- 21.Pasquier E, de Saint Martin L, Bohec C, et al. Enoxaparin for prevention of unexplained recurrent miscarriage: a multicenter randomized double-blind placebo-controlled trial. Blood 2015; 125:2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2014: http://www.cochrane-handbook.org/. [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duval S, Tweedie R. Practical estimates of the effect of publication bias in meta-analysis. Austral Epidemiol 1998; 5:14–17. [Google Scholar]
- 26.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23:3105–3124. [DOI] [PubMed] [Google Scholar]
- 27.Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc 2006; 101:447–459. [Google Scholar]
- 28.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29:932–944. [DOI] [PubMed] [Google Scholar]
- 29.Tu YK, Needleman I, Chambrone L, et al. A Bayesian network meta-analysis on comparisons of enamel matrix derivatives, guided tissue regeneration and their combination therapies. J Clin Periodontol 2012; 39:303–314. [DOI] [PubMed] [Google Scholar]
- 30.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 2009; 373:746–758. [DOI] [PubMed] [Google Scholar]
- 31.van der Valk R, Webers CA, Lumley T, et al. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol 2009; 62:1279–1283. [DOI] [PubMed] [Google Scholar]
- 32.Hartling L, Fernandes RM, Bialy L, et al. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta-analysis. BMJ 2011; 342:d1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64:163–171. [DOI] [PubMed] [Google Scholar]
- 34.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giancotti A, La Torre R, Spagnuolo A, et al. Efficacy of three different antithrombotic regimens on pregnancy outcome in pregnant women affected by recurrent pregnancy loss. J Matern Fetal Neonatal Med 2012; 25:1191–1194. [DOI] [PubMed] [Google Scholar]
- 36.Visser J, Ulander VM, Helmerhorst FM, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia. HABENOX: a randomised multicentre trial. Thromb Haemost 2011; 105:295–301. [DOI] [PubMed] [Google Scholar]
- 37.Clark P, Walker ID, Langhorne P, et al. SPIN (Scottish Pregnancy Intervention) study: a multicenter, randomized controlled trial of low-molecular-weight heparin and low-dose aspirin in women with recurrent miscarriage. Blood 2010; 115:4162–4167. [DOI] [PubMed] [Google Scholar]
- 38.Fawzy M, Shokeir T, El-Tatongy M, et al. Treatment options and pregnancy outcome in women with idiopathic recurrent miscarriage: a randomized placebo-controlled study. Arch Gynecol Obstet 2008; 278:33–38. [DOI] [PubMed] [Google Scholar]
- 39.Dendrinos S, Kalogirou I, Makrakis E, et al. Safety and effectiveness of tinzaparin sodium in the management of recurrent pregnancy loss. Clin Exp Obstet Gynecol 2007; 34:143–145. [PubMed] [Google Scholar]
- 40.Fouda UM, Sayed AM, Abdou AM, et al. Enoxaparin versus unfractionated heparin in the management of recurrent abortion secondary to antiphospholipid syndrome. Int J Gynaecol Obstet 2011; 112:211–215. [DOI] [PubMed] [Google Scholar]
- 41.Pattison NS, Chamley LW, Birdsall M, et al. Does aspirin have a role in improving pregnancy outcome for women with the antiphospholipid syndrome? A randomized controlled trial. Am J Obstet Gynecol 2000; 183:1008–1012. [DOI] [PubMed] [Google Scholar]
- 42.Martinelli I, Ruggenenti P, Cetin I, et al. Heparin in pregnant women with previous placenta-mediated pregnancy complications: a prospective, randomized, multicenter, controlled clinical trial. Blood 2012; 119:3269–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badawy AM, Khiary M, Sherif LS, et al. Low-molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. J Obstet Gynaecol 2008; 28:280–284. [DOI] [PubMed] [Google Scholar]
- 44.Mantha S, Bauer KA, Zwicker JI. Low molecular weight heparin to achieve live birth following unexplained pregnancy loss: a systematic review. J Thromb Haemost 2010; 8:263–268. [DOI] [PubMed] [Google Scholar]
- 45.Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ 2003; 327:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawood MY. Nonsteroidal antiinflammatory drugs and reproduction. Am J Obstet Gynecol 1993; 169:1255–1265. [DOI] [PubMed] [Google Scholar]
- 47.van der Weiden RM, Helmerhorst FM, Keirse MJ. Prostanoid excretion in incipient singleton and twin pregnancies. Am J Obstet Gynecol 1996; 174:1614–1617. [DOI] [PubMed] [Google Scholar]
- 48.van der Weiden RM, Helmerhorst FM, Keirse MJ. Influence of prostaglandins and platelet activating factor on implantation. Hum Reprod 1991; 6:436–442. [PubMed] [Google Scholar]
- 49.van der Weiden RM, Wouters JM. Infertility may sometimes be associated with non-steroidal anti-inflammatory drug consumption. Br J Rheumatol 1997; 36:605. [DOI] [PubMed] [Google Scholar]
- 50.Schisterman EF, Silver RM, Lesher LL, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014; 384:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


