Abstract
The Helicobacter species in the gut microbiota comprise Helicobacter pylori (H pylori) and enterohepatic Helicobacter species (EHS), which can colonize the intestinal mucosa. However, it is unclear whether EHS are associated with inflammatory bowel disease (IBD). Therefore, we conducted this meta-analysis to examine the association between EHS and IBD.
PubMed, Scopus, Cochrane Library, and Web of Science databases, as well as abstracts from conference proceedings were searched to identify studies that used polymerase chain reaction to detect Helicobacter species in intestinal samples from patients with IBD.
After screening, we carefully reviewed 20 of the 2955 identified studies, and performed a meta-analysis of the findings from 14 studies (11 adult studies and 3 pediatric studies) using STATA v12.0. These studies evaluated 1407 individuals, including 433 patients with Crohn's disease, 306 patients with ulcerative colitis, and 668 controls. The prevalence of Helicobacter species was higher among the patients with IBD, compared to that among the controls, which corresponded to a pooled risk ratio (RR) of 1.59 (95% confidence interval [CI]: 1.12–2.27). The RRs for adult and pediatric patients with IBD were 1.61 (95% CI: 1.03–2.52) and 1.76 (95% CI: 1.17–2.64), respectively. Compared to the controls, the patients with IBD tended to have a higher prevalence of EHS in the intestinal mucosa (RR: 2.01, 95% CI: 1.36–2.98), although the prevalence of H pylori was not significantly higher (RR: 1.22, 95% CI: 0.77–1.95). Compared to the controls, the RRs for EHS in patients with Crohn's disease and ulcerative colitis were 1.72 (95% CI: 1.20–2.47) and 3.27 (95% CI: 0.93–11.44), respectively.
It appears that EHS was associated with IBD, while intestinal H pylori infection was not significantly associated with IBD. Further studies are needed to determine the involvement of EHS in the microbiological etiology of IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a group of chronic idiopathic conditions that are related to gastrointestinal tract inflammation, which primarily involve Crohn's disease (CD) and ulcerative colitis (UC). Unfortunately, IBD is a growing health concern for patients around the world,1 and its etiology remains unknown. According to existing hypotheses, gastrointestinal microorganisms might trigger a deregulated immune response in genetically predisposed individuals, which might result in chronic inflammation.2 A group of spiral or helical microorganisms that are called enterohepatic Helicobacter species (EHS) have also recently been implicated as potential pathogens, as they can colonize the mucus layer within the gastrointestinal epithelium.3,4 These microorganisms induce an IBD-like colitis in an immunocompromised murine model,5–7 and may be involved in the pathogenesis of IBD.8
In 2005, 2 Australian scientists were awarded the Nobel Prize in Physiology and Medicine for their discovery that Helicobacter pylori (H pylori) was the direct causative agent of gastritis and peptic ulcer disease.9 A recent study has also reported an inverse association between gastric H pylori infection and IBD, which was due to a down-regulation of the pro-inflammatory response.10 Several meta-analyses have also suggested that H pylori infection provides a protective effect against IBD,11–13 including among Asian patients with IBD. Nevertheless, those meta-analyses only evaluated serological testing for H pylori-specific markers, and their data only reflect the gastric prevalence of H pylori. The role of other Helicobacter species in IBD, especially that of EHS, has not been sufficiently evaluated.
Thus, we performed the first meta-analysis to investigate the prevalence of intestinal Helicobacter species in specimens from patients with IBD. Using that information, we aimed to explore the association between intestinal EHS and IBD, in order to help our understanding of the role of EHS in the etiology of IBD.
METHODS
Search Strategy and Information Sources
This study's design was approved by the Medical Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University. The study was performed in accordance with the guidelines for meta-analyses of observational studies in epidemiology14 and the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.15 To detect relevant studies, we searched the PubMed, Scopus, Cochrane Library, and Web of Science databases for all relevant articles that were published before October 1, 2014. Additional studies were identified by searching abstracts from the Digestive Disease Week and European Crohn's and Colitis Organization annual meetings. The search strategy employed various Medical Subject Heading (MeSH) terms, as well as free text words to increase the search's sensitivity: “inflammatory bowel disease” (MeSH), “Crohn's disease” (MeSH), “ulcerative colitis” (MeSH), “Helicobacter” (MeSH), “gastrospirillum” (free text), “Helicobacter species” (MeSH), “H pylori” (MeSH), “Helicobacter mustelae” (free text), “Helicobacter hepaticus” (MeSH), “Helicobacter heilmannii” (free text), and “Helicobacter felis” (free text) (Supplementary File A). All references within each publication were carefully and manually evaluated to avoid omitting any pertinent study, and any additional studies were verified via email contact with study's investigators.
Eligibility Criteria
Studies were included in our screening if they focused on all Helicobacter species and IBD, compromising both intestinal EHS and H pylori. The studies were screened using the following criteria: peer reviewed articles that were published in English (to facilitate the review and evaluation of the full text), the study received ethical approval and all participants provided informed consent, the IBD and control groups were matched in terms of age, sex, and study area, Helicobacter species were identified via polymerase chain reaction (PCR) using biopsy specimens that were obtained from both the small and large intestine mucosa, and measurable outcomes were fully reported. Animal studies and single-case reports were excluded. Two reviewers (QY and LL) independently assessed the eligibility of each study, and disagreements were resolved via consensus after discussion with the senior investigators (SZ and MC). If the results of a study were reported in multiple publications (with possible overlap), only the most recent and informative publication was included in the analysis.
Quality Assessment
The Newcastle–Ottawa Scale (NOS)16 was used to assess the quality of the nonrandomized studies (details regarding this evaluation are shown in the Supplementary Material). A score of ≥6 stars indicates a high-quality study.17 Scoring for each study was performed independently by 2 investigators (QY and LL), and kappa statistics were used to assess the inter-rater agreement.18
Data Extraction and Assessment of the Risk Bias
Four investigators (QY, LL, YL, and HW) independently extracted the data, and agreement was reached via consensus for all items when a discrepancy was found. For each study, we recorded the author, year of publication, geographical location, patient enrollment, sample size, type of IBD, age, sex distribution, disease location, PCR method, and type of Helicobacter species (Table 1). Heterogeneity between the studies was assessed using the I2 test, and an I2 of ≥50% indicated the presence of at least moderate heterogeneity.19 The presence of publication bias and related bias were evaluated using funnel plots and Egger's test.20
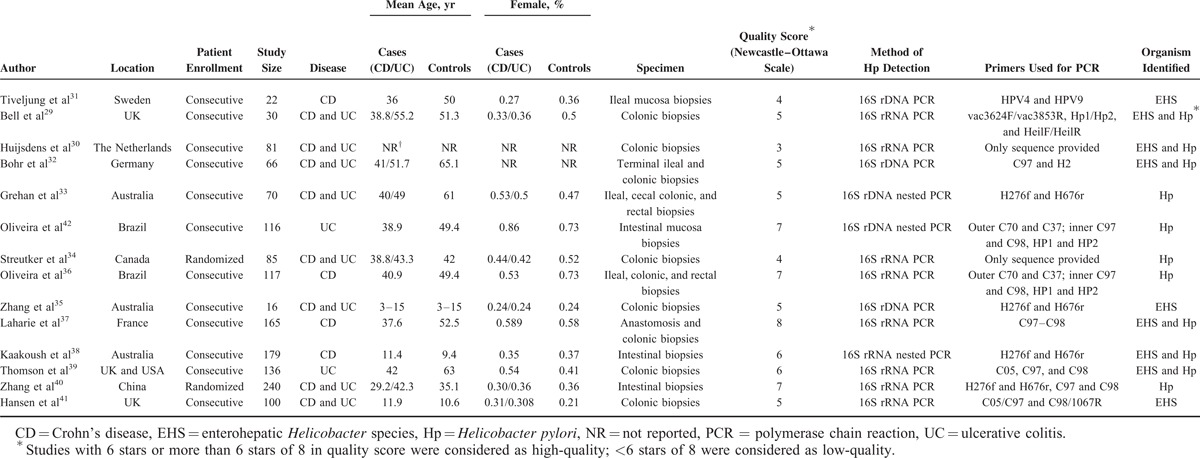
TABLE 1.
Basic Characteristics of 14 Case–Control Studies That Used PCR to Detect Intestinal Positivity of Helicobacter Species in Patients With Inflammatory Bowel Disease and in Controls
Statistical Analysis
We constructed 2 × 2 tables of the numbers of positive cases and total cases for both the IBD and control groups in the included studies. Pooled risk ratios (RRs) and the associated 95% confidence intervals (CIs) were estimated for the binary variables. The priori decision to use a fixed- or random-effects model (DerSimonian and Laird) was guided by the presence or absence of heterogeneity.21,22 STATA software (version 12.0; STATA Corporation, College Station, TX) was used for the main and subgroup meta-analyses. Subgroup analyses were performed according to patient age (adults vs children) and disease classification (UC vs CD) for H pylori and EHS. A P value of <0.05 was considered statistically significant.
RESULTS
Eligible Studies
The process for the study selection is shown in Figure 1. After applying the initial search strategy, we identified 2955 citations, and 20 potential studies were subjected to a detailed review. Among these studies, we excluded 3 studies for not using PCR to detect Helicobacter species (the culture method was used).23–25 Three additional studies were excluded for not reporting useful results to calculate the RR: 1 study included patients with other bowel diseases and did not report the exact number of patients with IBD,26 a 2nd study was excluded because the author did not reply to our enquiry email,27 and a 3rd article focused on T-cell clones instead of Helicobacter strains.28 Our meta-analysis included 14 studies that evaluated 1407 individuals. However, 2 studies did not find H pylori or EHS in the intestine samples from their patients with IBD or their controls.29,30 Therefore, only 12 studies31–42 were displayed in the final forest plot for analysis. The NOS quality of the included studies was 3 to 8 stars, and the inter-rater reliability was good (kappa = 0.70, P = 0.006).
FIGURE 1.
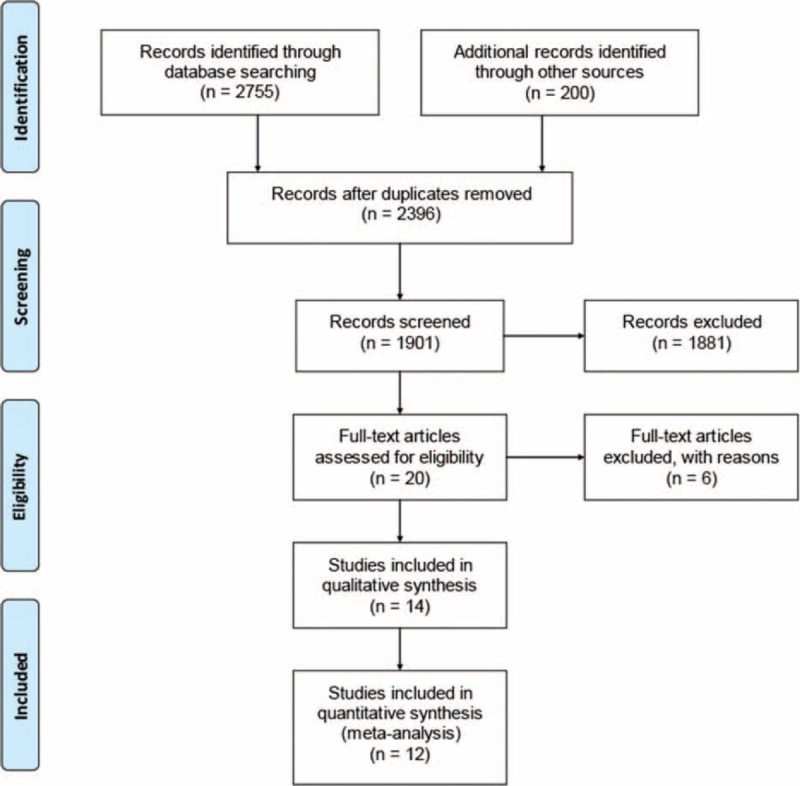
Flowchart of the article selection process.
Study Characteristics
The detailed characteristics of the 14 studies are shown in Table 1. Of 14 studies, 12 used consecutive participant recruitment, the remainders recruited participants randomly. Eleven studies included adult patients, while 3 studies included pediatric patients. Eight studies (8/14) included patients with both CD and UC; 4 studies only evaluated CD; and the other 2 studies only evaluated UC. Patients who were enrolled in the studies were diagnosed using the standard clinical diagnostic criteria for IBD,43 as well as radiography, endoscopy, and pathology findings. Ten studies used non-IBD patients as the control group; 3 studies used normal individuals; 2 studies used patients with culture-negative diarrhea or polyps; and only 1 study used patients with irritable bowel syndrome. The median year of publication was 2005 (range: 2004–2010), and the median sample size was 93 participants (range: 57–143). The median ages at IBD diagnosis for the patients with CD and UC were 38.8 years (range: 20.6–40.5 years) and 49.4 years (range: 22.9–56.8 years), respectively. The median age of the control patients was 49.4 years (range: 22.9–56.8 years). The studies included populations from various countries, including the UK (3 studies), Brazil (2 studies), Germany (2 studies), Sweden, France, Italy, the Netherlands, Canada, Australia, and China. All of the included studies were performed prospectively and used PCR to detect 16S rRNA or rDNA from Helicobacter species. In 9 studies, PCR products from the intestinal mucosa of patients with IBD were highly similar to H pylori, and 7 studies identified EHS via PCR and sequencing.
Analysis of the Association Between All Intestinal Helicobacter Species and IBD
Both H pylori and EHS were detected in the intestines of the patients with IBD. Given the evidence of moderate heterogeneity among the included studies (I2 = 61.1%), we used a random-effects model to calculate the RR. The overall prevalence of Helicobacter species was higher among the patients with IBD, compared to that among the controls, which corresponded to an overall pooled RR of 1.59 (95% CI: 1.12–2.27) (Fig. 2). We also analyzed the adult and pediatric studies separately, and observed a higher prevalence of Helicobacter species among both the adult patients with IBD (9 studies; RR: 1.61, 95% CI: 1.03–2.52) and the pediatric patients with IBD (3 studies; RR: 1.76, 95% CI: 1.17–2.64). There was significant heterogeneity among the studies of adult patients (I2 = 66.7%), although no heterogeneity was observed for the studies of pediatric patients (I2 = 0.0%).
FIGURE 2.
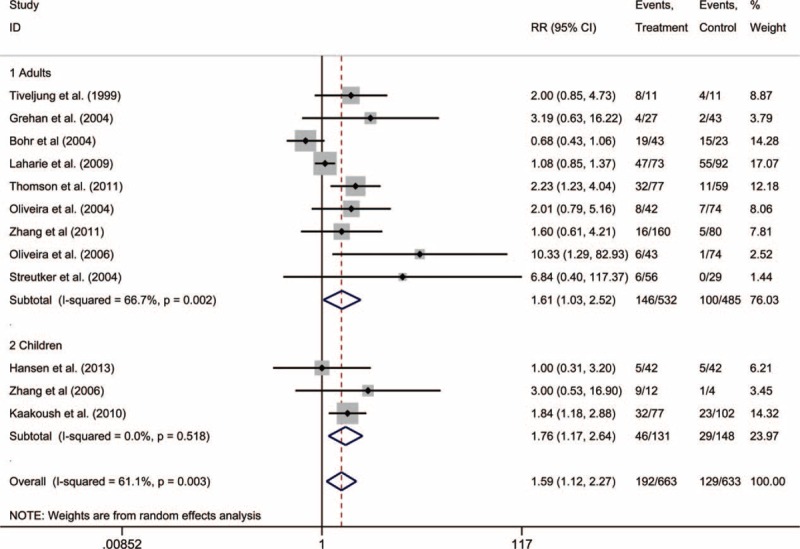
Forest plots of adult and pediatric studies that used polymerase chain reaction to detect Helicobacter species in patients with inflammatory bowel disease.
The results from the 2 studies that were not included in the forest plot corresponded to 2 zeros in the 2 × 2 table, which prevented us from calculating an RR. Furthermore, the analysis of the funnel plot indicated a possible publication bias in these studies (Fig. 3),34,36 which fell outside of the funnel plot due to the low number of controls that were positive for Helicobacter species (0 and 1 cases, respectively).
FIGURE 3.
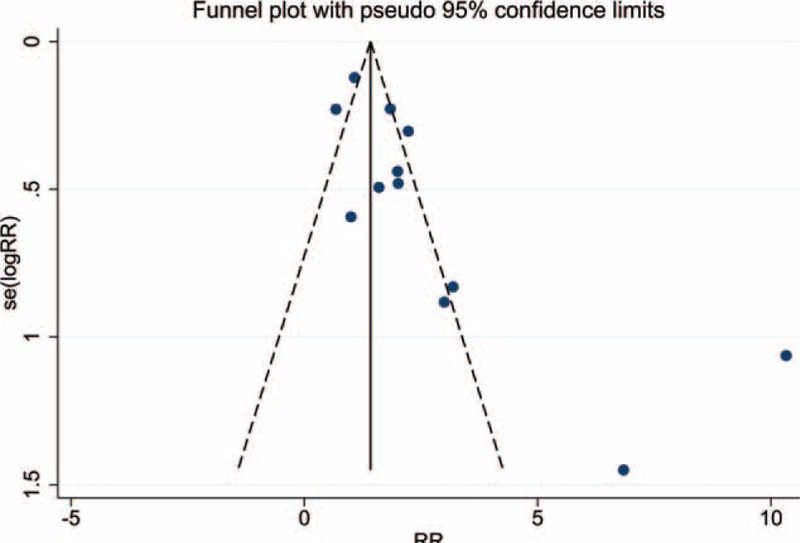
Funnel plot showing the publication bias in the included studies. Each solid circle represents one study.
Analysis of the Association Between Intestinal H Pylori and IBD
Nine studies of 1174 individuals (598 IBD cases) evaluated the prevalence of intestinal H pylori in patients with IBD. However, compared to the controls, the RR for intestinal H pylori infection was 1.22 (95% CI: 0.77–1.95), and this result was not statistically significant with the presence of moderate heterogeneity (I2 = 59.9%) (Fig. 4). Figure 4 also shows the subgroup analyses for H pylori infection in patients with CD or UC, compared to that in the controls (CD RR: 1.30, 95% CI: 0.74–2.27; UC RR: 1.13; 95% CI: 0.44–2.90). However, significant heterogeneity was also observed in these studies (CD: I2 = 57.5%; UC: I2 = 68.7%).
FIGURE 4.
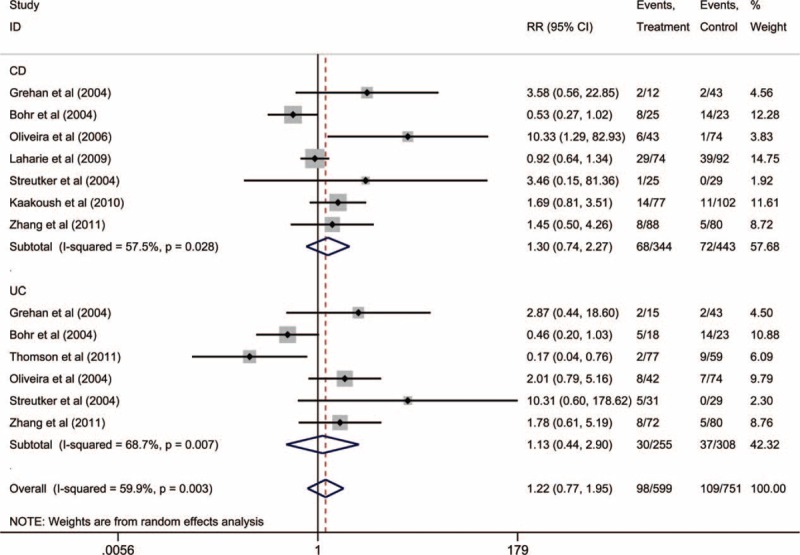
Subgroup meta-analysis of Helicobacter pylori detection via polymerase chain reaction in patients with Crohn's disease or ulcerative colitis.
Analysis of the Association Between Intestinal EHS and IBD
As shown in Figure 5, 7 studies of 580 individuals (281 IBD cases) evaluated the prevalence of intestinal EHS. The IBD cases were more likely to have EHS infection, compared to the controls (RR: 2.01, 95% CI: 1.36–2.98), and no significant heterogeneity was observed (I2 = 15.9%). Furthermore, the EHS studies exhibited a relatively symmetrical funnel plot, which suggested that publication bias was not present.
FIGURE 5.
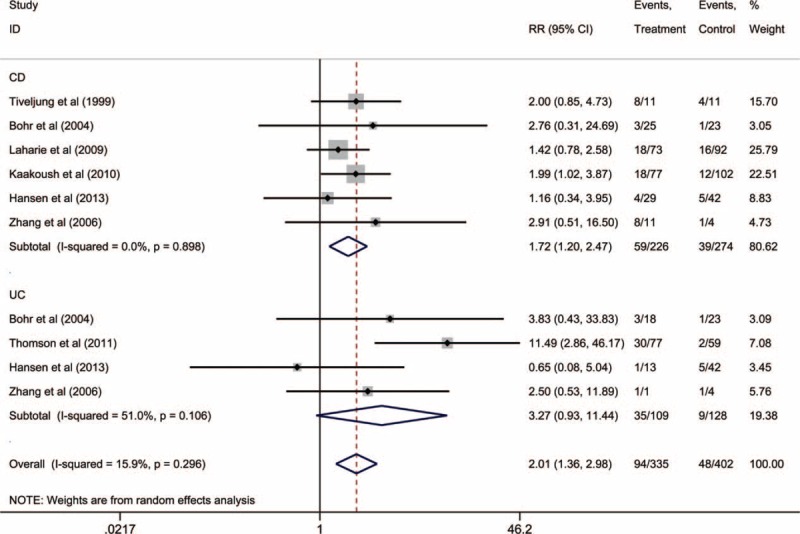
Subgroup meta-analysis of enterohepatic Helicobacter species detection via polymerase chain reaction in patients with Crohn's disease or ulcerative colitis.
We also performed subgroup analyses for the prevalence of EHS in patients with CD and UC. Compared to the controls, the patients with CD had a significantly higher prevalence of EHS (RR: 1.72, 95% CI: 1.20–2.47), and no heterogeneity was observed (I2 = 0.0%) (Fig. 5). In contrast, patients with UC did not exhibit a significantly higher prevalence of EHS, compared to the controls (RR: 3.27, 95% CI: 0.93–11.44), and a moderate level of heterogeneity was observed (I2 = 51.0%).
DISCUSSION
The present meta-analysis included 14 case–control studies from 9 different countries that investigated the association between intestinal Helicobacter species and IBD. The majority of the studies (11/14) reported an RR of >1, although the RR was only significant in 3 studies, and 1 study reported a nonsignificant RR of <1. No significant association between intestinal H pylori and IBD was found. The results of our meta-analysis suggest that non-H pylori EHS infection might be associated with IBD, although publication bias and a moderate level of heterogeneity may confound that interpretation.
There are several potential sources of the heterogeneity for the present meta-analysis. First, different PCR primers were used in various studies. Most included studies (9/14) used the C97–C98 pair of primers,32,36,37,39,41,42 as well as 1 excluded study,26 although 4 studies used the H276f–H676r primers.33,35,38,40 Furthermore, Tiveljung et al31 used the HPV4–HPV9 pair of primer, which can only identify EHS (not H pylori), and reported a higher prevalence of EHS in IBD (72.7%) compared to the controls (36.4%). Second, age may have confounded several studies, especially in the selection of the control groups. Third, we included studies from 9 different countries, with most of the studies being performed in Europe, America, and Australia; only 1 study was performed in Asia (China). However, geographical and population variations may affect the detected species of gut microbiota.44 Fourth, the sample size of the included studies ranged from 16 to 240 cases, which may have incorporated selection bias into the patient enrollment. These 4 factors may be responsible for the moderate level of heterogeneity in our analysis, and we recommend that additional studies with larger sample sizes should use the same PCR primers to minimize heterogeneity in future studies.
Several meta-analyses have reported that gastric H pylori was a protective factor against IBD.11–13 However, our data did not reveal any significant association between intestinal H pylori and IBD. In this context, H pylori may be transferred from the mouth to the colon in animals, which may explain why it is detectable in the intestinal tissues.45 However, H pylori could colonize the intestinal mucosa in a mouse model,46 which may partially explain the different prevalence of H pylori in the stomach and intestine. The different detection methods may also be responsible for this discrepancy, as gastric H pylori infection is usually diagnosed via serology, a urea breath test, or histology. We detected intestinal H pylori via PCR, which can more accurately differentiate between intestinal bacteria. Therefore, prospective high-quality controlled studies are needed to confirm our findings.
Unlike H pylori, EHS can persistently colonize the lower gastrointestinal tract and hepatobiliary system.47 Interestingly, all 7 included studies that evaluated EHS revealed a relationship with IBD,31,32,35,37–39,41 although it is not clear whether the presence of EHS is a risk factor or causative factor in the etiology of IBD. One multicenter cross-sectional study detected EHS in 24.7% of patients with CD, and found that Helicobacter pullorum and Helicobacter canadensis were the 2 most common species.37 In contrast, a German study found Helicobacter fennelliae and H pullorum in the colon samples from 12% of patients with CD, compared to in only 4% of the controls.32 These studies unanimously indicate a positive relationship between EHS and IBD. Based on our data, it appears that EHS were most responsible for the dissimilarity between the patients with IBD and the controls, although the role of intestinal H pylori in IBD cannot currently be determined. Nevertheless, our data are not sufficient to clearly demonstrate that EHS is a risk factor for IBD, and further studies are needed to address this issue.
In addition, EHS were not detected in the IBD or control groups in 2 studies.29,30 The absence of detectable EHS may be related to 3 confounding factors. First, 1 of the studies only evaluated 30 patients, which may preclude an extrapolation to the general patient population (eg, due to sampling error). Second, the gut flora might be contaminated by feces, which could influence the sensitivity of the PCR tests. Third, drug therapies (eg, immunosuppressants or other biologic agents) might inhibit the activation and growth of EHS.
Although we screened for studies that used similar techniques and adhered to strict inclusion criteria, several limitations still existed in our meta-analysis. First, the confounding factors within each study's group increased the heterogeneity, as this type of study is rarely conducted. For example, the baseline patient characteristics varied in terms of age, sex, life experience, and environmental factors. Furthermore, the selection bias for control selection may have also intensified the heterogeneity in this meta-analysis. Second, only PCR was used to identify the infection of Helicobacter species. As PCR detection of Helicobacter species has good concordance in different intestinal regions for 99.1% of patients,36 CD studies typically involve a biopsy of the ileal tissue and UC studies perform biopsy of the colonic tissue. Therefore, our subgroup analyses of CD and UC might partially reflect the effects of using different specimen locations. However, a single-site detection method has not become routine clinical practice, which may have led to further selection bias. Third, our assessment of study quality via NOS indicated a range of 3 to 8 stars, which suggested that there were discrepancies in the methodological quality of these studies.
Nevertheless, to our knowledge, the current meta-analysis is the first to reveal an association between intestinal Helicobacter species and patients with IBD. Future studies should examine this association using other diagnostic methods and sample sources, such as the patients’ stool and serum. Furthermore, these studies need to determine whether this association is causative, and what role EHS might play in the pathogenesis of IBD.
CONCLUSION
This meta-analysis revealed a statistically significant association of EHS with IBD using PCR detection of 16S RNA. Furthermore, we found no significant association between intestinal H pylori and IBD. However, well-organized prospective studies are needed to verify our findings and to identify the role of EHS in the immune mechanisms that are involved in the etiology of IBD.
UNCITIED REFERENCES
Footnotes
Abbreviations: CD = Crohn's disease, CI = confidence interval, EHS = enterohepatic Helicobacter species, H pylori = Helicobacter pylori, IBD = inflammatory bowel disease, MeSH = Medical Subject Heading, NOS = Newcastle–Ottawa Scale, PCR = polymerase chain reaction, RR = risk ratio, UC = ulcerative colitis.
QY and SZ have contributed equally to the study.
This work was supported by the major cultivation project of Sun Yat-Sen University (10ykjc23) and the National Natural Science Foundation of China (81270473, 81301769).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.M’Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol 2013; 14:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003; 361:512–519. [DOI] [PubMed] [Google Scholar]
- 3.Mateos-Munoz B, Perez-de-la-Serna J, Ruiz-de-Leon A, et al. Enterohepatic Helicobacter other than Helicobacter pylori. Rev Esp Enferm Dig 2013; 105:477–485. [DOI] [PubMed] [Google Scholar]
- 4.Frank DN, St AA, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007; 104:13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagalingam NA, Robinson CJ, Bergin IL, et al. The effects of intestinal microbial community structure on disease manifestation in IL-10−/− mice infected with Helicobacter hepaticus. Microbiome 2013; 1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang I, Eibach D, Kops F, et al. Intestinal microbiota composition of interleukin-10 deficient C57BL/6J mice and susceptibility to Helicobacter hepaticus-induced colitis. PLoS ONE 2013; 8:e70783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthupalani S, Ge Z, Feng Y, et al. Systemic macrophage depletion inhibits Helicobacter bilis-induced proinflammatory cytokine-mediated typhlocolitis and impairs bacterial colonization dynamics in a BALB/c Rag2−/− mouse model of inflammatory bowel disease. Infect Immun 2012; 80:4388–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manichanh C, Borruel N, Casellas F, et al. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012; 9:599–608. [DOI] [PubMed] [Google Scholar]
- 9.Megraud F. A humble bacterium sweeps this year's Nobel Prize. Cell 2005; 123:975–976. [DOI] [PubMed] [Google Scholar]
- 10.Luther J, Owyang SY, Takeuchi T, et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut 2011; 60:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luther J, Dave M, Higgins PD, et al. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis 2010; 16:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rokkas T, Gisbert JP, Niv Y, et al. The association between Helicobacter pylori infection and inflammatory bowel disease based on meta-analysis. UEG J 2015; doi: 10.1177/2050640615580889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu XW, Ji HZ, Yang MF, et al. Helicobacter pylori infection and inflammatory bowel disease in Asians: a meta-analysis. World J Gastroenterol 2015; 21:4750–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 17.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003; 7:1–173. [DOI] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748. [PubMed] [Google Scholar]
- 22.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28:105–114. [DOI] [PubMed] [Google Scholar]
- 23.Tomoda A, Miyazawa K, Tabuchi T. Prevention of carcinogenesis and development of gastric and colon cancers by 2-aminophenoxazine-3-one (Phx-3): direct and indirect anti-cancer activity of Phx-3. Int J Mol Sci 2013; 14:17573–17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang Z, Chen YP, Ye YF, et al. Helicobacter pylori and Crohn's disease: a retrospective single-center study from China. World J Gastroenterol 2013; 19:4576–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonnenberg A, Genta RM. Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35:469–476. [DOI] [PubMed] [Google Scholar]
- 26.Basset C, Holton J, Bazeos A, et al. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci 2004; 49:1425–1432. [DOI] [PubMed] [Google Scholar]
- 27.Bohr UR, Primus A, Zagoura A, et al. A group-specific PCR assay for the detection of Helicobacteraceae in human gut. Helicobacter 2002; 7:378–383. [DOI] [PubMed] [Google Scholar]
- 28.Duchmann R, Marker-Hermann E, Meyer ZBK. Bacteria-specific T-cell clones are selective in their reactivity towards different enterobacteria or H. pylori and increased in inflammatory bowel disease. Scand J Immunol 1996; 44:71–79. [DOI] [PubMed] [Google Scholar]
- 29.Bell SJ, Chisholm SA, Owen RJ, et al. Evaluation of Helicobacter species in inflammatory bowel disease. Aliment Pharmacol Ther 2003; 18:481–486. [DOI] [PubMed] [Google Scholar]
- 30.Huijsdens XW, Linskens RK, Koppes J, et al. Detection of Helicobacter species DNA by quantitative PCR in the gastrointestinal tract of healthy individuals and of patients with inflammatory bowel disease. FEMS Immunol Med Microbiol 2004; 41:79–84. [DOI] [PubMed] [Google Scholar]
- 31.Tiveljung A, Soderholm JD, Olaison G, et al. Presence of eubacteria in biopsies from Crohn's disease inflammatory lesions as determined by 16S rRNA gene-based PCR. J Med Microbiol 1999; 48:263–268. [DOI] [PubMed] [Google Scholar]
- 32.Bohr UR, Glasbrenner B, Primus A, et al. Identification of enterohepatic Helicobacter species in patients suffering from inflammatory bowel disease. J Clin Microbiol 2004; 42:2766–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grehan M, Danon S, Lee A, et al. Absence of mucosa-associated colonic Helicobacters in an Australian urban population. J Clin Microbiol 2004; 42:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streutker CJ, Bernstein CN, Chan VL, et al. Detection of species-specific helicobacter ribosomal DNA in intestinal biopsy samples from a population-based cohort of patients with ulcerative colitis. J Clin Microbiol 2004; 42:660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Day A, McKenzie G, et al. Nongastric Helicobacter species detected in the intestinal tract of children. J Clin Microbiol 2006; 44:2276–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira AG, Rocha GA, Rocha AM, et al. Isolation of Helicobacter pylori from the intestinal mucosa of patients with Crohn's disease. Helicobacter 2006; 11:2–9. [DOI] [PubMed] [Google Scholar]
- 37.Laharie D, Asencio C, Asselineau J, et al. Association between entero-hepatic Helicobacter species and Crohn's disease: a prospective cross-sectional study. Aliment Pharmacol Ther 2009; 30:283–293. [DOI] [PubMed] [Google Scholar]
- 38.Kaakoush NO, Holmes J, Octavia S, et al. Detection of Helicobacteraceae in intestinal biopsies of children with Crohn's disease. Helicobacter 2010; 15:549–557. [DOI] [PubMed] [Google Scholar]
- 39.Thomson JM, Hansen R, Berry SH, et al. Enterohepatic helicobacter in ulcerative colitis: potential pathogenic entities? PLoS ONE 2011; 6:e17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, Zhong B, Chao K, et al. Role of Helicobacter species in Chinese patients with inflammatory bowel disease. J Clin Microbiol 2011; 49:1987–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen R, Berry SH, Mukhopadhya I, et al. The microaerophilic microbiota of de-novo paediatric inflammatory bowel disease: the BISCUIT study. PLoS ONE 2013; 8:e58825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira AG, Das GPSM, Rocha GA, et al. Helicobacter species in the intestinal mucosa of patients with ulcerative colitis. J Clin Microbiol 2004; 42:384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60:571–607. [DOI] [PubMed] [Google Scholar]
- 44.Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013; 62:630–649. [DOI] [PubMed] [Google Scholar]
- 45.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 2006; 19:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cellini L, Marzio L, Ferrero G, et al. Transmission of Helicobacter pylori in an animal model. Dig Dis Sci 2001; 46:62–68. [DOI] [PubMed] [Google Scholar]
- 47.Fox JG. The non-H pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut 2002; 50:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


