Abstract
To determine the association between frontal lobe function and risk of hip fracture in patients with Alzheimer disease (AD).
Retrospective cohort study using multicenter hospital-based dementia registry and national health insurance claim data was done. Participants who had available data of neuropsychological test, national health insurance claim, and other covariates were included. A total of 1660 patients with AD were included based on Stroop Test results. A total of 1563 patients with AD were included based on the Controlled Oral Word Association Test (COWAT) results. Hip fracture was measured by validated identification criteria using national health insurance claim data. Frontal lobe function was measured by Stroop Test and COWAT at baseline.
After adjusting for potential covariates, including cognitive function in other domains (language, verbal and nonverbal memory, and attention), the Cox proportional hazard regression analysis revealed that risk of a hip fracture was decreased with a hazard ratio (HR) of 0.98 per one point of increase in the Stroop Test (adjusted HR = 0.98, 95% confidence interval [CI]: 0.97–1.00) and 0.93 per one point increase in COWAT (adjusted HR = 0.93, 95% CI: 0.88–0.99).
The risk of hip fracture in AD patients was associated with baseline frontal lobe function. The result of this research presents evidence of association between frontal lobe function and risk of hip fracture in patients with AD.
INTRODUCTION
Alzheimer disease (AD) and hip fracture are predominantly occurring diseases in the elderly (≥65 years). Their high morbidity and mortality give heavy burdens to both patients and their caregivers. A few prior studies have suggested that the elderly with AD have higher risk of hip fracture compared to cognitively intact elderly.1–7 However, some of these research studies were based on cross-sectional design that it was difficult to draw a conclusion on their etiologic association.3,5,8 Although some research studies were based on longitudinal design, only limited characteristics of AD such as Mini-mental state examination (MMSE) score or insufficient numbers of subjects were used. Therefore, it was difficult to find out specifically what factors of AD patients affected the occurrence of hip fracture.2,6,7
The main objective of the present study was to assess the longitudinal association of frontal lobe function and risk of hip fracture in AD patients. A few prior research studies have reported that frontal lobe function is associated with increased fall risk in cognitively intact elderly.9–11 We placed a finer focus on research subjects by involving exclusively patient with AD. Main outcome was not focused simply on falls, but more specifically on hip fracture that exerts great effect on morbidity and mortality. Such a specific research setting could secure a higher clinical significance. We hypothesized that impaired frontal lobe function would increase the risk of hip fracture in AD patients. To determine the association between frontal lobe function and the risk of hip fracture in AD patients, we analyzed longitudinally linked data of Clinical Research Center for Dementia of South Korea (CREDOS) which has a wide range of baseline information including standardized neuropsychological test results and National Health Insurance (NHI) Claims Database of the Health Insurance Review & Assessment Service (HIRA).
METHODS
Participants
The study population comprised CREDOS study participants aged 65 years and above who were diagnosed with AD. The CREDOS study registered on ClinicalTrials.gov (identifier: NCT01198093) recruited participants from university-affiliated hospitals who were diagnosed with normal cognition, subjective memory impairment, mild cognitive impairment, vascular cognitive impairment, subcortical ischemic vascular dementia, or AD by neurologist or psychiatrist. In the CREDOS study, we used the diagnostic criteria for probable AD issued by the National Institute of Neurological and Communicative Disorder and Stroke-Alzheimer's Disease and Related Disorder Association (NINCDS-ADRDA).12 A more detailed description of CREDOS study has been published previously.13,14 To identify the occurrence of hip fracture, we used the NHI Claims Database of the HIRA. South Korea has a universal national health coverage system that covers approximately 98% of overall South Korean population.15 A more detailed description of HIRA database is published previously.16
Of CREDOS study participants from January 1, 2008 to August 31, 2012, we retrospectively analyzed the NHI claim data from the time of participant's CREDOS study enrollment up to August 31, 2012 or the time of event (hip fracture or death). Participants who had available data of neuropsychological test, NHI claim, and other covariates were included. We excluded those who met following criteria: (1) history of hip fracture within 6 months at the time of CREDOS study enrollment; (2) history of significant hearing or visual impairment rendering participation in the interview difficult; (3) history of following neurologic disorder (brain tumor, intracranial hemorrhage, subarachnoid hemorrhage, epilepsy, hydrocephalus, encephalitis, and metabolic encephalopathy) or other neurologic conditions that could interfere with the study; (4) history of following psychiatric disorder (mental retardation, schizophrenia, and bipolar disorder) or other psychiatric conditions that could interfere with the study; (5) history of using psychoactive substances other than alcohol; (6) history of following physical illnesses or disorders (cancer, renal failure, hepatic failure, uncontrolled diabetes, severe asthma or chronic obstructive pulmonary disease, syphilis and abnormal thyroid function test) or other physical conditions that could interfere with the study.
Standard Protocol Approvals, Registrations, and Patient Consents
CREDOS study was approved by the institutional review board of the participating centers. All participants signed informed written consents. This study was approved by the institutional review boards at the clinical sites. We were provided with permission to use the NHI claim database by the HIRA and Ministry of Health & Welfare.
Primary Measurements
Cognitive function was assessed at baseline with a standardized neuropsychological battery of the Seoul Neuropsychological Screening Battery-Dementia Version (SNSB-D).17 The SNSB-D included tests of language, verbal/nonverbal memory, attention, and frontal lobe function domains. These domains were assessed using the following tests: the Korean short version of the Boston Naming Test for language, the Digit Span Test-backward for attention function, the Rey Complex Figure Test-delayed recall for visuospatial memory, the Seoul Verbal Learning Test-delayed recall for verbal memory, and Stroop Test and Controlled Oral Word Association Test (COWAT) for frontal lobe function. Stroop Test or COWAT scores below the one standard deviation (16th percentile) of the age, gender, and education specific norm were classified as impaired in this study based on SNSB-D reference standards.17
Occurrence of hip fracture was assessed by validated identification criteria for cases of hip fracture using NHI Claims Database.18 The criteria to identify hip fracture using a diagnostic code, a procedure code, the type, and number of medical service usages were developed via discussions among orthopedic and epidemiologic experts. The criteria were validated by using a hip fracture cohort. Fracture due to car accident was not covered by NHI but by automobile insurance system. Therefore, we did not conduct other exclusion process.
Covariates
Possible covariates included baseline age, gender, and education years. Comorbidity was assessed using Charlson Comorbidity Index.19–21 Body mass index (BMI) was calculated from direct height and weight measurements and categorized into 4 groups reflecting World Health Organization Asian standard.22 Parkinsonian gait was determined after physical examinations by neurologists or psychiatrists based on the presence of decreased arm swing, stooped posture, short step gait, festination, shuffling, and pivot turning. Depressive symptoms were assessed using the Korean version of 15-item Geriatric Depression Scale (GDS-15).23
Statistical Analyses
Categorical variables were compared using Chi-square tests. Continuous variables were compared using independent Student t tests between the group with frontal lobe function impairment and the group without frontal lobe function impairment. The incidence rate of hip-fracture was calculated for the subjects at the end of follow-up. We used Kaplan–Meier survival curves to plot the survival curve for incident hip fracture by the presence of frontal lobe impairment. Cox proportional hazards regression was created to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of hip-fracture after adjusting demographic factors, comorbidities, BMI, parkinsonian gait, GDS-15 score, and other cognitive function score. All statistical analyses were performed using Statistical Analysis System (SAS) version 9.3 (SAS Institute, Inc., Cary, NC). Statistical significance was considered when P-value was less than 0.05.
RESULTS
Baseline characteristics of participants are summarized in Table 1. Based on Stroop Test and COWAT, 1660 and 1563 patients with AD were included, respectively. One thousand four hundred fifty-nine participants who completed both tests were analyzed twice in each group, once as the Stroop Test group and once as the COWAT group.
TABLE 1.
Baseline Characteristics by Frontal Lobe Function Among Participants∗
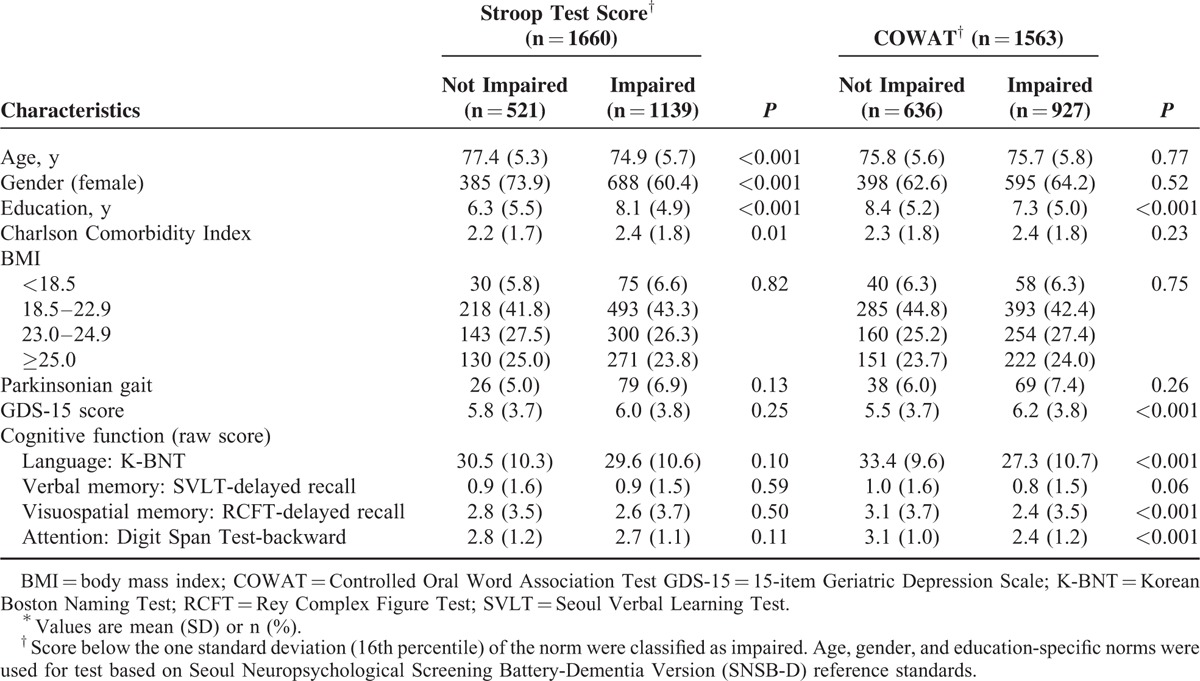
Based on Stroop Test result, the longest follow-up duration was 54.7 months. Over the follow-up, 44 (2.7%) participants developed incidents of hip fracture. AD patients who had impaired Stroop Test at baseline were more likely to develop hip fracture (n = 32, 2.8%) compared to those who did not have impaired Stroop Test at baseline (n = 12, 2.3%, Fig. 1). In the unadjusted Cox proportional hazard models, the risk of hip fracture was decreased with HR of 0.98 per one point increase in Stroop Test. Direction and strength of the association between the Stroop Test and risk of hip fracture in patient with AD remained even after adjusting for potential confounders including cognitive function in other domains (language, verbal and nonverbal memory, and attention). In the adjusted Cox proportional hazard models, the risk of hip fracture was decreased with HR of 0.98 per one point increase in Stroop Test (Table 2).
FIGURE 1.

Kaplan–Meier survival function of time to hip fracture by frontal lobe function testa. aScore below the one standard deviation (16th percentile) of the norm were classified as impaired. Age, gender, and education-specific norms were used for test based on Seoul Neuropsychological Screening Battery-Dementia Version (SNSB-D) reference standards. COWAT = Controlled Oral Word Association Test.
TABLE 2.
Cox Proportional Hazard Ratios for Time to Hip Fracture by One Point Increase of Frontal Lobe Function Test

Based on COWAT result, the longest follow-up duration was 54.7 months. Over the follow-up, 39 (2.5%) participants developed incidents of hip fracture. AD patients who had impaired COWAT at baseline were more likely to develop hip fracture (n = 31, 3.3%) compared to those who did not have impaired COWAT at baseline (n = 8, 1.3%, Fig. 1). In the unadjusted Cox proportional hazard models, the risk of hip fracture was decreased with HR of 0.94 per one point increase in COWAT. Direction and strength of the association between the COWAT and risk of hip fracture in patient with AD remained even after adjusting for potential confounders including cognitive function in other domains (language, verbal and nonverbal memory, and attention). In the adjusted Cox proportional hazard models, the risk of hip fracture was decreased with HR of 0.93 per one point increase in COWAT (Table 2).
DISCUSSION
The primary purpose of this study was to investigate the association between frontal lobe function and risk of hip fracture in patient with AD. Our study demonstrated that the risk of hip fracture in AD patients was associated with baseline frontal lobe function after adjusting for age, gender, and education. The association was still significant after adjusting for potential confounders, including comorbidities, BMI, parkinsonian gait, depressive symptoms, and other cognitive function domains.
The possible interpretation linking frontal lobe function impairment to increased risk of hip fracture in AD patients could be considered in several ways. First, gait disturbance and increased risk of fall, which could be associated with frontal lobe function impairment, might be a significant cause of increased risk for hip fractures. Prior research has revealed that AD patients show gait disturbance accompanied by slower walking velocity, decreased step length, and increased step length variation, which are all associated with an increased risk of fall.24–26 Meanwhile, some researchers reported that such gait disturbance of AD patients was associated with their frontal lobe function impairment.27 Recent review articles, and a few prospective studies about neuropsychological mechanisms of falls in elderly, also support similar ideas.10,28,29 In general, aging causes cognitive shifting from unconscious to increasingly conscious information processing, resulting in greater reliance on frontal lobe function.30 Consequently, seemingly easy and automatic tasks such as walking could become difficult and require greater conscious control.31 Such a change, however, is not a sufficiently stable process. It is accompanied by problems in motor performance increasing the risk of injury in the elderly.31–33 According to this concept, AD patients with frontal lobe function impairment are anticipated to show poor compensation for their cognitive shifting. They may show severe gait disturbance, the so-called frontal gait disorder, and are at a higher risk of falls.34,35 In the present research, however, gait measurement was insufficient and falls were not included as covariates in regression models. So such an association could not be clearly identified. Future research requires direct information about gait and history of falls and, based on them, an approach should be made to the relationship between frontal lobe function, gait disturbance, history of falls, and risk of hip fractures in AD patients. Second, general intelligence factor (G factor) associated with frontal lobe function may have contributed to the risk of hip fracture in AD patients. The concept of G factor is based on the presence of one dominant general intelligence factor that influences performance on narrow task-specific cognitive abilities.36 Recent researches have shown a close association between frontal lobe function and general intelligence.37 In the present research, the COWAT impairment group, which gave more significant results, also exhibited significantly lower scores in other cognitive domains, indicating the possibility of the contribution of G factor. To make an approach to such a concern, the analysis in our research involved an adjustment of other cognitive function domains. Yet, this can hardly be considered sufficient for addressing the independent effects of frontal lobe function and G factor. Follow-up researches would need a statistical approach, such as the structural equation model, that enables a more accurate assessment of independent effects. Third, frontal lobe function impairment could be a general indicator of dementia progression accompanying comorbidity, sarcopenia, and depression. These accompanying features were known to have influence on hip fracture development.38–40 However, our results were robust to adjustment for comorbidity score, parkinsonian gait, BMI, and depression. Fourth, restless or uncoordinated behavior associated with frontal lobe function impairment could have increased the risk of hip fracture. In previous researches, AD patients with frontal lobe function impairment have shown more behavioral problems such as agitation or aberrant motor behavior. Such problems could expose patients to more risks of hip fracture.41–43 Although Stroop Test and COWAT are known to be partly related to the extent of impulsivity and aggressiveness, the implications of these findings for hip fracture predictions are unclear.43,44
An important implication of this study is that various factors that should be considered in future research have been confirmed. Of them, the authors strongly felt the necessity of a more thorough executive function and gait performance test. An alternative for such a test could be the dual task test used in recent researches for measuring executive function on gait.45 In addition, the authors also strongly felt the need for a direct measurement of bone mass and sufficient information about fall history. A few prior research studies have suggested that AD could increase the risk of fracture by increasing the risk of falls and also by reducing the bone mineral density, probably through the degeneration in the hypothalamus.46,47 Clinical, neuropathological, and neuroimaging data together exhibited that the hypothalamus is affected in AD and undergoes neuronal loss, profound plaque and tangle formation, and overall atrophy.48–51 Also, atrophy of the hypothalamus and loss of hypothalamic neurons were associated with bone mineral density in AD patients.46 Therefore, the bone mineral density and hypothalamic degeneration should be considered as confounding factors in future research to provide etiologic evidence. Finally, the authors also strongly felt the necessity of a more direct measurement, rather than the indirect indices, of sarcopenia and frailty.
The possibility that AD patients at increased risk for hip fracture could be identified by a frontal lobe function test is another implication of this study that has been confirmed. Much research has been done on fall prevention for the elderly population. So, what is more important now is to whom the intervention should be applied to.52 In this regard, the present research presents further evidence-based information.
Our study has several strengths, including a longitudinal design with a long follow-up period up to 54.7 months and a relatively large sample of patient with AD. Also, diagnostic classification of our study was carried out directly by a neurologist or a psychiatrist who had abundant clinical experiences. Finally, corrections were also made for diverse potential covariates, including those that have previously been shown to be related to AD and hip fracture.
There are several limitations that should be taken into consideration when interpreting our study results. First, our study cannot provide a sufficient explanation regarding the various effects shown by the 2 different frontal lobe function tests. The Stroop Test is known to be associated with the dorsolateral prefrontal cortex and orbitofrontal cortex.53,54 The orbitofrontal cortex is known to be associated with sympathetico-vagal balance control through the central autonomic network.55 Also, recent research have suggested that sympathetic nervous output from the ventral hypothalamus directly regulates bone remodeling through activation of beta-2 adreno-receptors on the osteoblasts, resulting in reduced bone formation.56 These autonomous nervous system changes could be associated with increased risk of osteoporosis and hip fracture in AD patients. Whereas COWAT is known to reflect frontal lobe function, no sufficient consensus has yet been developed as to the specific interpretation or neuroanatomical correlates.57 Taking such a difference into consideration, we analyzed the Stroop Test and COWAT individually, and found that the Stroop Test was of borderline significance level, while the COWAT was significant. We could presume the reason for such a difference is that the 2 tests exert different effects on general intelligence, autonomic instability, neuroanatomical change, etc. However, since we could not conduct an evaluation to verify such a presumption, we cannot give a sufficient explanation for the difference. Though this was a major limitation of our study, it could be a point of special interest for follow-up researches. Second, there could be selection bias in our study, which is probably caused by little extensive and broad exclusion criteria, such as psychological history, neurologic or physical illnesses, and unperformed Stroop Test or COWAT, for those participants who were unable to complete both tests. Also, more young patients among impaired Stroop Test group indicate a possibility that there could be a selection bias which is probably caused by higher risk of premature death in impaired group. Third, BMI is an indirect and insufficient measure of one's nutritional status or sarcopenia. For instance, an elderly person with sarcopenic obesity is not distinguishable from a healthy muscular elderly person by BMI alone. Future work is greatly needed to attain a more specific measure, such as serum albumin concentration or lower limb strength. Fourth, score below the one standard deviation is not enough to be sure these patients have a definite clinical frontal lobe function deficit. However, in Stroop test group, 20% of subjects who developed hip fracture (n = 9) exhibited baseline standard deviation score between −1.5 and −1.0 of the norm, indicating the possibility of high risk of hip fracture in mild to moderate impairment of Stoop Test group. This could be associated with high-level activity and low-level dependency of mild to moderate AD patients, but our study cannot suggest sufficient explanation about this issue. Finally, no approach was made to frailty or osteoporosis or the use of psychotropic medication that were shown by preceding researches to be associated with increased risk of hip fracture in AD patients.31,58–61 In particular, medications that could influence the bone mineral density, such as vitamin D, bisphosphonates, antiepileptic drugs, selective serotonin reuptake inhibitor and proton pump inhibitor should be considered as important confounding factors in future research.
Through some preceding researches, it has been suggested that AD patients have relatively high risk of hip fracture.1–8 Yet the mechanism still remains unclear. No approach has been made to access respective involvement of different cognitive function domains. To our knowledge, the result of this research presents the first evidence of association between frontal lobe function and risk of hip fracture in patient with AD.
Acknowledgments
The authors thank the skillful and passionate members of Clinical Research Center for Dementia of South Korea (CREDOS) and Health Insurance Review & Assessment Service (HIRA). Collaboration of these 2 organizations enabled us to perform this study.
Footnotes
Abbreviations: AD = Alzheimer disease, BMI = body mass index, COWAT = Controlled Oral Word Association Test, CREDOS = Clinical Research Center for Dementia of South Korea, GDS-15 = 15-item Geriatric Depression Scale, HIRA = Health Insurance Review & Assessment Service, HR = hazard ratio, MMSE = Mini-mental state examination, NHI = National Health Insurance, NINCDS-ADRDA = National Institute of Neurological and Communicative Disorder and Stroke-Alzheimer's Disease and Related Disorder Association, SNSB-D = Seoul Neuropsychological Screening Battery-Dementia Version.
The authors have no conflicts of interest to disclose.
This study was supported by a grant (HI 10C2020) from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
REFERENCES
- 1.Zhao Y, Shen L, Ji HF. Alzheimer's disease and risk of hip fracture: a meta-analysis study. Scientific World J 2012; 2012:872173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker NL, Cook MN, Arrighi HM, et al. Hip fracture risk and subsequent mortality among Alzheimer's disease patients in the United Kingdom, 1988–2007. Age Ageing 2011; 40:49–54. [DOI] [PubMed] [Google Scholar]
- 3.Weller I, Schatzker J. Hip fractures and Alzheimer's disease in elderly institutionalized Canadians. Ann Epidemiol 2004; 14:319–324. [DOI] [PubMed] [Google Scholar]
- 4.Melton LJ, III, Beard CM, Kokmen E, et al. Fracture risk in patients with Alzheimer's disease. J Am Geriatr Soc 1994; 42:614–619. [DOI] [PubMed] [Google Scholar]
- 5.Weller II. The relation between hip fracture and Alzheimer's disease in the Canadian National Population Health Survey Health Institutions Data, 1994–1995. A cross-sectional study. Ann Epidemiol 2000; 10:461. [DOI] [PubMed] [Google Scholar]
- 6.Lai SW, Chen YL, Lin CL, et al. Alzheimer's disease correlates with greater risk of hip fracture in older people: a cohort in Taiwan. J Am Geriatr Soc 2013; 61:1231–1232. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Kanoko T, Satoh K, et al. Risk factors for hip fracture among elderly patients with Alzheimer's disease. J Neurol Sci 2004; 223:107–112. [DOI] [PubMed] [Google Scholar]
- 8.Weisenberg LB, Gaines J. The increased rate of fractures of the hip and spine in Alzheimer's patients. West J Med 1989; 151:206. [PMC free article] [PubMed] [Google Scholar]
- 9.Anstey KJ, Wood J, Kerr G, et al. Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology 2009; 23:500–508. [DOI] [PubMed] [Google Scholar]
- 10.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS ONE 2012; 7:e40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtzer R, Friedman R, Lipton RB, et al. The relationship between specific cognitive functions and falls in aging. Neuropsychology 2007; 21:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34:939–944. [DOI] [PubMed] [Google Scholar]
- 13.Hong YJ, Yoon B, Shim YS, et al. APOE epsilon4 allele status in Korean dementia patients with severe white matter hyperintensities. J Alzheimers Dis 2011; 24:519–524. [DOI] [PubMed] [Google Scholar]
- 14.Son SJ, Lee KS, Lee Y, et al. Association between white matter hyperintensity severity and cognitive impairment according to the presence of the apolipoprotein E (APOE) epsilon4 allele in the elderly: retrospective analysis of data from the CREDOS study. J Clin Psychiatry 2012; 73:1555–1562. [DOI] [PubMed] [Google Scholar]
- 15.Shin Y, Lee L. The health insurance system in Korea and its implications. World Hosp Health Serv 1995; 31:3–9. [PubMed] [Google Scholar]
- 16.Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health 2014; 36:e2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Y, Na DL. Seoul Neuropsychological Screening Battery (SNSB). Seoul:Human Brain Research & Consulting Co; 2003. [Google Scholar]
- 18.Park C, Jang S, Jang S, et al. Identification and validation of osteoporotic hip fracture using the National Health Insurance Database. J Korean Hip Soc 2010; 22:305–311. [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 20.D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med 1993; 32:382–387. [PubMed] [Google Scholar]
- 21.Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004; 57:1288–1294. [DOI] [PubMed] [Google Scholar]
- 22.Asia-Pacific Steering Committee. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Australia:Health Communications Australia; 2000. [Google Scholar]
- 23.Bae JN, Cho MJ. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J Psychosom Res 2004; 57:297–305. [DOI] [PubMed] [Google Scholar]
- 24.van Iersel MB, Hoefsloot W, Munneke M, et al. Systematic review of quantitative clinical gait analysis in patients with dementia. Z Gerontol Geriatr 2004; 37:27–32. [DOI] [PubMed] [Google Scholar]
- 25.Mazoteras Munoz V, Abellan van Kan G, Cantet C, et al. Gait and balance impairments in Alzheimer disease patients. Alzheimer Dis Assoc Disord 2010; 24:79–84. [DOI] [PubMed] [Google Scholar]
- 26.Wittwer JE, Webster KE, Menz HB. A longitudinal study of measures of walking in people with Alzheimer's disease. Gait Posture 2010; 32:113–117. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer's disease. Dement Geriatr Cogn Disord 2007; 24:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kearney FC, Harwood RH, Gladman JR, et al. The relationship between executive function and falls and gait abnormalities in older adults: a systematic review. Dement Geriatr Cogn Disord 2013; 36:20–35. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Chan JS, Yan JH. Neuropsychological mechanisms of falls in older adults. Front Aging Neurosci 2014; 6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leshikar ED, Gutchess AH, Hebrank AC, et al. The impact of increased relational encoding demands on frontal and hippocampal function in older adults. Cortex 2010; 46:507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fasano A, Plotnik M, Bove F, et al. The neurobiology of falls. Neurol Sci 2012; 33:1215–1223. [DOI] [PubMed] [Google Scholar]
- 32.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci 2013; 68:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J, Wu YD, Chan JS, et al. Cognitive aging affects motor performance and learning. Geriatr Gerontol Int 2013; 13:19–27. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura T, Meguro K, Yamazaki H, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Dis Assoc Disord 1997; 11:132–139. [DOI] [PubMed] [Google Scholar]
- 35.Thompson PD. Frontal lobe ataxia. Handb Clin Neurol 2012; 103:619–622. [DOI] [PubMed] [Google Scholar]
- 36.Spearman C. “General intelligence,” objectively determined and measured. Am J Psychol 1904; 15:201–292. [Google Scholar]
- 37.Royall DR, Palmer RF. “Executive functions” cannot be distinguished from general intelligence: two variations on a single theme within a symphony of latent variance. Front Behav Neurosci 2014; 8:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reyes C, Estrada P, Nogues X, et al. The impact of common co-morbidities (as measured using the Charlson index) on hip fracture risk in elderly men: a population-based cohort study. Osteoporos Int 2014; 25:1751–1758. [DOI] [PubMed] [Google Scholar]
- 39.Farmer ME, Harris T, Madans JH, et al. Anthropometric indicators and hip fracture. The NHANES I epidemiologic follow-up study. J Am Geriatr Soc 1989; 37:9–16. [DOI] [PubMed] [Google Scholar]
- 40.Wu Q, Liu J, Gallegos-Orozco JF, et al. Depression, fracture risk, and bone loss: a meta-analysis of cohort studies. Osteoporos Int 2010; 21:1627–1635. [DOI] [PubMed] [Google Scholar]
- 41.Chen ST, Sultzer DL, Hinkin CH, et al. Executive dysfunction in Alzheimer's disease: association with neuropsychiatric symptoms and functional impairment. J Neuropsychiatry Clin Neurosci Fall 1998; 10:426–432. [DOI] [PubMed] [Google Scholar]
- 42.Tekin S, Mega MS, Masterman DM, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol 2001; 49:355–361. [PubMed] [Google Scholar]
- 43.Back-Madruga C, Boone KB, Briere J, et al. Functional ability in executive variant Alzheimer's disease and typical Alzheimer's disease. Clin Neuropsychol 2002; 16:331–340. [DOI] [PubMed] [Google Scholar]
- 44.Kockler TR, Stanford MS. Using a clinically aggressive sample to examine the association between impulsivity, executive functioning, and verbal learning and memory. Arch Clin Neuropsychol 2008; 23:165–173. [DOI] [PubMed] [Google Scholar]
- 45.Plummer-D’Amato P, Altmann LJ, Reilly K. Dual-task effects of spontaneous speech and executive function on gait in aging: exaggerated effects in slow walkers. Gait Posture 2011; 33:233–237. [DOI] [PubMed] [Google Scholar]
- 46.Loskutova N, Honea RA, Brooks WM, et al. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer's disease. J Alzheimers Dis 2010; 20:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duthie A, Chew D, Soiza RL. Non-psychiatric comorbidity associated with Alzheimer's disease. QJM 2011; 104:913–920. [DOI] [PubMed] [Google Scholar]
- 48.de Lacalle S, Iraizoz I, Gonzalo LM. Cell loss in supraoptic and paraventricular nucleus in Alzheimer's disease. Brain Res 1993; 609:154–158. [DOI] [PubMed] [Google Scholar]
- 49.Standaert DG, Lee VM, Greenberg BD, et al. Molecular features of hypothalamic plaques in Alzheimer's disease. Am J Pathol 1991; 139:681–691. [PMC free article] [PubMed] [Google Scholar]
- 50.Saper CB, German DC. Hypothalamic pathology in Alzheimer's disease. Neurosci Lett 1987; 74:364–370. [DOI] [PubMed] [Google Scholar]
- 51.Callen DJ, Black SE, Gao F, et al. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology 2001; 57:1669–1674. [DOI] [PubMed] [Google Scholar]
- 52.Guo JL, Tsai YY, Liao JY, et al. Interventions to reduce the number of falls among older adults with/without cognitive impairment: an exploratory meta-analysis. Int J Geriatr Psychiatry 2014; 29:661–669. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein RZ, Tomasi D, Rajaram S, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience 2007; 144:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanagisawa H, Dan I, Tsuzuki D, et al. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010; 50:1702–1710. [DOI] [PubMed] [Google Scholar]
- 55.Hansel A, von Kanel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc Med 2008; 2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone 2008; 42:837–840. [DOI] [PubMed] [Google Scholar]
- 57.Malek-Ahmadi M, Small BJ, Raj A. The diagnostic value of Controlled Oral Word Association Test-FAS and category fluency in single-domain amnestic mild cognitive impairment. Dement Geriatr Cogn Disord 2011; 32:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rolland Y, Abellan van Kan G, Benetos A, et al. Frailty, osteoporosis and hip fracture: causes, consequences and therapeutic perspectives. J Nutr Health Aging 2008; 12:335–346. [DOI] [PubMed] [Google Scholar]
- 59.Albaba M, Cha SS, Takahashi PY. The Elders Risk Assessment Index, an electronic administrative database-derived frailty index, can identify risk of hip fracture in a cohort of community-dwelling adults. Mayo Clin Proc 2012; 87:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray WA, Griffin MR, Schaffner W, III, et al. Psychotropic drug use and the risk of hip fracture. N Engl J Med 1987; 316:363–369. [DOI] [PubMed] [Google Scholar]
- 61.Oderda LH, Young JR, Asche CV, et al. Psychotropic-related hip fractures: meta-analysis of first-generation and second-generation antidepressant and antipsychotic drugs. Ann Pharmacother 2012; 46:917–928. [DOI] [PubMed] [Google Scholar]


