Abstract
Preoperative chemoradiotherapy (CRT) is the standard of care for patients with stage II and III rectal cancer. This strategy leads to pathologic complete response (pCR) in a significant number of patients. Factors predictive of pCR are currently being extensively investigated. The aim of this study was to analyze clinical factors that might be predictive of pCR.
This study was a retrospective analysis of rectal cancer patients from January 2004 through December 2012. A total of 332 stage II and III patients with middle and low rectal cancer (≤10 cm) who received CRT and underwent curative total mesorectal excision were eligible. The median radiation dose was 50.4 Gy, and 72.6% of patients received infusional 5-fluorouracil with leucovorin, whereas 19.6% of patients received TS-1 with irinotecan, and 7.8% of patients received xeloda only. Pathologic complete response was confirmed by using pathologic specimens and analyzed based on predictive clinical factors.
Among the 332 patients, 27.4% (n = 91) achieved pCR. Age, sex, body mass index, clinical T and N stages, tumor differentiation, the chemotherapy agent for CRT, and the time interval between CRT and surgery did not differ between the pCR and non-pCR groups. Carcinoembryogenic antigen (CEA) levels before CRT were 4.61 ± 7.38 ng/mL in the pCR group and 10.49 ± 23.83 ng/mL in the non-pCR group (P = 0.035). Post-CRT CEA levels were 1.4 ± 1.07 ng/mL in the pCR group and 2.16 ± 2.8 ng/mL in the non-pCR group (P = 0.014), and the proportion of middle rectal cancer patients was higher in pCR group (54.9%, P = 0.028). The results from multivariate logistic regression analysis indicated that higher tumor location (odds ratio 2.151; P = 0.003) and low post-CRT CEA level (odds ratio 0.789; P = 0.04) were independent predictive factors for pCR.
Tumor location and post-CRT CEA level were predictive factors in pCR for rectal cancer patients. Therefore, these factors may be important determinants in achieving pCR, and may also be used to predict oncologic outcomes.
INTRODUCTION
Preoperative chemoradiotherapy (CRT) followed by surgery has become the standard treatment for patients with stage II and III rectal cancer, resulting in excellent local tumor control and possibly improved long-term survival.1 Moreover, pathologic complete response(pCR) is often reported for specimens obtained after CRT followed by surgery. Pathologic complete response, defined as complete tumor regression with no viable cancer cells in both primary tumors and regional lymph nodes, is a remarkable finding, as it shows favorable results compared with patients without pCR.2–5 It is also a critical issue for determining the optimal management of rectal cancer after CRT, in particular whether to perform surgery or not, as pCR is defined as having no cancer.6 Several reports on breast cancer treatment showed that responsiveness to preoperative treatment is critical as it may predict clinical outcome and increase breast conservation.7,8 Similarly, pCR can predict a good outcome in rectal cancer patients; however, surgery must be performed to confirm the presence of cancer, which can provide accurate and definite staging, although one should accept the risk of complications that may occur after surgery. If the patient chooses a “wait and see” policy, which involves declining surgery and relying on follow-up by observation, one might avoid the potential risk of surgery; however, accurate staging would not be available. Judging the tumor response before surgery is not a simple issue, but a complex problem. Although the disappearance of gross tumor after CRT has been found to be highly correlated with pCR, residual disease in the mesorectal lymph nodes has been reported in up to 17% of patients.9 Therefore, the discovery of relevant clinical markers for predicting pCR is an important issue. The aim of this study was to determine any differences in clinical factors that might occur between pCR and non-pCR groups after CRT in order for physicians to better predict pCR.
METHODS
We performed a retrospective review of prospectively collected records from the database of the Department of Surgery, Yonsei University Health System, Seoul, South Korea from January 2004 to December 2012. This study included 332 patients with radiologic stage II or III rectal cancer as indicated by the National Comprehensive Cancer Network guidelines. These patients received CRT followed by total mesorectal excision (TME). Strict selection criteria were tumor location ≤10 cm from the anal verge, no concurrent benign or malignant disease, and no history of familial adenomatous polyposis or hereditary nonpolyposis colorectal cancer. Upper rectal cancer with a tumor location of more than 10 cm was excluded, as most of such patients underwent surgery without CRT in our hospital. In total, 91 of the 332 patients comprised the pCR group and the remaining 241 patients comprised the non-pCR group. Pathologic complete response was defined based on tumor regression and fibrotic changes of pathologic specimen after CRT followed by surgery, using a grading system adapted from Mandard et al.10
The initial clinical stage was based on digital rectal examination, colonoscopy, endorectal ultrasound abdominal computed tomography scan, and pelvic magnetic resonance imaging. Tumor location was defined as the distance from the caudal margin of the tumor to the anal verge, measured by digital rectal examination, rigid proctoscopy, and pelvic magnetic resonance imaging measurement. An anal verge of less than 6 cm was defined as low rectal cancer and that of 6–10 cm was defined as middle rectal cancer. Chemoradiotherapy was administered to all patients with 3 types of chemotherapy options, 5-fluorouracil with leucovorin, TS-1 (tegafur, gimeracil, and oteracil potassium capsule) with irinotecan or xeloda only. 5-Fluorouracil was administered intravenously at a dose of 425 mg/m2/d and leucovorin was also administered intravenously at a dose of 20 mg/m2/d during the first and fifth weeks of radiotherapy. Five cycles of TS-1 and irinotecan were administered during the radiotherapy. Irinotecan was given intravenously at a dose of 40 mg/m2/d every once a week, and TS-1 was administered orally at a dose of 70 mg/m2/d for 25 days during the radiation therapy period. Xeloda was continuously administered orally at a dose of 1450 mg/m2/d twice daily during the radiation therapy period. Concurrent pelvic radiation treatment with a median radiation dose of 50.4 Gy (range 25–57.5 Gy) was administered; 45 Gy was applied to the whole pelvis in 25 fractions for 5 weeks, followed by 5.4 Gy boosts on the gross tumor area for 3 days. All patients underwent TME after the initial CRT. The time interval between CRT and surgery was recorded from the end date of radiotherapy to the day before surgery. Operations included low anterior resection (n = 167), low anterior resection with coloanal anastomosis (n = 107), and abdominoperineal resection (n = 58). The study was approved by the institutional review board of Severance Hospital (4-2015-0476).
Statistical Analysis
Data are summarized as frequencies and percentages for categorical variables, and medians and ranges are used for continuous variables. The χ2 test was used to evaluate categorical variables and Student's t-test was used for continuous variables. Multivariate analysis was performed using a logistic regression model with factors shown to have an effect based on univariate analysis. All other results were considered statistically significant if P < 0.05. We calculated receiver-operating characteristic curves to validate the prediction power of the logistic regression model. Statistical Package for the Social Sciences for Windows (version 20.0, Chicago, IL) was used for statistical analyses.
RESULTS
Clinical and pathologic characteristics of the population are described in Table 1. The median age was 58 years (range 27–85) and the number of men was 228 (68.7%). Among the 322 patients, 91 (27.4%) were confirmed to have pCR on pathologic specimen analysis after surgery. In comparing the characteristics of patients in the pCR and non-pCR groups, no statistically significant differences were found in age (P = 0.407), sex (P = 0.508), and American Society of Anesthesiologists class score (P = 0.093). Body mass index, however, was slightly higher in the pCR group (P = 0.053).
TABLE 1.
Patient, Tumor, and Treatment Characteristics

Clinical factors predicting pCR by univariate analysis are presented in Table 2. Carcinoembryogenic antigen levels before CRT were 4.61 ± 7.38 ng/mL in the pCR group and 10.49 ± 23.83 ng/mL in the non-pCR group (P = 0.035). Post-CRT CEA levels were 1.4 ± 1.07 ng/mL in the pCR group and 2.16 ± 2.8 ng/mL in the non-pCR group (P = 0.014), and the proportion of middle rectal cancer patients was higher in the pCR group (54.9%, P = 0.028). Other variables (age, sex, body mass index, pretreatment clinical T stage, initial tumor differentiation, pretreatment clinical N stage, time interval between CRT and surgery, and type of chemotherapy) showed no statistical differences between the two groups. Although statistically significant, pre-CRT CEA was to be excluded from multivariable analysis, as it had been obtained before CRT and was thus representative of the initial cancer rather than pCR. Multicollinearity between pre-CRT CEA and post-CRT CEA, however, indicated a low variance inflation factor (VIF = 1.449), thus, all factors were acceptable for inclusion in the multivariable analysis Table 3 shows tumor location (P = 0.003) and post-CRT CEA level (P = 0.04) as significant factors in multivariable logistic regression analysis, after applying effective variables with P value of less than 0.05 from univariable analysis. An acceptable receiver-operating characteristic curve was achieved after logistic regression model in Figure 1. The area under curve was 0.638, which indicated that the model was acceptable for predicting pCR.
TABLE 2.
Univariate Pathologic Complete Response Analysis
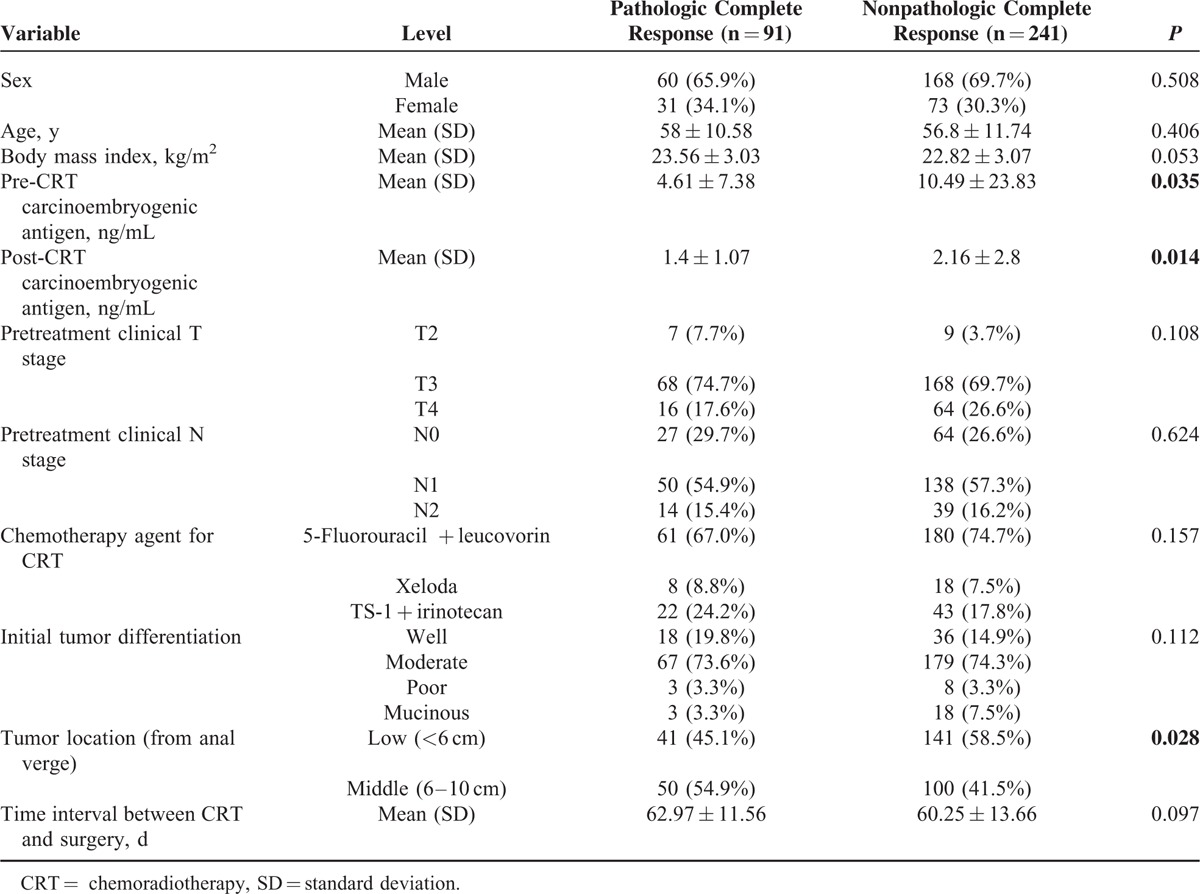
TABLE 3.
Multivariate Analysis of Variables Significant in Univariate Analysis for Pathologic Complete Response
FIGURE 1.
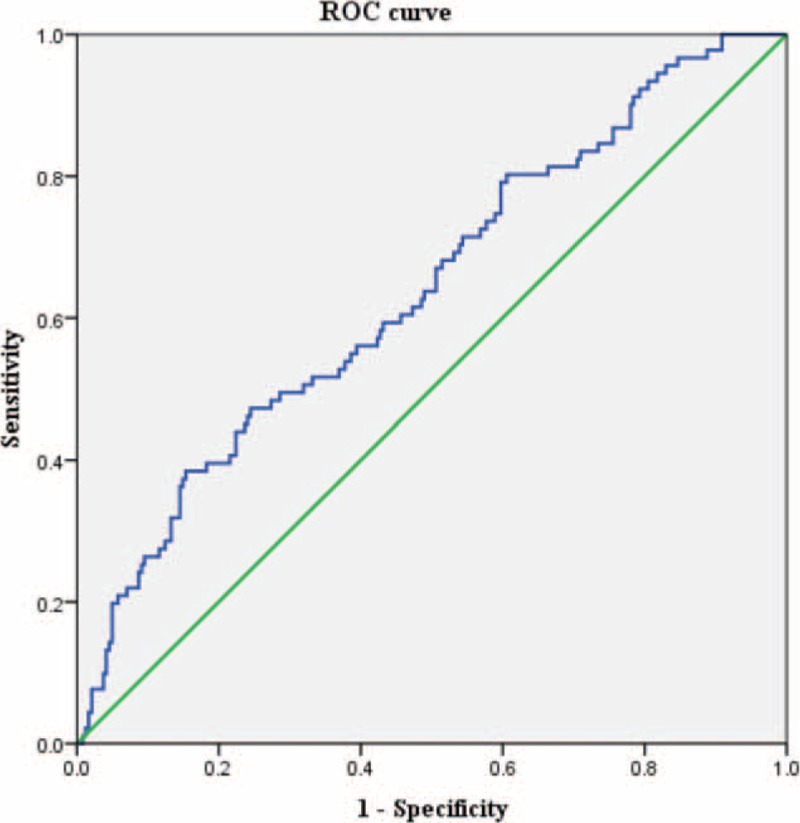
Receiver-operating characteristic curve. Area under curve = 0.638 (95% confidence interval, 0.571–0.705), P < 0.001.
DISCUSSION
Recently, there has been considerably more interest in devising a method to predict pCR before undertaking surgery, not only in the colorectal field but also in many other surgical fields. Physicians have investigated a variety of variables to find clinical factors that are predictive of pCR, such as tumor subtypes in breast cancer; biomarkers, serum CEA level, macroscopic ulceration status, and tumor circumference in rectal cancer; and smoking habits and tumor length in esophageal cancer.11–14 A definite prediction of pCR is important, as a recent study showed that tumor regression of near-pCR with ypT3 or ypN1/2 in rectal cancer is associated with poor clinical outcome.15 Moreover, performing surgery only to find a nonviable tumor creates unnecessary risk in terms of morbidity and mortality.
We found that post-CRT, CEA level and tumor location were significant factors associated with pCR. Carcinoembryogenic antigen is the most widely used serum marker for surveillance, particularly after colorectal cancer surgery, to monitor for recurrence. In addition, in rectal cancer, postoperative decrease in the CEA level has been found to be related to survival improvement.16 Thus, several studies have used this simple tool to predict pCR, and low serum CEA level has been reported as a significant factor associated with pCR.17–19 Based on the results of our study, a low post-CRT CEA level was associated with pCR. Pre-CRT CEA may therefore be more indicative of tumor biologic status than the response to CRT. Recently, Kleiman et al20 similarly reported that normalization of CEA level after CRT showed predictive value for pCR. In addition, Perez et al21 showed that CEA levels of less than 5 ng/dL were predictive for pCR as well as 5-year overall survival. There, however, is still some debate over whether pre-CRT CEA, post-CRT CEA, or even both, are predictive.22,23 We did not analyze the change between post-CRT CEA and postoperative CEA; however, the pCR group had a lower post-CRT CEA level than the non-pCR group, which may account for the marked reduction in the CEA level of the non-pCR group after CRT.
Interestingly, our results indicated a middle tumor level as a predictor for pCR in multivariable analysis. Most previous reports do not report tumor height as a significant factor associated with pCR. There is no definite borderline for tumor height that divides rectal cancer between low and high. Some consider tumors 5 to 6 cm above the anal verge to be low rectal cancer, although classification remains arbitrary.24,1 There is debate as to whether upper rectal cancer patients should undergo CRT before surgery or not, as they show lower local recurrence rates than those with middle or low rectal cancer, and certain reports also demonstrated no local recurrence differences between CRT and non-CRT groups in upper rectal cancer.1,25,26 Accordingly, a low tumor location may increase the risk of local recurrence in postradiation status.27 Kapiteijn et al1 reported a 1% 2-year local recurrence rate in a radiotherapy with surgery group with tumor locations in the range of 5 to 10 cm, and a 10.1% recurrence rate in a surgery alone group with the same tumor locations; conversely a 5.8% 2-year local recurrence rate was found in the radiotherapy with surgery group with tumor locations at less than 5 cm, and a 10.0% recurrence rate was found in the surgery alone group with the same tumor locations. In addition, Das28 et al reported that a tumor distance from the anal verge of more than 5 cm was independently predictive of tumor downstaging. From a Dutch rectal trial, middle rectal cancer showed lower local recurrence rate after short-course radiotherapy followed by TME than after TME alone.25 Although these reports do not completely explain rectal cancer pCR in terms of tumor location, they are based on the expected effects of radiotherapy, such as tumor or lymph nodes sterilization; thus, a mid-level tumor location may be the most effective radiation field for rectal cancer. Further studies, however, are needed to confirm our findings.
CONCLUSIONS
Given its retrospective nature, our study had several limitations. First, there were no genetic data or biologic markers with relevant clinical variables. There were also several reports demonstrating that epidermal growth factor receptor and vascular endothelial growth factor expression predicted decreased pCR after CRT. The addition of such clinical data may have enriched our results when predicting pCR. Second, as these results were derived from a single institution, further prospective studies are necessary to fully evaluate the predictive factors for pCR in rectal cancer.
Footnotes
Abbreviations: CEA = carcinoembryogenic antigen, CRT = chemoradiotherapy, pCR = pathologic complete response, TME = total mesorectal excision.
All the authors have no conflicts of interest or financial ties to disclose.
REFERENCES
- 1.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345:638–646. [DOI] [PubMed] [Google Scholar]
- 2.Habr-Gama A, Perez RO, Nadalin W, et al. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg 2005; 9:90–99.discussion 99–101. [DOI] [PubMed] [Google Scholar]
- 3.Yeo SG, Kim DY, Kim TH, et al. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg 2010; 252:998–1004. [DOI] [PubMed] [Google Scholar]
- 4.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010; 11:835–844. [DOI] [PubMed] [Google Scholar]
- 5.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol 2011; 18:1590–1598. [DOI] [PubMed] [Google Scholar]
- 6.Dedemadi G, Wexner SD. Complete response after neoadjuvant therapy in rectal cancer: to operate or not to operate? Dig Dis 2012; 30:109–117. [DOI] [PubMed] [Google Scholar]
- 7.Spanheimer PM, Carr JC, Thomas A, et al. The response to neoadjuvant chemotherapy predicts clinical outcome and increases breast conservation in advanced breast cancer. Am J Surg 2013; 206:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teshome M, Hunt KK. Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin N Am 2014; 23:505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes R, Glynne-Jones R, Grainger J, et al. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? Int J Colorectal Dis 2006; 21:11–17. [DOI] [PubMed] [Google Scholar]
- 10.Mandard AM, Dalibard F, Mandard JC, et al. Pathological assessment of tumor-regression after preoperative chemoradiotherapy of esophageal-carcinoma. Cancer 1994; 73:2680–2686. [DOI] [PubMed] [Google Scholar]
- 11.Hur H, Kim NK, Min BS, et al. Can a biomarker-based scoring system predict pathologic complete response after preoperative chemoradiotherapy for rectal cancer? Dis Colon Rectum 2014; 57:592–601. [DOI] [PubMed] [Google Scholar]
- 12.Gulben K, Berberoglu U, Kinas V, et al. Breast cancer subtypes can be a predictor of pathologic complete response and survival in the neoadjuvant setting for T4 noninflammatory breast cancer. Acta Chir Belg 2014; 114:153–159. [DOI] [PubMed] [Google Scholar]
- 13.Huang RW, Chao YK, Wen YW, et al. Predictors of pathological complete response to neoadjuvant chemoradiotherapy for esophageal squamous cell carcinoma. World J Surg Oncol 2014; 12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh JW, Kim HR, Kim YJ. Clinical prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum 2013; 56:698–703. [DOI] [PubMed] [Google Scholar]
- 15.Swellengrebel HAM, Bosch SL, Cats A, et al. Tumour regression grading after chemoradiotherapy for locally advanced rectal cancer: a near pathologic complete response does not translate into good clinical outcome. Radiother Oncol 2014; 112:44–51. [DOI] [PubMed] [Google Scholar]
- 16.Park YA, Lee KY, Kim NK, et al. Prognostic effect of perioperative change of serum carcinoembryonic antigen level: a useful tool for detection of systemic recurrence in rectal cancer. Ann Surg Oncol 2006; 13:645–650. [DOI] [PubMed] [Google Scholar]
- 17.Moreno Garcia V, Cejas P, Blanco Codesido M, et al. Prognostic value of carcinoembryonic antigen level in rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2009; 24:741–748. [DOI] [PubMed] [Google Scholar]
- 18.Yang KL, Yang SH, Liang WY, et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat Oncol 2013; 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallin U, Rothenberger D, Lowry A, et al. CEA: a predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum 2013; 56:859–868. [DOI] [PubMed] [Google Scholar]
- 20.Kleiman A, Al-Khamis A, Farsi A, et al. Normalization of CEA levels post-neoadjuvant therapy is a strong predictor of pathologic complete response in rectal cancer. J Gastrointest Surg 2015; 19:1106–1112. [DOI] [PubMed] [Google Scholar]
- 21.Perez RO, Sao Juliao GP, Habr-Gama A, et al. The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon Rectum 2009; 52:1137–1143. [DOI] [PubMed] [Google Scholar]
- 22.Song SS, Hong JC, McDonnell SE, et al. Combined modality therapy for rectal cancer: the relative value of posttreatment versus pretreatment CEA as a prognostic marker for disease recurrence. Ann Surg Oncol 2012; 19:2471–2476. [DOI] [PubMed] [Google Scholar]
- 23.Jang NY, Kang SB, Kim DW, et al. The role of carcinoembryonic antigen after neoadjuvant chemoradiotherapy in patients with rectal cancer. Dis Colon Rectum 2011; 54:245–252. [DOI] [PubMed] [Google Scholar]
- 24.Battersby NJ, How P, Moran B, et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II Study. Ann Surg 2015; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007; 246:693–701. [DOI] [PubMed] [Google Scholar]
- 26.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 27.Syk E, Torkzad MR, Blomqvist L, et al. Local recurrence in rectal cancer: anatomic localization and effect on radiation target. Int J Radiat Oncol Biol Phys 2008; 72:658–664. [DOI] [PubMed] [Google Scholar]
- 28.Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 2007; 109:1750–1755. [DOI] [PubMed] [Google Scholar]


