Abstract
Biliopancreatic cancer is one of the most aggressive solid neoplasms, and incidence is rising worldwide. It is known that ATF6α is one of the transmembrane proteins that acts crucially in endoplasmic reticulum stress response, and knockdown induces apoptosis of pancreatic cells. Apart from this, p-p38 has been previously correlated with better outcome in pancreatic cancer. Interestingly, ATF6α knockdown pancreatic cells showed increased p-p38. The aim of this study was to evaluate the expression of these 2 proteins, p-p38 and ATF6α, and their correlation with the outcome of biliopancreatic adenocarcinoma patients.
Samples from patients with biliopancreatic adenocarcinoma that underwent pancreaticoduodenectomy from 2007 to 2013 were used to construct a tissue microarray to evaluate p-p38 and ATF6α proteins by immunohistochemistry.
We observed that both markers showed a tendency to impact in the time to recurrence; then a combination of these 2 proteins was analyzed. Combination of ATF6αhigh and p-p38low was strongly associated with a higher risk of recurrence (hazard ratio 2.918, P = 0.013). This 2-protein model remained significant after multivariate adjustment.
We proposed a 2-protein signature based on ATF6αhigh and p-p38low as a potential biomarker of risk of recurrence in resected biliopancreatic adenocarcinoma patients.
INTRODUCTION
Biliopancreatic cancer is the fourth leading cause of cancer death in both sexes in the USA. It is estimated that 46,420 new cases of this cancer are diagnosed in 2014—23,530 in men and 22,890 in women. The estimated death number in USA in 2014 was 39,590 cases,1 and 227,000 deaths per year worldwide.2 Furthermore, statistical analysis from 2001 to 2010 indicates that death rates are rising.1
In this kind of cancer, survival can be improved when tumors are detected at an early stage. It has been reported that 5-year survival rate is of 50% when tumors are <2 cm3 and close to 100% for tumors <1 cm.4 Although these data are encouraging, biliopancreatic cancer is usually asymptomatic, and disease only becomes apparent after the tumor invades surrounding tissues or metastasizes to distant organs.5
Up to date, the unique prognostic biomarker approved by the US Food and Drug Administration (FDA) for resectable biliopancreatic cancer is the preoperative levels of carbohydrate antigen 19-9 (CA19-9).6 This marker shows a relatively high sensitivity and specificity for this cancer,7 which is superior compared with other markers, such as carcinoembryonic antigen (CEA), carbohydrate antigen 50 (CA-50), and pancreatic cancer-associated antigen 2.8,9 However, the applicability of this marker is not obvious since other clinical events such as biliary obstruction can increase CA19-9 serum levels,10 and up to 10% of the population cannot synthesize CA19-9.11 Nevertheless, CA19-9 is considered the best serum marker for biliopancreatic cancer.12
To date, surgical resection remains the best option to manage with biliopancreatic cancer in surgically amenable tumors, and survival can be predicted based on pathological characteristics like tumor size, grade of differentiation, and lymph node status.13 However, after surgery, there is a lack of validated prognostic or predictive markers to be used in the patient management.14 In this sense, there have been several reports on some prognostic molecular biomarkers, such as mothers against decapentaplegic homolog 4 (Smad4) or mucin 1 (MUC1), and also predictive markers including secreted protein acidic and rich in cysteine (SPARC), human antigen R (HuR), or members of the breast cancer type 2 (BRCA2) family.15–17
Very recently, it has been reported that high phospho-p38 (p-p38) levels are correlated with a significant overall survival advantage in biliopancreatic cancer. In fact, inhibition of p38 increased growth of pancreatic tumour cells lines in vitro, suggesting that p-p38 expression constrained cell growth through negative regulation of cell cycle at the G1/S and G2/M transitions.18,19 Interestingly, p-p38 was elevated in ATF6α knockdown pancreatic cells. Indeed, ATF6α knockdown resulted in an apoptotic phenotype given by a p38 phosphorylation.20 The same authors suggested that p38 is an important contributor to nuclear translocation and transcriptional activation of ATF6α.
ATF6α is one of the transmembrane proteins needed to induce response to endoplasmic reticulum (ER) stress caused by hypoglycemia, hypoxia, or accumulation of unfolded proteins during protein synthesis.21 Actually, ER stress is critical for pancreatic cells dysfunction and death.22,23 ATF6 has 2 isoforms—α and β, and it has been described that double ATF6α and ATF6β- knockout mice die in the embryonary period,24,25 which indicates that the presence of at least 1 isoform is essential for survival.
ATF6α is activated in Golgi apparatus,22,23 migrates to the nucleus, and stimulates transcription of survival genes to neutralize ER stress avoiding apoptosis and promoting cell survival.24,25 Apart from this, ATF6α is expressed in nonstressed pancreatic cancer cell lines and primary rodent islets, and apoptosis is induced after ATF6α knockdown.26 Independently of ER stress, ATF6α is considered an important component in the vascular endothelial growth factor (VEGF)-induced vascularization, inducing survival and angiogenesis.27 Moreover, high expression of ATF6α has been implicated in the pathogenesis of high-grade hepatocellular carcinomas28–30 and chemotherapy resistance.31,32
The goal of this study was to analyze the expression of these 2 proteins—p-p38 and ATF6α—with outcome of biliopancreatic adenocarcinoma patients.
METHODS
Patient Samples
A total of 53 patients with biliopancreatic adenocarcinoma who underwent pancreaticoduonenectomy from 2007 to 2013 at the Hepatobiliary and Pancreatic Surgery Unit, General and Digestive Tract Surgery Department, University Hospital Fundación Jiménez Díaz, were assessed for eligibility. For this study, 8 patients were excluded because samples had not enough quality to perform immunohistochemistry (n = 4), and patients presented loss of follow-up (n = 3) or duodenum origin (n = 1). Half of the tumors (51%) were originated in the pancreas and the rest were originated in the intrapancreatic bile duct (27%) or in the ampulla (22%). Gemcitabine was used alone or in combination with radiotherapy and 5-fluorouracil (5FU) as adjuvant treatment in 40% of cases. Histopathological grading of the tumors was based on the recommendations by the College of American Pathologists.33 A 2-tiered system has been used to grade tumors in 2 groups: low grade was defined as greater than or equal to 50% of gland formation in the tumor and high grade as less than 50% gland formation.
Ethics committee of clinical research of Fundacion Jimenez Diaz Hospital (CEIC-FJD) has evaluated this study and approved it on December 9, 2014, by the act number 17/14. CEIC-FJD also certified this study belongs to RNA-Reg Consolider-Ingenio CSD2009–0080. All patients gave written informed consent for the use of their biological samples for research purposes.
Tissue Microarray
Samples from 45 patients were used to construct a paraffin block containing 90 cores (2 cores per patient) to allow immunohistochemistry analysis. A hollow needle was used to obtain a tissue core of 0.6 mm in diameter from selected tumor regions in formalin-fixed paraffin-embedded (FFPE) tissues. These tissue cores were then inserted in a recipient paraffin block in a precisely spaced resembling an array pattern. Sections from this FFPE block were cut in a microtome and mounted on a microscope slide to be analyzed by immunohistochemistry.
Immunohistochemistry and Quantification
Immunohistochemical staining was conducted in 2-μm FFPE tumour sections. Slides were deparaffinized by incubation at 60°C. Biopsies were cut and incubated with PT-Link (Dako) for 20 minutes at 95°C in a high pH-buffered solution. To block endogenous peroxidase holders were incubated with peroxidase blocking reagent (Dako). Biopsies were stained for 20 minutes with a 1:750 dilution of ATF6α antibody (AP08853PU-N, Acris Antibodies) and 1:150 of p-p38 (ab38238, Abcam), followed by incubation with the appropriate anti-Ig horseradish peroxidase-conjugated polymer (EnVision, Dako) to detect antigen-antibody. Sections were then visualized with 3,3’-diaminobenzidine as a chromogen for 5 minutes and counterstained with hematoxylin. Immunoreactivity was quantified as the percentage of positively stained cells over total tumor cells. Quantification for each patient was calculated with the average of both cores by 2 independent pathologists.
Statistical Analysis
The association of ATF6α or p-p38 expression their combination with time to recurrence after resection and overall survival was assessed. Time to recurrence was defined as the interval between the dates of surgery and recurrence (local or distant). Overall survival was defined as the interval between the dates of surgery and death from any cause. Survival curves were estimated using the Kaplan–Meier method, and significant survival differences between groups were determined by the log-rank test. Univariate and multivariate Cox proportional-hazard models were used to assess the hazard ratios (HRs) and confidence intervals (CIs) of both molecular and clinical variables. In the multivariate analysis, only those variables that were statistically significant in the univariate analysis were included. P values ≤0.05 were considered significant. All statistics were performed with the IBM SPSS statistics 20.0.
RESULTS
Patient Characteristics
The clinical features of the resected biliopancreatic cancer patients are summarized in Table 1. The sex distribution in our cohort was 40% of men and 60% of women. The median age for this cohort of patients was 66 years (range 37–82 y).
TABLE 1.
Clinical Characteristics of Biliopancreatic Adenocarcinoma

Most of the tumors were low grade (76%). Metastasis appeared in lymph nodes in 64% of patients; in addition most of the patients had neural and vascular invasion (71% and 69%, respectively). Survival analysis according to tumor origin did not reveal any statistical difference between pancreas, bile duct, or ampulla localization for both time to progression after surgery or overall survival (P = 0.956 and P = 0.892, respectively, data not shown).
Combination of High ATF6α and Low p-p38 Levels is Associated with Poor Prognosis in Resected Biliopancreatic Cancer Patients
ATF6α staining had not only basically nuclear localization but also was diffusely detected in the cytoplasm of tumor cells (Fig. 1A). In the cases with high expression, ATF6α was also detected in the nucleus of some stromal cells, although most of the cases showed stronger staining in tumor cells than in stroma. The correlation of ATF6α with outcome of the patients was assessed. For this, patients were stratified into tertiles and the first tertile was established as the cut-off point. Patients with high expression of ATF6α (ATF6αhigh) showed a trend to decreased time to recurrence and overall survival (P = 0.1 and P = 0.07, respectively) (data not shown).
FIGURE 1.
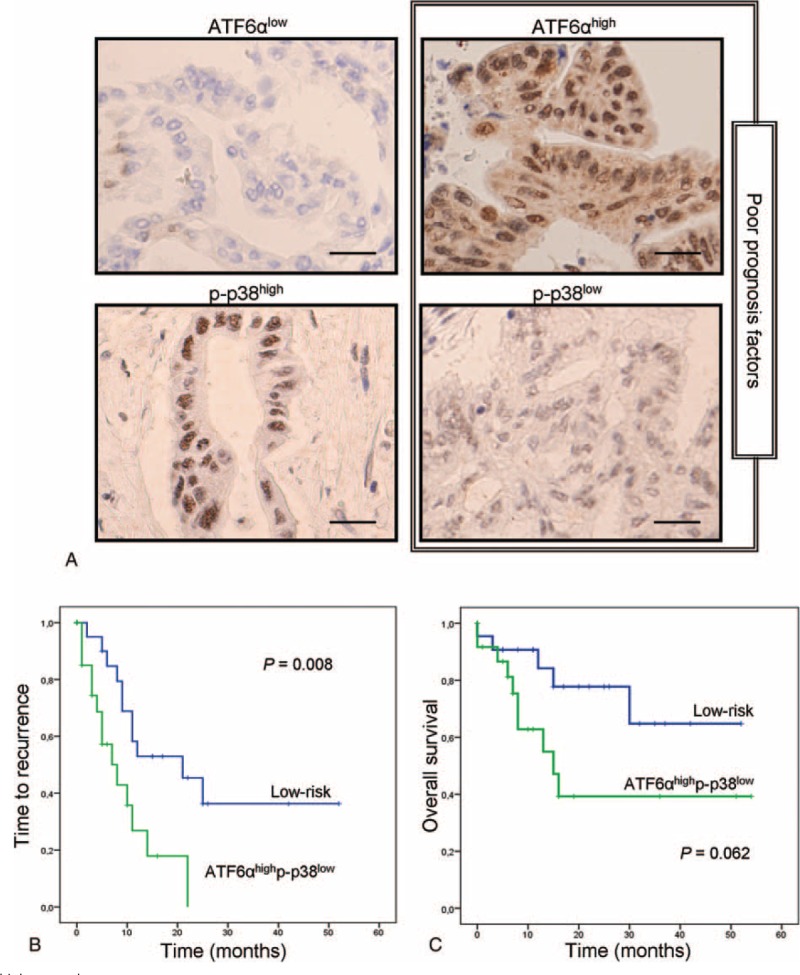
ATF6αhighp-p38low signature predicts shorter time to recurrence after surgery. A, Four representative immunostaining of ATF6α and p-p38 showing differential expression pattern. B, Kaplan–Meier analysis for time to recurrence after surgery of patients showing ATF6αhighp-p38low expression (green line) versus low-risk patients (blue line). C, Kaplan–Meier analysis for overall survival of patients with ATF6αhighp-p38low (green line) versus low-risk patients (blue line).
Expression of p-p38 was seen preferentially in tumor cells with a clear nuclear localization, and also it was detected in isolated fibroblasts (Fig. 1A). In this case, patients were stratified in low or high-expression groups using the median as cut-off point. Patients with low p-p38 (p-p38low) levels showed a trend to reduced time to recurrence (P = 0.09) (data not shown).
As both markers showed a tendency to impact in the time to recurrence, a combination of these 2 proteins was analyzed. For this purpose, the patients were grouped as high-risk (ATF6αhighp-p38low) and low-risk (remaining combinations). The combination of ATF6αhigh and p-p38low was strongly associated with a higher risk of recurrence (HR 2.918, 95% CI 1.259–6.761, P = 0.013). Survival curve showed statistically significant differences for time to recurrence between these 2 groups of patients (P = 0.008; Fig. 1B). The median time to recurrence for the patients expressing ATF6αhighp-p38low was 8 months (range 3–13) compared with 21 months (range 6–36) for the low-risk group.
In the univariate analysis of clinical variables, only tumor grade (HR 3.256, 95% CI 1.283–8.266, P = 0.013) remained significantly associated with time to recurrence in conjunction with ATF6αhighp-p38low signature (HR 2.918, 95% CI 1.259–6.761, P = 0.013) (Table 2). Therefore, tumor grade was the only covariate used for adjustment. After multivariate Cox regression analysis, the combination of ATF6αhigh and p-p38low (HR 2.705, 95% CI 1.148–6.376, P = 0.023) and the tumor grade (HR 2.886, 95% CI 1.113–7.484, P = 0.029) remained significant (Table 2).
TABLE 2.
Univariate and Multivariate Analysis for Time to Recurrence
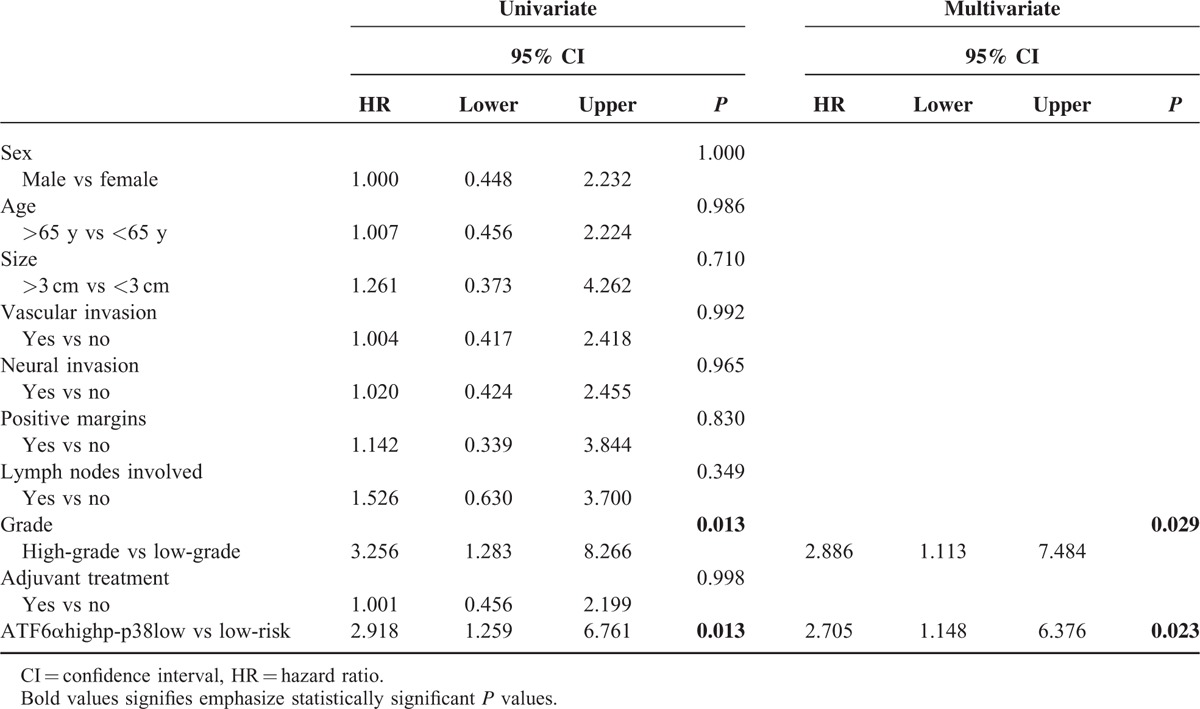
Furthermore, we performed Kaplan–Meier analysis for overall survival. The correlation of patients expressing ATF6αhigh and p-p38low with lower overall survival was close to be significant (P = 0.062, Fig. 1C). Median overall survival of patients expressing ATF6αhigh and p-p38low was 15 months (range 6–23), although low-risk patients did not reach the median value. Although ATF6αhigh and p-p38low did not achieve significance in overall survival analysis, this result supports the role of this protein signature as a high-risk factor for these patients.
These results suggested that the combined analysis of ATF6α and p-p38 could be used as a potential biomarker for high risk of recurrence in resected biliopancreatic adenocarcinoma patients.
DISCUSSION
Up to date, biliopancreatic cancer is one of the most aggressive solid neoplasms, and incidence is rising worldwide. Prognosis of this cancer is still determined by the histopathological grading,34,35 and surgical resection is the best option so far to improve survival.36 Therefore, adjuvant therapy, usually based on gemcitabine, has a scarce benefit in survival, being mostly used as a palliative intent.26,37 Indeed, adjuvant therapy is administered after surgical resection without any clinical consensus and depends on oncologist know-how advised by a multidisciplinary team of experts. The current US FDA-approved marker for biliopancreatic carcinoma, CA19-9, is not recommended for its use in disease recurrence nor for response to therapy prediction.27 Thus, the decision whether or not to treat these patients and the type of adjuvant chemotherapy is always compromising for an oncologist. For this reason, medical community demands biomarkers to improve and personalize the treatment.28
ATF6α is a factor of ER stress response considered a biomarker involved in multiple pathways, such as survival and angiogenesis,27 and it has been related to high-grade transformation29,32 and chemotherapy resistance.31,32 Furthermore, ATF6α knockdown induced apoptosis of pancreatic cells after p38 phosphorylation,20 so ATF6α seems to be crucial for pancreatic cell survival maintenance. Independently, it has been described that overexpression of p-p38 in biliopancreatic cancer patients significantly improved median overall survival compared with those with low expression.18 Moreover, in patients who had completed adjuvant therapy, median overall survival was significantly higher for patients overexpressing p-p38.18
In this study, survival analysis was performed to analyze the correlation of ATF6α and p-p38 expression with outcome of the patients. Expression of both markers showed a trend to be related to the time to recurrence. We consider that significance was not reached due to the limited sample size. For this reason, we combined both markers to improve the predictive ability of our model. The 2-marker prognostic signature, based on ATF6αhigh and p-p38low levels, showed highly significant association with time to recurrence (P = 0.008) and a high trend with overall survival (P = 0.062). Cox hazard model showed not only ATF6αhigh and p-p38low signature (HR 2.705, P = 0.023) associated with poor outcome but also tumor grade (HR 2.886, P = 0.029).
To the best of our knowledge, adjuvant chemotherapy is an important factor to indicate early recurrence. Statistical analysis revealed no significant differences between treated and untreated arms. This result might be expected since there is no proof of any advantage of adjuvant or additive chemoradiation.34,38 Concerning positive margins and lymph node involvement, they did not achieve significance in our study and they should normally be significantly associated to poor outcome; however, this is not always the case. Neither positive margins nor lymph node involvement remained significant for time to progression and overall survival analysis in a phase II clinical trial which enrolled 48 resectable biliopancreatic cancer patients.35 Moreover, Andren-Sandberg39 classed lymph node positivity as a controversial variable for predicting survival.
These results suggest the combination of ATF6αhigh and p-p38low as a novel marker associated with poor outcome in resected biliopancreatic cancer patients. We propose a model in which the high level of ATF6α and low levels of p-p38 could influence directly over cell survival (Fig. 2). In normal conditions, ATF6α is linked to glucose-regulating protein 78 (GRP78), but in ER stress, which is critical for tumor cells, both proteins dissociate. GRP78 prevents tumor cells from apoptosis, and ATF6α translocates to Golgi where it is processed and migrates to the nucleus where it stimulates transcription of survival genes.25 On the contrary, p38 is activated by phosphorylation through mitogen-activated protein kinase (MAPK) signaling pathway, then p-p38 translocates to the nucleus. Whereas high levels of p-p38 induce cell apoptosis, low levels of p-p38 promote cell survival.40 The joint effect of both proteins on cell survival would impact the outcome of biliopancreatic cancer patients.
FIGURE 2.
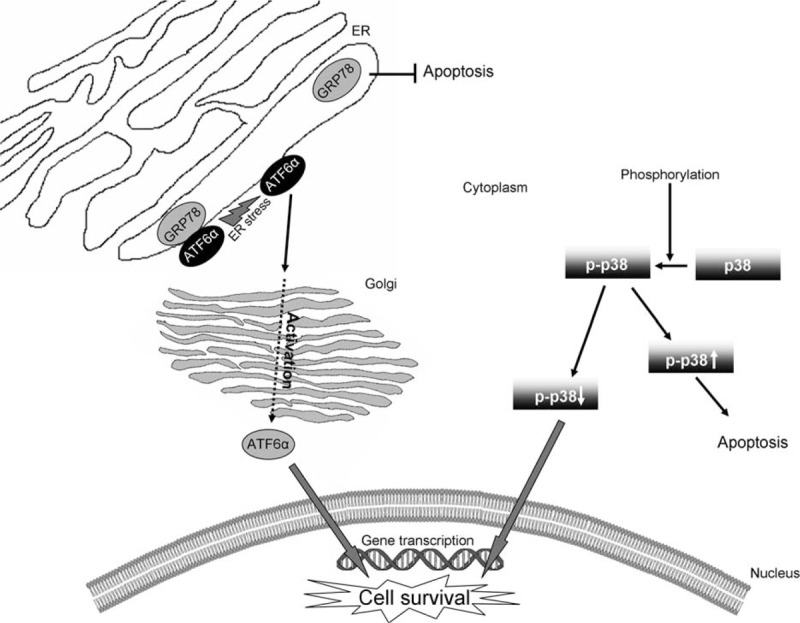
Proposed model by which ATF6α and p-p38 could modulate pancreatic cancer survival. ER = endoplasmic reticulum.
Acknowledgments
We thank Alberto Puime-Otin from Department of Pathology from University Hospital Fundacion Jimenez Diaz (Madrid, Spain) for his appreciated technical support and Oliver Shaw and Ana Martin for revising the manuscript. We also thank the BioBank of the Fundacion Jimenez Diaz-Universidad Autonoma de Madrid (RD09/0076/00101 - Spain) for supply clinical samples.
Footnotes
Abbreviations: 5FU = 5-fluorouracil, ATF6α = activating transcription factor 6 isoform α, BRCA2 = breast cancer type 2, CA19-9 = carbohydrate antigen 19-9, CA-50 = carbohydrate antigen 50, CEA = carcinoembryonic antigen, CI = confidence interval, DUPAN-2 = pancreatic cancer-associated antigen 2, ER = endoplasmic reticulum, FFPE = formalin-fixed paraffin-embedded tissues, GRP78 = glucose regulating protein 78, HR = hazard ratio, HuR = human antigen R, MAPK = mitogen-activated protein kinase, MUC1 = mucin 1, p-p38 = phosphorylated-p38 (cytokinin specific binding protein), Smad4 = mothers against decapentaplegic homolog 4, SPARC = secreted protein acidic and rich in cysteine, VEGF = Vascular endothelial growth factor.
This work has been carried out with the support of the RNA-Reg CONSOLIDER-Consortium (CSD2009-00080).
The authors declare no conflict of interest.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009; 6:699–708. [DOI] [PubMed] [Google Scholar]
- 3.Egawa S, Takeda K, Fukuyama S, et al. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004; 28:235–240. [DOI] [PubMed] [Google Scholar]
- 4.Ariyama J, Suyama M, Satoh K, et al. Imaging of small pancreatic ductal adenocarcinoma. Pancreas 1998; 16:396–401. [DOI] [PubMed] [Google Scholar]
- 5.Kelsen DP, Portenoy R, Thaler H, et al. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery 1997; 122:53–59. [DOI] [PubMed] [Google Scholar]
- 6.Maithel SK, Maloney S, Winston C, et al. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol 2008; 15:3512–3520. [DOI] [PubMed] [Google Scholar]
- 7.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol 2007; 33:266–270. [DOI] [PubMed] [Google Scholar]
- 8.Bosman FT, Carneiro F, Hruban RH, Theise ND. IARC Press, World Health Organization Classification of Tumours of the Digestive. System. Lyon:2010. [Google Scholar]
- 9.Klöppel G, Hruban RH, Longnecker DS, Adler G, Kern SE, Partanen TJ. Ductal adenocarcinoma of the pancreas. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon:IARC Press; 2000. [Google Scholar]
- 10.Kim JE, Lee KT, Lee JK, et al. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol 2004; 19:182–186. [DOI] [PubMed] [Google Scholar]
- 11.Kawai S, Suzuki K, Nishio K, et al. Smoking and serum CA19-9 levels according to Lewis and secretor genotypes. Int J Cancer 2008; 123:2880–2884. [DOI] [PubMed] [Google Scholar]
- 12.Duffy MJ, Sturgeon C, Lamerz R, et al. Tumour markers in pancreatic cancer: a European Group on Tumour Markers (EGTM) status report. Ann Oncol 2010; 21:441–447. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362:1605–1617. [DOI] [PubMed] [Google Scholar]
- 14.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010; 7:163–172. [DOI] [PubMed] [Google Scholar]
- 15.Fong ZV, Winter JM. Biomarkers in pancreatic cancer: diagnostic, prognostic, and predictive. Cancer J 2012; 18:530–538. [DOI] [PubMed] [Google Scholar]
- 16.Reddy DN, Sriram PV, Das G, et al. Endoscopic treatment of pancreatic disorders. Trop Gastroenterol 2001; 22:149–154. [PubMed] [Google Scholar]
- 17.Wang Q, Ding Q, Dong Z, et al. Downregulation of mitogen-activated protein kinases in human colon cancers. Anticancer Res 2000; 20 (1A):75–83. [PubMed] [Google Scholar]
- 18.Zhong Y, Naito Y, Cope L, et al. Functional p38 MAPK identified by biomarker profiling of pancreatic cancer restrains growth through JNK inhibition and correlates with improved survival. Clin Cancer Res 2014; 20:6200–6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrosino C, Nebreda AR. Cell cycle regulation by p38 MAP kinases. Biol Cell 2001; 93:47–51. [DOI] [PubMed] [Google Scholar]
- 20.Teodoro T, Odisho T, Sidorova E, et al. Pancreatic beta-cells depend on basal expression of active ATF6alpha-p50 for cell survival even under nonstress conditions. Am J Physiol Cell Physiol 2012; 302: C992-1003. [DOI] [PubMed] [Google Scholar]
- 21.Sommer T, Jarosch E. BiP binding keeps ATF6 at bay. Dev Cell 2002; 3:1–2. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem 2002; 277:13045–13052. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Chen X, Hendershot L, et al. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 2002; 3:99–111. [DOI] [PubMed] [Google Scholar]
- 24.Parmar VM, Schroder M. Sensing endoplasmic reticulum stress. Adv Exp Med Biol 2012; 738:153–168. [DOI] [PubMed] [Google Scholar]
- 25.Adachi Y, Yamamoto K, Okada T, et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct 2008; 33:75–89. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004; 363:1049–1057. [DOI] [PubMed] [Google Scholar]
- 27.Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013; 107:15–22. [DOI] [PubMed] [Google Scholar]
- 28.Crane CH, Iacobuzio-Donahue CA. Keys to personalized care in pancreatic oncology. J Clin Oncol 2012; 30:4049–4050. [DOI] [PubMed] [Google Scholar]
- 29.Shuda M, Kondoh N, Imazeki N, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol 2003; 38:605–614. [DOI] [PubMed] [Google Scholar]
- 30.Arai M, Kondoh N, Imazeki N, et al. Transformation-associated gene regulation by ATF6alpha during hepatocarcinogenesis. FEBS Lett 2006; 580:184–190. [DOI] [PubMed] [Google Scholar]
- 31.Higa A, Taouji S, Lhomond S, et al. Endoplasmic reticulum stress-activated transcription factor ATF6alpha requires the disulfide isomerase PDIA5 to modulate chemoresistance. Mol Cell Biol 2014; 34:1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol 2011; 54:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adsay NV, Basturk O, Bonnett M, et al. A proposal for a new and more practical grading scheme for pancreatic ductal adenocarcinoma. Am J Surg Pathol 2005; 29:724–733. [DOI] [PubMed] [Google Scholar]
- 34.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 35.Herman JM, Fan KY, Wild AT, et al. Phase 2 study of erlotinib combined with adjuvant chemoradiation and chemotherapy in patients with resectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2013; 86:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004; 91:586–594. [DOI] [PubMed] [Google Scholar]
- 37.Abbruzzese JL. New applications of gemcitabine and future directions in the management of pancreatic cancer. Cancer 2002; 95 (4 Suppl):941–945. [DOI] [PubMed] [Google Scholar]
- 38.Seufferlein T, Bachet JB, Van Cutsem E, et al. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 2012:vii33–40. [DOI] [PubMed] [Google Scholar]
- 39.Andren-Sandberg A. Prognostic factors in pancreatic cancer. N Am J Med Sci 2012; 4:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulavin DV, Fornace AJ., Jr p38 MAP kinase's emerging role as a tumour suppressor. Adv Cancer Res 2004; 92:95–118. [DOI] [PubMed] [Google Scholar]


