Abstract
Tyrosine kinase inhibitors (TKIs) such as vandetanib have shown clinical effectiveness in advanced medullary thyroid cancer (MTC). During TKI treatment, fluctuations in the tumor markers carcinoembryonic antigen (CEA) and calcitonin (CTN) are frequently observed. Their role for treatment monitoring and the decision-making process has not been fully elucidated yet.
Twenty-one patients (male, 16, female, 5; mean age, 49 ± 13 years) with progressive MTC receiving vandetanib (300 mg orally per day) were considered. Tumor restaging was performed every 3 months including contrast-enhanced computed tomography (CT). Response was assessed according to recent criteria (Response Evaluation Criteria in Solid Tumors, RECIST 1.1). Additionally, CEA and CTN were measured at the day of CT imaging and alterations observed in tumor markers were compared to respective imaging findings (partial response, PR; stable disease, SD; progressive disease, PD).
During long-term follow-up (510 ± 350 days [range, 97–1140 days]), CTN and CEA levels initially dropped in 71.4% and 61.9% of the patients followed by fluctuations in serum marker levels. A rise in CTN ≥39.5% between 2 subsequent measurements (defined by ROC analysis) had a sensitivity of 70.6% and a specificity of 83.2% in predicting PD with an accuracy of 82.0% (area under the curve (AUC), 0.76). Oscillations in CEA levels were not predictive for PD.
Whereas tumor marker fluctuations in MTC patients undergoing TKI treatment are a frequent phenomenon, a significant rise in CTN ≥40% turns out to as an early indicator of tumor progression.
INTRODUCTION
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor of the parafollicular cells of the thyroid gland that secretes both calcitonin (CTN) and carcinoembryonic antigen (CEA). It accounts for approximately 5% of thyroid carcinomas.1,2 Due to its origination, MTC is not iodine-responsive and surgery remains the only curative option in early stages.3 Patients with unresectable local disease and/or distant metastases are candidates for systemic treatment.
Recently, the tyrosine kinase inhibitors (TKIs) vandetanib and cabozantinib have been approved for use in MTC.4 Although almost all patients respond initially with significant decreases in serum tumor marker levels during the first weeks after treatment initiation,5 tumor escape to alternate pathways frequently occurs.6 Therefore, early detection of progressive disease (PD) is crucial, facilitating treatment with alternative TKIs in case of apparent resistance to treatment. Serum tumor marker assessment, including measurement of both CTN and CEA, is a simple and well-established means of disease monitoring; however, the role of their kinetics in the long-term course of TKI treatment has not been fully elucidated yet. Fluctuations in both CTN and CEA without clinical relevance have recently been described.5 Given the rising importance and more widespread clinical use of TKI in MTC patients outside the setting of controlled clinical trials, detection of the appropriate time point to modify the treatment in individual patients due to changes in serum tumor markers will be of growing importance in a clinical setting.
Therefore, we assessed the value of both CEA and CTN for prediction of tumor progression in MTC patients treated with vandetanib.
METHODS
Between April 2007 and April 2013, 21 patients (16 male, 5 female; mean age, 49 ± 13 years) received vandetanib (300 mg orally per day) due to advanced MTC on a compassionate use basis at the University Hospital of Würzburg, Germany. All patients underwent a number of previous treatments including surgery (n = 20/21; 95.3%), chemotherapy (n = 3/21; 14.3%), and radiation therapy (n = 3/21; 14.3%). All patients gave written informed consent to the therapeutic and diagnostic procedures. As this study is a retrospective analysis of single-center data, the local ethic committee has waived the need for further approval. Detailed patient information including clinical factors is given in Table 1.
TABLE 1.
Detailed Patients’ Characteristics

Tumor Response Assessment
Onset of tumor progression (PD) was defined according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 based on computed tomography (CT) performed every 3 months (9 ± 6 examinations per patient).7 RECIST measurements were confirmed by both an attending nuclear medicine physician and radiologist. All scans were performed using a 64-slice spiral CT (SOMATOM Sensation 64, Siemens Medical Solutions, Erlangen, Germany) with intravenous contrast enhancement (care dose modulation with a quality reference of 210 mAs, 120 kV, a 512 × 512 matrix, 5 mm slice thickness) or without (40 mAs, 120 kV, a 512 × 512 matrix, 5 mm slice thickness), covering the base of the skull to the proximal thighs.
Tumor Markers
CEA (μg/L) and CTN (pg/ml) were measured at baseline and at each restaging time point using dedicated radioimmunoassays (electro-chemiluminescence immunoassay, DPC-Biermann-Siemens, Bad Nauheim, Germany [CEA] and immunoluminometric assay, DPC-Biermann-Siemens, Bad Nauheim Germany [CTN]). Intra- and interassay comparisons were performed regularly. The upper reference-limit for CTN is 18.2 pg/ml and for CEA 5.0 μg/l (nonsmokers) and 6.5 μg/l (smokers), respectively.
Analysis and Statistics
A potential relationship between imaging findings and serum tumor marker levels was investigated. Statistical analyses were performed using PASW Statistics software (version 22.0; SPSS, Inc., Chicago, IL). Quantitative values were expressed as mean ± standard deviation and range as appropriate. The 2-tailed paired Student t test was used to compare differences between 2 dependent groups, and the 2-tailed independent Student t test for differences between independent groups. A P-value of less than 0.05 was assumed to be statistically significant. Cut-off values for the prediction of imaging-based PD were determined for CTN by receiver operating characteristic (ROC) analysis.8 Additionally, in order to test a correlation between tumor markers and clinical factors, Pearson correlation was performed. A P-value of 0.05 or less was considered to be statistically significant. Other observations described are of descriptive nature.
RESULTS
Patients
At baseline CT, performed before initiation of therapy with vandetanib, all patients presented with progressive metastatic/locally advanced disease. Metastatic sites included lymph nodes (n = 16/21; 76.2%), lung (n = 13/21; 61.9%), liver (n = 10/21; 47.6%), and bone (n = 5/21, 23.8%). Mean follow-up of subjects was 510 ± 350 days (range, 97–1140 days). Information on RET mutation status was available in 12/21 patients (57.1%). A germline RET mutation was present in 2/12 subjects, whereas the remaining 10/12 patients tested were negative for both germline and sporadic mutations.
Tumor Marker Kinetics
Before treatment, all but 1 patient (Patient #19) had elevated CTN and CEA levels with median baseline values of 13,657 pg/ml (range, 2.5–135,100) and 458 μg/L (range, 2.8–1014.8), respectively. An initial decrease of CTN (at 3 months) was recorded in 15/21 (71.4%) of the subjects, whereas 6/21 (28.6%) patients did not show biochemical response to therapy. In the course of treatment, CTN levels dropped to a nadir in 14/21 (66.6%) with a median drop of 45.7% (range, 11–98%). The nadir was reached after 268 ± 208 days (range, 5–777 days). Thereafter, fluctuations in CTN could be observed in all 14 subjects, temporarily exceeding baseline in 9/14 cases (64.3%). Of the remaining 7 patients, 5 presented with continuously declining, 2 with continuously rising tumor marker levels during follow-up.
At 3 months, CEA levels decreased in 13/21 subjects (61.9%). In the course of vandetanib treatment, CEA levels dropped to a nadir in 7/21 (33.3%) subjects (median drop of 18.9% [range, 6–56%]). The nadir was reached after 368 ± 273 days (range, 5–776 days). Thereafter, alterations were recorded in all of these 7 patients with CEA spiking above baseline in 1/7 (14.1%). Of the remaining 14 patients, 4 demonstrated continuously increasing, 10 with continuously decreasing tumor marker levels.
Tumor Response
During a follow-up period of 17 ± 11.7 months, 13/21 (61.9%) patients could be classified as having stable disease (SD) and 2/21 (9.5%) achieved a partial response (PR) according to RECIST. On average 517 ± 461 days (range, 97–1449 days) after pretherapeutic baseline CT, 6/21 (28.6%) patients (28.5%) developed PD. None of the patients died from their cancer within follow-up. Of note, in contrast to the patients with controlled disease, CTN demonstrated a more continuous pattern in tumor marker increases in the subjects with PD. This pattern was not as distinct for CEA. The respective time curves of CTN and CEA levels for both patient groups (progressive vs. controlled) are given in Figure 1.
FIGURE 1.
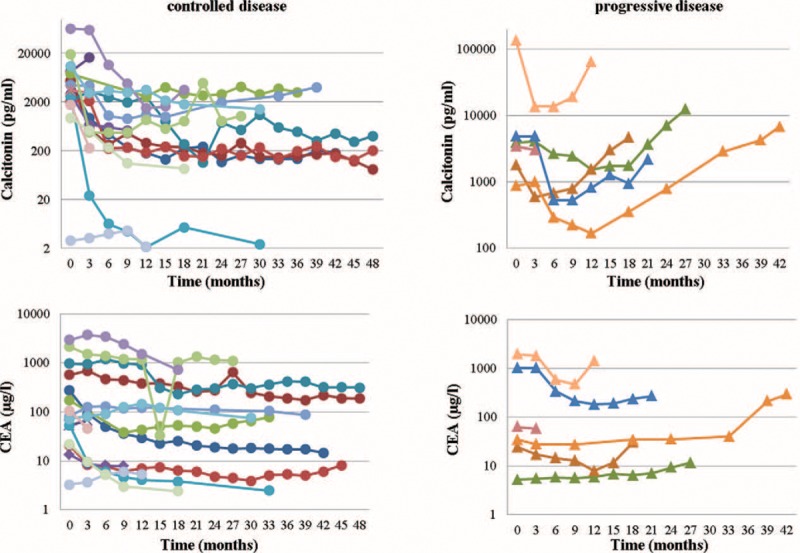
Time curves (of every patient) for calcitonin (CTN) and carcinoembryonic antigen (CEA) for patients with controlled (stable disease or partial response; left) or progressive disease (right). Almost all patients display an initial decline in serum tumor markers after initiation of treatment with vandetanib, whereas the patients with long-term disease control just present with transient fluctuations in CTN and CEA levels (left column), the subjects with true progressive disease experience a continuous rise in tumor markers (right column). This distinction is more prominent for CTN than for CEA.
Correlation of Tumor Markers With Age, Sex, and Imaging Findings
Changes in CEA moderately correlated with changes in CTN (r = 0.405, P < 0.001), independent from age and sex.
ROC Analysis
Whereas changes in CEA levels were not predictive for PD, a rise in CTN with a cut-off value of ≥14.5% predicted morphologic progression with a sensitivity of 82.4% and a specificity of 70.2%, resulting in an accuracy of 71.3% (area under the curve (AUC)) 0.76; positive predictive value (PPV), 22.6%; negative predictive value (NPV), 97.3%. A cut-off value ≥39.5% yielded a sensitivity of 70.6% and a specificity of 83.2% with an accuracy of 82.0% (PPV, 30.8%, NPV, 96.4%; Table 2; Fig. 2).
TABLE 2.
Overview of Results of ROC Analysis for CTN

FIGURE 2.
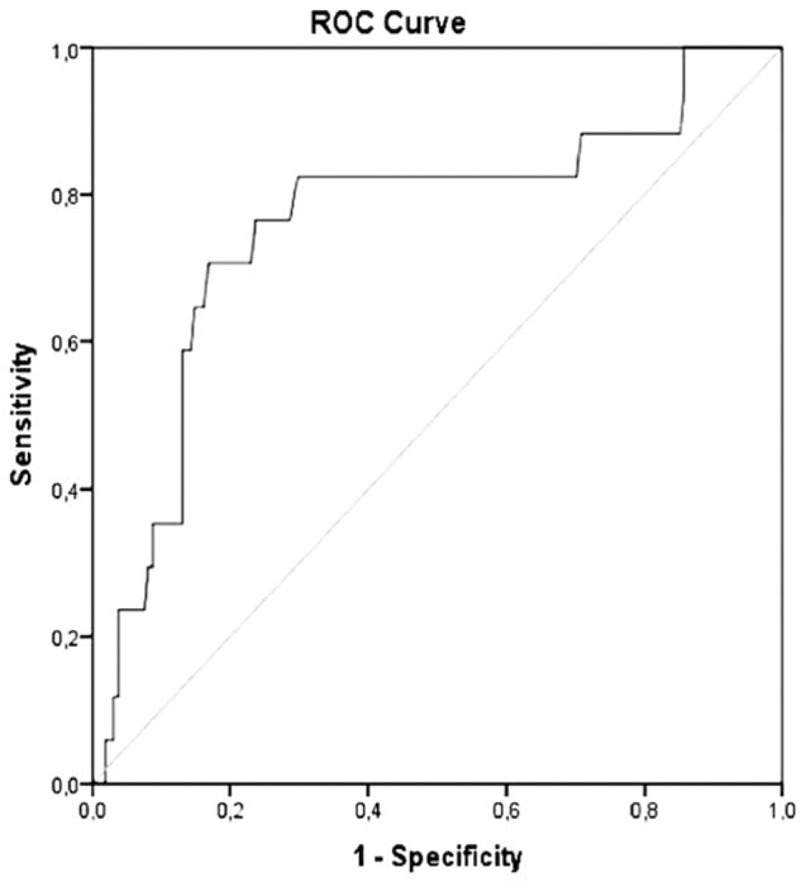
Receiver operating characteristic (ROC) curves of calcitonin for prediction of progressive disease. Area under the curve = 0.76.
DISCUSSION
The clinical utility of both tumor markers CTN and CEA in the management of MTC is well accepted.9 RET inhibition therapy using TKIs has been reported to be associated with an initial rapid decline in tumor marker levels and a subsequent phase of oscillation without necessarily indicating relapse, resistance to treatment or PD.5 Controlling a pathway responsible for CTN gene transcription and protein secretion, RET inhibitors may influence serum tumor marker levels without any reference to tumor burden or growth inhibition. However, true tumor escape cannot always be excluded. In our cohort enrolling patients with advanced disease not adequately responding to 1st or 2nd line therapy, PD developed more than 1 year after initiation of TKI therapy. Given the increasing use of this class of drugs, the rising number of long-term treatments and the growing numbers of RET- or other kinase-directed medications, it is important to discern the optimal time point for further work-up and, potentially, a change in treatment management.
In our study comprising 21 MTC vandetanib treated MTC patients, a rise in CTN ≥40% predicted PD (as detected by CT) with an accuracy of 82%. This cut-off value might serve treating physicians outside the scenario of controlled studies as an indicator when to order further diagnostic work-up or when to consider a change in patient management. Short-term fluctuations lower than this cut-off could be observed before taking further diagnostic or therapeutic steps.
In contrast to CTN, CEA did not prove to have any value for prediction of morphologic progression. The heterogeneity of CEA secretion in MTC has already been reported.10,11 Given the variety of conditions that are associated with an elevated CEA, this tumor marker appears inferior to CTN.
The present study underlines the fact that changes in tumor markers not always reflect changes in tumor size. In analogy to previously published studies, we could confirm a solid initial decrease of both tumor markers upon treatment initiation with a TKI with subsequent fluctuations in the majority of patients.5,12,13 Compared to other trials investigating different TKIs, a homogenous patient cohort exclusively under vandetanib followed-up up to almost 7 years was enrolled in this study.5 The fluctuations in both tumor marker levels compared to imaging-based findings clearly indicate that short-term variations may not reflect treatment response in MTC patients undergoing vandetanib treatment.
This study has some limitations. First, only a small number of patients could be included, thereby limiting statistical power of analyses. Especially type II error concerning the value of CEA cannot be excluded. Future studies in larger cohorts are needed to fully elucidate the significance of CEA oscillations for patient management. Second, this is a retrospective analysis and a prospective approach is necessary to strengthen our preliminary findings. Third, in almost 50% of subjects the RET mutation status was not known and only 2 patients with RET mutations could be enrolled.
In summary, in MTC patients undergoing treatment with vandetanib, an increase of 40% in serum CTN levels predicts objective PD with good accuracy and should trigger further diagnostic work-up. Whereas all patients on TKI present with oscillating tumor markers after an initial nadir, changes in CTN < 40% can be monitored before taking further actions, that is, discontinuing medication. CEA levels do not seem to be of prognostic value for TKI monitoring. Larger prospective studies are needed to fully elucidate the association between tumor marker levels and tumor biology.
CONCLUSIONS
Tumor marker fluctuations in MTC patients undergoing vandetanib treatment are a frequently observed phenomenon and should not prompt drug discontinuation. A rise in CTN ≥40% indicates tumor progression suggesting further diagnostic work-up. The value of CEA in the setting of TKI response monitoring seems to be rather limited.
Acknowledgments
We thank all members of the laboratory of the nuclear medicine department Würzburg for their assistance. Additionally, we express our gratitude to Johanna Vogt for her assistance in data analysis.
Footnotes
Abbreviations: AUC = area under the curve, CEA = carcinoembryonic antigen, CT = computed tomography, CTN = calcitonin, MTC = medullary thyroid carcinoma, NPV = negative predictive value, PD = progressive disease, PPV = positive predictive value, RECIST = Response Evaluation Criteria in Solid Tumors, ROC = receiver operating characteristic, TKIs = tyrosine kinase inhibitors.
Authors’ contributions: AKB, KH, CL, and RAW contributed to conception and design. CL, RAW, DOM, IG, and JSS acquired, analyzed, and interpreted the data. CL, RAW, KL, and DOM drafted the manuscript. HH, TH, KH, CR, and AKB revised the manuscript critically. All authors read and approved the final manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Schlumberger M, Carlomagno F, Baudin E, et al. New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab 2008; 4:22–32. [DOI] [PubMed] [Google Scholar]
- 2.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008; 93:682–687. [DOI] [PubMed] [Google Scholar]
- 3.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 2008; 93:3943–3949. [DOI] [PubMed] [Google Scholar]
- 4.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012; 30:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurzrock R, Atkins J, Wheler J, et al. Tumor marker and measurement fluctuations may not reflect treatment efficacy in patients with medullary thyroid carcinoma on long-term RET inhibitor therapy. Ann Oncol 2013; 24:2256–2261. [DOI] [PubMed] [Google Scholar]
- 6.Niedzwiecki S, Stepien T, Kopec K, et al. Angiopoietin 1 (Ang-1), angiopoietin 2 (Ang-2) and Tie-2 (a receptor tyrosine kinase) concentrations in peripheral blood of patients with thyroid cancers. Cytokine 2006; 36:291–295. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228–247. [DOI] [PubMed] [Google Scholar]
- 8.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation 2007; 115:654–657. [DOI] [PubMed] [Google Scholar]
- 9.Cohen R, Campos JM, Salaun C, et al. Preoperative calcitonin levels are predictive of tumor size and postoperative calcitonin normalization in medullary thyroid carcinoma. Groupe d’Etudes des Tumeurs a Calcitonine (GETC). J Clin Endocrinol Metab 2000; 85:919–922. [DOI] [PubMed] [Google Scholar]
- 10.Akeno-Stuart N, Croyle M, Knauf JA, et al. The RET kinase inhibitor NVP-AST487 blocks growth and calcitonin gene expression through distinct mechanisms in medullary thyroid cancer cells. Cancer Res 2007; 67:6956–6964. [DOI] [PubMed] [Google Scholar]
- 11.Busnardo B, Girelli ME, Simioni N, et al. Nonparallel patterns of calcitonin and carcinoembryonic antigen levels in the follow-up of medullary thyroid carcinoma. Cancer 1984; 53:278–285. [DOI] [PubMed] [Google Scholar]
- 12.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011; 29:2660–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong DS, Sebti SM, Newman RA, et al. Phase I trial of a combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in advanced malignancies. Clin Cancer Res 2009; 15:7061–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]


