Abstract
Diverticular disease and acute coronary syndrome (ACS) are common disorders that share several risk factors. Few researchers have evaluated the association between diverticular disease and ACS. We aimed to assess the risk of ACS in patients with diverticular disease.
A nationwide retrospective cohort study was conducted by analyzing data from the National Health Insurance Research Database in Taiwan. All patients aged ≥20 years with a diagnosis of diverticular disease from January 1, 2000, to December 31, 2011, were included in this study. For comparison, patients without diverticular disease were randomly selected and matched with the study cohort at a 4:1 ratio according to age, sex, and the year of the diagnosis of diverticular disease. Patients with incomplete age or sex information and a history of cardiovascular diseases were excluded from the study. All patients were followed until an ACS event, withdrawal from the insurance program, or December 31, 2011.
In this study, 52,681 patients with diverticular disease and 210,724 patients without diverticular disease were included. Men accounted for 56.1% of patients and 57.8% of patients were ≥50 years old. The overall incidence density of ACS in patients with diverticular disease (45.5 per 10,000 person-years) was significantly higher than in those without diverticular disease (30.3 per 10,000 person-years), with an adjusted hazard ratio (HR) of 1.23 (95% confidence interval [CI], 1.14–1.32) after adjustment for age, sex, and comorbidities. The cumulative incidence of ACS in patients with diverticular disease was significantly higher than that in the control cohort (log-rank test, P < 0.001). The adjusted HRs for the development of ACS were 1.25 (95% CI, 1.15–1.37) and 1.19 (95% CI, 1.07–1.32) in patients with diverticulitis and diverticulosis, respectively. The adjusted HRs of ACS in patients with diverticular disease additionally increased from 1.97 (95% CI, 1.73–2.23) in patients with 1 comorbidity to 5.51 (95% CI, 3.88–7.84) in those with ≥5 comorbidities.
This large population-based retrospective study revealed an association between diverticular disease and ACS. Further research is warranted to determine the exact mechanism of the link between these diseases.
INTRODUCTION
Both diverticular disease and cardiovascular disease are common worldwide and are generally considered distinct entities with different pathogeneses. However, several studies have provided evidence suggesting a link between diverticular disease and cardiovascular disease.1–3 Although the association between these diseases is difficult to conceptualize, they share several risk factors including low dietary fiber intake, smoking, hypertension, obesity, physical inactivity, and aging.1,4–11 The pathogeneses of diverticular disease and cardiovascular disease are multifactorial and complex. Chronic inflammation contributes to both of these diseases.12–14 Diverticular disease is known to be associated with segmental colitis.15 Chronic inflammation could result in intestinal microbiota transformation and cause systemic inflammation, followed by arterial atherosclerosis and then cardiovascular disease.16–17
Acute coronary syndrome (ACS), including ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and unstable angina, is a life-threatening form of cardiovascular disease.11 Recently, a large retrospective study in Denmark involving ∼77,000 patients reported that diverticular disease slightly increased the risk of acute myocardial infarction.1 The prevalence and clinical manifestation of diverticular disease vary between Asian and Western countries.18–21 However, based on our research, no study has assessed the association between diverticular disease and the risk of ACS in Asian populations. Therefore, we conducted a nationwide retrospective cohort study to determine the subsequent risk of ACS in patients with diverticular disease by analyzing data from the National Health Insurance Research Database (NHIRD) of Taiwan.
METHODS
Data Source
The National Health Insurance (NHI) program of Taiwan is a government-operated, compulsory enrollment, and single-payer system that was launched on March 1, 1995, and has since provided coverage for ∼99% of the 23 million residents of Taiwan.22 The National Health Research Institutes manages the NHIRD claims data, which have been released to researchers in electronically encrypted form since 1999. Demographic data, medications, treatments, and diagnoses based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) are included in the NHIRD. To ensure the accuracy of disease diagnosis, the Bureau of NHI randomly reviews the medical charts of 1 of every 100 outpatient and 1 of every 20 inpatient claims. For this study, a subset of the NHIRD containing health care data of inpatient claims and the Registry of Beneficiaries was used. This study was approved by the Ethics Review Board of China Medical University Hospital (CMU-REC-101-012).
Sampled Participants
People in Taiwan are mainly an ethnically homogenous Han Chinese population, accounting for 98% of all residents.23 All patients aged ≥20 years with a diagnoses of diverticular disease, namely diverticulosis (ICD-9-CM codes 562.10, 562.12) and diverticulitis (ICD-9-CM codes 562.11, 562.13), from January 1, 2000, to December 31, 2011, were included in this study. A comparison cohort of patients without diverticular disease was randomly selected from all NHI beneficiaries and frequency matched with the study cohort at a 4:1 ratio according to age (in 5-year bands), sex, and the year of diverticular disease diagnosis. The index date was defined as the date of the initial diagnosis of diverticular disease. Patients with incomplete age or sex information and diagnosed with a history of cardiovascular diseases (ICD-9-CM codes 410-414), namely acute myocardial infarction (ICD-9-CM code 410), other acute and subacute forms of ischemic heart disease (ICD-9-CM code 411), old myocardial infarction (ICD-9-CM code 412), angina pectoris (ICD-9-CM code 413), and other forms of chronic ischemic heart disease (ICD-9-CM code 414), before the index date were excluded from the study.
We analyzed the comorbidities of patients, including diabetes mellitus (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401-405), hyperlipidemia (ICD-9-CM code 272), stroke (ICD-9-CM codes 430-438), chronic obstructive pulmonary disease (ICD-9-CM codes 490-496), heart failure (ICD-9-CM code 428), chronic kidney disease (ICD-9-CM code 585), atrial fibrillation (ICD-9-CM code 427.31), cancer (ICD-9-CM codes 140-208), liver cirrhosis (ICD-9-CM codes 571.2, 571.5, 571.6), depression (ICD-9-CM codes 296.2, 296.3, 300.4, 311), autoimmune diseases (ICD-9-CM codes 710.0-710.4, 714), and alcoholism (ICD-9-CM codes 291, 303, 305.0x, 357.5, 425.5, 571.0-571.3, V11.3).
Outcome
The primary outcome was the occurrence of ACS (ICD-9-CM codes 410, 411.1, 411.8) during the follow-up period. All the patients in our study were followed from the index date to the date of a primary outcome, withdrawal from the NHI program, or December 31, 2011.
Statistical Analysis
The baseline distributions of demographic characteristics and comorbidities were evaluated using a chi-square test for categorical variables and a 2-sample t test for the mean age and mean follow-up between the study and comparison cohorts. The cumulative incidence of ACS was assessed using the Kaplan–Meier method, and the difference of incidence between the cohorts was assessed using a log-rank test. The overall sex-, age-, and comorbidity-specific incidence density rates (per 10,000 person-years) were calculated for each cohort. Univariate and multivariate Cox proportional hazard regression analyses were used to assess the risk of ACS in patients with diverticular disease compared with those without diverticular disease. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using a Cox proportional hazards model. We used a Wald chi-square test to assess the significance of each variable in the model. The joint effects of diverticular disease and comorbidities on the development of ACS were evaluated in a multivariate model. All statistical analyses were performed using SAS statistical software, Version 9.4 (SAS Institute Inc., Cary, NC). A 2-tailed probability was considered statistically significant at P < 0.05.
RESULTS
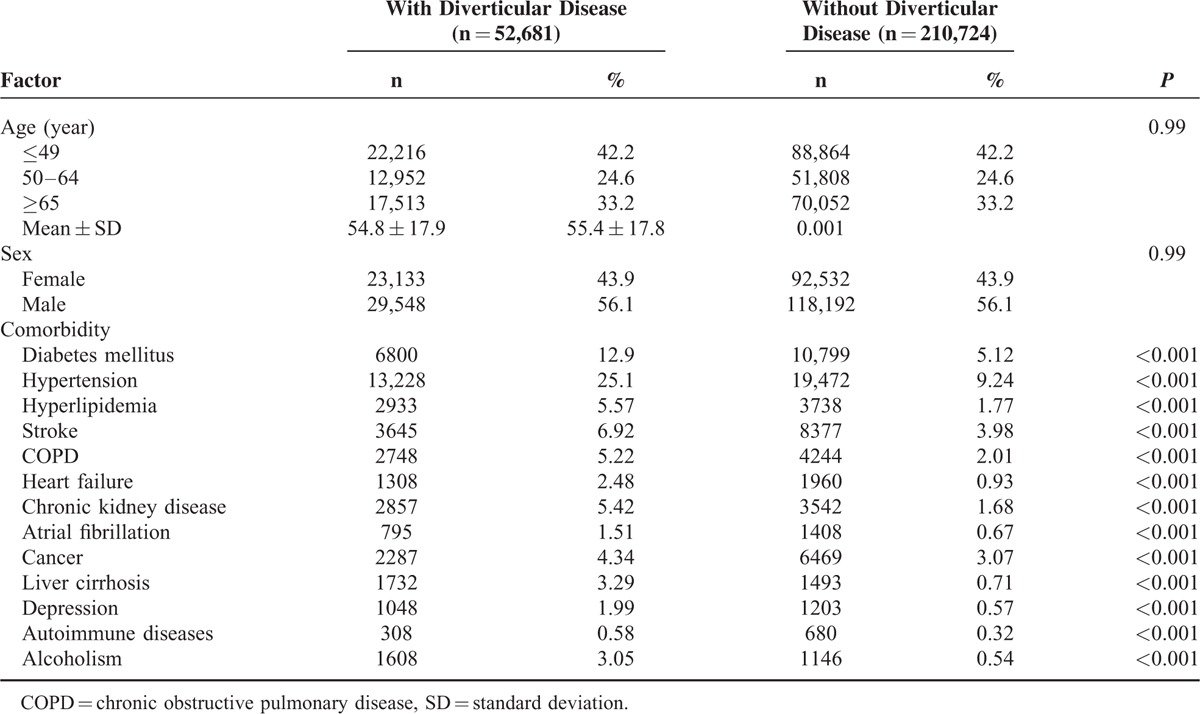
This study investigated 52,681 patients with diverticular disease and 210,724 patients without diverticular disease. Table 1 shows the demographics and comorbidities of both enrolled cohorts. Of the patients with diverticular disease, 57.8% of them were ≥50 years old, and 56.1% were men. The mean ± standard deviation (SD) ages of patients with and without diverticular disease were 54.8 ± 17.9 years and 55.4 ± 17.8 years, respectively. Compared with those without diverticular disease, patients with diverticular disease had a significantly higher prevalence of all included comorbidities (P < 0.001).
TABLE 1.
Characteristics of Age, Sex, and Comorbidities of Patients in this Study
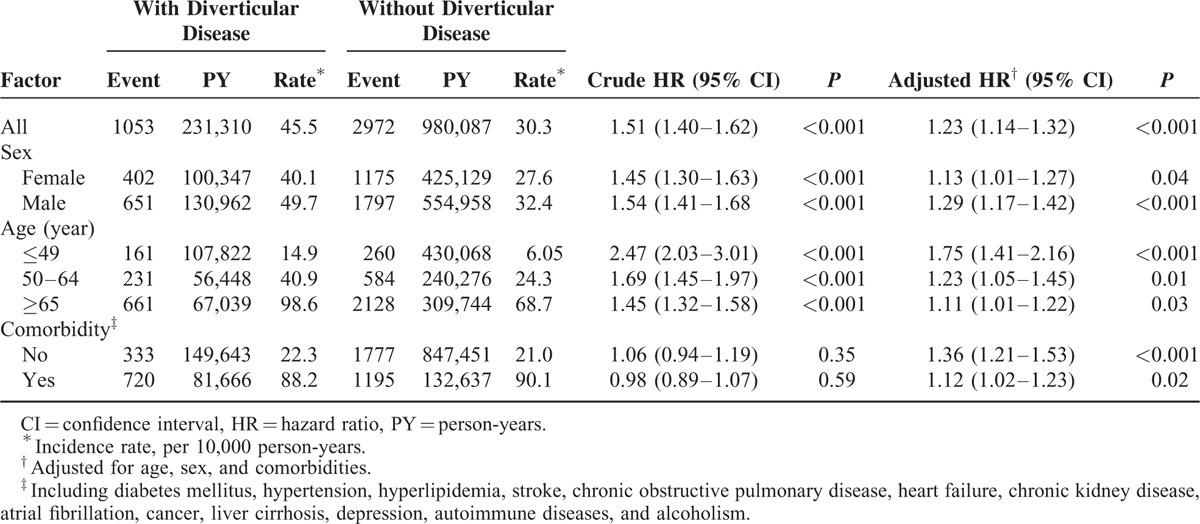
The mean ± SD follow-up periods were 4.39 ± 3.25 years and 4.65 ± 3.25 years in the study cohort and the comparison cohort, respectively. The cumulative incidence of ACS in patients with diverticular disease was significantly higher than in those without diverticular disease (log-rank test, P < 0.001) (Fig. 1). The overall incidence density rate of ACS in patients with diverticular disease (45.5 per 10,000 person-years) was significantly higher than in those without diverticular disease (30.3 per 10,000 person-years), with an adjusted HR of 1.23 (95% CI, 1.14–1.32) after adjustment for age, sex, and comorbidities (Table 2). The risk of developing ACS was consistently higher in the study cohort compared with that of the control cohort, regardless of sex or age group. However, the increased hazard of ACS declined when the patients’ ages were higher. The comorbidity-specific analysis showed that the increased risk of ACS in patients with diverticular disease was significant, irrespective of whether there was comorbidity.
FIGURE 1.
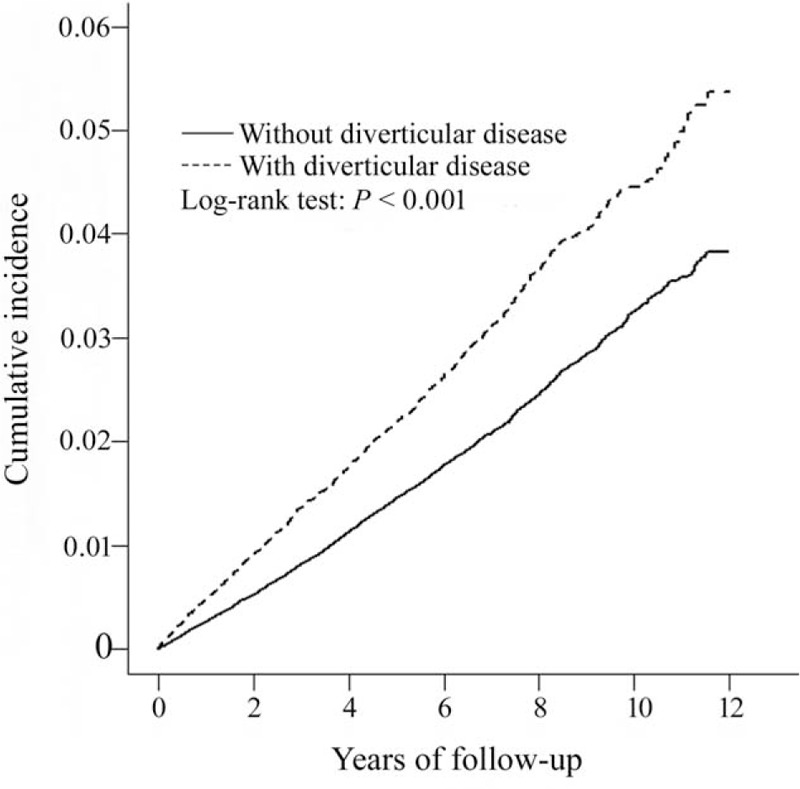
Cumulative incidence of acute coronary syndrome in patients with and without diverticular disease.
TABLE 2.
Incidence and Hazard Ratio of Acute Coronary Syndrome in Patients With and Without Diverticular Disease
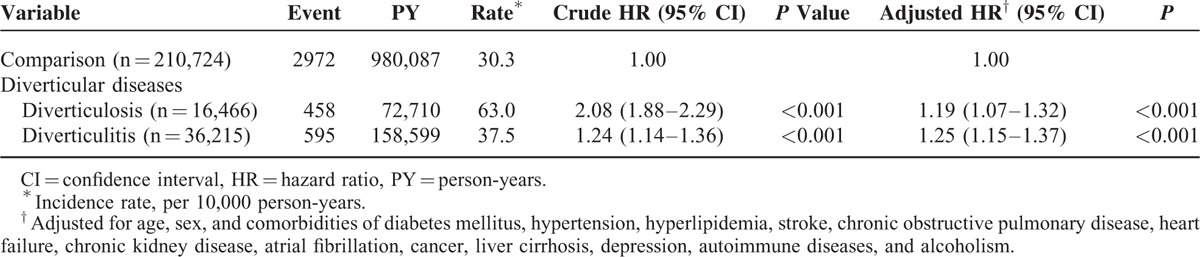
We further evaluated the association of ACS with diverticulosis and diverticulitis (Table 3). Compared with the normal population, patients with diverticulosis had a 1.19-fold risk of ACS (95% CI, 1.07–1.32), whereas those with diverticulitis exhibited a 1.25-fold risk of developing ACS (95% CI, 1.15–1.37).
TABLE 3.
Incidence and Hazard Ratio of Acute Coronary Syndrome in Patients With Different Entities of Diverticular Disease Compared With Those Without Diverticular Disease
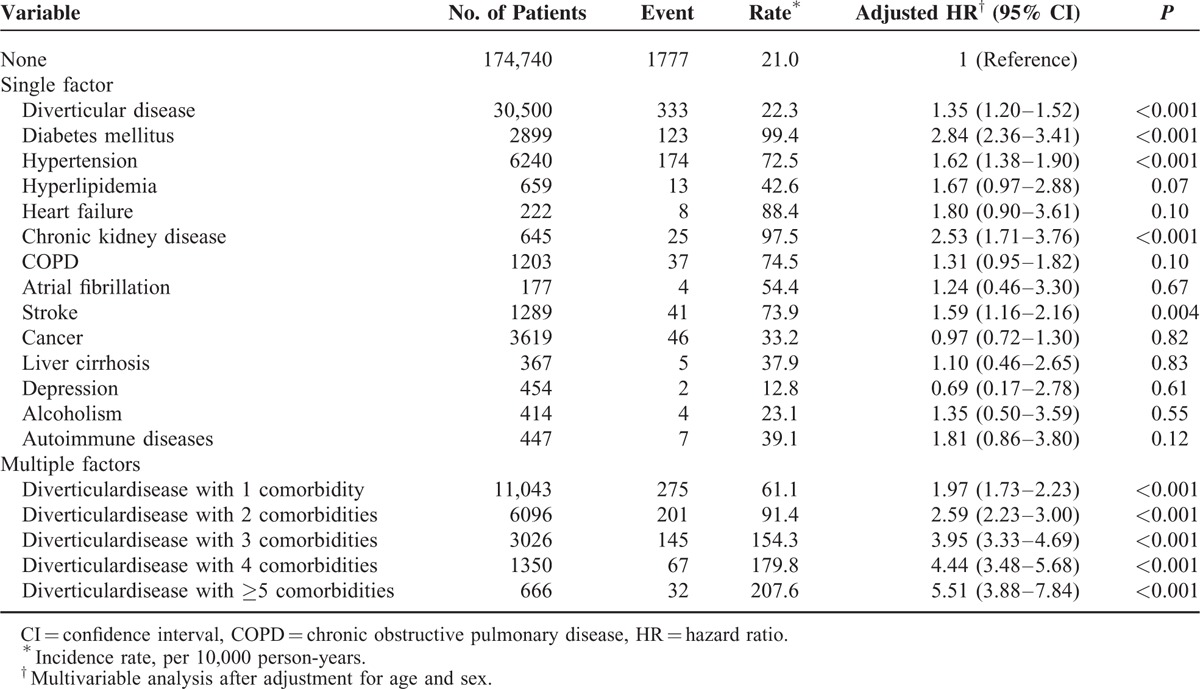
The risk of ACS in patients with diverticular disease based on comorbidities is shown in Table 4. Compared with the control cohort, diverticular disease, diabetes mellitus, hypertension, chronic kidney disease, and stroke significantly increased the risk of developing ACS. The risk elevated further in patients with diverticular disease as the number of comorbidities increased. The adjusted HR of ACS rose from 1.97 (95% CI, 1.73–2.23) to 5.51 (95% CI, 3.88–7.84) in patients with diverticular disease when the comorbidity increased from 1 to >5.
TABLE 4.
Joint Effects for the Risk of Acute Coronary Syndrome in Patients With Diverticular Disease and the Associated Risk Factors
DISCUSSION
This nationwide population-based retrospective cohort study revealed that patients with diverticular disease exhibited a 1.23-fold increased risk of ACS compared with those without diverticular disease, after adjustment for age, sex, and relevant comorbidities. With the increased number of comorbidities, the risk of ACS gradually rose in patients with diverticular disease.
Few studies have evaluated the association between diverticular disease and ACS. A prospective study performed ∼4 decades ago surveyed the prevalence of diverticular disease in male patients within 3 to 4 months after acute myocardial infarction.2 This previous study found a significantly higher prevalence of diverticular disease among patients with myocardial infarction (57%) than among the normal control patients (25%).2 However, only 28 patients with ischemic heart disease were surveyed for diverticular disease in this previous study. Another small matched case-control study analyzed the risk factors for diverticular hemorrhage in Japan and demonstrated a higher prevalence of ischemic heart disease in patients with diverticular bleeding compared with that of control patients.3 In addition to the small sample of 103 patients, the study from Japan only included patients with diverticular bleeding. The real prevalence of ischemic heart disease remains unknown in patients with asymptomatic diverticulitis or diverticulosis without inflammation in that study.
Recently, a large Danish study demonstrated that patients with diverticular disease had an increased incidence rate ratio of 1.11 (95% CI, 1.07–1.14) for acute myocardial infarction when compared with population cohort members. The incidence rate ratios for venous thromboembolism and subarachnoid hemorrhage also increased in patients with diverticular disease. Compared with the Danish study, our study showed a stronger association between ACS and diverticular disease (adjusted HR, 1.23; 95% CI, 1.14–1.32). The prevalence of diverticular disease differs among various regions, races, and ethnicities.18 The prevalence of diverticular disease in Asian populations is ∼1% to 5%, which is much lower than that of Western population (13.3%–52%).18 In addition to the lower prevalence of diverticular disease in Asian populations compared with Western populations, the clinical characteristics of diverticular disease also vary between these populations. For example, colonic diverticular disease occurs more frequently in the right-side colon in Asian populations, whereas this disease is more common in the left hemicolon in Western populations.19–21 The closer link between diverticular disease and ACS shown in our study compared with that shown in the Danish study may have been caused by the variations in race, ethnicity, culture, or dietary habits between the patients examined in both studies.
Diverticular disease consists of diverticulosis and diverticulitis. The majority of patients with diverticulosis remain asymptomatic, but 10% to 25% of patients may experience clinical symptoms of infection, obstruction, fistula formation, bleeding, and perforation.24–26 Diverticulitis is the most common manifestation of diverticular disease. However, based on our research, the association between diverticulitis and ACS has not been evaluated. In the present study, we compared the risk of developing ACS between patients with diverticulosis and those with diverticulitis, observing a higher hazard of ACS in patients with diverticulitis compared with those with diverticulosis, after adjusting for age, sex, and comorbidities (adjusted HR, 1.25 vs 1.19).
In this study, we found that patients with diverticular disease were more likely to have comorbidities than those without diverticular disease. Many comorbidities, such as diabetes mellitus, hypertension, and hyperlipidemia, are the core components of metabolic syndrome.27–28 These comorbidities could increase the risk of cardiovascular disease. Therefore, a positive correlation between diverticular disease and ACS is reasonable. In our study, we observed that the risk of developing ACS in patients with diverticular disease significantly rose when the number of comorbidities increased. In addition to the influence of diverticular disease, our study demonstrates the joint effect of diverticular disease and comorbidity illnesses on the development of ACS.
The main strengths of this study were a large-scale nationwide sample and the collection of comprehensive demographic information. However, our study still had limitations. First, several risk factors for ACS, such as obesity, tobacco smoking, and physical exercise habits, were not available in the NHIRD. These factors could have biased the results of our study. Second, diverticular disease may be an incidental finding in the routine examination or screening of lower gastrointestinal endoscopy. The number of patients with diverticular disease, particularly asymptomatic diverticulosis, may thus have been underestimated in our study. Third, we could not analyze manifestations of diverticular disease, such as bleeding, perforation, obstruction, and fistula formation, because there are no specific diagnostic codes for these complications. Fourth, the inpatient data set and registry of the beneficiaries in the NHIRD do not include information on medications. Some drugs, such as antiplatelet medications, anticoagulants, and statins, could influence the occurrence of ACS. Fifth, the diagnoses in our study were based on the ICD-9-CM codes in the NHIRD. The ICD-9-CM codes may not be accurate; however, disease experts in the Bureau of NHI regularly evaluate the accuracy of NHIRD claims files. Inaccurate coding of diseases results in no reimbursement for patients and fines for health care providers. Therefore, the diagnostic codes in the NHIRD are highly accurate and several studies have proven their accuracy.29–30 Sixth, this study was a retrospective cohort study. Such an epidemiologic study lacks biological plausibility and cannot determine the exact mechanism of the link between diverticular disease and ACS. Finally, the correlational relationship between these diseases was revealed. However, the causal relationship remains to be unknown.
In conclusion, this large nationwide retrospective population study revealed an association between diverticular disease and ACS, even after adjustment for demographics and relevant comorbidities. These findings may indicate that diverticular disease has implications for disorders beyond those of the gastrointestinal tract. Further research is warranted to determine the exact mechanism of the link between diverticular disease and ACS.
Footnotes
Abbreviations: ACS = acute coronary syndrome, CI = confidence interval, COPD = chronic obstructive pulmonary disease, HR = hazard ratio, ICD-9- = CMInternational Classification of Diseases Ninth Revision Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, SD = standard deviation.
Author Contributions: All authors have contributed significantly, and all authors are in agreement with the content of the manuscript—conception/design: Jiun-Nong Lin, Chia-Hung Kao; provision of study materials: Chia-Hung Kao; collection and/or assembly of data: all authors; data analysis and interpretation: all authors; manuscript writing: all authors; final approval of manuscript: all authors.
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Strate LL, Erichsen R, Horváth-Puhó E, et al. Diverticular disease is associated with increased risk of subsequent arterial and venous thromboembolic events. Clin Gastroenterol Hepatol 2014; 12:1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster KJ, Holdstock G, Whorwell PJ, et al. Prevalence of diverticular disease of the colon in patients with ischaemic heart disease. Gut 1978; 19:1054–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Uchiyama S, Imajyo K, et al. Risk factors for colonic diverticular hemorrhage: Japanese multicenter study. Digestion 2012; 85:261–265. [DOI] [PubMed] [Google Scholar]
- 4.Aldoori WH, Giovannucci EL, Rockett HR, et al. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr 1998; 128:714–719. [DOI] [PubMed] [Google Scholar]
- 5.Aldoori WH, Giovannucci EL, Rimm EB, et al. A prospective study of alcohol, smoking, caffeine, and the risk of symptomatic diverticular disease in men. Ann Epidemiol 1995; 5:221–228. [DOI] [PubMed] [Google Scholar]
- 6.Hjern F, Wolk A, Håkansson N. Smoking and the risk of diverticular disease in women. Br J Surg 2011; 98:997–1002. [DOI] [PubMed] [Google Scholar]
- 7.Hjern F, Wolk A, Håkansson N. Obesity, physical inactivity, and colonic diverticular disease requiring hospitalization in women: a prospective cohort study. Am J Gastroenterol 2012; 107:296–302. [DOI] [PubMed] [Google Scholar]
- 8.Humes DJ, Fleming KM, Spiller RC, et al. Concurrent drug use and the risk of perforated colonic diverticular disease: a population-based case-control study. Gut 2011; 60:219–224. [DOI] [PubMed] [Google Scholar]
- 9.Strate LL, Liu YL, Aldoori WH, et al. Physical activity decreases diverticular complications. Am J Gastroenterol 2009; 104:1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosemar A, Angerås U, Rosengren A. Body mass index and diverticular disease: a 28-year follow-up study in men. Dis Colon Rectum 2008; 51:450–455. [DOI] [PubMed] [Google Scholar]
- 11.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015; 131:e29–322. [DOI] [PubMed] [Google Scholar]
- 12.Strate LL, Modi R, Cohen E, et al. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. Am J Gastroenterol 2012; 107:1486–1493. [DOI] [PubMed] [Google Scholar]
- 13.Floch MH, White J. Diverticulitis: new concepts and new therapies. J Clin Gastroenterol 2005; 39:355–356. [DOI] [PubMed] [Google Scholar]
- 14.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004; 351:2599–2610. [DOI] [PubMed] [Google Scholar]
- 15.Imperiali G, Meucci G, Alvisi C, et al. Segmental colitis associated with diverticula: a prospective study. Gruppo di Studio per le Malattie Infiammatorie Intestinali (GSMII). Am J Gastroenterol 2000; 95:1014–1016. [DOI] [PubMed] [Google Scholar]
- 16.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003; 361:512–519. [DOI] [PubMed] [Google Scholar]
- 17.Drosos I, Tavridou A, Kolios G. New aspects on the metabolic role of intestinal microbiota in the development of atherosclerosis. Metabolism 2015; 64:476–481. [DOI] [PubMed] [Google Scholar]
- 18.Korzenik JR. Case closed? Diverticulitis: epidemiology and fiber. J Clin Gastroenterol 2006; 40 Suppl 3:S112–116. [DOI] [PubMed] [Google Scholar]
- 19.Markham NI, Li AK. Diverticulitis of the right colon – experience from Hong Kong. Gut 1992; 33:547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura S, Kodaira S, Shatari T, et al. Recent trends in diverticulosis of the right colon in Japan: retrospective review in a regional hospital. Dis Colon Rectum 2000; 43:1383–1389. [DOI] [PubMed] [Google Scholar]
- 21.Fischer MG, Farkas AM. Diverticulitis of the cecum and ascending colon. Dis Colon Rectum 1984; 27:454–458. [DOI] [PubMed] [Google Scholar]
- 22.Center for Biomedical Resources of National Health Research Institutes. National Health Insurance Research Database. Available from: http://nhird.nhri.org.tw/en/index.html Accessed 19 May 2015). [Google Scholar]
- 23.Shaw CK, Chen LL, Lee A, et al. Distribution of HLA gene and haplotype frequencies in Taiwan: a comparative study among Min-nan, Hakka, Aborigines and Mainland Chinese. Tissue Antigens 1999; 53:51–64. [DOI] [PubMed] [Google Scholar]
- 24.Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol 1975; 4:53–69. [PubMed] [Google Scholar]
- 25.Almy TP, Howell DA. Medical progress. Diverticular disease of the colon. N Engl J Med 1980; 302:324–331. [DOI] [PubMed] [Google Scholar]
- 26.Stollman N, Raskin JB. Diverticular disease of the colon. Lancet 2004; 363:631–639. [DOI] [PubMed] [Google Scholar]
- 27.Stone NJ, Robinson JG, Lichtenstein AH, et al. Circulation 2014; 129:S1–45. [DOI] [PubMed] [Google Scholar]
- 28.Alexander CM, Landsman PB, Teutsch SM, et al. Third National Health and Nutrition Examination Survey (NHANES III), National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003; 52:1210–1214. [DOI] [PubMed] [Google Scholar]
- 29.Yu YB, Gau JP, Liu CY, et al. A nationwide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb Haemost 2012; 108:225–235. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]


