Abstract
Psoriasis is recently characterized by a specific microRNAs (miRNAs) expression profile, which guides the researchers’ efforts to explore the therapeutic targets and objective biomarkers that reflect the diagnosis and disease activity in clinical use for psoriasis.
The paper presents a state-of-the-art review of expression and function of miRNAs in psoriasis along with its clinical implications.
We analyzed all literature searched by keywords “microRNA” and “psoriasis” in PubMed (Medline) from inception up to July 2015, and the references in the literature searched were also considered.
Relevant literature was chosen according to the objective of this review. Relevant literature was searched by 3 independent investigators, and experts in the field of miRNAs and psoriasis were involved in analyzing process.
We included any study in which role of miRNAs in psoriasis was examined in relation to disease pathogenesis, diagnosis, and treatment.
The specific miRNAs profile has been identified from human psoriatic skin, blood, and hair samples. It is found that genetic polymorphisms related to some of specific miRNAs, miR-146a for example, are associated with psoriasis susceptibility. Key roles of several unique miRNAs, such as miR-203 and miR-125b, in inflammatory responses and immune dysfunction, as well as hyperproliferative disorders of psoriatic lesions have been revealed. Moreover, circulating miRNAs detected from blood samples have a potential of clinic application to be the biomarkers of diagnosis, prognosis, and treatment responses. Additionally, a new layer of regulatory mechanisms mediated by miRNAs is to some extent revealed in pathogenesis of psoriasis.
The dramatically altered mRNA expression profiles are displayed in psoriasis, and some of these may become disease markers and therapeutic targets. Herein, this work underscores the potential importance of miRNAs to diagnosis, prognosis, and treatment of psoriasis. However, further study in this field is worth doing in the future, as the exact roles of miRNAs in psoriasis have not been fully elucidated.
Systematic review registration number is not registered.
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease that involves the skin or joints or both in adults, with an overall prevalence of 2% to 3% of population worldwide and a substantial negative impact on the quality of patients’ life.1,2 There are 5 types of psoriasis, including plaque psoriasis (also known as psoriasis vulgaris), guttate or eruptive psoriasis, inverse psoriasis, pustular psoriasis, and erythrodermic psoriasis, among which psoriasis vulgaris is the most common one and accounts for about 90% of cases.3 However, pathogenesis of this disease is still poorly understood, as psoriasis is a complex multifactorial disease.3 It is widely accepted that the etiology of psoriasis involves genetic susceptibility, environmental, as well as sex and age-related factors.4 Recently, genetic and epigenetic anomalies, particularly genetic regulation by aberrant expressed microRNAs (miRNAs), are indicated to be causative elements in psoriasis.5 Current knowledge of epigenetic involvement of miRNA deregulation in psoriasis is surveyed here.
MiRNAs have emerged as key mediators of post-transcriptional gene silencing in both pathogenic and pathological aspects of disorders during the last decade.6 Additionally, a new paradigm of gene regulations and the pathways involved in pathogenesis of autoimmune disorders and malignant diseases has been revealed by previous research on miRNAs.7 MiRNAs are short, single-stranded, noncoding RNAs molecules about 22 to 25 nucleotides in length, capable of negatively modulate gene expression by binding to the 3’ untranslated region (UTR) of target messenger RNAs (mRNAs), leading to the degradation or translational repression of their target mRNAs based on the degree of complementarity.8 MiRNAs have been shown to play pivotal roles in diverse developmental and cellular processes implicated in a variety of many autoimmune diseases including psoriasis.9,10
It is well known that psoriasis is a chronic inflammatory skin disorder mediated by a complex interplay among epidermal keratinocytes, immune cells, and inflammatory mediators.11 It is now clear that miRNAs can regulate differentiation, proliferation, and cytokine responses of keratinocytes, activation, and survival of T cells, as well as the crosstalk between immunocytes and keratinocytes.12 However, objective biomarkers that reflect diagnosis and disease activity have not yet been in clinical application, although differential expression profile of miRNAs is described in psoriasis as compared with that of healthy skin.13 Understanding role of miRNAs in psoriasis may lead to future insights into disease pathogenesis, diagnosis, and treatment. In this work, we review all studies focusing on role of miRNAs in psoriasis in order to provide the information for further researches in this field and the possible clinical implications of miRNAs in psoriasis.
METHODS
We searched articles indexed in PubMed (MEDLINE) database using Medical Subject Heading (MeSH) or Title/Abstract words (“microRNA” and “psoriasis”) from inception up to July 2015. There were no limitations imposed on language and study types. The additional reports from the reference list of seminal reviews were also identified. We included any study in which role of miRNAs in psoriasis was examined in relation to disease pathogenesis, diagnosis, and treatment.
The searching process was conducted by 3 independent investigators. Experts in the field of miRNAs and dermatology were involved in discussion and analyzing process.
ETHICAL REVIEW
The present study is a systemic literature review. We do not involve human beings or experimental subject in this study, and no any identifiable private information is collected.
Results and Discussion
Unique miRNAs Identified in Human Samples
During the last decade, unique miRNA expression profile has been described in psoriasis. However, only few cell- and region-specific miRNAs have been identified in psoriatic lesions, as illustrated in Tables 1 and 2 .
TABLE 1.
Several Key miRNAs Specifically Over-Expressed in Psoriasis
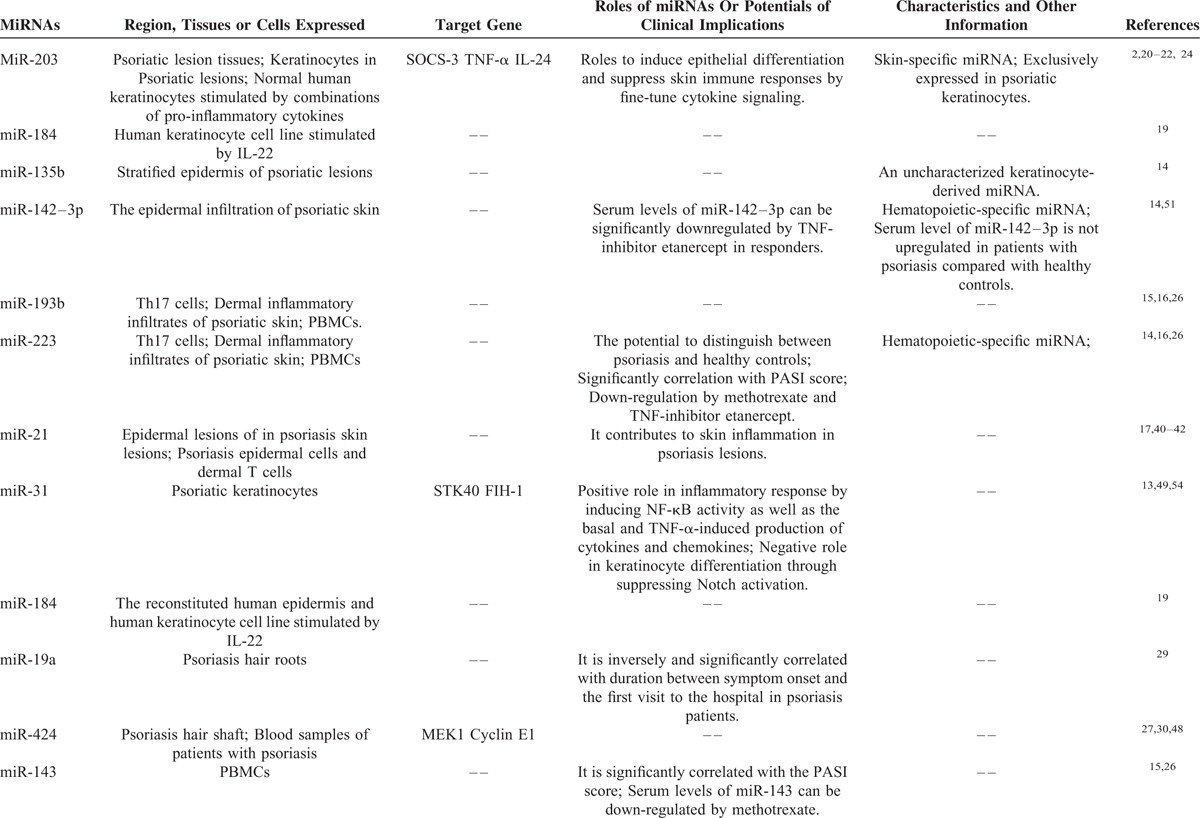
TABLE 1 (Continued).
Several Key miRNAs Specifically Over-Expressed in Psoriasis
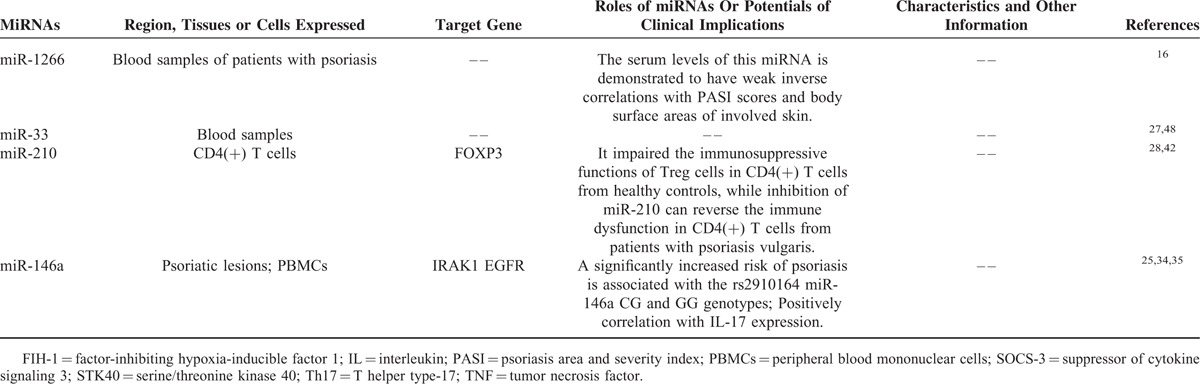
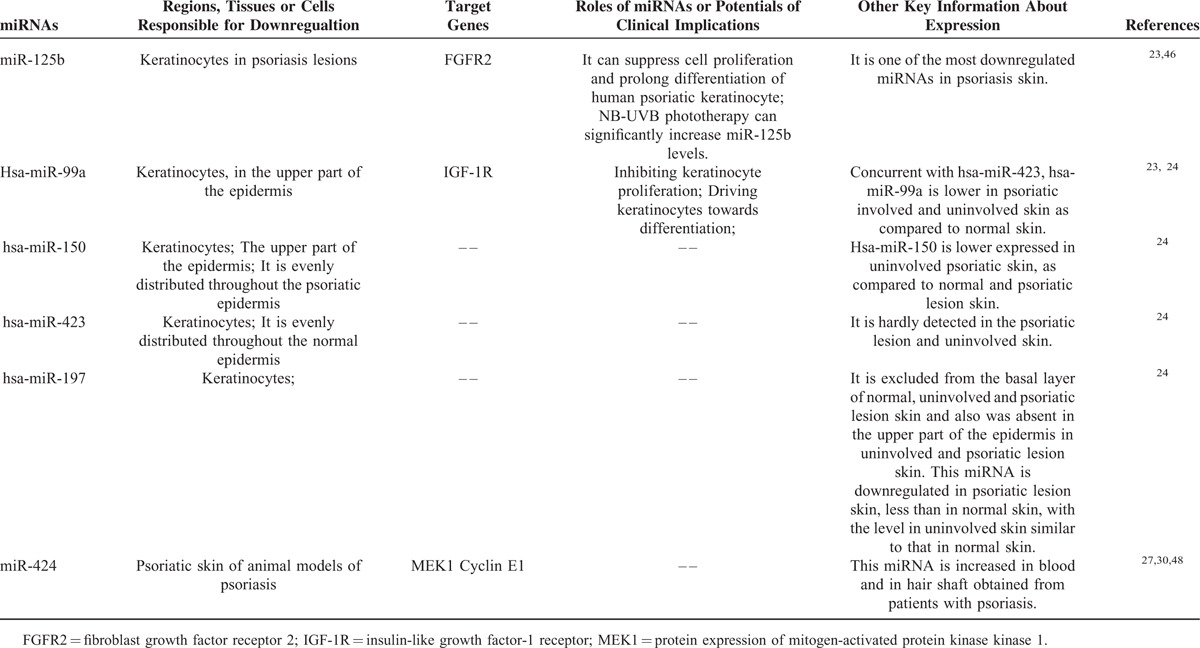
TABLE 2.
The Known miRNAs Specifically Downregulated in Psoriasis
First, we address specific miRNA expression profiles in psoriatic skin. By a comprehensive analysis of miRNAome in normal and psoriatic skin, the most highly upregulated miRNAs in psoriatic skin was identified.14 These miRNAs are skin-specific microRNA (miR)-203, hematopoietic-specific miRNAs, including miR-142-3p and miR-223/223∗, and angiogenic miRNAs, including miR-21, miR-378, miR-100, and miR-31.14 The study also revealed stratified epidermal expression of an uncharacterized keratinocyte-derived miRNA, miR-135b, as well as the epidermal infiltration of hematopoietic-specific miRNA, miR-142-3p, in psoriatic lesions,14 suggesting that miRNA deregulation is involved in pathogenesis of psoriasis and contributes to dysfunction of the cross-talk between resident and infiltrating cells, as described previously.2 It is believed that altered local miRNA changes seen in dermal inflammatory infiltrates are reflected in circulating immune cells.15 For example, both miR-193b and miR-223 are identified to be presented in dermal inflammatory infiltrates of psoriatic skin, but they are also expressed in T helper type-17 (Th17) cells,15 suggesting a role of these miRNAs in pathogenesis of psoriasis, as psoriasis has attracted attention for its characteristics as a Th17 disease.16 Furthermore, it is reported that miR-21 is significantly increased in epidermal lesions of in psoriasis skin lesions,17,18 and miR-31 is markedly overexpressed in psoriatic keratinocytes.13 Also, expression of miR-184 can be dramatically increased by proinflammatory mediators like IL-22 in reconstituted human epidermis and human keratinocyte cell line.19
Among those miRNAs, of particular significance is miR-203, which has been identified to be exclusively expressed by keratinocytes and upregulated in psoriatic lesions with skin-specific properties,2,20 Additionally, in normal human keratinocytes, the increased miR-203 is reported to be induced by combinations of proinflammatory cytokines, such as interleukin (IL)-1α, IL-17A, IL-6, and tumor necrosis factor (TNF)-α,21 which coupled with a critical role of miR-203 in epithelial differentiation,22 suggesting that miR-203 is crucially implicated in hyperproliferative phenotype of psoriatic lesion.
Some miRNAs are downregulated in psoriasis-affected skin (Table 2). When compared with healthy skin, miR-125b is suppressed in keratinocytes in psoriasis lesions, being one of the most downregulated miRNAs in psoriasis skin.23 The differentially expressed miRNAs profile has been identified recently in normal skin versus psoriatic involved and uninvolved skin, and it is revealed 4 dramatically differential distributed miRNAs that are hsa-miR-99a, hsa-miR-150, hsa-miR-423, and hsa-miR-197.24 Expressions of hsa-miR-99a and hsa-miR-423 were lower in psoriatic involved and uninvolved skin as compared with normal skin. Hsa-miR-150 was down regulated in uninvolved psoriatic skin, as compared with normal and psoriatic lesion skin. Hsa-miR-197 was under expressed in psoriatic lesion skin, less than in normal skin, with the level in uninvolved skin similar to that in normal skin. This study confirmed that miR-203 was upregulated in psoriasis lesion samples as compared with normal skin samples, and intriguingly a significant down regulation of miR-203 in the uninvolved psoriatic skin as compared with psoriatic lesion and normal skin was revealed.24
Second, expression of miRNAs in whole blood, plasma, and peripheral blood mononuclear cells (PBMCs) from patients with psoriasis and healthy controls has been explored in several studies. It is found that expression of miR-146a was up-regulated both in lesions and PBMCs of psoriatic patients, and positively correlated with IL-17 expression.25 MiR-193b, miR-223, and miR-143 are found to be significantly upregulated in the PBMCs from patients with psoriasis, as compared with healthy controls.26,15 The serum miR-1266 levels are considerably higher in psoriasis patients than in healthy control subject, and importantly, the level of this miRNA is demonstrated to have weak inverse correlations with Psoriasis Area Severity Index (PASI) scores and body surface areas of involved skin.16 Moreover, in patients with plaque psoriasis, the plasma levels of miRNA-33 were significantly higher than in controls,27 and miR-210 expression is increased significantly in CD4 (+) T cells.28 These observations suggest that circulating miRNAs may have potentials for the diagnostic, prognosis, and treatment markers of psoriasis.
Third, the profile of miRNAs expression was also examined in hair root and shaft obtained from patients with psoriasis, healthy controls, and patients with atopic dermatitis, another chronic inflammatory skin disorder.29,30 The studies have demonstrated that hair root levels of miR-19a are significantly up-regulated only in psoriasis compared with normal controls, and relative miR-19a levels are inversely and significantly correlated with duration between symptom onset and the first visit to the hospital in psoriasis patients.29 Additionally, hair shaft miR-424 levels are found to be significantly upregulated only in patients with psoriasis compared with normal controls and those with atopic dermatitis.30 These findings suggest that either hair root miR-19a levels or hair shaft miR-424 levels are effective as disease marker of psoriasis.
Altogether, these differentially expressed miRNAs may function as regulators of gene expression in skin or other tissues/organs and potentially play a role in psoriasis pathogenesis (depicted in Table 1 ).31 We will discuss these issues in the following sections, as unique miRNAs identified in human samples provide important new avenues for therapeutic interventions in psoriasis.
Genetic Polymorphisms Related to miRNAs Contributes to Psoriasis Susceptibility
Psoriasis has a strong genetic background, and it has become apparent that genetic polymorphisms in miRNA genes and/or in miRNA binding sites of target genes can affect miRNA activity and contribute to disease susceptibility,12,32 which can be supported by the following studies addressed. For instance, in a genome-wide interaction analysis, it has been identified 4 single nucleotide polymorphisms (SNPs) in miRNA (miR-324-3p, miR-433, and miR-382) target sites which interact with 5 SNPs to contribute to psoriasis.33 These 5 interacting pairs of SNPs in genes leucocyte-specific transcript 1 (LST1)/natural cytotoxicity-triggering receptor 3 (NCR3), C-X-C chemokine receptor type 5 (CXCR5)/B-cell CLL/lymphoma 9-like(BCL9L), and phosphate-activated glutaminase (GLS2) are located in the target sites of miR-324-3p, miR-433, and miR-382, respectively.33
The miR-146a rs2910164 SNP was genotyped in a total of 521 Han Chinese patients with psoriasis and 582 healthy controls, and it is found that a significantly increased risk of psoriasis is associated with the rs2910164 miR-146a CG and GG genotypes [adjusted odds ratio (OR), 1.38; 95% confidence interval (CI), 1.06–1.80].34 A further study has elucidated the possible mechanisms account for the increased risk of psoriasis, as evidenced by the data showing that the rs2910164G allele in miR-146a weakens its suppression on the proliferation of keratinocytes through the decreased inhibition of the target gene, epidermal growth factor receptor (EGFR), which is overexpressed in psoriatic lesions and contributes to hyperproliferation of keratinocytes in psoriasis.34 Additionally, interleukin-1 receptor-associated kinase 1 (IRAK1) is another miR-146 gene target, and it is reported that IRAK1 rs3027898 polymorphism is associated with susceptibility of psoriatic arthritis,35 and inability of miR-146a inhibiting target gene IRAK1 may contribute to the persistent inflammation in lesions of psoriasis.25
Extracellular matrix metalloproteinase inducer (CD147) is a member of the immunoglobulin superfamily expressed ubiquitously in circulating immune cell populations. It is observed that expression level of CD147 in PBMCs is elevated in psoriasis patients, while the rs8259 T allele of CD147 is associated with significantly decreased psoriasis susceptibility.36 Interestingly, the rs8259 polymorphism of CD147 is located in a seed region for miR-492 binding.36 Therefore, this study suggests that miR-492 may physiologically suppress CD147 expression and the CD147 rs8259 polymorphism is associated with decreased psoriasis susceptibility through affecting miR-492 binding.36
Additionally, by using TaqMan Genotyping Assay, rs9264942T > C polymorphism in HLA-C gene was genotyped in 292 patients with psoriasis vulgaris and 254 controls, and the results demonstrated that these HLA alleles are associated with psoriasis vulgaris.37 In addition, rs9264942C allele has been described to be in strong linkage disequilibrium (LD) with another SNP, rs67384697 ins/del, which by affecting a miRNA binding is responsible for regulating HLA-C expression.37
Role of miRNAs in Inflammatory Response and Immune Dysfunction
In disorders of inflammatory responses, immune cells of the innate and/or adaptive immune system are activated and recruited to the site of inflammation.38 Attraction and activation of immune cells is regulated by a variety of different cytokines and chemokines, which can affect or be affected by miRNAs in autoimmune diseases like psoriasis.38 A characteristic miRNAs profile in psoriasis skin suggests putative functions of these miRNAs in perturbed cytokine production and signaling during chronic inflammatory skin conditions in psoriasis. As stated previously, miR-203 is a well-known miRNA identified to be uniquely expressed in psoriasis keratinocyte. Up-regulation of miR-203 in psoriatic plaques is shown to be concurrent with down-regulation of an evolutionary conserved target of miR-203, suppressor of cytokine signaling 3 (SOCS-3), which is involved in inflammatory responses and keratinocyte functions.2 By screening a panel of cytokines that are upregulated in psoriatic skin for regulation by miR-203, it has been established the genes encoding the proinflammatory cytokines TNF-α and IL-24 as direct targets of miR-203 in primary keratinocytes.20 These findings suggest a role of that miR-203 serves to fine-tune cytokine signaling and may dampen skin immune responses by repressing key proinflammatory cytokines.20
In contrast to miR-203, overexpression of miR-31 contributes to skin inflammation in psoriasis lesions by regulating production of inflammatory mediators and leukocyte chemotaxis to the skin, as evidenced by a study showing that, in human primary keratinocytes, specific inhibition of miR-31 suppressed the basal and TNF-α-induced production of IL-1β, chemokine (C-X-C motif) ligand 1 (CXCL1)/growth-related oncogene-α, CXCL5/epithelial-derived neutrophil-activating peptide 78, and CXCL8/IL-8.13 Moreover, this study also showed that inhibition of endogenous miR-31 in keratinocytes decreased the capability of keratinocytes to activate endothelial cells and attract leukocytes.13 Furthermore, both in vitro and in vivo studies demonstrated that, in psoriatic keratinocytes, miR-31 expression was up-regulated by transforming growth factor (TGF)-β1,13 which is a cytokine highly expressed in psoriasis epidermis.39 Additionally, it has been demonstrated that miR-21 can regulate a variety of immune cells.40 For example, in activated human T cells, specific inhibition of miR-21 increased the apoptosis rate,17 and thus, overexpression of miR-21 may contribute to skin inflammation in psoriasis lesions, as psoriasis is a T cell-meditated autoimmune skin disease.41
Several lines of evidence clarified that functions of circulating immune cells are regulated by miRNAs in psoriasis. It is well known that an immune dysfunction manifested by abnormally activated T cells and defective regulatory T (Treg) cells may play an important role in pathogenesis of psoriasis vulgaris.28 The immune dysfunction in patients with psoriasis vulgaris is found to be induced by overexpression of miR-210, whose target gene is forkhead box P3 (FOXP3).42 It is reported that overexpression of miR-210 inhibited FOXP3 expression and impaired immunosuppressive functions of Treg cells in CD4 (+) T cells from healthy controls, while on the other hand, inhibition of miR-210 increased FOXP3 expression and reversed immune dysfunction in CD4(+) T cells from patients with psoriasis vulgaris.28
The above-addressed miRNAs, miR-31, miR-21, and miR-210 play a positive role in inflammatory response and immune dysfunction of psoriasis, suggesting that inhibition of these miRNAs may be a potential therapeutic option in psoriasis, while, intriguingly, miR-138 may have protective effects against immune dysfunction in psoriasis. It is reported that transfection with miR-138 inhibitor into CD4(+) T cells from healthy controls resulted in the increased expression of Runt-related transcription factor 3 (RUNX3), which is a susceptibility gene for psoriasis and a target gene of miR-138, and the increased ratio of T helper type-1 cells (Th1)/T helper type-2 cells (Th2). Moreover, transfection with miR-138 mimic into CD4(+) T cells from psoriasis patients led to suppression of RUNX3 and the decreased ratio of Th1/Th2.41 Therefore, it is suggested that miR-138 plays a protective role by regulating the balance of Th1/Th2 via inhibiting RUNX3 expression in psoriasis.
Role of miRNAs in Hyperproliferative Phenotype of Psoriatic Lesions
Psoriasis is well known to be a hyperproliferative skin disorder, characterized by intense proliferation and abnormal differentiation of keratinocytes.43,44 Because miRNAs are important posttranscriptional regulators of keratinocyte gene expression, they assist in modulating the fine balance between cell proliferation and differentiation in psoriatic skin.20,45 For instance, based on the miRNA and mRNA profiles, miR-21, miR-205, miR-221, and miR-222 are found to have the following potential mRNA targets in psoriatic skin: programmed cell death protein 4 (PDCD4), tropomyosin alpha-1 chain (TPM1), P57, mast/stem cell growth factor receptor (C-KIT), reticulon-4 (RTN4), phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase (SHIP), metalloproteinase inhibitor 3 (TIMP3), reversion-inducing-cysteine-rich protein with kazal motifs (RECK), and nuclear factor I/B (NFIB), all of which are likely to be involved in cellular growth, proliferation, apoptosis, and degradation of the extracellular matrix and further contribute to phenotype of psoriatic skin lesion.46
As stated above, miR-125b is one of the most downregulated miRNAs in psoriasis skin.23 Skin biopsies from 12 patients with psoriasis were collected before, during, and after narrow bound Ultra Violet B Light (NB-UVB) therapy, and it is shown that NB-UVB phototherapy significantly increased miR-125b levels.47 The other studies showed that transfection with miR-125b precursor RNA into human primary keratinocytes to overexpress miR-125b significantly suppressed proliferation and induced the expression of miR-203, which is a differentiation-induced miRNA with suppressing effects on skin inflammation.23 Conversely, inhibition of endogenous miR-125b using miR-125b inhibitor oligonucleotide promoted cell proliferation and delayed differentiation.23 Mechanism study has revealed that miR-125b suppresses cell proliferation of psoriatic keratinocytes through inhibiting its directly target gene fibroblast growth factor receptor 2 (FGFR2),23 a receptor expressed on keratinocytes and reported to be upregulated in lesional psoriasis skin.48
In addition to miR-125b, studies also showed that overexpression of miR-99a inhibited keratinocyte proliferation through directly targeting insulin-like growth factor-1 receptor (IGF-1R), which is involved in skin development and pathogenesis of psoriasis.24 Moreover, overexpression of hsa-miR-99a in keratinocytes drives them toward differentiation, as evidenced by increased expression of Keratin 10,24 an early differentiation marker.23 However, interestingly, expression of miR-99a can be increased by IGF1 in PHK cell line,24 suggesting that miR-99a acts together with IGF1 signals to maintain the balance between keratinocyte proliferation and differentiation.
Protein expression of mitogen-activated protein kinase kinase 1 (MEK1), which is a signal kinase of cell proliferation and a predicted target gene of miR-424, is increased in psoriatic skin, as miR-424 level is markedly downregulated in psoriasis skin in vivo.49 The in vitro studies showed that transfection of specific inhibitor of miR-424 in normal human keratinocytes led to upregulation of MEK1 protein, and resulted in increased cell proliferation.49
Finally, the genetic deficiency of miR-31 in keratinocytes inhibits their hyperproliferation, decreases acanthosis, and reduces the disease severity in psoriasis mouse models,50 and such effects are supported by another report addressing that interference with endogenous miR-31 decreased the ability of keratinocytes to activate endothelial cells and attract leukocytes.13
Taken together, these findings validated that the aberrant microRNA expression contributes to the skin phenotype of psoriasis, mainly including keratinocytes hyperproliferation and abnormal skin differentiation. Undoubtedly, investigation of the regulatory mechanisms of keratinocyte proliferation by miRNAs may lead to new treatments and disease activity markers.
Potentials of Serum MiRNAs as the Biomarkers in Psoriasis
MiRNAs found in the blood are considered to be relevant as disease biomarkers,26 since miRNAs are present in circulation in a stable form and their levels are altered in diseases.51 MiR-223 and miR-143 have been identified to be significantly upregulated in PBMCs from patients with psoriasis compared with healthy controls.26 Moreover, serum levels of miR-424 and miR-33 have been also demonstrated to be increased in patients with psoriasis compared with healthy controls.27,49 It is therefore suggested that these miRNAs may have the potential to distinguish between psoriasis and healthy populations. Importantly, miR-223 and miR-143 are found to be significantly correlated with the PASI score,26 suggesting that these 2 miRNAs may serve as novel biomarkers for disease activity in psoriasis.
Additionally, there is a study showing that after 3 to 5 weeks of treatment with methotrexate following a significant decrease in psoriasis severity, miR-223 and miR-143 were significantly downregulated in PBMCs from patients with psoriasis.26 In another study, it is reported that serum levels of miR-106b, miR-26b, miR-142–3p, miR-223, and miR-126 can be significantly downregulated by TNF-inhibitor etanercept in responders (PASI change >50%), while serum levels of these miRNAs are not upregulated in patients with psoriasis compared with healthy controls.52 Though it is not related to disease severity, change of the serum levels of miRNAs by anti-TNF-α therapy may reflect a previously unknown effect of treatment with anti-TNF-α agents. Nevertheless, these observations strongly suggest that some of serum miRNAs may also function as the potential biomarkers for therapy response in psoriasis.
Possible Signaling Mechanisms Underlying miRNAs Action
MiRNAs are endogenous small regulatory RNAs that stabilize cellular phenotypes and fine-tune signal transduction feedback loops through regulation of gene networks, thereby regulating the development of inflammatory cell subsets and have a significant impact on the magnitude of inflammatory responses.12 However, only a few reports have addressed signaling mechanisms underlying mRNAs actions, studies about the signaling mechanisms involved in function of miRNAs in psoriasis are still on the way.
In the course of psoriatic inflammation, attraction and activation of immune cells is regulated by a variety of different cytokines and chemokines, which are predominantly regulated by transcription factors such as nuclear factor (NF)-κB, signal transducer and activator of transcription 3 (STAT3), and Jun/activating protein 1 (AP-1).27 NF-κB is constitutively activated in psoriatic epidermis, and the NF-κB activation triggered by inflammatory mediators like TGF-β1 induces transcription of miR-31 in keratinocytes and mouse model of psoriasis.13,50 Protein phosphatase 6 (ppp6c), a negative regulator that restricts the G1 to S phase progression, is diminished in human psoriatic epidermis and is directly targeted by miR-31. NF-κB activation inhibits ppp6c expression directly through induction of miR-31, and enhances hyperproliferation of epidermis in psoriasis.50 In turn, miR-31 can induce the basal and TNF-α-induced production of cytokines and chemokines, as stated previously, through stimulating NF-κB activity in human primary keratinocytes.13 Serine/threonine kinase 40 (STK40), a negative regulator of NF-κB signaling, was identified to be a direct target for miR-31. Mechanism study demonstrated that silencing of STK40 rescued suppressive effect of miR-31 inhibition on cytokine/chemokine expression, indicating that miR-31 regulates cytokine/chemokine expression via targeting STK40 in psoriasis keratinocytes.13
However, little is known about the factors responsible for intracellular signaling between ligand activation and NF-κB activation. Notch plays a critical role in various disorders through interplaying with or depending on NF-κB signals,53,54 and importantly Notch signaling is a feature of psoriasis.55 Notch can undergo hydroxylation by factor-inhibiting hypoxia-inducible factor 1 (FIH-1). MiR-31 is an endogenous negative regulator of FIH-1 expression that results in keratinocyte differentiation, mediated by Notch activation. Therefore, a mechanism is defined for keratinocyte fate decisions where Notch signaling potential is, in part, controlled through a miR-31/FIH-1 nexus.55 Taken together, it is elucidated a signal transduction feedback loop, in which miR-31 and NF-κB play a key role, and this feedback loop contributes to inflammatory responses and skin phenotype of psoriasis finally (Fig. 1A).
FIGURE 1.
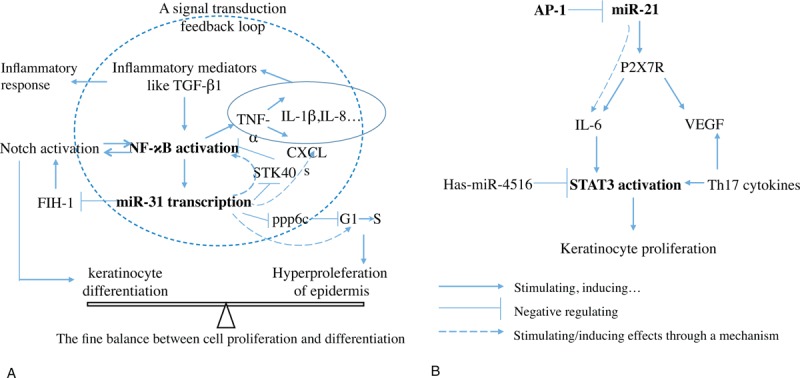
Possible signaling mechanisms underlying miRNAs action with the examples of miR31 and miR-21. A, a signal transduction feedback loop, in which miR-31 and NF-κB play a key role, has been delineated. This feedback loop contributes to inflammatory responses and skin phenotype of psoriasis. B, a complex network formed by miR-21 activity and STAT3 activation contributes to keratinocytes proliferation in psoriasis. The detailed information of these signals is seen in the related body text. AP-1 = Jun/activating protein 1; CXCLs = chemokine (C-X-C motif) ligands; FIH-1 = factor-inhibiting hypoxia-inducible factor 1; IL = interleukin; NF-κB = nuclear factor κB; ppp6c = protein phosphatase 6c; P2X7R = receptor of purinergic recepter p2x, ligand-gated ion channel,7; STAT3 = signal transducer and activator of transcription 3; TGF-β1 = transforming growth factor β1; Th17 = T helper type-17; VEGF = vascular endothelial growth factor.
In addition to NF-κB activation, STAT3 is also crucial to the development of psoriasis, as STAT3 phosphorylation and translocation to nucleus results in keratinocyte proliferation.45 Ectopic expression of hsa-miR-4516 directly targets STAT3 protein by binding to its 3’UTR in human keratinocytes, which is supported by a report showing that overexpression of hsa-miR-4516 downregulated STAT3 and p-STAT3 and induced apoptosis in human keratinocytes.56 Using patient-derived skin samples and mouse models of psoriasis, a study demonstrates that miR-21 expression is increased in epidermal lesions of patients with psoriasis, and the increased miR-21 may be a consequence of impaired transcriptional activity of Jun/activating protein 1 (AP-1), leading to activation of the IL-6/STAT3 pathway.18 Additionally, it is revealed that a P2X7R (receptor of purinergic recepter p2x, ligand-gated ion channel, 7)-dependent mir-21 angiogenesis pathway that leads to the expression of vascular endothelial growth factor (VEGF) and IL-6, which may be involved in development of psoriatic lesions.57 Of note, both STAT3 and VEGF are Th17-related cytokines and positively correlated with expression of Th17 in psoriasis.45 Now, a complex network critically involved by miR-21 and STAT3 is delineated in psoriasis keratinocytes (Fig. 1B).
Conclusions and Future Directions
In summary, psoriasis is a chronic and complex inflammatory skin disease with lesions displaying dramatically altered mRNA expression profiles, and some of these may become disease markers and therapeutic targets. In this review, we present an overview of what is currently known about role of miRNAs in psoriasis. Specifically, we focus on the differential expression profile, immune and inflammatory responses, and hyperproliferative skin disorder associated with miRNAs in psoriasis. Although the exact roles of miRNAs in psoriasis have not been fully elucidated, a new layer of regulatory mechanisms mediated by miRNAs is revealed in the pathogenesis of psoriasis. The goal of drawing clinically relevant conclusions about role of miRNAs in psoriasis will be aided by some novel methods that enable fast and sensitive epigenomic profiling in the future.
Additionally, considering a fact that objective diagnostic markers have not been in clinical use for psoriasis, miRNAs levels could be useful biomarkers for diagnosis, prognosis, and therapeutic value in psoriasis. It is because miRNAs are easily detected in a variety of sources, including tissues, serum, and other body fluids.7 In particular, it has been proved that miRNAs detection in human skin is robust irrespective of preservation method, as miRNAs are less prone to RNA degradation than mRNAs due to lack a poly-A tail.58
Anyway, our systematic literature review highlights the potential of miRNAs aberrantly expressed in psoriasis in development of novel therapeutic strategies and useful biomarkers for disease diagnosis and prognosis. However, although specific miRNAs profile in psoriasis has been to some extent identified, signal transduction mediated by specific miRNAs and the relationships among these specific miRNAs in psoriasis are largely unknown. Further studies in this field could be promising research directions leading to better understanding in psoriasis pathogenesis.
Acknowledgment
This study was supported by National Natural Science Foundation of China (No. 81473681), the joint special fund of Guangdong Provincial Department of Science and Technology-Guangdong Provincial Academy of Chinese Medical Sciences (No. 2014A020221028), as well as Chinese Medical Science and Technology research funding from Guangdong Provincial Hospital of Chinese Medicine (No. YN2014ZH04).
Footnotes
Abbreviations: AP-1 = Jun/activating protein 1, BCL9L = B-cell CLL/lymphoma 9-like, CD147 = extracellular matrix metalloproteinase inducer, CI = confidence interval, C-KIT = Mast/stem cell growth factor receptor, CXCL1 = chemokine (C-X-C motif) ligand 1, CXCR5 = C-X-C chemokine receptor type 5, EGFR = epidermal growth factor receptor, FIH-1 = factor-inhibiting hypoxia-inducible factor 1, GLS2 = phosphate-activated glutaminase, IL = interleukin, IRAK1 = interleukin-1 receptor-associated kinase 1, LD = linkage disequilibrium, LST1 = leucocyte-specific transcript 1, microRNA = miRNA, mRNAs, messenger RNAs, NCR3 = natural cytotoxicity-triggering receptor 3, NF-κB = nuclear factor-κB, NFIB = Nuclear factor I/B, OR = Adjusted odds ratio, P2X7R = receptor of purinergic recepter p2x, ligand-gated ion channel,7, P57 = passenger 57, PASI = psoriasis Area And Severity Index, PBMCs = peripheral blood mononuclear cells, PDCD4 = programmed cell death protein 4, ppp6c = protein phosphatase 6c, RECK = reversion-inducing-cysteine-rich protein with kazal motifs, RTN4 = reticulon-4, RUNX3 = runt-related transcription factor 3, SHIP = phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase, SNPs = single nucleotide polymorphisms, SOCS-3 = suppressor of cytokine signaling 3, STAT3 = signal transducer and activator of transcription 3, STK40 = serine/threonine kinase 40, TGF = transforming growth factor, Th1/Th2 = T helper type-1 cells/T helper type-2 cells, Th17 = T helper type-17, TIMP3 = Metalloproteinase inhibitor 3, TNF = tumor necrosis factor, TPM1 = tropomyosin alpha-1 chain, Treg cells = defective regulatory T cells, UTR = 3’ untranslated region, VEGF = vascular endothelial growth factor.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Zhao YK, Zhang YQ, Wang F, et al. Developing Shingles-Induced Koebner Phenomenon in a Patient With Psoriasis: A Case Report. Medicine 2015; 94:e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS One 2007; 2:e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehncke WH, Schön MP. Psoriasis. Lancet 2015; 386:983–994. [DOI] [PubMed] [Google Scholar]
- 4.Chandra A, Ray A, Senapati S, et al. Genetic and epigenetic basis of psoriasis pathogenesis. Mol Immunol 2015; 64:313–323. [DOI] [PubMed] [Google Scholar]
- 5.Trowbridge RM, Pittelkow MR. Epigenetics in the pathogenesis and pathophysiology of psoriasis vulgaris. J Drugs Dermatol 2014; 13:111–118. [PubMed] [Google Scholar]
- 6.Jinnin M. [microRNA in autoimmune disorders]. Nihon Rinsho Meneki Gakkai Kaishi 2011; 34:439–446. [DOI] [PubMed] [Google Scholar]
- 7.Deng X, Su Y, Wu H, et al. The role of microRNAs in autoimmune diseases with skin involvement. Scand J Immunol 2015; 81:153–165. [DOI] [PubMed] [Google Scholar]
- 8.Ivey KN, Srivastava D. microRNAs as developmental regulators. Cold Spring Harb Perspect Biol 2015; 7:a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh RP, Massachi I, Manickavel S, et al. The role of miRNA in inflammation and autoimmunity. Autoimmun Rev 2013; 12:1160–1165. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez SA, Piera-Velazquez S. Potential role of human-specific genes, human-specific microRNAs and human-specific non-coding regulatory RNAs in the pathogenesis of systemic sclerosis and Sjogren's syndrome. Autoimmun Rev 2013; 12:1046–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinberg-Bleyer Y, Dainichi T, Oh H, et al. Cutting edge: NF-kappaB p65 and c-Rel control epidermal development and immune homeostasis in the skin. J Immunol 2015; 194:2472–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pivarcsi A, Stahle M, Sonkoly E. Genetic polymorphisms altering microRNA activity in psoriasis-a key to solve the puzzle of missing heritability? Exp Dermatol 2014; 23:620–624. [DOI] [PubMed] [Google Scholar]
- 13.Xu N, Meisgen F, Butler LM, et al. MicroRNA-31 is overexpressed in psoriasis and modulates inflammatory cytokine and chemokine production in keratinocytes via targeting serine/threonine kinase 40. J Immunol 2013; 190:678–688. [DOI] [PubMed] [Google Scholar]
- 14.Joyce CE, Zhou X, Xia J, et al. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Hum Mol Genet 2011; 20:4025–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovendorf MB, Mitsui H, Zibert JR, et al. Laser capture microdissection followed by next-generation sequencing identifies disease-related microRNAs in psoriatic skin that reflect systemic microRNA changes in psoriasis. Exp Dermatol 2015; 24:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichihara A, Jinnin M, Oyama R, et al. Increased serum levels of miR-1266 in patients with psoriasis vulgaris. Eur J Dermatol 2012; 22:68–71. [DOI] [PubMed] [Google Scholar]
- 17.Meisgen F, Xu N, Wei T, et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Exp Dermatol 2012; 21:312–314. [DOI] [PubMed] [Google Scholar]
- 18.Guinea-Viniegra J, Jimenez M, Schonthaler HB, et al. Targeting miR-21 to treat psoriasis. Sci Transl Med 2014; 6:225re221. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JC, Warren RB, Griffiths CE, et al. Expression of microRNA-184 in keratinocytes represses argonaute 2. Hum Mol Genet 2013; 228:2314–2323. [DOI] [PubMed] [Google Scholar]
- 20.Primo MN, Bak RO, Schibler B, et al. Regulation of pro-inflammatory cytokines TNFalpha and IL24 by microRNA-203 in primary keratinocytes. Cytokine 2012; 60:741–748. [DOI] [PubMed] [Google Scholar]
- 21.Bracke S, Desmet E, Guerrero-Aspizua S, et al. Identifying targets for topical RNAi therapeutics in psoriasis: assessment of a new in vitro psoriasis model. Arch Dermatol Res 2013; 305:501–512. [DOI] [PubMed] [Google Scholar]
- 22.Nissan X, Denis JA, Saidani M, et al. miR-203 modulates epithelial differentiation of human embryonic stem cells towards epidermal stratification. Dev Biol 2011; 356:506–515. [DOI] [PubMed] [Google Scholar]
- 23.Xu N, Brodin P, Wei T, et al. MiR-125b, a microRNA downregulated in psoriasis, modulates keratinocyte proliferation by targeting FGFR2. J Invest Dermatol 2011; 131:1521–1529. [DOI] [PubMed] [Google Scholar]
- 24.Lerman G, Avivi C, Mardoukh C, et al. MiRNA expression in psoriatic skin: reciprocal regulation of hsa-miR-99a and IGF-1R. PLoS One 2011; 6:e20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia P, Fang X, Zhang ZH, et al. Dysregulation of miRNA146a versus IRAK1 induces IL-17 persistence in the psoriatic skin lesions. Immunol Lett 2012; 148:151–162. [DOI] [PubMed] [Google Scholar]
- 26.Lovendorf MB, Zibert JR, Gyldenlove M, et al. MicroRNA-223 and miR-143 are important systemic biomarkers for disease activity in psoriasis. J Dermatol Sci 2014; 75:133–139. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Rodriguez S, Arias-Santiago S, Orgaz-Molina J, et al. abnormal levels of expression of plasma microRNA-33 in patients with psoriasis. Actas Dermosifiliogr 2014; 105:497–503. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Wang LT, Liang GP, et al. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin Immunol 2014; 150:22–30. [DOI] [PubMed] [Google Scholar]
- 29.Hirao H, Jinnin M, Ichihara A, et al. Detection of hair root miR-19a as a novel diagnostic marker for psoriasis. Eur J Dermatol 2013; 23:807–811. [DOI] [PubMed] [Google Scholar]
- 30.Tsuru Y, Jinnin M, Ichihara A, et al. miR-424 levels in hair shaft are increased in psoriatic patients. J Dermatol 2014; 41:382–385. [DOI] [PubMed] [Google Scholar]
- 31.Xia J, Joyce CE, Bowcock AM, et al. Noncanonical microRNAs and endogenous siRNAs in normal and psoriatic human skin. Hum Mol Genet 2013; 22:737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sethupathy P, Collins FS. MicroRNA target site polymorphisms and human disease. Trends Genet 2008; 24:489–497. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Dong H, Luo L, et al. A novel statistic for genome-wide interaction analysis. PLoS Genet 2010; 6:e1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Yi X, Guo S, et al. A single-nucleotide polymorphism of miR-146a and psoriasis: an association and functional study. J Cell Mol Med 2014; 18:2225–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatzikyriakidou A, Voulgari PV, Georgiou I, et al. The role of microRNA-146a (miR-146a) and its target IL-1R-associated kinase (IRAK1) in psoriatic arthritis susceptibility. Scand J Immunol 2010; 71:382–385. [DOI] [PubMed] [Google Scholar]
- 36.Wu LS, Li FF, Sun LD, et al. A miRNA-492 binding-site polymorphism in BSG (basigin) confers risk to psoriasis in central south Chinese population. Hum Genet 2011; 130:749–757. [DOI] [PubMed] [Google Scholar]
- 37.Majorczyk E, Matusiak L, Nowak I, et al. A single nucleotide polymorphism -35 kb T > C (rs9264942) is strongly associated with psoriasis vulgaris depending on HLA-Cw(∗)06. Hum Immunol 2014; 75:504–507. [DOI] [PubMed] [Google Scholar]
- 38.Schonthaler HB, Guinea-Viniegra J, Wagner EF. Targeting inflammation by modulating the Jun/AP-1 pathway. Ann Rheum Dis 2011; 70 Suppl 1:i109–112. [DOI] [PubMed] [Google Scholar]
- 39.Kitoh A, Nomura T, Kabashima K. TGFbeta1, an epidermal controller of skin dendritic cell homeostasis. J Invest Dermatol 2013; 133:9–11. [DOI] [PubMed] [Google Scholar]
- 40.Xu WD, Pan HF, Li JH, et al. MicroRNA-21 with therapeutic potential in autoimmune diseases. Expert Opin Ther Targets 2013; 17:659–665. [DOI] [PubMed] [Google Scholar]
- 41.Fu D, Yu W, Li M, et al. MicroRNA-138 regulates the balance of Th1/Th2 via targeting RUNX3 in psoriasis. Immunol Lett 2015; 166:55–62. [DOI] [PubMed] [Google Scholar]
- 42.Fayyad-Kazan H, Rouas R, Fayyad-Kazan M, et al. MicroRNA profile of circulating CD4-positive regulatory T cells in human adults and impact of differentially expressed microRNAs on expression of two genes essential to their function. J Biol Chem 2012; 287:9910–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi HR, Nam KM, Park SJ, et al. Suppression of miR135b increases the proliferative potential of normal human keratinocytes. J Invest Dermatol 2014; 134:1161–1164. [DOI] [PubMed] [Google Scholar]
- 44.Guo S, Zhang W, Wei C, et al. Serum and skin levels of miR-369-3p in patients with psoriasis and their correlation with disease severity. Eur J Dermatol 2013; 23:608–613. [DOI] [PubMed] [Google Scholar]
- 45.Xia J, Zhang W. MicroRNAs in normal and psoriatic skin. Physiol Genomics 2014; 46:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zibert JR, Lovendorf MB, Litman T, et al. MicroRNAs and potential target interactions in psoriasis. J Dermatol Sci 2010; 58:177–185. [DOI] [PubMed] [Google Scholar]
- 47.Gu X, Nylander E, Coates PJ, et al. Effect of narrow-band ultraviolet B phototherapy on p63 and microRNA (miR-21 and miR-125b) expression in psoriatic epidermis. Acta Derm Venereol 2011; 91:392–397. [DOI] [PubMed] [Google Scholar]
- 48.Finch PW, Murphy F, Cardinale I, et al. Altered expression of keratinocyte growth factor and its receptor in psoriasis. Am J Pathol 1997; 151:1619–1628. [PMC free article] [PubMed] [Google Scholar]
- 49.Ichihara A, Jinnin M, Yamane K, et al. microRNA-mediated keratinocyte hyperproliferation in psoriasis vulgaris. Br J Dermatol 2011; 165:1003–1010. [DOI] [PubMed] [Google Scholar]
- 50.Yan S, Xu Z, Lou F, et al. NF-kappaB-induced microRNA-31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nat Commun 2015; 6:7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y, Pan HF, Ye DQ. microRNAs function in CD8 + T cell biology. J Leukoc Biol 2015; 97:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pivarcsi A, Meisgen F, Xu N, et al. Changes in the level of serum microRNAs in patients with psoriasis after antitumour necrosis factor-alpha therapy. Br J Dermatol 2013; 169:563–570. [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Zhang H, Rodriguez S, et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-kappaB-dependent manner. Cell stem cell 2014; 15:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baldoni S, Sportoletti P, Del Papa B, et al. NOTCH and NF-kappaB interplay in chronic lymphocytic leukemia is independent of genetic lesion. Int J Hematol 2013; 98:153–157. [DOI] [PubMed] [Google Scholar]
- 55.Peng H, Kaplan N, Hamanaka RB, et al. microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc Natl Acad Sci U S A 2012; 109:14030–14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol 2014; 229:1630–1638. [DOI] [PubMed] [Google Scholar]
- 57.Killeen ME, Ferris L, Kupetsky EA, et al. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol 2013; 190:4324–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovendorf MB, Zibert JR, Hagedorn PH, et al. Comparison of microRNA expression using different preservation methods of matched psoriatic skin samples. Exp Dermatol 2012; 21:299–301. [DOI] [PubMed] [Google Scholar]


