Abstract
Follicular helper T (Tfh) cells are recognized as a distinct CD4+helper T cell subset, and mainly dysregulated in the autoimmune disease, whether it plays a role in the infectious mononucleosis (IM) diseases is unknown. In this study, we found that the CD4+CXCR5+ Tfh cells were not significantly changed, but the CD4+CXCR5+ICOS+ and CD4+CXCR5+ICOS+PD1+ Tfh subsets were significantly increased in the IM patients, and all these cells were significantly changed after antiviral therapy. Second, only the numbers of CD4+CXCR5+ICOS+PD1+ Tfh cells correlated with the Epstein-Barr virus (EBV) DNA load, negatively correlated with the numbers of naive B cells and amount of IL-21, and positively correlated with the numbers of plasma cells, memory B cells, and atypical lymphocytes. Third, the frequency of CD4+CXCR5+ICOS+PD1+ Tfh subset was significantly higher in lymphadenectasis or hepatosplenomegaly patients, and associated with the level of alanine aminotransferase (ALT). All together, our findings discovered this CD4+CXCR5+ICOS+PD1+ Tfh cell subset might play an important role in the pathogenesis of IM.
INTRODUCTION
Infectious mononucleosis (IM) is usually a benign self-limiting disease, which results most often from a primary Epstein-Barr virus (EBV) infection. EBV, which is a member of the Herpes virus family, and the acute EBV infection is the most frequent clinical manifestation of IM disease. Primary EBV infection in children is usually asymptomatic. In symptomatic children, mild fever, a sore throat, and swollen lymph nodes in the neck area are the first signs of symptomatic infection; then a large number of atypical lymphoblasts, mainly CD8+ T cell origin, will appear in the blood.1 Moreover, some children could develop hepatitis or spleen swelling and need the antiviral treatment.2
Recently, follicular helper T (Tfh) cells have been described as a new subset regulating the development of antigen-specific B-cell immunity.3–6 Expression of CXCR5, along with the loss of the T-cell zone homing chemokine receptor CCR7, allows Tfh cells to relocate from the T-cell zone to the B-cell follicles, where they are positioned to directly support B-cell expansion and differentiation.7 Additionally, antibody production by B lymphocytes requires “help” from Tfh cells in the form of cytokines and many surface molecules.8 Among them, the CD28 family members, programmed death-1 (PD-1) and inducible costimulator (ICOS), are 2 distinguishing molecules closely related to the function of Tfh cells.9,10 Furthermore, the IL-21 cytokine is also critical for the formation of germinal centers and the development of Tfh cells.5 Together, these molecules promote the growth, differentiation, and class switching of B cells.5,10 In recent years, abnormal Tfh cells frequency and certain molecules highly expressed by Tfh cells have been observed in mice and human with autoimmune diseases,5 which included systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), sjogrens syndrome (SS), autoimmune thyroid disease (AITD).
Additionally, recent research found that circulating Tfh cells were dysregulated in patients with hepatitis B (HBV) or human immunodeficiency virus (HIV) infection.11,12 However, study in EBV-related IM disease is lacking. Herein, these Tfh cells were investigated in peripheral blood from 61 IM patients. Among them, the CD4+CXCR5+ Tfh cells were not significantly changed, CD4+CXCR5+ICOS+ and CD4+CXCR5+ICOS+PD1+ Tfh subset cells were all significantly increased in IM patients compared with the health controls. Furthermore, only the numbers of CD4+CXCR5+ICOS+PD1+ Tfh cells correlated with the level of EBV DNA load, and significantly negative correlated with the numbers of naive B cells and amount of IL-21, positively correlated with the numbers of memory B cells and plasma cells. Moreover, these CD4+CXCR5+ICOS+PD1+ Tfh cells were positively correlated with atypical lymphocytes or CD8+CD38+ cells. At last, we found that the level of CD4+CXCR5+ICOS+PD1+ with lymphadenectasis or hepatosplenomegaly IM patients was significantly higher than those patients without lymphadenectasis or hepatosplenomegaly, and also positively correlated with the ALT, an indicator for the liver function damage.
MATERIAL AND METHODS
Ethics Statement
All patients and healthy controls gave their written informed consent by their parents on behalf of themselves for their sample analysis in accordance with the Declaration of Helsinki. The study was approved by the Zhejiang Provincial People's Hospital Review Board.
Patient Characteristics and Samples
The diagnosis criteria of infectious mononucleosis was as reference.13 Peripheral blood samples were collected from 61 untreated IM patients (37 boys and 24 girls; mean age, 41.35 ± 28.74 months) and 21 age-matched healthy controls (12 boys and 9 girls; mean age, 37.71 ± 29.36 months) were included in this study who had not been treated at the time and the samples were collected for the first diagnose in our hospital. Samples were collected on the 3rd day after the children had a fever where the 3rd day was the typical acute EBV infection phase and recover patients samples were collected on the 15th day after the children had a fever. Additionally, for the recover IM patients, they were given Ganciclovir (5 mg/kg, ivgtt, q12 h) antiviral drugs for 3 to 5 days depends on the individual patient. All these samples were screened by the serological tests which include herpes simplex virus 2, rubella virus, cytomegalovirus, toxoplasma, rotavirus, coxsackie virus, mycoplasma, chlamydia, and hepatitis A, B, C, D to exclude other virus or bacteria infection. Additionally, any children with immune and chronic infectious diseases were also excluded. Peripheral blood mononuclear cells (PBMCs) were collected and isolated by density-gradient centrifugation using Ficoll-Hypaque solution.
Clinical Parameters
Lymphocyte absolute number analysis was done by the Sysmex XE-2100 Automated Hematology System. The absolute number of each lymphocyte subset was calculated by the frequency multiply the lymphocyte absolute number.
Flow Cytometry
The following antibodies were used for flow cytometry: PerCP-CD3 (SK7, BD Bioscience), PE-CD4 (13B8.2, Beckman Coulter), Alexa Fluor 488-CXCR5 (RF8B2, BD Bioscience), PerCP-Cy5.5-PD-1 (EH12.1, eBioscience), APC-ICOS (ISA-3, eBioscience), PE-CD27 (M-T271, BD Bioscience), FITC-CD8 (SK1, BD Bioscience), FITC-CD19 (J3.11P, Beckman Coulter), PE-CD19 (J4.11P, Beckman Coulter), FITC-CD23 (9P25, Beckman Coulter), PE-CD38 (HB7, BD Bioscience). Appropriate isotype controls were used. Cells stained with separate antibodies were defining CD3+ T cells (CD3+), CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B cells (CD3−CD19+), activated B cell (CD19+CD23+), naive B cell (CD19+CD27−), memory B cell (CD19+CD27+), and plasma cell (CD19+CD27hi). Isotype-matched Ab controls were used in all procedures. All the staining was performed according to manufacturers’ protocol. The stained cells were then analyzed by using a FACS CanoII flow cytometer and diva software (Becton Dickinson, Sparks, MD).
EBV Serology and EBV-DNA Quantification
Serum samples were screened for the presence of immunoglobulin (Ig) M (IgM) antibodies to viral-capsid antigen (VCA) by using commercially available enzyme immunoassays (Liason Liason VCA IgM, DiaSorin S.p.A.) according to the manufacturer's instructions. EBV-DNA was measured in the PBMCs of 57 IM patients at the time of initial diagnosis, using by a commercial real-time PCR kit, amplifying a 191 bp region of the EBNA-1 gene (BioQuant EBV, Biodiversity), according to the manufacturer's protocol, and detected by the ABI PRISM 7500 Sequencer Detection System (Applied Biosystems, USA).
Enzyme-Linked IMMUNOSORBENT Assay (ELISA)
Serum samples were stored at –80°C. The levels of the serum IL-21 in individual IM patients and healthy controls were measured by ELISA using Human IL-21 Platinum ELISA Kit with Pre-coated Plates ELISA kits (eBioscience, 85-BMS2043) according to the manufacturer's instructions. All samples were run in triplicate.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5.01 software. Statistical tests for data analysis included the Mann–Whitney U test. Correlations between variables were determined using Spearman's correlation coefficient. The quantitative data were presented as the mean values ± standard deviations (SD). Differences were considered to be statistically significant at values of ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
RESULTS
CD3+T and CD3+CD8+T Cells Were Significantly Increased in the Peripheral Blood of IM Patients
It is well known that infectious mononucleosis is associated with dramatic disturbances of the immune system including raised CD8+ T cell and NK cell numbers. Herein, we found that the frequencies of CD3+T (% lymphocyte) and CD3+CD8+T (% CD3+ T cells) cells were significantly increased in the peripheral blood of IM patients when compared with the healthy controls (P < 0.0001), similar results were also found in the absolute number of these cells (Fig. 1A, B). In contrast, the frequencies of CD3+CD4+T (% CD3+ T cells) and CD3−CD19+B (% lymphocyte) cells were significantly decreased in the peripheral blood of IM patients when compared with the healthy controls (P < 0.0001); however, the absolute numbers of CD3+CD4+T and CD3-CD19+B cells were not significantly different (Fig. 1A, B). Furthermore, these results were consistent with the previous finding,14 where the IM disease is characterized with the robust CD8+ T cells immune response to the EBV.
FIGURE 1.
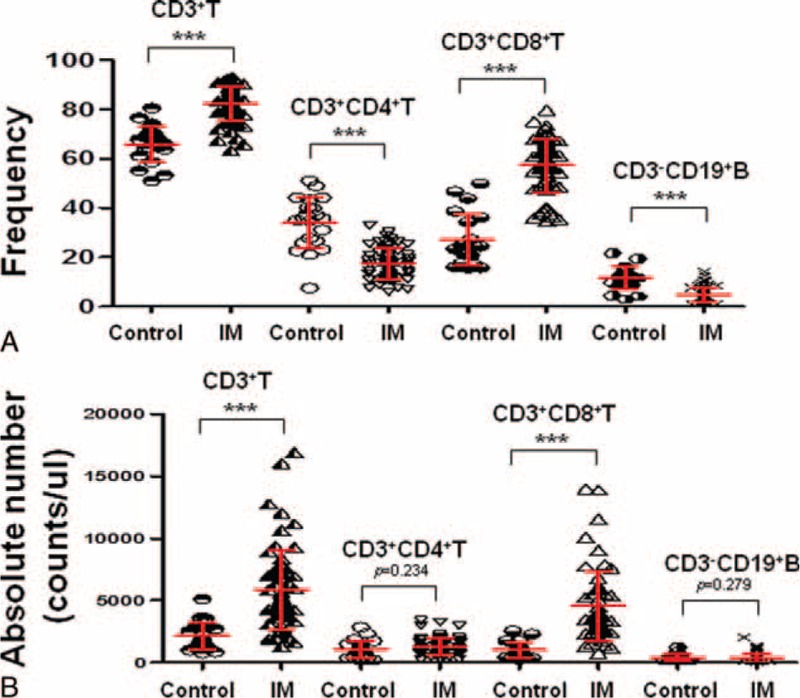
CD3+T and CD3+CD8+T cells were significantly increased in the peripheral blood of IM patients. A, The frequencies of CD3+T (% lymphocyte), CD3+CD4+T (% CD3+ T cells), CD3+CD8+T (% CD3+ T cells), and CD3-CD19+B (% lymphocyte) cells among the 21 healthy controls and 61 new onset IM patients are shown. B, The absolute numbers of CD3+T, CD3+CD4+T, CD3+CD8+T, and CD3−CD19+B cells among the 21 healthy controls and 61 new onset IM patients are shown. Each data point represents an individual subject. ∗∗∗P < 0.001. NS = no significant; Mann–Whitney U test.
CD4+CXCR5+ Tfh Cells Were Not Significantly Changed in the Peripheral Blood of IM Patients
Peripheral blood lymphocytes from 61 IM patients and 21 healthy individuals were analyzed by flow cytometry. CD4+ T cells were identified and then different Tfh cell markers were used to identify the Tfh subsets (Fig. 2A). Among the different subsets of Tfh cells, the frequency of CD4+CXCR5+ was significantly decreased in IM patients compared with healthy controls (P < 0.0001) (Fig. 2B, C). However, when these IM patients receive the antiviral treatment, the frequency of CD4+CXCR5+ was significantly increased (P < 0.0001) (Fig. 2B, C). Moreover, when we analyzed the absolute number of these cells, we found that there is no significant change between the IM patients and healthy controls (Fig. 2D), but significantly elevated when these IM patients receive the antiviral treatment compared with the new onset IM patients (P < 0.001) (Fig. 2D).
FIGURE 2.
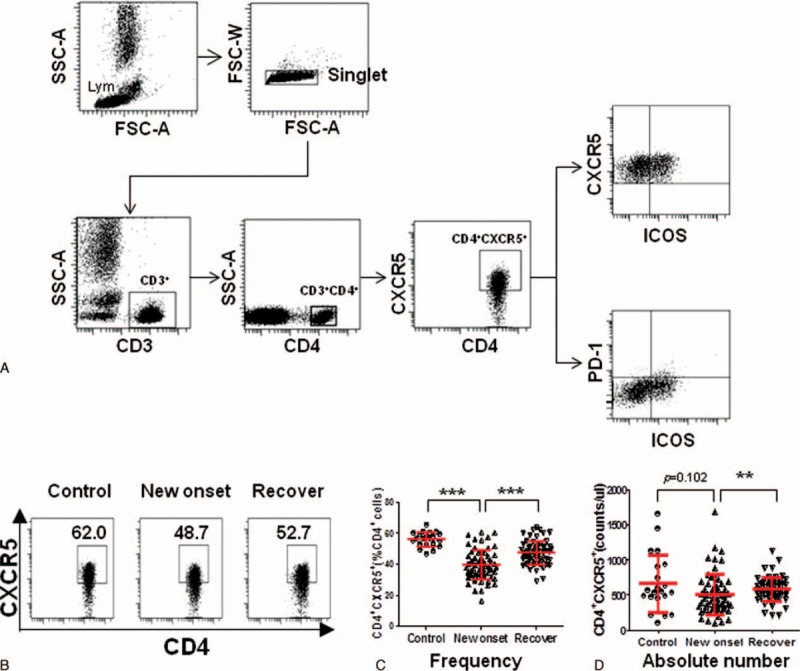
The CD4+CXCR5+ Tfh cells were not significantly changed in the peripheral blood of IM patients. A, Representative dot plots of CD4+CXCR5+, CD4+CXCR5+ICOS+, and CD4+CXCR5+ICOS+PD-1+ cells and gating criteria from the peripheral blood of IM patients are shown. Values in the upper right quadrant correspond to the percentage of CD4+CXCR5+, CD4+CXCR5+ICOS+ cells, or CD4+CXCR5+ICOS+PD-1+ Tfh cells. We used isotype controls to determine the positive cells, and all the values are gated on the CD4+cells. B, Representative dot plots of CD4+CXCR5+ Tfh cells among the healthy controls, new onset IM patients, and after antiviral treatment IM patients are shown. C, The percentage of CD4+CXCR5+ Tfh cells was compared among the 21 healthy controls, 61 new onset IM patients, and 61 after antiviral treatment IM patients. D, The absolute number of CD4+CXCR5+ Tfh cells was compared among the 21 healthy controls, 61 new onset IM patients, and 61 after antiviral treatment IM patients. Each data point represents an individual subject. The bars indicate the mean values. ∗∗P < 0.01, ∗∗∗P < 0.001, Mann–Whitney U test.
CD4+CXCR5+ICOS+ and CD4+CXCR5+ICOS+PD1+ Tfh Cells Were Increased in the Peripheral Blood of IM Patients
Typical features of Tfh cells are the expression of CXCR5, ICOS, and PD-1.12 Thus, other Tfh subsets, such as CD4+CXCR5+ICOS+ and CD4+CXCR5+ICOS+PD-1+ Tfh cells, were also investigated in IM patients. To our surprise, the frequency and absolute number of all these cells were all significantly increased in the peripheral blood of IM patients when compared with the healthy controls (P < 0.0001) (Fig. 3A–F). Moreover, when these IM patients receive antiviral treatment, the frequency and absolute number of CD4+CXCR5+ICOS+ and CD4+CXCR5+ICOS+PD-1+ cells were all significantly decreased (P < 0.01, P < 0.0001) (Fig. 3A–F).
FIGURE 3.
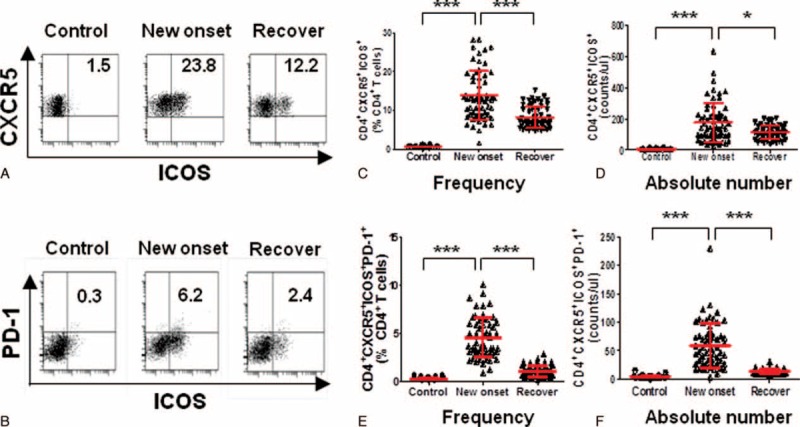
The CD4+CXCR5+ICOS+ and CD4+CXCR5+ICOS+PD1+ cells were increased in the peripheral blood of IM patients. Representative dot plots of CD4+CXCR5+ICOS+ (A), CD4+CXCR5+ICOS+PD1+ (B) cells among the 21 healthy controls, 61 new onset IM patients, and 61 after antiviral treatment IM patients are shown. The frequency and absolute number of CD4+CXCR5+ICOS+ (C, D), CD4+CXCR5+ICOS+PD1+ (E, F) cells was compared among the 21 healthy controls, 61 new onset IM patients, and 61 after antiviral treatment IM patients. Each data point represents an individual subject. The bars indicate the mean values. ∗P < 0.05, ∗∗∗P < 0.001. Mann–Whitney U test.
Upregulation of CD4+CXCR5+ICOS+PD1+ Tfh Cells Was Positively Correlated With the EBV DNA Load in IM Patients
Since all the Tfh subsets were dysregulated in the IM patient, this phenomenon drives us to know whether these Tfh subsets are associated with the EBV DNA load of IM patients, where the IM disease is mainly caused by the EBV and the clinical symptoms are provoked by the strong immune response. Strikingly, Spearman's correlation analysis revealed that only the CD4+CXCR5+ICOS+PD1+ Tfh subset was positively correlated with EBV DNA load (r = 0.333, P = 0.011) (Fig. 4A–C), although there is no correlation with CD4+CXCR5+ and CD4+CXCR5+ICOS+ cells (Fig. 4A, B). Additionally, primary EBV infection can trigger the production of antibodies against the VCA in IM patients, and EBV infection was diagnosed in 94% of patients by the VCA capsid antigen.15 Herein, we divided all the IM patients into 2 groups: VCA IgM(+) group and VCA IgM(−) group. However, statistical analysis shows that there is no significant difference between the VCA IgM(−) and VCA IgM(+) subgroups in the frequency of CD4+CXCR5+ICOS+PD1+ Tfh cells (P = 0.105) (Fig. 4D).
FIGURE 4.
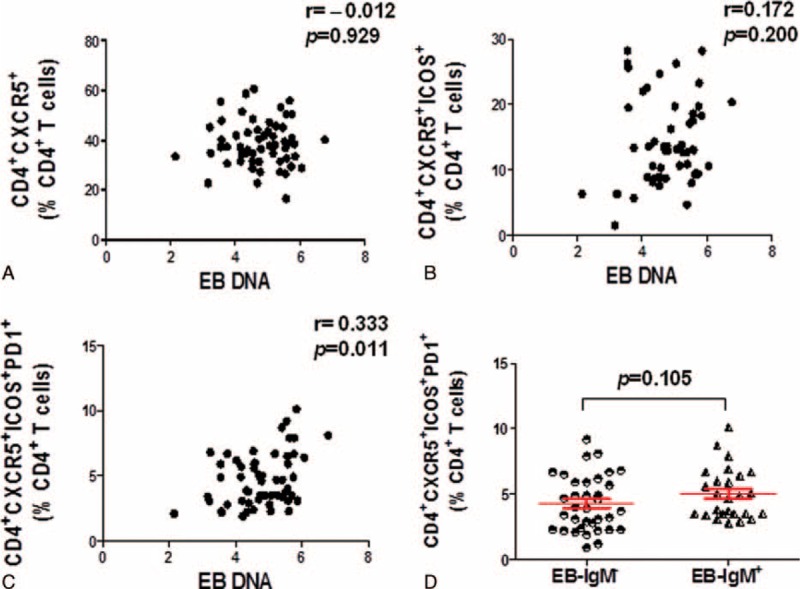
The percentage of CD4+CXCR5+ICOS+PD1+ Tfh cells was significantly correlated with the EB DNA load in IM patients. The correlation between the percentage of CD4+CXCR5 (A), CD4+CXCR5+ICOS+ (B), CD4+CXCR5+ICOS+PD-1+ (C) T cells with the log value of EB DNA load in PBMC of IM patients (n = 57), respectively. D, The percentage of CD4+CXCR5+ICOS+PD-1+ Tfh cells was compared with the 35 EB-IgM negative and 26 EB-IgM positive IM patients. Mann–Whitney U test.
Upregulation of CD4+CXCR5+ICOS+PD1+ Tfh Cells Was Significantly Negatively Correlated With Naive B Cell and IL-21, and Positively Correlated With Memory B Cell and Plasma Cell
Whether these Tfh cells are correlated with the different B subsets in IM patients with acute EBV infection is unknown. Herein, the different B subsets were detected, which included CD19+CD23+ activated B cell, CD19+CD27− naive B cell, CD19+CD27+ memory B cell, and CD19+CD27hi plasma cell. Most importantly, our Spearman's correlation analysis revealed that only the numbers of CD4+CXCR5+ICOS+PD1+ Tfh cells were significantly negatively correlated with the numbers of CD19+CD27− naive B cells (r = –0.7463, P < 0.0001), positively correlated with the numbers of CD19+CD27+ memory B cells (r = 0.7463, P < 0.0001), and CD19+CD27hi plasma cells (r = 0.6774, P < 0.0001) (Fig. 5A–D). Moreover, previous studies indicated that IL-21 play a critical role in Tfh differentiation and B-cell immunity.5 Thus, the IL-21 of the peripheral blood sample of the IM patients were analyzed and the result shows that the CD4+CXCR5+ICOS+PD1+ Tfh cells were negatively correlated with the IL-21 expression (r = –0.3021, P = 0.022) (Fig. 5E).
FIGURE 5.
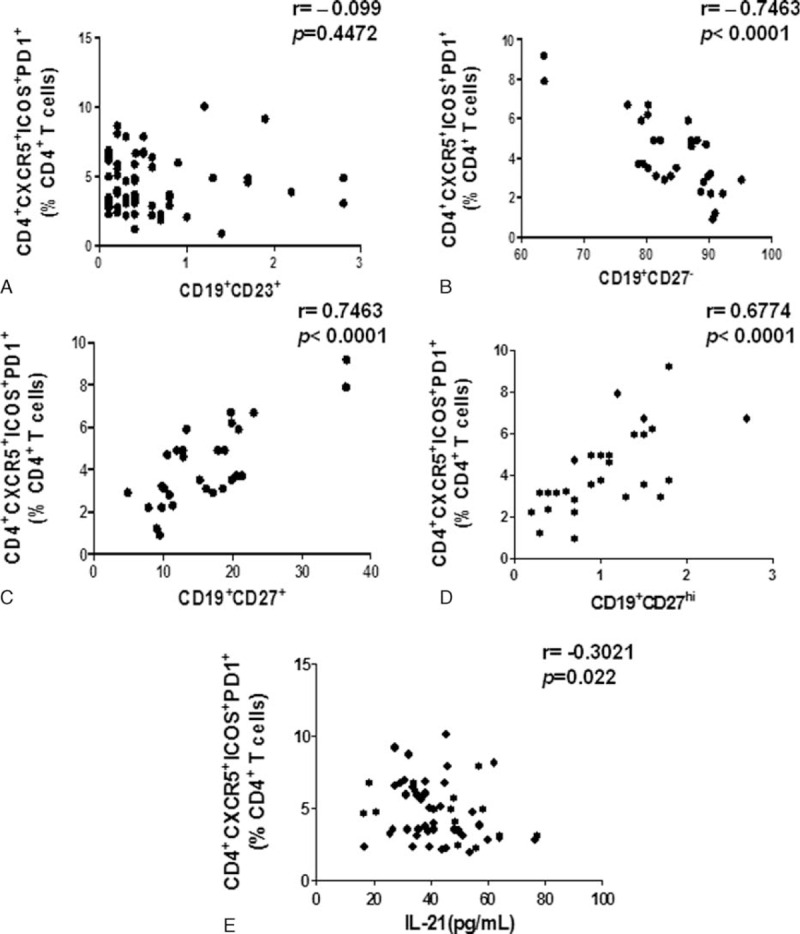
The numbers of CD4+CXCR5+ICOS+PD1+ Tfh cells were significantly negatively correlated with the numbers of naive B cells and the amount of IL-21, and positively correlated with the numbers of memory B cells and plasma cells in IM patients. The correlation between the numbers of CD4+CXCR5+ICOS+PD-1+ Tfh cells with the numbers of CD19+ CD23+ active B cells (n = 61) (A), CD19+CD27− naive B cells (n = 29) (B), CD19+CD27+ memory B cells (n = 29) (C), CD19+CD27hi plasma cells (n = 29) (D), and the amount of IL-21(n = 57) (E) in IM patients.
Upregulation of CD4+CXCR5+ICOS+PD1+ Tfh Cells Was Positively Correlated With Atypical Lymphocytes and CD8+CD38+ Cells
The IM patient is diagnosed hematologically by the presence of large numbers of atypical lymphocytes in the blood, where the CD8+CD38+ activated T cells are regarded as the atypical lymphocytes in the IM patients.16 Thus, the Spearman's correlation analysis was used to analyze the relationship between the number of Tfh cells and atypical lymphocytes. Herein, the CD4+CXCR5+ICOS+PD1+ Tfh cells were positively correlated with atypical lymphocytes (P = 0.009, r = 0.334) (Fig. 6A), also with the CD8+CD38+ activated T cells (P = 0.011, r = 0.32) (Fig. 6B). Together, these data suggest that this Tfh subset is associated with the immune response of the IM patients.
FIGURE 6.
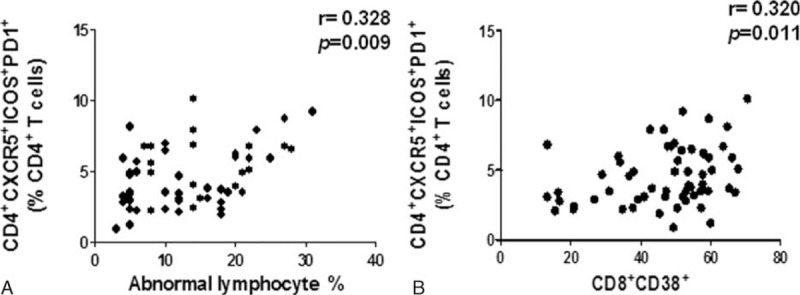
The percentage of CD4+CXCR5+ICOS+PD1+ Tfh cells was significantly correlated with the atypical lymphocytes and CD8+CD38+ T cells in IM patients. The correlation between the percentage of CD4+CXCR5+ICOS+PD-1+ Tfh cells with atypical lymphocytes (A) and CD8+CD38+ (B) in IM patients (n = 61).
Upregulation of CD4+CXCR5+ICOS+PD1+ Tfh Cells Was Correlated With the IM Disease Progression
In the clinic, some IM patients will develop lymphadenectasis and hepatosplenomegaly and need the antiviral treatment. This phenomenon drives us wonder that whether these CD4+CXCR5+ICOS+PD1+Tfh cells play an important role in the clinical progression of IM patients. Subsequently, when comparing IM patients grouped according to the lymphadenectasis, the level of CD4+CXCR5+ICOS+PD1+ in lymphadenectasis IM patients was significantly higher than other IM patients without lymphadenectasis (P = 0.033) (Fig. 7A), and this Tfh subset was also higher in hepatosplenomegaly subgroup than other IM patients without hepatosplenomegaly (P < 0.0001) (Fig. 7B). Moreover, when we compare the other clinical characteristics that included alanine aminotransferase (ALT), aspartate transaminase (AST), creatine kinase (CK), and creatine kinase MB isoenzyme (CK-MB) with these CD4+CXCR5+ICOS+PD1+ Tfh cells (Fig. 7C–F), we found that these Tfh cells were only significant positively correlated with the ALT index (P = 0.007).
FIGURE 7.
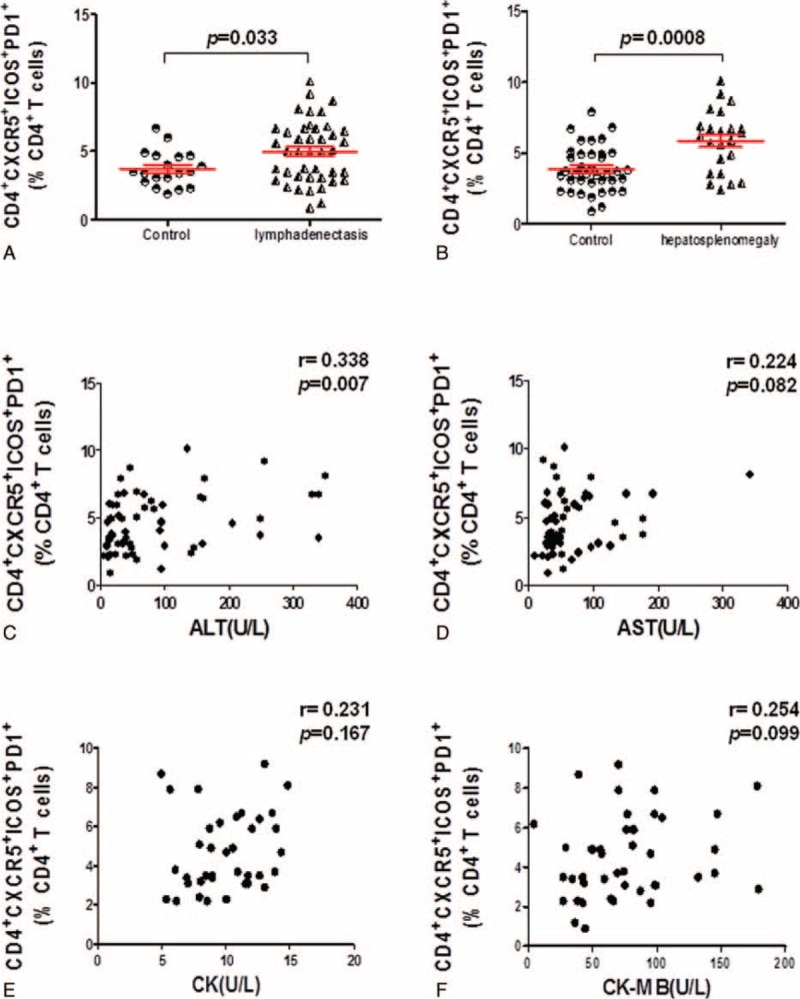
The percentage of CD4+CXCR5+ICOS+PD1+ Tfh cells was significantly increased in lymphadenectasis or hepatosplenomegaly IM patients. A, The percentage of CD4+CXCR5+ICOS+PD-1+ Tfh cells in lymphadenectasis IM patients (n = 42) was significantly elevated compared with other IM patients without lymphadenectasis (n = 19) (P = 0.033). B, The percentage of CD4+CXCR5+ICOS+PD-1+ Tfh cells was significantly elevated in hepatosplenomegaly patients (n = 23) compared with other IM patients without hepatosplenomegaly (n = 38) (P = 0.0008). C–F, The correlation between the percentage of CD4+CXCR5+ICOS+PD-1+ Tfh cells with the clinical index which includes ALT (n = 61) (A), AST (n = 61) (B), CK (n = 37) (C), and CK-MB (n = 43) (D) in IM patients.
DISCUSSION
In this study, we confirmed that the CD3+T and CD3+CD8+T cells were significantly increased in the peripheral blood of IM patients, but showed that the absolute numbers of CD3−CD19+B, CD3+CD4+T, and CD4+CXCR5+ Tfh cells were not significantly changed. However, the frequency of CD4+CXCR5+ Tfh cells was significantly decreased in IM patients compared with healthy controls. Interestingly, elevated frequency of CD4+CXCR5+ Tfh cells had been reported in other virus infection patients, such as hepatitis B virus, hepatitis C virus, and Enterovirus 71.11,17,18 In contrast, in this study, we found that unlike the increased frequency of the CD4+CXCR5+ Tfh cells in these virus infection patients, the frequency of CD4+CXCR5+ Tfh cells was significantly decreased in IM patients compared with the healthy controls, when these IM patients receive antiviral treatment, the frequency of these CD4+CXCR5+ Tfh cells was significantly elevated.
These interesting phenomena drove us to further analyze other Tfh subsets in the infectious mononucleosis patients and show that the frequency and absolute number of CD4+CXCR5+ICOS+ and CD4+CXCR5+ICOS+PD-1+ cells were all significantly increased in IM patients compared with the healthy controls. Also, when the IM patients were treated with antiviral drugs, the frequency and absolute number of these cells were all significantly decreased. Most importantly, only the CD4+CXCR5+ICOS+PD1+ Tfh cells were correlated with the level of EB DNA load, and significantly negatively correlated with the numbers of naive B cells and the amount of IL-21, positively correlated with the numbers of memory B cells and plasma cells. Moreover, only the CD4+CXCR5+ICOS+PD1+ Tfh subset was positively correlated with atypical lymphocytes or CD8+CD38+ cells, and significantly higher in lymphadenectasis or hepatosplenomegaly patients than other IM patients, with a correlation to the ALT levels. Traditionally, the interaction between Tfh cell with the B cell typically occurs in germinal centers (GCs) located within the secondary lymphoid organ sites, where the Tfh cells also produce factors essential for B-cell selection and maturation into memory B cells or long-lived antibody-secreting plasma cells.19 When aberrantly regulated, Tfh cells can migrate to the periphery, where they augment inflammation, as occurs in the human tonsil or the kidney in SLE or in the brain in multiple sclerosis, or in the asthmatic lung.19,20 Although much progress has been made in understanding the cues that control B-cell migration in and out of the different compartments in secondary lymphoid organs, including germinal centers, little is known regarding the key determinants of Tfh cell migration, persistence and exit from germinal centers, besides the role of CXCR5. Whether human blood CD4+CXCR5+ Tfh cells originate from cells that migrated out of GCs or Tfh-committed extrafollicular helper cells is still unknown.21 For IM patients, the increase in numbers of CD4+CXCR5+ICOS+PD1+ Tfh cells in peripheral blood could be due to release from germinal centers into the blood rather than actual increases in numbers of cells in the body. However, current data cannot verify this hypothesis, only further study can verify it.
In this study, we collect peripheral blood samples on the 3rd day after the children had a fever, and the IM patients with the serologic test alone are not sensitive enough since the time for the VCA IgM antibody varies differently in the different IM patients, and this may explain the phenomenon that there is no significant difference between the EB-IgM− or EB-IgM+ IM patients in the frequency of CD4+CXCR5+ICOS+PD1+ Tfh cells.
Previously, Collins and Speck22 found that in the mouse model of murine gammaherpesvirus 68 (MHV68) infection, MHV68 resembles EBV, differentiation of naive T cells into Tfh cells is critical for providing helper functions to GC B cells infected with MHV68. In the absence of Tfh cells, MHV68-infected GC B cells are unable to proliferate. However, the human EBV is thought to be able to accomplish this without the need for CD4 T cell help by expressing viral proteins that mimic signals from CD4 T cells–LMP1 mimics constitutive CD40 signaling23 whereas LMP2A provides survival signals that recapitulate those provided by the BCR.24 Additionally, the EBV gene BCRF1 also encodes an IL10 analog that can promote the B-cell growth.25 Therefore, in this observation study, although CD4+CXCR5+ICOS+PD1+ Tfh cells were correlated with the different B cell subsets, we cannot conclude that these Tfh cells can provide help to the EBV-infected B cell; further study is needed to investigate the function of this elevated Tfh subset. Also we cannot rule out the possibility that these IM patients with more severe symptoms are likely to have greater general immune disturbance and so it might be that they would also have the greatest frequency and absolute number of CD4+CXCR5+ICOS+PD1+ Tfh cells, if these cells are also affected by the overall immune disturbance.
Overall, for the first time, we indicate that dysregulated CD4+CXCR5+ICOS+PD1+ Tfh cell subsets may play an important role in the pathogenesis of IM disease. However, in vitro experiment for the study of the function of CD4+CXCR5+ICOS+PD1+Tfh cell will be necessary to validate these findings and it will provide new insight into the understanding of immune mechanism of the acute EBV infection IM patients.
Acknowledgment
For LQ: supported by Zhejiang Provincial Natural Science Fund (No: LY12H16019), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (2012) and Zhejiang Provincial Health Bureau (No: 2010KYA015). For JL: supported by National Natural Science Foundation of China (No: 81502472), Zhejiang Provincial Natural Science Fund (No: LQ14H100001), Zhejiang Provincial Health Bureau (No: 2014KYA015), and Outstanding Youth Foundation of Zhejiang Provincial People's Hospital (No: 2015 A level).
Footnotes
Abbreviations: ALT = alanine aminotransferase, EBV = Epstein-Barr virus, ICOS = inducible costimulator, IM = infectious mononucleosis, PD-1 = programmed death-1, Tfh cells = follicular helper T cells, VCA = viral-capsid antigen.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
XL and GC managed the clinic and recruited the IM patient cohort; JL and LQ performed the experimental work; QY, ZZ, HW, YC, ZZ, and MW helped the experimental work; JL, YZ, and LQ analyzed the data; JL, YZ, and LQ designed the study; and JL and LQ wrote the article with input from all authors. All authors reviewed the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Clute SC, Watkin LB, Cornberg M, et al. Cross-reactive influenza virus-specific CD8 + T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest 2005; 115:3602–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hislop AD, Taylor GS, Sauce D, et al. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol 2007; 25:587–617. [DOI] [PubMed] [Google Scholar]
- 3.Fazilleau N, Mark L, McHeyzer-Williams LJ, et al. Follicular helper T cells: lineage and location. Immunity 2009; 30:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29:621–663. [DOI] [PubMed] [Google Scholar]
- 5.Tangye SG, Ma CS, Brink R, et al. The good, the bad and the ugly: TFH cells in human health and disease. Nat Rev Immunol 2013; 13:412–426. [DOI] [PubMed] [Google Scholar]
- 6.Yu D, Batten M, Mackay CR, et al. Lineage specification and heterogeneity of T follicular helper cells. Curr Opin Immunol 2009; 21:619–625. [DOI] [PubMed] [Google Scholar]
- 7.Schaerli P, Willimann K, Lang AB, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 2000; 192:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol 2009; 9:757–766. [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Ma J, Liu Y, et al. Increased frequency of follicular helper T cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 2012; 97:943–950. [DOI] [PubMed] [Google Scholar]
- 10.Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol 2011; 12:472–477. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Lu L, Hua C, et al. High frequency of CD4 + CXCR5 + TFH cells in patients with immune-active chronic hepatitis B. PLoS One 2011; 6:e21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol 2014; 92:64–71. [DOI] [PubMed] [Google Scholar]
- 13.Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med 2010; 362:1993–2000. [DOI] [PubMed] [Google Scholar]
- 14.Long HM, Taylor GS, Rickinson AB. Immune defence against EBV and EBV-associated disease. Curr Opin Immunol 2011; 23:258–264. [DOI] [PubMed] [Google Scholar]
- 15.Hohaus S, Santangelo R, Giachelia M, et al. The viral load of Epstein-Barr virus (EBV) DNA in peripheral blood predicts for biological and clinical characteristics in Hodgkin lymphoma. Clin Cancer Res 2011; 17:2885–2892. [DOI] [PubMed] [Google Scholar]
- 16.Odumade OA, Hogquist KA, Balfour HH., Jr Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev 2011; 24:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu TT, Song XF, Lei Y, et al. Expansion of circulating TFH cells and their associated molecules: involvement in the immune landscape in patients with chronic HBV infection. Virol J 2014; 11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Cui D, Yang X, et al. Increased frequency of circulating follicular helper T cells in children with hand, foot, and mouth disease caused by enterovirus 71 infection. J Immunol Res 2014; 2014:651872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol 2012; 8:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bentebibel SE, Schmitt N, Banchereau J, et al. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4 + T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci U S A 2011; 108:E488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins CM, Speck SH. Expansion of murine gammaherpesvirus latently infected B cells requires T follicular help. PLoS Pathog 2014; 10:e1004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 1999; 286:300–303. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell RG, Wilson JB, Anderson SJ, et al. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 1998; 9:405–411. [DOI] [PubMed] [Google Scholar]
- 25.Jochum S, Moosmann A, Lang S, et al. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog 2012; 8:e1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]


