There is still no evidence that neoadjuvant chemotherapy followed by interval debulking surgery is superior to primary debulking surgery in advanced ovarian cancer. Clinical status, tumor biology, and chemosensitivity should be taken into account to individualize surgical approach.
Keywords: Stage III–IV; Debulking; Randomized trials; Ovarian cancer, type 1 and 2; Categories
Abstract
Background.
Standard treatment of stage III and IV advanced ovarian cancer (AOC) consists of primary debulking surgery (PDS) followed by chemotherapy. Since the publication of the European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada trial, clinical practice has changed and many AOC patients are now treated with neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS). The best option remains unclear. Ovarian cancer is a heterogenic disease. Should we use the diversity in biology of the tumor and patterns of tumor localization to better stratify patients between both approaches?
Methods.
This analysis was based on results of five phase III randomized controlled trials on PDS and IDS in AOC patients, three Cochrane reviews, and four meta-analyses.
Results.
There is still no evidence that NACT-IDS is superior to PDS. Clinical status, tumor biology, and chemosensitivity should be taken into account to individualize surgical approach. Nonserous (type 1) tumors with favorable prognosis are less chemosensitive, and omitting optimal PDS will lead to less favorable outcome. For patients with advanced serous ovarian cancer (type 2) associated with severe comorbidity or low performance status, NACT-IDS is the preferred option.
Conclusion.
We propose stratifying AOC patients into five categories according to patterns of tumor spread (reflecting the biologic behavior), response to chemotherapy, and prognosis to make a more rational decision between PDS and NACT-IDS.
Implications for Practice:
Trial results regarding effect and timing of debulking surgery on survival of patients with advanced ovarian cancer have been inconsistent and hence difficult to interpret. This review examines all randomized trials on primary and interval debulking surgery in advanced ovarian cancer, including the results of the newly published CHORUS (chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer) trial. On the basis of findings presented in this review and in view of recent molecular data on the heterogeneity of ovarian tumors, we propose prognostic categorization for patients with advanced ovarian cancer to better distinguish those who would optimally benefit from primary debulking from those who would better benefit from interval debulking following neoadjuvant chemotherapy.
Introduction
With an estimated worldwide annual incidence of about 204,000 and causing 125,000 deaths [1], epithelial ovarian cancer (EOC) remains the leading cause of death in gynecological cancer. Because of its insidious onset without early specific symptoms and the lack of efficient screening techniques, two thirds of patients will present with advanced ovarian cancer (AOC)—stage III or IV according to the International Federation of Gynecology and Obstetrics (FIGO) [1, 2]. Despite advances in surgery, chemotherapy, and radiotherapy, the resulting 5-year overall survival (OS) is about 40% [1, 2]. Recent molecular studies showed that EOC is a heterogenic disease that varies markedly in biologic behavior, chemotherapy response, and prognosis [3, 4].
Primary debulking surgery (PDS) has been the standard of care [1]. Neoadjuvant chemotherapy (NACT) followed by an interval debulking surgery (IDS) is an alternative that has gained popularity, stimulated by the results of several randomized trials (RCTs) [5–8].
The aim of this study was to determine whether PDS and NACT followed by IDS are equivalent approaches with regard to patient outcome and whether the seemingly lower morbidity reported with NACT-IDS would favor that approach. To facilitate a more rational decision between both approaches, we propose stratifying AOC into 5 categories based on recent molecular data revealing heterogeneity within EOC. This heterogeneity is clinically reflected in different patterns of spread, biologic behavior, response to chemotherapy, and survival.
Methods
We searched PubMed for relevant articles published between January 1, 1985, and May 30, 2015. Potential articles reporting on primary or interval debulking in patients with AOC were identified by using the following PubMed search strategy: ((((((neoadjuvant) OR neoadjuvant therapy[MeSH Major Topic]) OR adjuvant) OR “chemotherapy, adjuvant”[MeSH Major Topic])) AND ((debulking) OR “cytoreduction surgical procedures”[MeSH Major Topic])) AND ((“ovarian neoplasms”[MeSH Major Topic]) OR ((ovar*) AND ((carc*) OR cancer*))) Abstracts were reviewed for relevance to the review subject. If the relevance was not clear in the abstract, the full text was assessed. All historical series, randomized trials, meta-analyses, and systematic reviews were included. All non-English articles and all reports from meetings, case reports, and editorials were excluded. If multiple publications from the same institution were available, the most recent publication was selected.
Results and Discussion
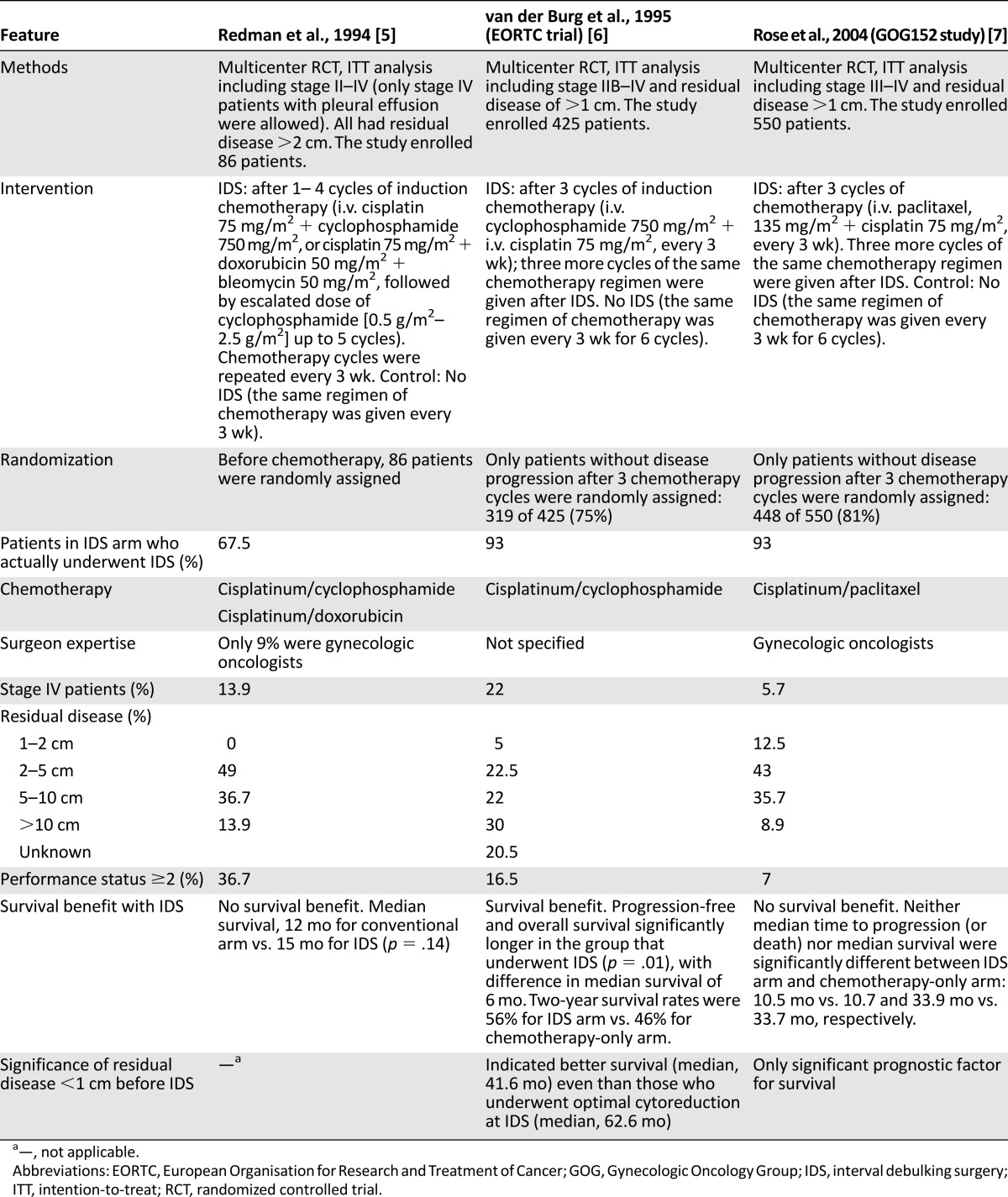
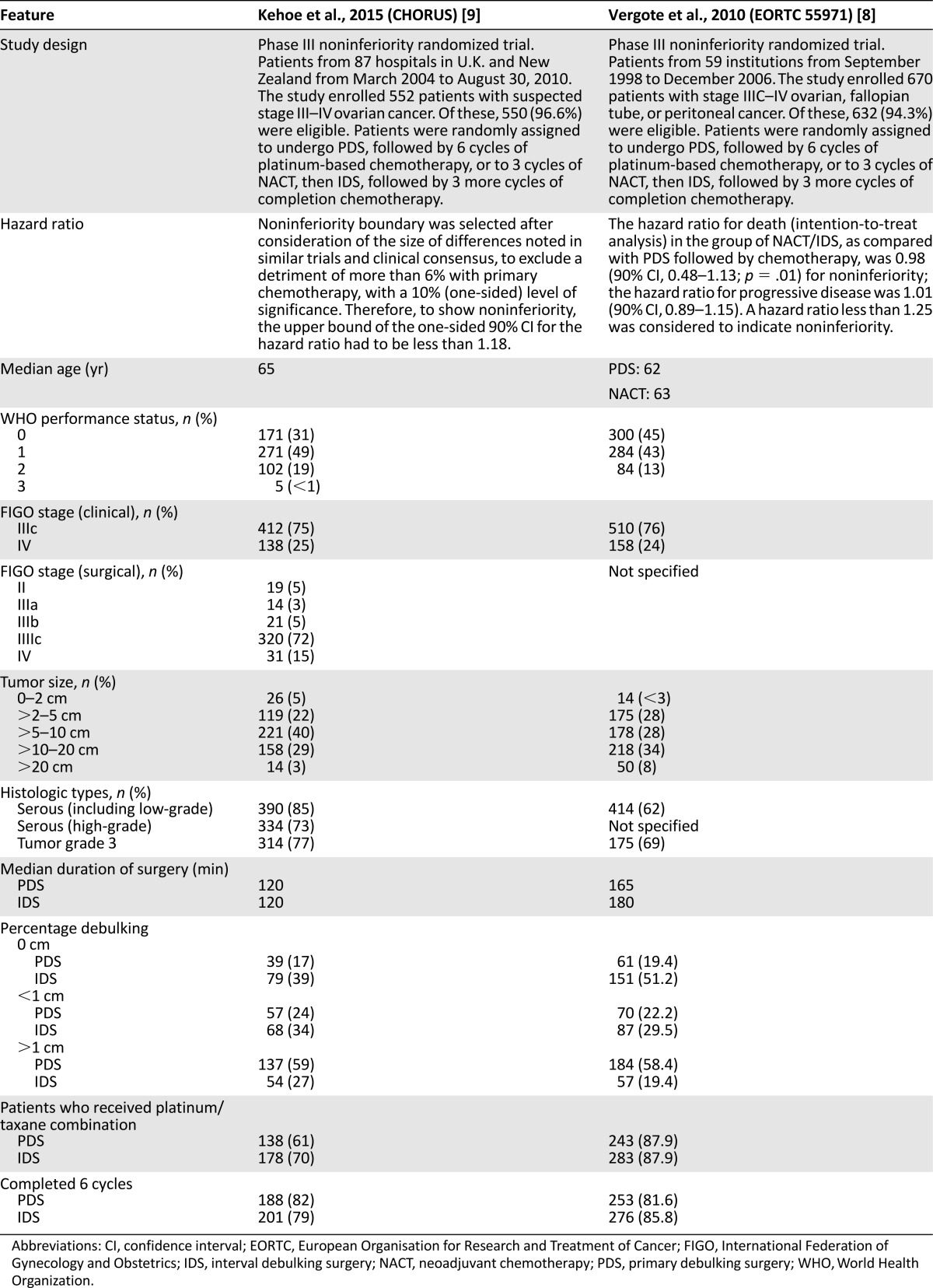
Using the PubMed search strategy, we identified 543 records. After screening the abstracts, assessing the full-text articles, and identifying additional records through the reference lists of selected articles, we included 5 RCTs [5–9], 3 meta-analyses, and 3 Cochrane reviews [10–16] in this review. Three RCTs compared NACT-IDS with chemotherapy only [5–7]. Two RCTs compared PDS with NACT-IDS [8, 9]. Details on the inclusion/exclusion criteria, patient characteristics, and results of these RCTs can be found in Tables 1 and 2.
Table 1.
Comparison among three randomized trials evaluating interval debulking surgery vs. no surgery in patients with stage III and IV ovarian cancer
Table 2.
Comparison between CHORUS (chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer) and European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada randomized controlled trials
Optimal Debulking Surgery Milestones
Munnell observed that a “definitive operation” cytoreduction before chemotherapy led to improved survival compared with “partial removal” or “biopsy only” [17]. In 1975, Griffiths [18] proved (n = 102) that if residual disease (RD) was ≤1.5 cm, survival improved as tumor size decreased. Survival duration was 39, 29, 18, and 11 months for patients with RD of 0 cm to <0.5 cm, 0.6–1 cm, 1.1–1.5 cm, and >1.5 cm, respectively. The prognosis was worst if RD was >1.5 cm [19]. In agreement, Bertelsen [20] (Danish Ovarian Study Group trial; n = 361; FIGO stage III and IV) and several others [19, 21–24] showed that suboptimal cytoreduction resulted in as poor prognosis as an explorative laparotomy.
Single-institution trials showing that complete debulking to no macroscopic RD (0 cm) implied the best prognosis [25–29] and led to even more aggressive PDS. A retrospective review of six Gynecologic Oncology Group (GOG) studies (n = 1,895; FIGO stage III; PDS plus six cycles of carboplatinum-taxol) [27] showed that for patients with RD ≤1 cm, those left with no RD obtained the best prognosis. OS was 71.9 months, 42.4 months, and 35 months for those left with RD of 0 cm, ≤1 cm, and >1 cm, respectively [27]. This was confirmed in a Cochrane analysis [14] in which complete cytoreduction during PDS for AOC was recommended. The authors of the Cochrane analysis also proposed new definitions to describe tumor state after debulking as follows: optimal, near-optimal, and suboptimal for those left with 0 cm, ≤1 cm, and >1 cm RD, respectively [14].
Although systemic lymphadenectomy is part of the staging procedure in EOC [30–34], two large RCTs in AOC failed to show a significant survival advantage of systemic lymphadenectomy [33, 34]. However, both trials showed a trend toward a longer progression-free interval. Complete lymphadenectomy is advised when optimal debulking is possible in the peritoneal cavity [1, 33, 34].
The development of new surgical techniques, such as the retroperitoneal dissection technique [35, 36], diaphragmatic stripping, splenectomy, and gastrointestinal and partial liver resection [25, 26, 37–40], further facilitated optimal cytoreduction. The importance of cytoreduction of the diaphragm was illustrated in 181 patients with tumors involving the diaphragm. Diaphragm surgery led to a significantly improved 5-year OS (53% vs. 15%) [39, 40], even in cases of optimal cytoreduction (55% vs. 28%) [39].
Moving from standard intraperitoneal surgery toward retroperitoneal en bloc radical resection in the mid-1990s led to a 40% increase in surgical radicality and an improved median OS of at least 10% [41]. The subsequent extensive upper abdominal approach improved radicality up to 55%, translated to a further 10% improvement in OS [41]. This is in correlation with the conclusion of the meta-analysis of Bristow et al. [10] that every 10% incremental increase of cytoreduction to residual nodules <2 cm enhanced the median OS of a patient cohort by 5.5%. Increasing radicality of cytoreduction from 25% to 75% would be associated with a 50% increase in median survival (from 22.7 months to 33.9 months).
These techniques were soon adopted by large-volume hospitals and stimulated the growth of subspecialization in gynecologic oncology. Surgery performed by a gynecologic oncologist is associated with better staging, optimal cytoreduction, lower morbidity, and better survival. Both large-volume hospitals and subspecialization are independent prognostic factors for survival [42–47]. Through training and implementation of a more extensive surgical approach, PDS is possible in up to 85% of cases [10, 38, 41, 44, 45]. Despite all evidence, up to 60%–80% of AOC patients still undergo suboptimal debulking surgery [8, 9, 23].
PDS Versus NACT-IDS
NACT-IDS Versus No Surgery
Three RCTs have compared NACT-IDS with no surgery (Table 1) [5–7]. The study of Redman et al. [5], published in 1994, failed to a show a survival benefit or an increase in the number of operable cases (79% of patients had RD >2 cm at IDS). Only 67% of randomly assigned patients underwent IDS.
Patients in the European Organisation for Research and Treatment of Cancer (EORTC) study published by van der Burg et al. [6] were randomly assigned only if they showed no disease progression after NACT (319 of 425). A total of 140 patients were randomly assigned to IDS; 93% of these had surgery. IDS was associated with lower morbidity and a 33% mortality rate reduction. However, IDS did not markedly increase the percentage of optimal debulking; 55% of patients were left with tumor >1 cm. Interestingly, the prognosis of patients who had tumor <1 cm before IDS had superior survival (median, 46.6 months) compared with those who were optimally cytoreduced at IDS (median, 26.6 months). Approximately 25% of patients lost the opportunity for surgery because of significant adverse effects and refractory disease [6].
The GOG152 study, reported by Rose et al. [7], failed to show a survival benefit. As occurred in the EORTC trial, only patients who did not progress under NACT were randomly assigned.
Table 1 shows the substantial differences between the EORTC and GOG152 studies, which might explain the differences in outcome. The EORTC study accrued more patients with stage IV disease (21% vs. 7%) and/or performance status 2 and had more patients with bulky RD after IDS. In the GOG152 study, patients were operated on by gynecologic oncologists, which is (as are the other three differences) a prognostic factor for survival.
A Cochrane analysis that included these RCTs was unable to provide a conclusion [10] because of heterogeneity between the trials. The authors stated that PDS remained the standard of care and IDS was of no benefit in patients who underwent primary maximal debulking efforts by a gynecologic oncologist (GOG152). The subgroup that benefits from NACT-IDS consists of patients without tumor progression under NACT in whom primary debulking was not possible, because of factors such as older age and low performance status, or in whom PDS was not performed under optimal conditions or by a gynecologic oncologist (EORTC and GOG152).
Bristow and Chi [11] reviewed the role of platinum-based NACT-IDS for AOC in 51 phase I/II studies (n = 835 patients). Survival of patients who had NACT after an attempt of primary surgery was inferior to those who had PDS. The authors claimed that survival is inversely proportional to the number of NACT cycles; each additional cycle of NACT leads to a 4.1-month reduction in median survival. In a later systematic review that included 3 RCTs, 6 non-RCTs, and 26 retrospective and phase I/II studies, Bristow et al. [46] stated that IDS after a suboptimal attempt of upfront cytoreduction did not appear to have an appreciable effect on survival.
On the contrary, a meta-analysis [12] showed that patients who received NACT had a lower risk for suboptimal cytoreduction. However, the increased rate of optimal cytoreduction in NACT cohorts did not fully translate into an improved OS.
NACT-IDS Versus PDS
Trials
Details concerning the 2 RCTs are summarized in Table 2. The first RCT, performed by the EORTC/National Cancer Institute of Canada (NCIC) [8], randomly assigned 632 AOC patients to PDS or NACT-IDS. Most patients had stage IIIc or IV disease at PDS, with lesions >5 cm and >10 cm in 74.5% and 61.6% of patients, respectively. After PDS, 41.6% were left with RD ≤1 cm compared with 80.6% after NACT-IDS [8].
Postoperative adverse events and death (<28 days after surgery) tended to be higher after PDS. Grade 3/4 hemorrhage occurred in 7.4% (vs. 4.1%), infection in 8.1% (vs. 1.7%), venous complications in 2.6% (vs. 0%), and death in 2.5% (vs. 0.7%) of patients. Survival and quality of life (QOL) were similar with both approaches [8]. Complete resection of all microscopic disease, both for PDS and NACT-IDS, was the strongest independent prognostic factor. The authors concluded that for stage IIIC–IV AOC patients, NACT/IDS was not inferior to PDS followed by chemotherapy. Complete resection of all macroscopic disease remained the objective, independent of the timing of debulking [8].
The EORTC/NCIC study was included in a Cochrane review [16] and a meta-analysis [13]. Both concluded that PDS is standard of care for stage IIIa/IIIb patients. NACT-IDS is considered a reasonable alternative. The timing of surgery should be tailored to the patient, with consideration of resectability, age, histologic features, stage, performance status, and underlying morbidity [16].
The results of the multicentric CHORUS (chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer) phase III RCT (87 hospitals; n = 552) were recently published [9]. As in the EORTC/NCIC trial, the CHORUS study demonstrated that NACT-IDS was noninferior and associated with lower morbidity. Grade 3/4 postoperative adverse events and deaths (<28 days after surgery) were more common in the PDS group (24% vs. 14% and 6% vs. <1%, respectively). More NACT-IDS patients reported nonsignificant improvement in QOL at 6 and 12 months. This study also found that NACT-IDS significantly increased the incidence of optimal cytoreduction (RD <1 cm): 73% versus 41%. This increase in optimal cytoreductions (NACT-IDS arm) did not translate into a significant improvement of progression-free survival (PFS) or median survival (22.6 months vs. 24.1 months for PDS and NACT-IDS, respectively). The authors attributed these low figures to older median age of recruited patients and high percentage of grade 3 tumors. Up to 34% of patients in the CHORUS study received single-agent carboplatin (39% in the PDS arm vs. 30% in the NACT-IDS arm) [9].
For both RCTs, critics have arisen; even the authors of the CHORUS trial questioned whether patients would have benefitted from more aggressive attempts of surgery [9]. First, the median operation time in the EORTC/NCIC trial was shortest for PDS (165 vs. 180 minutes) [8]. The median operation time (both arms) was even shorter in the CHORUS trial (120 minutes) [9]. This might suggest suboptimal efforts at PDS, supported by the fact that only 40% of patients were left with tumor ≤1 cm after PDS in both trials. This rate is much lower than in the single-institutions studies (GOG and Arbeitsgemeinschaft Gynaekologische Onkologie [AGO] studies [25–29]).
Second, both arms of the EORTC/NCIC trial underwent little or no upper abdominal surgery [8]. Lesion sizes were measured before and after surgery. For both groups, the greatest reduction in RD (to <1 cm) was seen in the omentum, pelvis, and adnexa; no change was seen in the upper abdomen [25, 47]. In the CHORUS trial [9], 27% of PDS patients did not have hysterectomy and 24% did not have bilateral salpingo-oophorectomy. Patients underwent little or no upper abdominal surgery (supra- and infracolic omentectomy in 48% at PDS and in 58% at IDS) or lymphadenectomy (8%; complete pelvic lymphadenectomy at PDS in only 3% and complete para-aortic lymphadenectomy in only 1%). As discussed before, increasing the radicality of surgery (including the upper abdomen) in PDS translates to a direct proportional improvement in survival [10]. In contrast to these observations, increasing the complete resection rate from <20% to >50% at NACT-IDS did not improve prognosis [10, 25, 47]. These results indicate that only improvement in the radicality of PDS can further improve survival [10, 25, 44, 47, 48].
Third, for the EORTC/NCIC trial [8], the PFS and OS of the primary debulked patients were substantially lower than those reported in previous studies. Chi et al. [25] used identical inclusion criteria and treated 285 patients with PDS during the same period. They achieved cytoreduction to ≤1 cm in 71%, resulting in a PFS of 17 months and OS of 50 months compared with 41%, 17 months, and 29 months, respectively, for the PDS arm in the EORTC/NCIC study. This improvement (compared with the EORTC/NCIC study) could partly be explained by the higher rate of optimal cytoreduction but also by the smaller number of stage IV patients (13% vs. 23%) and higher chemotherapy administration rate (99% vs. 81.6%).
Fourth, over 8 years, 59 institutions included 670 patients in the EORTC/NCIC trial (median accrual per institution, 5 patients [range, 1–125] [8]. This raises questions about interinstitutional variation in the adequacy of surgical debulking and/or selection bias.
Fifth, both the EORTC/NCIC and CHORUS trials were designed to prove noninferiority of NACT-IDS. In the EORTC/NCIC trial, a higher mortality rate up to 25% would be considered noninferior [8]. In practice, an increase of this magnitude cannot be ignored [25, 47]. A subgroup analysis including tumors ≤5 cm that underwent optimal radical surgery (RD, 0 cm) showed a significantly better median survival for the PDS arm (45 months vs. 38 months) [8]. As in previous studies [19, 49, 50], a recent post hoc analysis [51] showed that stage IIIC EOC with metastatic tumors up to 45 mm had more benefit from PDS. The subgroup of the CHORUS trial that has been cytoreduced to RD <1 cm and >0 cm had a better median survival in the PDS arm (36.8 months vs. 23.2 months in the NACT-IDS arm).
Finally, only stage IIIc and IV patients were included. Thus, the results should not be extrapolated to all patients with AOC.
Pros and Cons
The most important effect of PDS is to improve the effect of chemotherapy by removing poorly perfused tumor portions that are receiving inadequate doses of chemotherapy and phenotypically resistant cells [1, 52, 53]. In addition, the spontaneous mutation rate of the tumor toward drug-resistance phenotypes is lower in the case of small RD.
Rauh-Hain et al. looked at the relapsed patients who were retreated with platinum-based chemotherapy and showed that 88.8% in the NACT-IDS group were considered platinum-resistant (recurrence within 6 months) compared with 55.3% in the PDS group (p < .001). The authors concluded that NACT-IDS appears to increase the risk for platinum resistance.
Induction of more platinum-resistant clones might explain the fact that, in both RCTs, NACT-IDS did not further improve OS despite the almost doubling of optimally debulked patients [54]. Rauh-Hain et al. [55] looked at the relapsed patients who were retreated with platinum-based chemotherapy and showed that 88.8% in the NACT-IDS group were considered platinum-resistant (recurrence within 6 months) compared with 55.3% in the PDS group (p < .001). The authors concluded that NACT-IDS appears to increase the risk for platinum resistance [55]. Drug resistance after NACT correlates with in vitro drug resistance [56–59]. A second possible explanation for why NACT-IDS has not been able to further improve OS might be the violation of the dose-density effect by the interruption of chemotherapy with IDS [60].
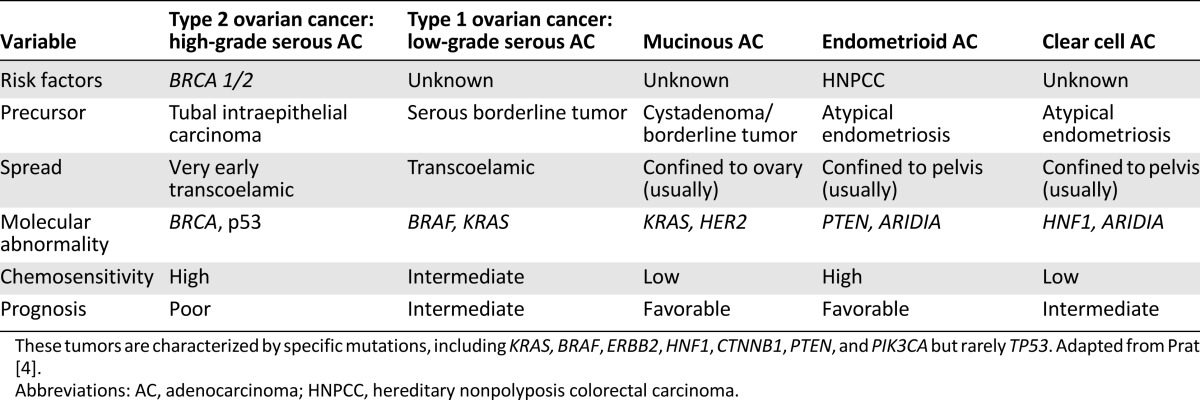
The NACT-IDS concept is based on the fact that EOC is a highly chemosensitive tumor, with a rate of response to standard chemotherapy (platinum and taxanes) of more than 80%, even in the case of massive ascites and diffuse dissemination. Sadly, it does not consider the heterogeneity of EOC (which correlates with chemosensitivity) [3, 4, 54]. As shown in Table 3, low-grade and nonserous tumors have low chemosensitivity but favorable prognosis, while high-grade serous tumors, despite their high chemosensitivity, have less favorable outcome [4, 23, 54, 61, 62]. Omitting PDS in the treatment of nonserous tumors will take a toll on survival. In 294 stage III/IV patients, Hosono et al. [61] showed that suboptimal debulking (>1 cm RD) in nonserous tumors was associated with an increased risk for death.
Table 3.
New concepts on the origin of ovarian adenocarcinomas
Evaluation of the radicality of surgery is mainly based on the surgeon’s visual estimation. Whether the surgeon’s statement of complete tumor resection in PDS and IDS is equal remains unclear. Hynninen et al. [63] evaluated the perioperative visual assessment of tumor dissemination at the start of PDS/diagnostic laparotomy (220 biopsies) or IDS (92 biopsies) and proved statistically significant (p < .001) worse sensitivity and accuracy in case of IDS. NACT before surgery causes fibrosis and adhesions in the peritoneal cavity; microscopically carcinomatous areas more often have a benign visual appearance than at PDS. This interferes with the perioperative evaluation of tumor spread. NACT may therefore lead to incomplete resection of tumor in potentially resectable areas and might increase incidence of platinum-resistant cell clones [63].
Important Prognostic Factors for Surgical Decision Making
Heterogeneity of Ovarian Cancer From Histologic Subtypes to Molecular Biology
Despite their low sensitivity to chemotherapy, grade 1 tumors are associated with better prognosis than grade 3 tumors [1, 23, 24]. Histologic subtype is also a significant prognostic factor [1, 23, 24]. Endometrioid tumors have the best prognosis and clear cell and undifferentiated tumors, the worst [1, 4, 8, 23, 24, 51]. Mucinous tumors have excellent prognosis when completely excised; none of patients left with RD survived 24 months [25, 26, 58].
The heterogeneity of clinical behavior related to histologic subtypes became easier to understand in view of recent data regarding molecular origin of EOC [3, 4]. According to these data, EOC can be divided into two types that develop independently along different molecular pathways and differ markedly in biological behavior and prognosis. Both types develop outside the ovary and involve it secondarily [3]. Type 1 EOC (nonserous or low- grade serous tumors) is generally indolent, presents in stage I, and develops from well-established precursors, so-called borderline ovarian tumors. They are relatively genetically stable. Type II EOC (high-grade serous) is composed of tumors that are aggressive, present in advanced stage, and develop from intraepithelial carcinomas in the fallopian tube. They are genetically highly unstable (Table 3).
This heterogeneity also explains differences in platinum sensitivity and has elicited the need to select chemotherapy regimens in terms of function of histologic subtypes [23, 54, 58, 59, 61, 62]. Several biomarkers and methods for predicting response to chemotherapy and identifying patients most likely to benefit from NACT-IDS have been investigated but never used widely [54, 57–59].
Even within high-grade serous tumors, assumed to form a homogenous entity, recent studies on a molecular basis showed a certain degree of heterogeneity. Tumors with low intraepithelial CD8+ T cells or high Ki-67 benefit from aggressive surgical debulking [64]. Prognosis of patients with serous tumors expressing high CD8+ T cells did not improve with optimal debulking efforts [64].
Tumors with low intraepithelial CD8+ T cells or high Ki-67 benefit from aggressive surgical debulking. Prognosis of patients with serous tumors expressing high CD8+ T cells did not improve with optimal debulking efforts.
The Cancer Genome Atlas project has analyzed messenger RNA expression, microRNA expression, promoter methylation, and DNA copy number in 489 high-grade serous ovarian adenocarcinomas and the DNA sequences of exons from coding genes in 316 of these tumors. Here we report that high-grade serous ovarian cancer is characterized by TP53 mutations in almost all tumors (96%); they have low prevalence but are statistically recurrent: somatic mutations in nine further genes, including NF1, BRCA1, BRCA2, RB1, and CDK12; 113 significant focal DNA copy number aberrations; and promoter methylation events involving 168 genes. Analyses delineated 4 ovarian cancer transcriptional subtypes, 3 microRNA subtypes, 4 promoter methylation subtypes, and a transcriptional signature associated with survival duration and shed new light on the effect that tumors with BRCA1/2 (BRCA1 or BRCA2) and CCNE1 aberrations have on survival [65]
FIGO Substages
The Norwegian Radium Hospital (n = 455 patients with stage III cancer) showed a direct correlation between survival and optimal debulking (≤2 cm RD) in all FIGO substages. FIGO substage was an independent prognostic factor, with substage IIIc having a significantly lower OS. Substage had no effect on OS in patients without optimal debulking [23].
Age and Performance Status
Older age and low performance index are independent prognostic factors for OS [1, 8, 9, 21, 23, 29, 45]. Multivariate analysis performed in a subgroup with homogeneous histology and complete surgical data within the AGO–Ovarian Cancer Study Group 3 study (a prospective, randomized phase III trial with 686 FIGO stage IIB–IV patients receiving either cisplatin-paclitaxel or carboplatin-paclitaxel) revealed age as an independent prognostic factor for survival [45]. Wimberger et al. [45] created 3 categories: younger (<50 years), middle-aged (50–65 years), and elderly (>65 years). No residual tumor after PDS was achieved significantly more often in the young patient group, resulting in an improved median OS of 60.7 months, compared with 41.3 and 33.2 months in the middle-aged and elderly groups, respectively. The survival advantage of young patients remained in completely debulked patients [45].
Intestinal Resections
Prognosis may be less favorable when optimal debulking necessitates intestinal resection [21, 66].
Peritoneal Carcinomatosis
Peritoneal carcinomatosis worsens prognosis [21, 67]. In addition, the number of peritoneal deposits left after nearly optimal debulking (residual deposits <0.5 cm) affects prognosis [21, 38, 67]. Patients who are left with >40 nodules have the worst prognosis (median survival of 16 months).
Size of Tumor in Upper Abdomen at Start of Surgery and Presence of Massive Ascites
Both the size of the tumor in the upper abdomen [23, 24, 48, 49] and the presence of massive ascites [1, 19, 23, 48–50] are independent poor prognostic factors. Hacker et al. reported that in 47 stage III–IV patients with tumors >10 cm and massive ascites (>10 L), cytoreduction to 1.5 cm RD had a weak (6 months) effect on survival [49, 50]. Hoskins et al. [22] (GOG study in stage III patients) showed that in the case of optimal debulking, the presence of gross disease in the omentum and at extrapelvic sites had a negative effect on prognosis.
Preoperative CA-125 Levels
Preoperative CA-125 levels are directly correlated to FIGO stage and extent of peritoneal metastases and are significantly lower in low-grade tumors. Outcome of patients with high CA-125 levels before surgery was worse than that of patients with lower levels [1, 68]. Postoperative CA-125 levels are independent prognostic factors for survival and allow early identification of nonresponders during chemotherapy [68, 69].
Conclusion
NACT-IDS or PDS?
NACT-IDS is noninferior but also not superior to PDS. IDS can be considered an acceptable treatment choice in AOC patients with low performance status, underlying morbidity, or older age or those for whom the optimal situation for radical surgery is not available (e.g., no gynecologic oncologist available or low-volume hospital).
NACT-IDS is associated with lower morbidity than PDS. Further study is necessary to evaluate morbidity and the usefulness of new agents, such as bevacizumab, for NACT-IDS.
NACT-IDS is associated with a possible loss of opportunity for surgery in case of significant adverse effects and/or refractory disease. Nonserous tumors with favorable prognosis are less chemosensitive, and excluding optimal PDS will lead to less favorable outcome. NACT interferes with the perioperative visual evaluation of tumor spread, which can lead to incomplete resection of tumor and might result in a higher incidence of platinum-resistant recurrences.
Data from pooled GOG studies and single-institution studies show that the 5-year survival rate of PDS is approximately 50% and even higher if a complete gross resection can be achieved. With appropriate training, support, and commitment, optimal debulking rates of >70%–75% can be achieved. Surgery for AOC should be restricted to high-volume hospitals and gynecologic oncologists who have undergone special training.
Categorization of Stage III Ovarian Cancer
Stage III ovarian cancer can be classified in different categories depending on patterns of tumor spread that reflects the biologic behavior and prognosis. The first three categories often represent type 1 EOC with low chemosensitivity. The fifth category often represents high-grade chemosensitive serous tumors (type 2).
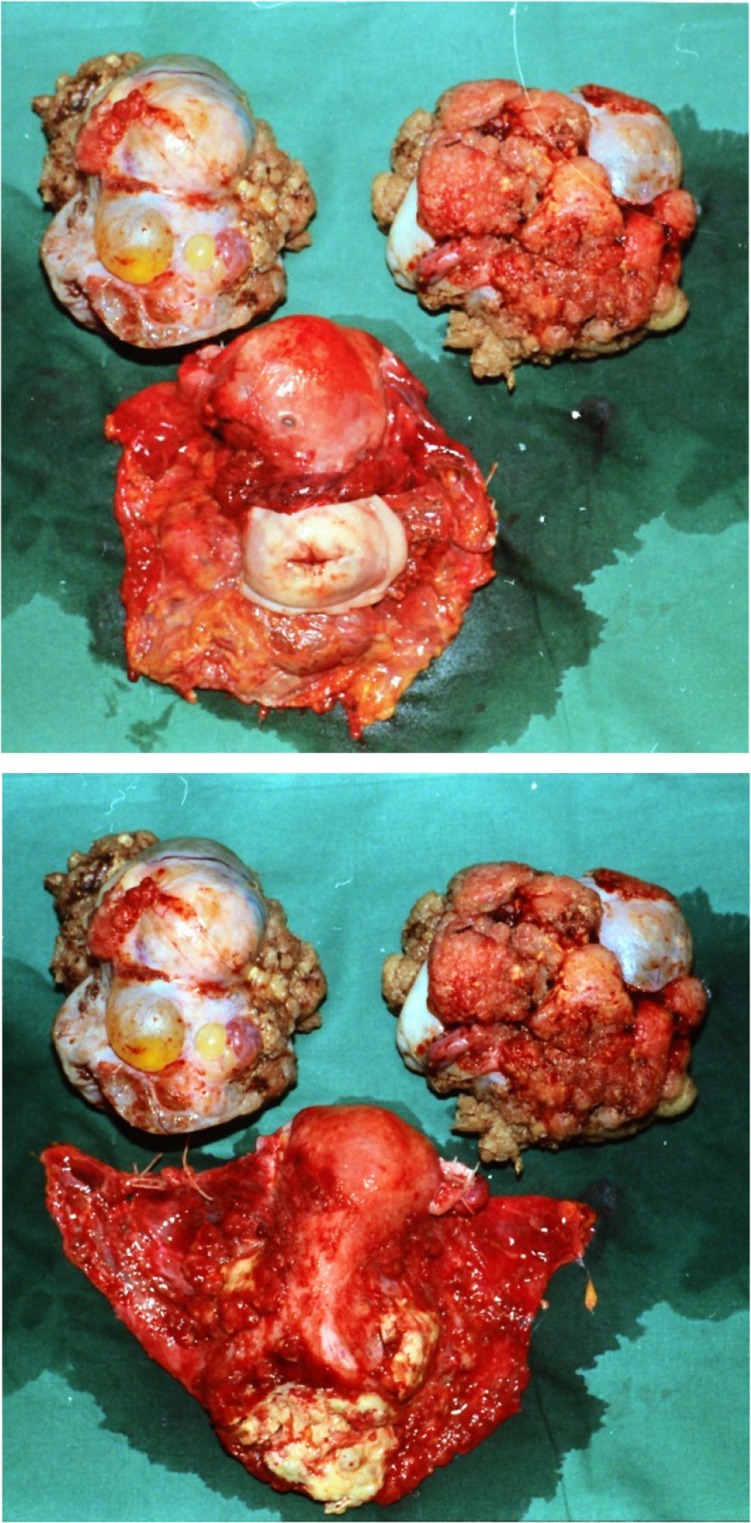
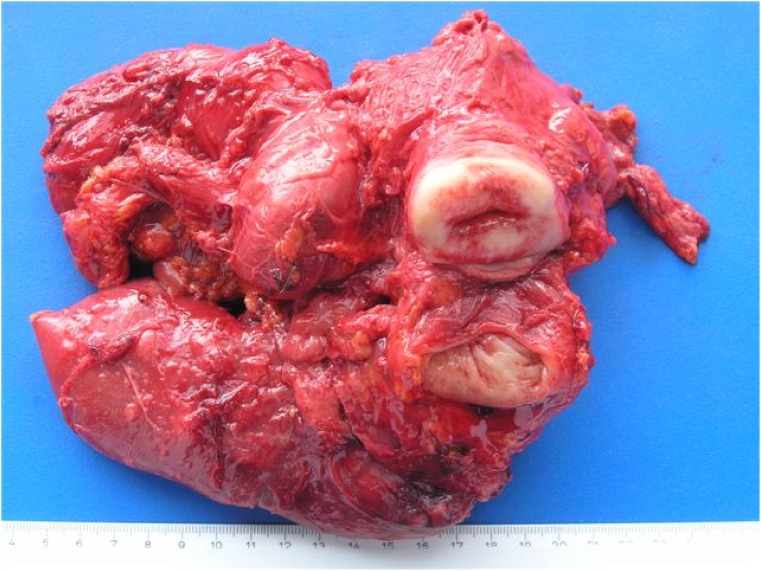
Category 1: The main tumor bulk is located in the small pelvis, with no massive ascites. No intestinal resection is required (Fig. 1). These tumors pose the best prognosis. PDS is recommended.
Figure 1.
Category 1. The main tumor bulk was located in the small pelvis. Radical tumor excision did not require intestinal resection. Note that complete resection of an invaded parametrium, peritoneum of the pelvic side wall, and the Douglas cavity was achieved by extraperitoneal dissection, as described by Barnes et al. [35].
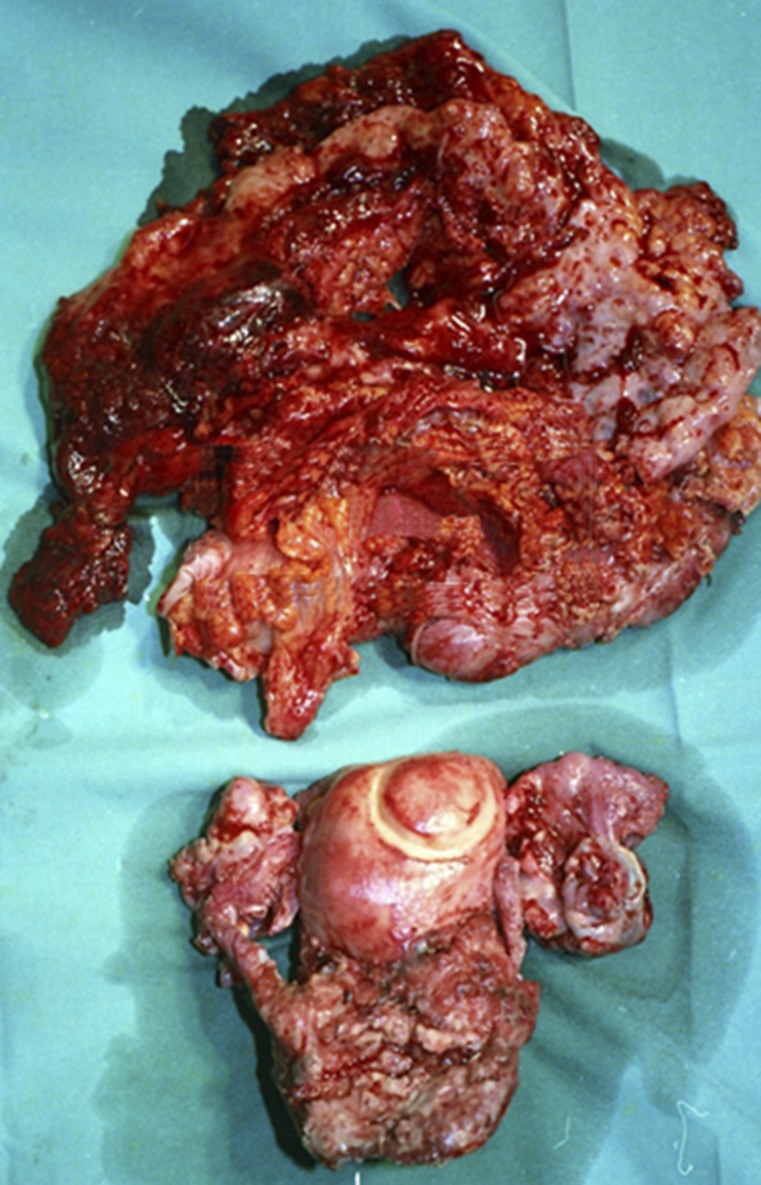
Category 2: The main tumor bulk is located in the small pelvis, with no massive ascites. Radical tumor excision requires intestinal resection (Fig. 2). The prognosis is less favorable than in category 1, even if no RD is left. PDS is recommended.
Figure 2.
Category 2. The main tumor bulk was located in the small pelvis, but radical tumor excision required intestinal resection. Note en bloc resection of the tumor with invaded segment of descending colon through extraperitoneal dissection, as described by Eisenkop et al. [36].
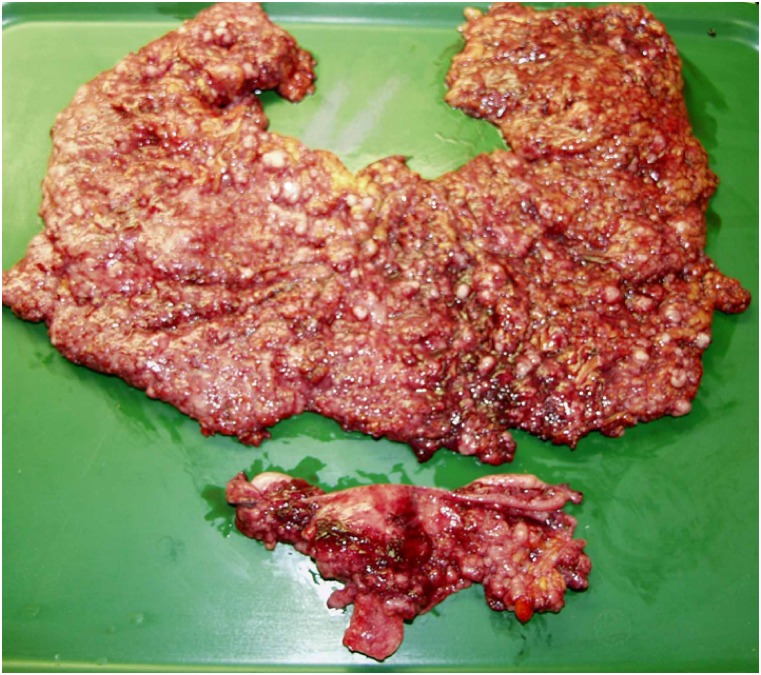
Category 3: The main tumor bulk is located in the upper abdomen, with no massive ascites. No intestinal resection is required (Fig. 3). Prognosis is less favorable than in category 2. PDS is recommended.
Figure 3.
Category 3. The main tumor bulk was located in the upper abdomen, with no massive ascites. Tumor excision did not require intestinal resection.
Category 4: The main tumor bulk is located in the upper abdomen, with no massive ascites. Radical tumor excision requires intestinal resection (Fig. 4). Prognosis is less favorable than in category 3. PDS is recommended. However, in case of low performance status, underlying morbidity, or older age, IDS following three cycles of NACT might be preferred. Most tumors within this category are high-grade serous subtypes, and molecular biology could assist subgroups that might not benefit from optimal PDS.
Figure 4.
Category 4. The main tumor bulk was in the upper abdomen, with no massive ascites. Tumor excision required intestinal resection.
Category 5: Main tumor bulk is restricted to the upper abdomen and is associated with massive ascites or the presence of miliary spread and/or massive mesenterial metastases. This type has the worst prognosis. PDS requires multiple intestinal resections. These tumors are usually associated with higher CA-125 levels. NACT-IDS is preferable.
Stage IV Ovarian Cancer
Stage IV ovarian cancer is not a contraindication for PDS. NACT-IDS is preferable in case of multiple intrahepatic/lung metastases or massive ascites with miliary spread.
Future Studies
Additional studies regarding type of surgery and choice of chemotherapy should be designed in view of molecular and genetic aspects of the tumor to allow for patient-individualized therapy. Recent data on molecular aberrations that cause ovarian cancer will be critical in selecting treatment strategies and deploying therapies.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Amin P. Makar, Claes G. Tropé
Provision of study material or patients: Amin P. Makar
Collection and/or assembly of data: Amin P. Makar, Katrien Vandecasteele
Data analysis and interpretation: Amin P. Makar, Katrien Vandecasteele
Manuscript writing: Amin P. Makar, Claes G. Tropé, Philippe Tummers, Hannelore Denys, Katrien Vandecasteele
Final approval of manuscript: Amin P. Makar, Claes G. Tropé, Philippe Tummers, Hannelore Denys, Katrien Vandecasteele
Disclosures
The authors indicated no financial relationships.
References
- 1.Chen LM, Berek JS. Epithelial carcinoma of the ovary, fallopian tube, and peritoneum: Epidemiology and risk factors. UpToDate. 2015 . http://www.uptodate.com. Accessed June 15, 2015. [Google Scholar]
- 2.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(suppl 10):x111–x117. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 5.Redman CW, Warwick J, Luesley DM, et al. Intervention debulking surgery in advanced epithelial ovarian cancer. Br J Obstet Gynaecol. 1994;101:142–146. doi: 10.1111/j.1471-0528.1994.tb13080.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Burg ME, van Lent M, Buyse M, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. N Engl J Med. 1995;332:629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 7.Rose PG, Nerenstone S, Brady MF, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351:2489–2497. doi: 10.1056/NEJMoa041125. [DOI] [PubMed] [Google Scholar]
- 8.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 9.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 10.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: A meta-analysis. Gynecol Oncol. 2006;103:1070–1076. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16:2315–2320. doi: 10.1245/s10434-009-0558-6. [DOI] [PubMed] [Google Scholar]
- 13.Dai-yuan M, Bang-xian T, Xian-fu L, et al. A meta-analysis: Neoadjuvant chemotherapy versus primary surgery in ovarian carcinoma FIGO stageIII and IV. World J Surg Oncol. 2013;11:267–270. doi: 10.1186/1477-7819-11-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elattar A, Bryant A, Winter-Roach BA, et al. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;8:CD007565. doi: 10.1002/14651858.CD007565.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangjitgamol S, Manusirivithaya S, Laopaiboon M, et al. Interval debulking surgery for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2013;4:CD006014. doi: 10.1002/14651858.CD006014.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Morrison J, Haldar K, Kehoe S, et al. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2012;8:CD005343. doi: 10.1002/14651858.CD005343.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munnell EW. The changing prognosis and treatment in cancer of the ovary. A report of 235 patients with primary ovarian carcinoma 1952-1961. Am J Obstet Gynecol. 1968;100:790–805. doi: 10.1016/s0002-9378(15)33580-8. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 19.Heintz AP, Van Oosterom AT, Trimbos JB, et al. The treatment of advanced ovarian carcinoma (I): Clinical variables associated with prognosis. Gynecol Oncol. 1988;30:347–358. doi: 10.1016/0090-8258(88)90249-1. [DOI] [PubMed] [Google Scholar]
- 20.Bertelsen K. Tumor reduction surgery and long-term survival in advanced ovarian cancer: A DACOVA study. Gynecol Oncol. 1990;38:203–209. doi: 10.1016/0090-8258(90)90042-j. [DOI] [PubMed] [Google Scholar]
- 21.Webb MJ. Cytoreduction in ovarian cancer: Achievability and results. Baillieres Clin Obstet Gynaecol. 1989;3:83–94. doi: 10.1016/s0950-3552(89)80044-6. [DOI] [PubMed] [Google Scholar]
- 22.Hoskins WJ, Bundy BN, Thigpen JT, et al. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 1992;47:159–166. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 23.Makar AP, Baekelandt M, Tropé CG, et al. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. 1995;56:175–180. doi: 10.1006/gyno.1995.1027. [DOI] [PubMed] [Google Scholar]
- 24.Makar AP, Kisic J, Tropé CG. Controversies in surgical management of advanced ovarian cancer (stage III-IV) Eur J Gynaecol Oncol. 2000;21:449–460. [PubMed] [Google Scholar]
- 25.Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124:10–14. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Colombo PE, Mourregot A, Fabbro M, et al. Aggressive surgical strategies in advanced ovarian cancer: A monocentric study of 203 stage IIIC and IV patients. Eur J Surg Oncol. 2009;35:135–143. doi: 10.1016/j.ejso.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 28.du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 29.Winter WE, 3rd, Maxwell GL, Tian C, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2008;26(1):83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 30.Chen SS, Lee L. Incidence of para-aortic and pelvic lymph node metastases in epithelial carcinoma of the ovary. Gynecol Oncol. 1983;16:95–100. doi: 10.1016/0090-8258(83)90013-6. [DOI] [PubMed] [Google Scholar]
- 31.Burghardt E, Girardi F, Lahousen M, et al. Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991;40:103–106. doi: 10.1016/0090-8258(91)90099-q. [DOI] [PubMed] [Google Scholar]
- 32.Chan JK, Kapp DS. Role of complete lymphadenectomy in endometrioid uterine cancer. Lancet Oncol. 2007;8:831–841. doi: 10.1016/S1470-2045(07)70275-9. [Review] [DOI] [PubMed] [Google Scholar]
- 33.Panici PB, Maggioni A, Hacker N, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: A randomized clinical trial. J Natl Cancer Inst. 2005;97:560–566. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]
- 34.Maggioni A, Benedetti Panici P, Dell’Anna T, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. 2006;95:699–704. doi: 10.1038/sj.bjc.6603323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes W, Johnson J, Waggoner S, et al. Reverse hysterocolposigmoidectomy (RHCS) for resection of panpelvic tumors. Gynecol Oncol. 1991;42:151–155. doi: 10.1016/0090-8258(91)90336-4. [DOI] [PubMed] [Google Scholar]
- 36.Eisenkop SM, Nalick RH, Teng NN. Modified posterior exenteration for ovarian cancer. Obstet Gynecol. 1991;78:879–885. [PubMed] [Google Scholar]
- 37.Juretzka MM, Barakat RR. Pelvic cytoreduction with rectosigmoid resection. Gynecol Oncol. 2007;104(suppl 1):40–44. doi: 10.1016/j.ygyno.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: A prospective study. Gynecol Oncol. 1998;69:103–108. doi: 10.1006/gyno.1998.4955. [DOI] [PubMed] [Google Scholar]
- 39.Aletti GD, Dowdy SC, Podratz KC, et al. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol. 2006;100:283–287. doi: 10.1016/j.ygyno.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 40.Fanfani F, Fagotti A, Gallotta V, et al. Upper abdominal surgery in advanced and recurrent ovarian cancer: role of diaphragmatic surgery. Gynecol Oncol. 2010;116:497–501. doi: 10.1016/j.ygyno.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 42.Bristow RE, Chang J, Ziogas A, et al. Impact of national cancer institute comprehensive cancer centers on ovarian cancer treatment and survival. J Am Coll Surg. 2015;220:940–950. doi: 10.1016/j.jamcollsurg.2015.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bristow RE, Chang J, Ziogas A, et al. High-volume ovarian cancer care: Survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132:403–410. doi: 10.1016/j.ygyno.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 45.Wimberger P, Lehmann N, Kimmig R, et al. Impact of age on outcome in patients with advanced ovarian cancer treated within a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR) Gynecol Oncol. 2006;100:300–307. doi: 10.1016/j.ygyno.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 46.Bristow RE, Eisenhauer EL, Santillan A, et al. Delaying the primary surgical effort for advanced ovarian cancer: A systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecol Oncol. 2007;104:480–490. doi: 10.1016/j.ygyno.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Chi DS, Bristow RE, Armstrong DK, et al. Is the easier way ever the better way? J Clin Oncol. 2011;29:4073–4075. doi: 10.1200/JCO.2011.35.9935. [DOI] [PubMed] [Google Scholar]
- 48.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103:1083–1090. doi: 10.1016/j.ygyno.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 49.Hacker NF, Berek JS, Lagasse LD, et al. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet Gynecol. 1983;61:413–420. [PubMed] [Google Scholar]
- 50.Hacker N. Surgical management of advanced ovarian cancer. In: Lawton F, Neijt JP, Swenerton KD, editors. Epithelial Cancer of the Ovary. London: BMJ Publishing Group; 1995. p. 144. [Google Scholar]
- 51.van Meurs HS, Tajik P, Hof MH, et al. Which patients benefit most from primary surgery or neoadjuvant chemotherapy in stage IIIC or IV ovarian cancer? An exploratory analysis of the European Organisation for Research and Treatment of Cancer 55971 randomised trial. Eur J Cancer. 2013;49:3191–3201. doi: 10.1016/j.ejca.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979;63:1727–1733. [PubMed] [Google Scholar]
- 53.De Vita VT. Principles of chemotherapy. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 4th ed. Philadelphia, PA: Lippincot; 1993. p. 276. [Google Scholar]
- 54.Sato S, Itamochi H. Neoadjuvant chemotherapy in advanced ovarian cancer: Latest results and place in therapy. Ther Adv Med Oncol. 2014;6:293–304. doi: 10.1177/1758834014544891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauh-Hain JA, Rodriguez N, Growdon WB, et al. Primary debulking surgery versus neoadjuvant chemotherapy in stage IV ovarian cancer. Ann Surg Oncol. 2012;19:959–965. doi: 10.1245/s10434-011-2100-x. [DOI] [PubMed] [Google Scholar]
- 56.Sato S, Itamochi H, Kigawa J, et al. Combination chemotherapy of oxaliplatin and 5-fluorouracil may be an effective regimen for mucinous adenocarcinoma of the ovary: A potential treatment strategy. Cancer Sci. 2009;100:546–551. doi: 10.1111/j.1349-7006.2008.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuo K, Bond VK, Im DD, et al. Prediction of chemotherapy response with platinum and taxane in the advanced stage of ovarian and uterine carcinosarcoma: A clinical implication of in vitro drug resistance assay. Am J Clin Oncol. 2010;33:358–363. doi: 10.1097/COC.0b013e3181af30d3. [DOI] [PubMed] [Google Scholar]
- 58.Matsuo K, Eno ML, Im DD, et al. Chemotherapy time interval and development of platinum and taxane resistance in ovarian, fallopian, and peritoneal carcinomas. Arch Gynecol Obstet. 2010;281:325–328. doi: 10.1007/s00404-009-1121-1. [DOI] [PubMed] [Google Scholar]
- 59.Lim MC, Song YJ, Seo SS, et al. Residual cancer stem cells after interval cytoreductive surgery following neoadjuvant chemotherapy could result in poor treatment outcomes for ovarian cancer. Onkologie. 2010;33:324–330. doi: 10.1159/000313823. [DOI] [PubMed] [Google Scholar]
- 60.Traina TA, Dugan U, Higgins B, et al. Optimizing chemotherapy dose and schedule by Norton-Simon mathematical modeling. Breast Dis. 2010;31:7–18. doi: 10.3233/BD-2009-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosono S, Kajiyama H, Mizuno K, et al. Comparison between serous and non-serous ovarian cancer as a prognostic factor in advanced epithelial ovarian carcinoma after primary debulking surgery. Int J Clin Oncol. 2011;16:524–532. doi: 10.1007/s10147-011-0223-5. [DOI] [PubMed] [Google Scholar]
- 62.Ledermann JA, Luvero D, Shafer A, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for mucinous ovarian carcinoma. Int J Gynecol Cancer. 2014;24(suppl 3):S14–S19. doi: 10.1097/IGC.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 63.Hynninen J, Lavonius M, Oksa S, et al. Is perioperative visual estimation of intra-abdominal tumor spread reliable in ovarian cancer surgery after neoadjuvant chemotherapy? Gynecol Oncol. 2013;128:229–232. doi: 10.1016/j.ygyno.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Adams SF, Levine DA, Cadungog MG, et al. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115:2891–2902. doi: 10.1002/cncr.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Potter ME, Partridge EE, Hatch KD, et al. Primary surgical therapy of ovarian cancer: how much and when. Gynecol Oncol. 1991;40:195–200. doi: 10.1016/0090-8258(90)90277-r. [DOI] [PubMed] [Google Scholar]
- 67.Farias-Eisner R, Teng F, Oliveira M, et al. The influence of tumor grade, distribution, and extent of carcinomatosis in minimal residual stage III epithelial ovarian cancer after optimal primary cytoreductive surgery. Gynecol Oncol. 1994;55:108–110. doi: 10.1006/gyno.1994.1257. [DOI] [PubMed] [Google Scholar]
- 68.Makar AP, Kristensen GB, Kaern J, et al. Prognostic value of pre- and postoperative serum CA 125 levels in ovarian cancer: New aspects and multivariate analysis. Obstet Gynecol. 1992;79:1002–1010. [PubMed] [Google Scholar]
- 69.Makar AP, Kristensen GB, Børmer OP, et al. Serum CA 125 level allows early identification of nonresponders during induction chemotherapy. Gynecol Oncol. 1993;49:73–79. doi: 10.1006/gyno.1993.1089. [DOI] [PubMed] [Google Scholar]