This study explored predictors of timely oncology care and whether being engaged in the medical system for HIV care improved time to access. According to records and interviews of cancer patients in Botswana, the median time from first symptom to specialized oncology care was 13 months. HIV status did not affect time to oncology care; however, advanced cancer stage and use of traditional medicine/healers was associated with earlier oncology access.
Keywords: HIV, Botswana, Cancer, Timely oncology care, Advanced-stage cancer
Abstract
Background.
Three-quarters of cancer deaths occur in resource-limited countries, and delayed presentation contributes to poor outcome. In Botswana, where more than half of cancers arise in HIV-infected individuals, we sought to explore predictors of timely oncology care and evaluate the hypothesis that engagement in longitudinal HIV care improves access.
Methods.
Consenting patients presenting for oncology care from October 2010 to September 2014 were interviewed and their records were reviewed. Cox and logistic models were used to examine the effect of HIV and other predictors on time to oncology care and presentation with advanced cancer (stage III or IV).
Results.
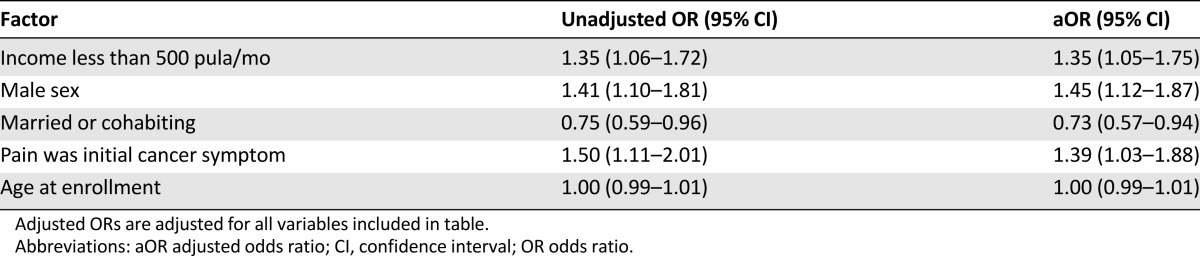
Of the 1,146 patients analyzed, 584 (51%) had HIV and 615 (54%) had advanced cancer. The initial clinic visit occurred a mean of 144 days (median 29, interquartile range 0–185) after symptom onset, but subsequent mean time to oncology care was 406 days (median 160, interquartile range 59–653). HIV status was not significantly associated with time to oncology care (adjusted hazard ratio [aHR] 0.91, 95% confidence interval [CI] 0.79–1.06). However, patients who reported using traditional medicine/healers engaged in oncology care significantly faster (aHR 1.23, 95% CI 1.09–1.40) and those with advanced cancer entered care earlier (aHR 1.48, 95% CI 1.30–1.70). Factors significantly associated with advanced cancer included income <$50 per month (adjusted odds ratio [aOR] 1.35, 95% CI 1.05–1.75), male sex (aOR 1.45, 95% CI 1.12–1.87), and pain as the presenting symptom (aOR 1.39, 95% CI 1.03–1.88).
Conclusion.
Longitudinal HIV care did not reduce the substantial delay to cancer treatment. Research focused on reducing health system delay through coordination and navigation is needed.
Implications for Practice:
The majority (54%) of patients in this large cohort from Botswana presented with advanced-stage cancer despite universal access to free health care. Median time from first symptom to specialized oncology care was 13 months. For HIV-infected patients (51% of total), regular longitudinal contact with the health system, through quarterly doctor visits for HIV management, was not successful in providing faster linkages into oncology care. However, patients who used traditional medicine/healers engaged in cancer care faster, indicating potential for leveraging traditional healers as partners in early cancer detection. New strategies are urgently needed to facilitate diagnosis and timely treatment of cancer in low- and middle-income countries.
Introduction
The global burden of cancer is increasing, with a more than 50% increase in cancer deaths since 1990 [1]. The increasing global cancer burden is caused partly by population growth and demographic shifts in many developing countries and partly by modifiable risk factors associated with cancer such as smoking and obesity [2–4]. Strikingly, more than half of new cancer diagnoses and 72% of cancer deaths occur in resource-limited settings [5]. For women in Africa, the lifetime risk of dying from cancer by the age of 65 is almost double that of western European women [6].
Botswana, a middle-income country in southern Africa, faces a multifaceted challenge in controlling cancer nationally. Demographic shifts resulting in lifestyle changes that often carry carcinogenic risks [5] have been compounded by a severe HIV epidemic—22% of adults (age 15–49) are infected [7]—further raising risks of cancer, particularly immune- and infection-mediated cancers [8]. Botswana has achieved higher HIV treatment coverage than nearly all high-income countries [9], but despite this robust access to combination antiretroviral therapy (ART), HIV-infected individuals remain three to five times more likely to develop cancer than age-matched uninfected counterparts [9, 10]. Individuals receiving ART are seen quarterly by a doctor and present to clinics monthly for medication refills. The HIV-infected population in Botswana provides an opportunity to evaluate the impact of engagement in regular medical care on the timeliness of oncology care.
Early detection and prompt treatment are critical to improving health outcomes in cancer patients [11, 12]. Previous work has targeted a reduction in time to diagnosis to improve survival [13]. In more developed settings, the median time from symptom onset to cancer treatment ranges from 3 to 5 months, with delays being both patient and system driven and varying by cancer type [14–16]. However, resource-limited settings often lack screening infrastructure, sufficient treatment centers, and therapeutic options, and patients tend to be diagnosed with later-stage cancers, which cumulatively has negative effects on survival [2, 17]. In addition, in settings suffering from high HIV prevalence (such as Botswana), it is important to understand the potential role of HIV and related factors in shaping cancer care trajectories and outcomes.
We sought to describe barriers and facilitators to timely oncology care in a resource-limited country with high HIV burden. We hypothesized that longitudinal care of HIV-infected individuals would facilitate care and reduce delays to initiation of specialized oncology treatment care.
Methods
Sampling and Data Collection
The data analyzed were taken directly from a prospective cohort of patients entering specialized cancer treatment in Botswana (the Botswana Prospective Cancer Cohort [BPCC]), which has previously been described [18]. We included adult patients enrolled at Princess Marina Hospital (October 2010 to September 2014) and Gaborone Private Hospital (November 2012 to September 2014). These hospitals provide oncology care for the 1.3 million residents living in southern Botswana, or 65% of the national population. Consenting patients are interviewed about demographic characteristics and cancer risk factors, medical records are abstracted, data regarding symptom/treatment history and potential cancer risk factors are collected, and patients are followed prospectively. History of symptoms of cancer and diagnostic evaluation is obtained retrospectively at the time of enrollment. Study staff conduct HIV testing for patients who have not received an HIV test in the previous 6 months, unless they refuse.
To develop an estimate of the cancer cases arising during the study period, we used the Botswana National Cancer Registry, a high-quality registry as noted by the African Cancer Registry Network [19]. We obtained the number of monthly recorded cancer cases in the catchment of the study facilities [20], and we assumed that this number remained constant during the study period. We also assumed that if all cases obtained specialized cancer care that the cases would be split 70:30 between Princess Marina Hospital and Gaborone Private Hospital (estimated from BPCC enrollment distribution).
Analytical Methods
Covariate Descriptions
Thirteen covariates from the cohort baseline survey were chosen for analysis and are described as follows. For the purposes of this analysis, participants earning an income of less than 500 pula/month (currently approximately US$52/month) were classified as below poverty level, which best approximates “moderate poverty” of US$2/day as designated by the World Bank [21]. Other covariates included education level, HIV status, biological sex, marital status, setting of residence, use of traditional medicine, and family history of cancer. Covariates regarding participants’ health and cancer included site of cancer, initial presenting symptom, restricted function (low performance status), bodily pain at time of enrollment in cancer care, and cancer stage at enrollment in cancer care. Eastern Cooperative Oncology Group performance status was assessed by trained research assistants and assigned on a 6-point scale, from 0 (asymptomatic) to 5 (death) [22]. Patients who were classified as symptomatic (1 or higher) at the time of entry into oncology care were considered to have restricted function, as we sought to identify the association of any symptom progression (versus no symptoms) with entry into care. Cancer diagnosis was made using a variety of available information, including histology of primary site (815), histology of metastasis (14), cytology/hematology (21), clinical assessment only (125), clinical assessment and radiology report (14), autopsy with histology (5), biochemical/immunological test (1), and surgical report (2). Cancers were staged according to the tumor-node-metastatis staging system of the American Joint Committee on Cancer [23]. Staging evaluation varied by cancer site, but routinely included an exam and imaging with chest x-ray and abdominal ultrasound. Cancers were categorized as localized (stages I and II) and advanced (stages III and IV). Kaposi sarcoma categorized as poor prognosis by the AIDS Clinical Trials Group was considered advanced [24]. Unstaged esophageal cancers that were deemed inoperable were classified as advanced. For univariate analyses including HIV status, participants with unknown HIV status were excluded. For analyses in which HIV status is included as a control, patients who were HIV uninfected or with unknown HIV status were combined to represent those with no known HIV infection. In those cases, sensitivity analyses resulted in similar effect estimates and justified the inclusion of participants with unknown HIV status with those confirmed to be HIV negative.
Time to Enrollment in Oncology Care
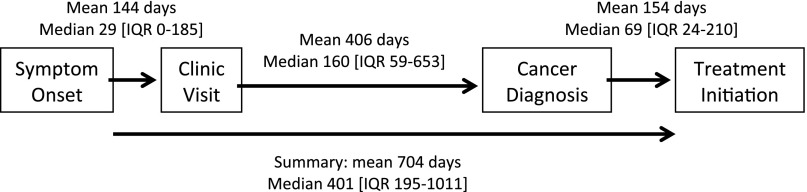
Four interval periods described the time from cancer symptom onset to treatment. These four time frames include (a) onset of first cancer symptom to first local clinic visit; (b) first local clinic visit to cancer diagnosis; (c) cancer diagnosis to cancer treatment initiation; and (d) summation of the previous three (onset of first cancer symptom to cancer treatment initiation) (see Fig. 1). The fourth interval, days until treatment, was the primary outcome. Enrollment in oncology care was defined as enrollment into the BPCC cohort, as patients are recruited from their initial visits with specialized oncology providers. Negative time intervals, indicating inconsistent chronology obtained during interviews, were excluded from analysis, and intervals were truncated at 95% to minimize the impact of several extreme values.
Figure 1.
Time to enrollment in oncology care. Mean, median, and IQR for all participants for four time intervals in days. Interval 1 is from cancer symptom onset to first clinic visit, with a mean of 144 and median of 29 days. Interval 2 is from first clinic visit to cancer diagnosis, with a mean of 406 and median of 160 days. Interval 3 is from cancer diagnosis to cancer treatment initiation, with mean of 154 and median of 69 days. Interval 4 is a summation of all time intervals from symptom onset to cancer treatment initiation, and has a mean of 704 and median 401 days.
Abbreviations: IQR, interquartile range.
Analytic Methods
Data were analyzed using Stata statistical software version 12.1 (College Station, TX, http://www.stata.com). The primary outcome in these analyses was days from onset of first cancer symptom to enrollment in oncology care. Differences in median time delays by HIV status were tested using Wilcoxon rank sum. Individual tests were run for the four different time intervals, stratified by each covariate above to assess associations between delays in care and participant characteristics.
Survival analysis was conducted with time to the primary outcome defined as the number of days from first cancer symptom to enrollment in oncology care. Kaplan-Meier survival curves were generated for all covariates, and univariate log-rank tests were assessed for each. All covariates with p value <0.20 in univariate analysis were included in the model building for a Cox proportional hazards model. This Cox regression included the covariates HIV, use of traditional medicine, moderate/severe pain, poverty, advanced-stage cancer, and dummy variables for cancer site. Age at enrollment into care was included in all models, as age is a strong determinant of cancer risk and outcome.
Because our hypothesis regarding the impact of HIV status relates to the assumption that HIV-positive patients receive more regular contact with health systems, the variable ART for at least 6 months before care was examined, which indicates whether a patient had been receiving ART treatment at the time before entry into oncology care when their cancer symptoms were likely developing. In the survival model, an interaction term between HIV status and ART for at least 6 months before care was assessed to rule out the potential confounding of ART treatment on the relationship between HIV status and days from initial symptom to entry into specialized oncology care.
Factors Associated With Advanced Cancer
Stepwise backward selection was used to fit a logistic regression model examining factors associated with advanced cancer at enrollment in oncology care. Type I error was set to α = 0.05, and all covariates were assessed. The final model included poverty, cancer site, biological sex, marital status, age, and pain as initial symptom. Interaction terms were tested to assess differences by biological sex in the association between other covariates and odds of presenting with advanced cancer.
Ethics
The study received approval for research on human subjects from the Health Research Development Committee (Ethics Committee) at the Botswana Ministry of Health and the institutional review board of the Harvard T.H. Chan School of Public Health. Participants provided written informed consent.
Results
Demographics
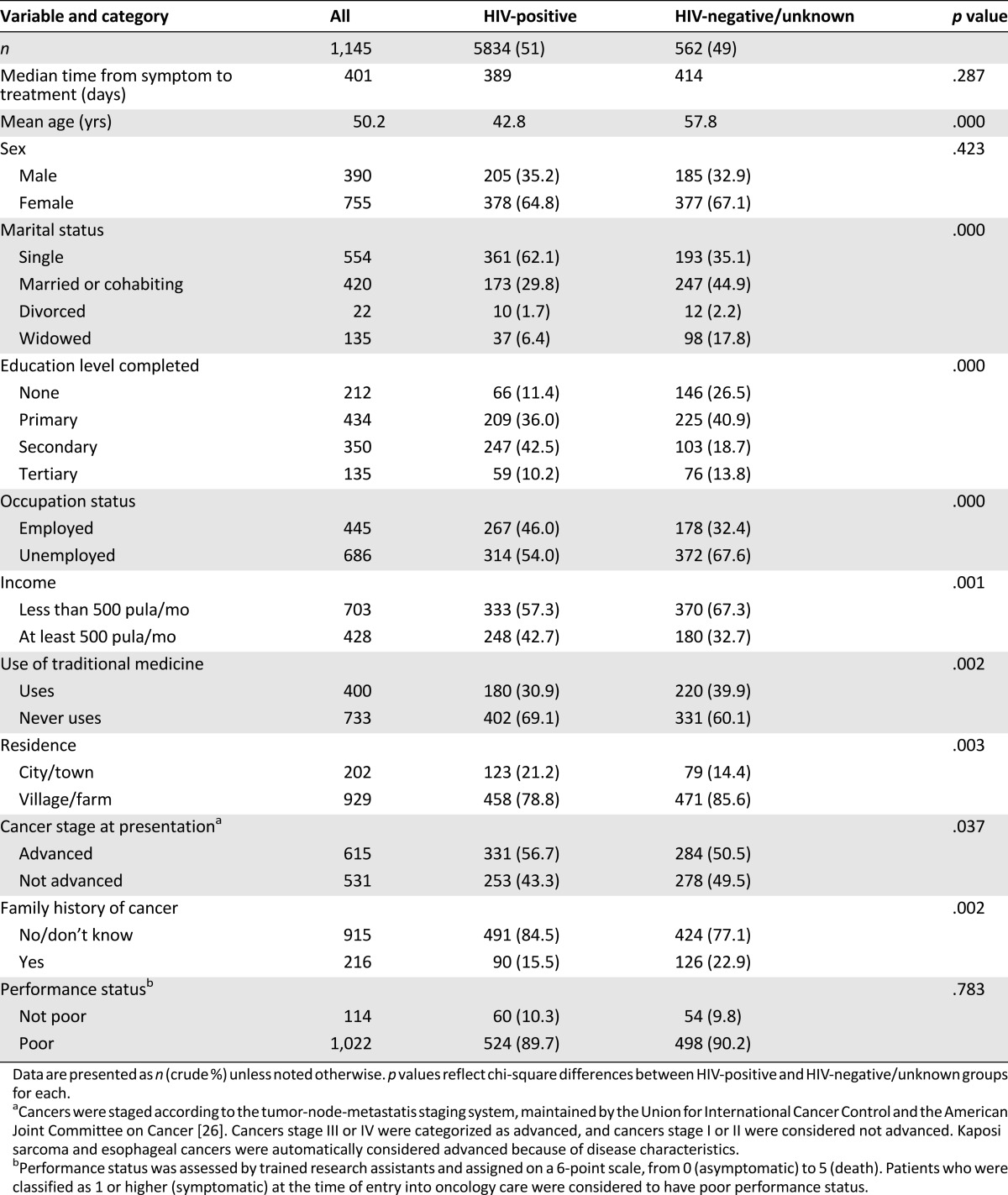
A total of 1,146 patients presenting for specialized cancer care were included in the analysis of an estimated 2,947 cancers that would be expected to have arisen in the catchment during the study period. Enrolled patients included 584 (51.0%) HIV infected, 417 (36.4%) HIV uninfected, and 145 (12.6%) with unknown HIV status (never tested, refused testing, or unwilling to report). Of HIV-infected patients, 424 (72.6%) were receiving ART at time of enrollment, 56 (9.6%) were not on ART, and 104 (17.8%) had insufficient documentation to determine ART status. Compared with HIV-uninfected participants, HIV-infected patients were younger (p < .001) and more likely to have monthly income of more than 500 pula (p = .05), to have no reported traditional medicine use (p < .01), to be married (p < .001), to have had at least some secondary-level education (p < .001), and to be employed (p = .001). Key characteristics are summarized by HIV status in Table 1.
Table 1.
Participant characteristics by HIV status
Time to Enrollment in Oncology Care
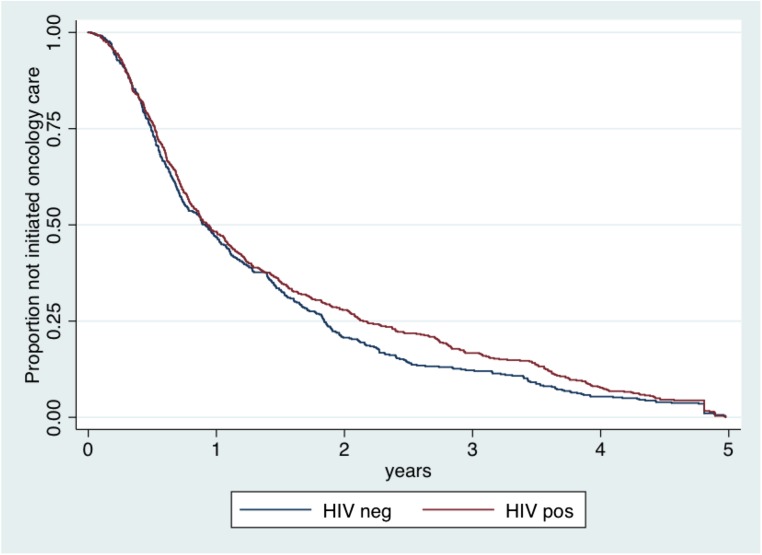
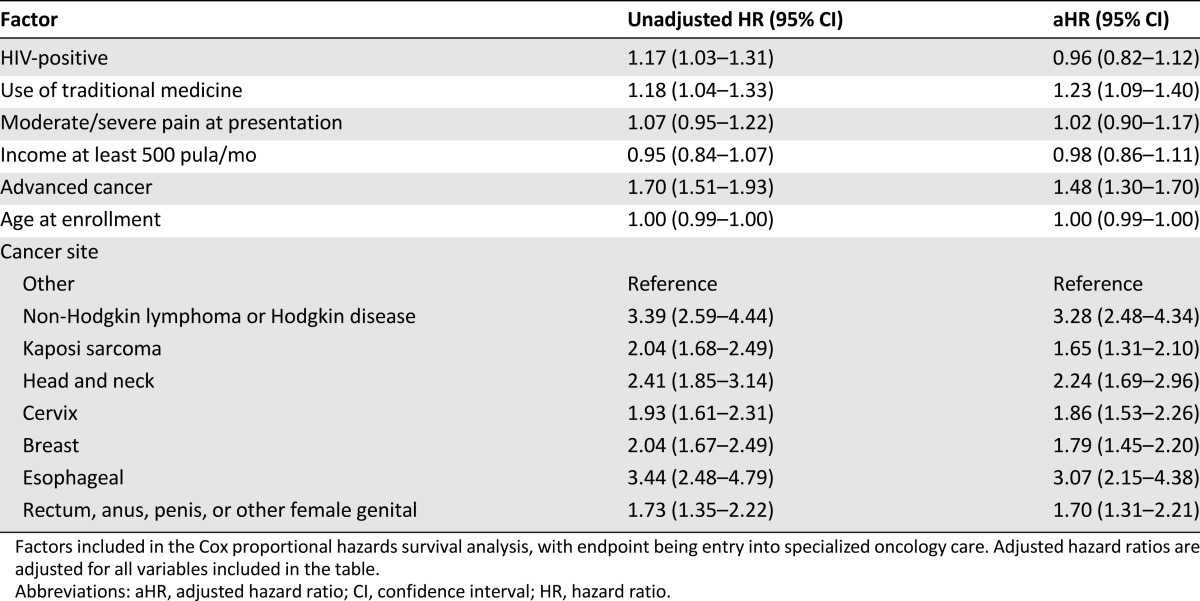
There were no significant differences in the median time from onset of cancer symptoms to enrollment in specialized care between individuals who were HIV infected versus HIV uninfected: median 389 days (interquartile range [IQR] 196–1,002) and 414 days (IQR 193–1,040), respectively (p = .29) (Table 1). There was no significant difference in time to entry into oncology care by HIV status (adjusted hazard ratio [aHR] 0.96, 95% confidence interval [CI] 0.82–1.12; p = .58) (Fig. 2), and HIV status was not associated with any of the four time intervals. Use of traditional medicine (aHR 1.23, 95% CI 1.09–1.40, p = .001) and advanced-stage cancer (aHR 1.48, 95% CI 1.30–1.70, p < .001) were associated with a shorter time to initiation of oncology care (Table 2).
Figure 2.
Kaplan-Meier failure curve for time from symptom onset to enrollment in specialized oncology care by HIV status. The x-axis is truncated at 5 years to show effect. Patients with unknown HIV status were excluded. Differences were not significant, p > .05.
Table 2.
Factors associated with time to enrollment in oncology care
The interaction term used to test for confounding of ART treatment on the relationship between HIV status and days from initial symptom to entry into specialized oncology care showed that there was no difference in time to entry into care for those engaged in ART (p = .69).
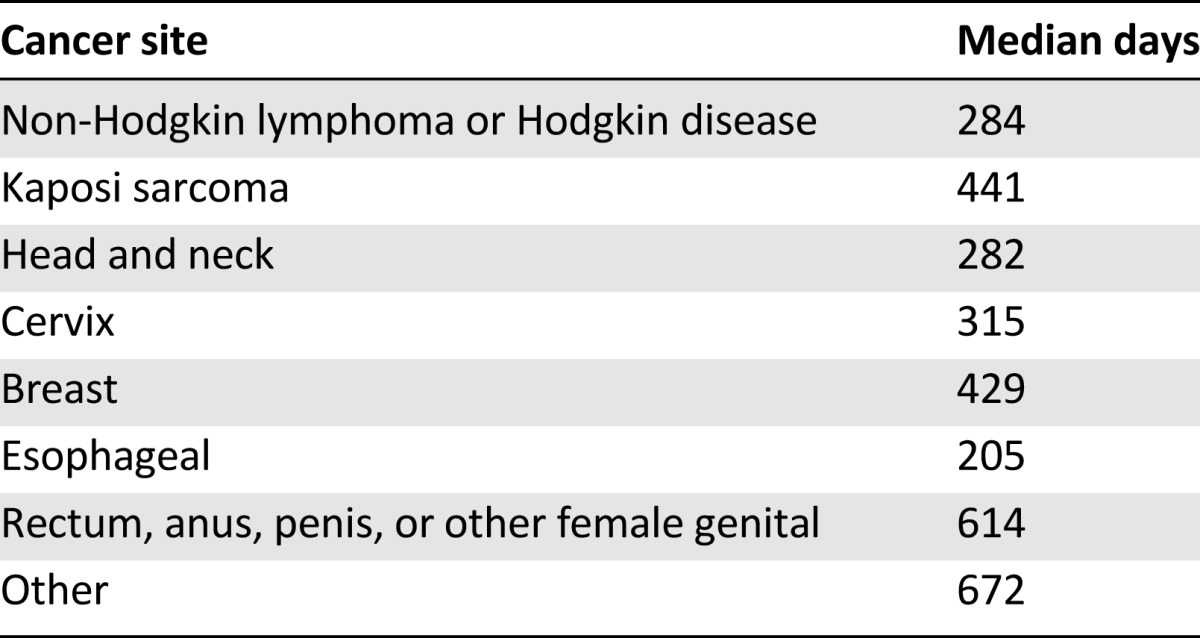
Mean and median days of the four time intervals were calculated for all participants, with longest delays seen between the first clinic visit and cancer diagnosis (Fig. 1). Median time from cancer symptom onset to cancer treatment initiation was 401 days, or approximately 13 months. Median time from cancer symptom onset to cancer treatment initiation is shown by cancer site in Table 3.
Table 3.
Median days to enrollment in oncology care by cancer site
Factors Associated With Advanced Cancer
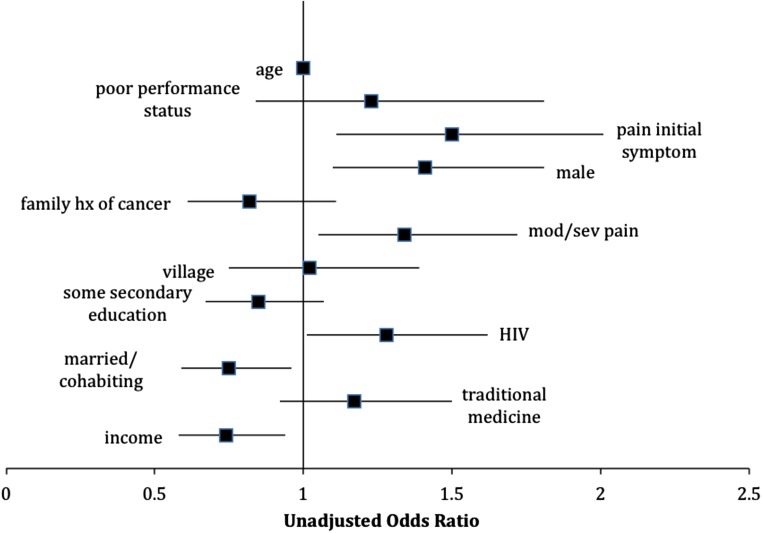
The majority (54%) of patients in this cohort presented with advanced cancer despite universal access to free health care. Bivariate analyses for factors associated with advanced stage can be seen in Figure 3, which depicts a forest plot with unadjusted odds ratios (ORs). As seen in the multivariate analysis depicted in Table 4, when holding included factors constant, those with household income less than 500 pula per month had 35% higher odds of presenting with advanced cancer (aOR 1.35, 95% CI 1.05–1.75, p = .019). Men had 45% greater odds than women of presenting with advanced cancer (aOR 1.45, 95% CI 1.12–1.87, p = .005), and those married or cohabiting had 27% decreased odds (aOR 0.73, 95% CI 0.57–0.94, p = .016). Finally, those patients who reported pain as the first cancer symptom had 1.39 times the odds of presenting with advanced cancer (aOR 1.39, 95% CI 1.03–1.88, p = .032). Interaction terms between biological sex and other model covariates were all nonsignificant.
Figure 3.
Factors associated with advanced cancer at presentation. Unadjusted ORs; bars represent 95% CIs. Cancers stage III or IV according to the tumor-node-metastatis staging system were categorized as advanced, and cancers stage I or II were considered not advanced. Kaposi sarcoma and esophageal cancers were automatically considered advanced because of disease characteristics.
Abbreviations: CI, confidence interval; hx, history; mod/sev, moderate to severe; OR, odds ratio.
Table 4.
Factors associated with advanced cancer at presentation
Discussion
Among patients needing specialized oncology care in Botswana, substantial delays were encountered, irrespective of HIV status. HIV positivity was higher in this cohort than in the general population, as would be expected because of established links between HIV and cancer [8], but HIV positivity was not significantly associated with decreased time to entry into specialized cancer care. Notably, less than half the expected number of cancer cases in the catchment population were enrolled in the BPCC, suggesting that large proportions of patients with cancer never receive any specialized cancer care.
These findings refute the hypothesis that HIV-positive patients enter into specialized cancer care with fewer delays, in spite of regularly scheduled appointments with health care professionals for ART review. This finding is surprising, as Botswana national HIV treatment guidelines [25] advise vigilance for cancer. Possible reasons for lack of impact of longitudinal care could be low clinical suspicion that hinders earlier detection of cancer among HIV-infected patients, unfamiliarity with cancer symptoms among health care providers or patients, or conflating of cancer symptoms with HIV outcomes.
Studies in other settings have demonstrated the importance of cancer detection in HIV patients and have documented that HIV-infected patients presenting with symptoms of lung cancer to HIV clinics experience delays in cancer diagnosis because cancer is not entertained as a likely etiology [26, 27]. We expect this may be the case for some HIV-associated cancers in Botswana, especially when symptoms overlap with more common AIDS-defining conditions such as tuberculosis. Regular ART review poses a unique place to implement routine point-of-care cancer screening and oncology referral for HIV-infected patients, but hinges on the training and awareness of health care professionals. Although time from symptom onset to entry into oncology care was not affected by HIV status, these findings nonetheless demonstrate the need for faster diagnosis and referral to oncology for all cancer patients in Botswana.
Median time from cancer symptom onset to cancer treatment initiation was substantially longer among this population than in other settings. This population experienced a median delay of 13 months, contrasting with more developed areas that have reported time frames of approximately 3–5 months [14–16]. The greatest time interval was found to be between first clinic visit and cancer diagnosis, indicating the importance of addressing delays once patients have entered into health care as a result of their cancer symptoms. Most of this time is likely to be accounted for by care patients receive at local clinics, although some may be accounted for by care received in larger referral hospitals, and future research should delineate more precisely in which clinic settings these delays occur.
Both the use of traditional medicine and advanced cancer upon diagnosis were associated with decreased delays into specialized oncology care. Patients who reported ever using traditional medicine experienced less time to treatment initiation. This finding challenges assumptions that use of traditional healers hinders entry into formalized medical care [28, 29]; a possible explanation is that consideration of traditional medicine use is a marker of health-seeking behavior more generally. Patients with rapidly progressing symptoms and advanced disease entered into specialized care earlier, perhaps because the patients were more likely to seek care and/or the local clinics were more likely to refer the patient to specialized oncology departments; however, this association should be examined further. These findings may suggest an issue around cancer symptom recognition at the clinic level and triage of cancer care based on presenting symptoms. The patients receiving the most timely care are those with advanced disease and symptom progression. Although it is essential to continue to diagnose and quickly treat these patients, additional efforts are needed to identify disease among patients with early-stage cancers to prevent disease progression and improve outcomes among the larger population.
Finally, considering the role of early detection on favorable treatment outcomes, we assessed factors associated with advanced cancers. Men and patients citing pain as the first cancer symptom were more likely to present with advanced disease. Other studies have demonstrated sex differences in health-seeking behaviors and cancer outcomes among cancer patients [13, 30, 31], with men more likely to use gender-specific rationales to explain reluctance in seeking care [13]. Investigation into gender dynamics in Botswana and the ways in which these impact health and cancer outcomes could further elucidate these findings. Participants reporting income less than 500 pula per month were more likely to present with advanced disease, which corroborates existing data linking low income and socioeconomic status to worse cancer outcomes and late-stage presentation in various settings [32–36]. Targeted screening and improved access to care for patients with low socioeconomic status is critical for more rapid diagnosis of cancer and improved treatment outcomes in Botswana.
There are several limitations of these analyses. A key limitation is that only patients who receive some specialized oncology care are enrolled into the cohort (i.e., not patients treated for Kaposi sarcoma with ART alone in their HIV clinic), as recruitment occurs within these oncology clinics. The design does not allow assessment of barriers experienced by patients who never receive a diagnosis or the large fraction who never present to oncology care. Future work is urgently needed to describe the experiences of this group and potential strategies for engagement. Some patients may have received surgical care after diagnosis and before entering into specialized oncology care clinics, so days to treatment initiation in these cases may be slightly overestimated, although likely only by several days to a couple of weeks. Another limitation is that presenting symptom and date of presenting symptom were collected retrospectively and were most commonly self-reported. Validation occurs through examination of patient medical records, but the effect of recall bias should be considered for these two factors. Finally, at the time of analysis, the cohort was not yet enrolling patients in Francistown, so the data may be most reflective of Gaborone and surrounding areas.
Despite these limitations, these data are the first to identify factors associated with delayed entry into cancer care and presentation with advanced-stage disease in Botswana. To improve cancer outcomes in Botswana, increased emphasis should be placed on screening and early detection of symptoms. Training clinic staff about cancer symptoms and disease progression may lead to increased rates of referral and improved outcomes for cancer patients. Additionally, public health awareness targeted to patients, particularly those at higher risk of cancer due to HIV infection, family history, or engagement in risky health behaviors, should be promoted. Because of the finding that the longest delays were encountered after the initial clinic visit, efforts to promote cancer symptom awareness among health care staff should focus largely on decentralized clinic settings. Additionally, the possibility of including traditional healers in educational campaigns about cancer symptoms and linkages to specialized oncology care could be explored in Botswana, as traditional healers could be leveraged as referral tools and partners in early cancer detection. Interestingly, HIV status did not play a role in timeliness of cancer care, demonstrating that despite a successful, comprehensive ART program in Botswana, regular clinic visits for people living with HIV do not necessarily translate into improved health outcomes for other conditions such as cancer.
Substantial delays in entry into oncology treatment were encountered in Botswana, but by implementing targeted screening interventions for those most vulnerable and promoting cancer symptom awareness among health care providers, cancer treatment outcomes and survival could improve drastically.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This article was made possible with help from the Harvard University (P30 AI060354) and University of Pennsylvania (P30 AI045008) Centers for AIDS Research (CFAR), an NIH funded program supported by the following NIH cofunding and participating institutes and centers: National Institute of Allergy and Infectious Diseases; National Cancer Institute; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; National Institute on Drug Abuse; National Institute of Mental Health; National Institute on Aging; Fogarty International Center; and Office of Regulatory Affairs. The work was also conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award 8UL1TR000170-05, and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the NIH.
Author Contributions
Conception/Design: Gita Suneja, Neo Tapela, Malebogo Pusoentsi, Mompati Mmalane, Doreen Ramogola-Masire, Memory Nsingo-Bvochora, Mukendi Kayembe, Jason Efstathiou, Shahin Lockman, Scott Dryden-Peterson
Provision of study material or patients: Neo Tapela, Mompati Mmalane, Zola Musimar, Memory Nsingo-Bvochora, Mukendi Kayembe, Jason Efstathiou, Shahin Lockman, Scott Dryden-Peterson
Collection and/or assembly of data: Carolyn A. Brown, Abigail Mapes, Ryan Hodgeman, Matthew Boyer, Memory Nsingo-Bvochora, Mukendi Kayembe, Scott Dryden-Peterson
Data analysis and interpretation: Carolyn A. Brown, Gita Suneja, Surbhi Grover, Shahin Lockman, Scott Dryden-Peterson
Manuscript writing: Carolyn A. Brown, Gita Suneja, Neo Tapela, Abigail Mapes, Malebogo Pusoentsi, Mompati Mmalane, Ryan Hodgeman, Matthew Boyer, Zola Musimar, Doreen Ramogola-Masire, Surbhi Grover, Memory Nsingo-Bvochora, Mukendi Kayembe, Jason Efstathiou, Shahin Lockman, Scott Dryden-Peterson
Final approval of manuscript: Carolyn A. Brown, Scott Dryden-Peterson
Disclosures
The authors indicated no financial relationships.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, DeLancey JO, Center MM, et al. The global burden of cancer: Priorities for prevention. Carcinogenesis. 2010;31:100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolin KY, Carson K, Colditz GA. Obesity and cancer. The Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tunstall-Pedoe H. Preventing Chronic Diseases: A Vital Investment. Available at: http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed March 22, 2016.
- 6.Parkin DM, Sitas F, Chirenje M, et al. Part I: Cancer in Indigenous Africans—Burden, distribution, and trends. Lancet Oncol. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS. HIV and AIDS Estimates (2014). Available at: http://www.unaids.org/en/regionscountries/countries/botswana. Accessed March 22, 2016.
- 8.Cooley TP. Non-AIDS-defining cancer in HIV-infected people. Hematol Oncol Clin North Am. 2003;17:889–899. doi: 10.1016/s0889-8588(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 9.Suneja G, Ramogola-Masire D, Medhin HG, et al. Cancer in Botswana: Resources and opportunities. Lancet Oncol. 2013;14:e290–e291. doi: 10.1016/S1470-2045(13)70283-3. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health Botswana. Botswana National Cancer Registry 2001–2010. Gaborone, Botswana: 2010.
- 11.Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer. 2003;3:243–252. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 12.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 13.Smith LK, Pope C, Botha JL. Patients’ help-seeking experiences and delay in cancer presentation: A qualitative synthesis. Lancet. 2005;366:825–831. doi: 10.1016/S0140-6736(05)67030-4. [DOI] [PubMed] [Google Scholar]
- 14.Salomaa E-R, Sällinen S, Hiekkanen H, et al. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128:2282–2288. doi: 10.1378/chest.128.4.2282. [DOI] [PubMed] [Google Scholar]
- 15.Myrdal G, Lambe M, Hillerdal G, et al. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59:45–49. [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen RP, Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment of cancer: A cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11:284. doi: 10.1186/1472-6963-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: A population-based study. Lancet Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 18.Dryden-Peterson S. HIV and Malignancy in Botswana: An Observational Study of Incidence, Toxicity of Concurrent Treatment, and Clinical Outcomes. Available at http://www.bhp.org.bw/research/. Accessed March 22, 2016.
- 19.Gakunga R, Parkin DM. Cancer registries in Africa 2014: A survey of operational features and uses in cancer control planning. Int J Cancer. 2015;137:2045–2052. doi: 10.1002/ijc.29668. [DOI] [PubMed] [Google Scholar]
- 20.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS One. 2015;10:e0135602. doi: 10.1371/journal.pone.0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The World Bank. Poverty: Overview. Available at: http://www.worldbank.org/en/topic/poverty/overview. Accessed March 2016.
- 22.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC et al. AJCC Cancer Staging Manual. New York: Springer, 2010.
- 24.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: A proposal for uniform evaluation, response, and staging criteria. J Clin Oncol. 1989;7:1201–1207. doi: 10.1200/JCO.1989.7.9.1201. [DOI] [PubMed] [Google Scholar]
- 25. Ministry of Health. Botswana Guidelines on Antiretroviral Treatment. Gaborone, Botswana: Department of HIV/AIDS Prevention and Care, Botswana Ministry of Health, 2012.
- 26.Brock MV, Hooker CM, Engels EA, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: Implications for patient care. J Acquir Immune Defic Syndr. 2006;43:47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 27.Cadranel J, Garfield D, Lavolé A, et al. Lung cancer in HIV infected patients: Facts, questions and challenges. Thorax. 2006;61:1000–1008. doi: 10.1136/thx.2005.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills E, Singh S, Wilson K, et al. The challenges of involving traditional healers in HIV/AIDS care. Int J STD AIDS. 2006;17:360–363. doi: 10.1258/095646206777323382. [DOI] [PubMed] [Google Scholar]
- 29.Smyth A, Martin M, Cairns J. South Africa’s health. Traditional healers may cause dangerous delays. BMJ. 1995;311:948. doi: 10.1136/bmj.311.7010.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: An analysis of the surveillance, epidemiology, and end results database. J Clin Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 31.Henschke CI, Yip R, Miettinen OS. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296:180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 32.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 33.Wells BL, Horm JW. Stage at diagnosis in breast cancer: Race and socioeconomic factors. Am J Public Health. 1992;82:1383–1385. doi: 10.2105/ajph.82.10.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Malley MS, Earp JA, Hawley ST, et al. The association of race/ethnicity, socioeconomic status, and physician recommendation for mammography: Who gets the message about breast cancer screening? Am J Public Health. 2001;91:49–54. doi: 10.2105/ajph.91.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madison T, Schottenfeld D, James SA, et al. Endometrial cancer: Socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–2111. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]