This systemic review and two-step meta-analysis examined existing literature to assess neoadjuvant chemotherapy (NCT) for muscle-invasive bladder cancer (MIBC). Findings support the use of cisplatin-based combination NCT to treat MIBC, but further investigation is warranted.
Keywords: Chemotherapy, Bladder cancer, Neoadjuvant, Platinum, Survival
Abstract
Background.
Platinum-based neoadjuvant chemotherapy has been shown to improve survival outcomes in muscle-invasive bladder cancer patients. We performed a systematic review and meta-analysis to provide updated results of previous findings. We also summarized published data to compare clinical outcomes of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) versus gemcitabine and cisplatin/carboplatin (GC) in the neoadjuvant setting.
Methods.
A meta-analysis of 15 randomized clinical trials was performed to compare neoadjuvant chemotherapy plus local treatment with the same local treatment alone. Because no randomized trials have investigated MVAC versus GC in the neoadjuvant setting, a meta-analysis of 13 retrospective studies was performed to compare MVAC with GC.
Results.
A total of 3,285 patients were included in 15 randomized clinical trials. There was a significant overall survival (OS) benefit associated with cisplatin-based neoadjuvant chemotherapy (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.79–0.96). A total of 1,766 patients were included in 13 retrospective studies. There was no significant difference in pathological complete response between MVAC and GC. However, GC was associated with a significantly reduced overall survival (HR, 1.26; 95% CI, 1.01–1.57). After excluding carboplatin data, GC still seemed to be inferior to MVAC in OS (HR, 1.31; 95% CI, 0.99–1.74), but the difference was no longer statistically significant.
Conclusion.
These results support the use of cisplatin-based combination neoadjuvant chemotherapy in muscle-invasive bladder cancer. Although GC and MVAC had similar treatment response rates, the different survival outcome observed in this study requires further investigation.
Implications for Practice:
Platinum-based neoadjuvant chemotherapy (NCT) has been shown to improve survival outcomes in muscle-invasive bladder cancer (MIBC) patients, but the optimal neoadjuvant regimen has not been established. Methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) and gemcitabine and cisplatin/carboplatin (GC) are two of the most commonly used chemotherapy regimens in modern oncology. In this two-step meta-analysis, an updated and more precise estimate of the survival benefit of cisplatin-based NCT in MIBC is provided. This study also demonstrated that MVAC might have superior overall survival compared with GC (with or without carboplatin data) in the neoadjuvant setting. The findings suggest that NCT should be standard care in MIBC, and MVAC could be the preferred neoadjuvant regimen.
Introduction
In the United States, bladder cancer is the fourth most common cancer and the eighth leading cause of cancer-related death in humans [1]. Internationally, the incidence and mortality of bladder cancer varies substantially, with highest rates in Europe, North America, and Egypt [2]. Muscle invasion remains a poor prognostic factor probably because of occult metastasis at the time of diagnosis. Despite radical cystectomy, approximately half of patients with deep, muscle-invasive bladder cancer (MIBC) involving the muscularis propria (≥T2) develop metastatic disease within 2 years of diagnosis and usually succumb to their disease [3].
Several randomized clinical trials have shown that platinum-based combination neoadjuvant chemotherapy (NCT) can improve survival outcomes, compared with locoregional treatment alone. For example, the SWOG trial, including 307 patients, showed a 33% reduced death risk in the methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) plus cystectomy arm, compared with cystectomy alone [4]. The updated BA06 30894 trial, including 976 patients, showed a statistically significant 16% reduced death risk in the arm of cisplatin, methotrexate, and vinblastine (CMV) plus cystectomy and/or radiotherapy, as well as a 6% increase in survival at 10 years [5]. In contrast, most prospective studies failed to prove a survival benefit probably because of insufficient power. Therefore, quantitative analyses by combining the results of all relevant randomized trials were attempted [6–8]. The most recent meta-analysis in 2005 included 11 randomized trials and showed a 5% absolute improvement in overall survival (OS) at 5 years (hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.77–0.95) in the arm of cisplatin-based NCT [7]. Based on these results, platinum-based combination neoadjuvant chemotherapy has been recommended as standard care for MIBC.
Notably, most of those clinical trials included in previous meta-analysis were designed in the 1990s and used MVAC or CMV as NCT regimens, although there has been an increased interest in a gemcitabine and cisplatin/carboplatin (GC) regimen recently due to its favorable toxicities. Although no randomized trials are available to compare GC with MVAC in the neoadjuvant setting, a large randomized trial showed that GC was noninferior to MVAC in metastatic settings [9]. As a result, MVAC and GC are the two most commonly used chemotherapy regimens for bladder cancer in modern oncology. Since 2005, results of a number of new randomized trials have been published, and three large randomized trials (Nordic I, Nordic II, and BA06 30894) presented updated results with longer follow-up time. Since 2007, a growing number of studies have compared clinical outcomes of GC versus MVAC as neoadjuvant regimens; however, all of these studies were limited by their small sample sizes. In light of the new evidence, we decided to perform a two-step systematic review and meta-analysis aiming to provide (a) an updated and more precise estimate of the effect of NCT on survival in MIBC and (b) quantitative evidence to compare GC with MVAC in the neoadjuvant setting, because no published meta-analysis was available.
Methods
Literature Search
We conducted this systematic review in accordance with the guidelines of the Cochrane Collaboration. We searched for relevant papers published before August 30, 2015, by using the electronic PubMed database, Cochrane database, and Ovid database with the following terms: “neoadjuvant,” “bladder cancer” or “carcinoma” or “tumor” or “neoplasm.” References of the retrieved articles were also screened for earlier original studies. The inclusion criteria were as follows: patients with (a) biopsy-proven MIBC who intended to receive local definitive treatment (defined as radical cystectomy or radiotherapy) with or without NCT, (b) chemotherapy to be platinum based and given systemically, and (c) no distant metastasis. In the first step of the meta-analysis (NCT plus locoregional therapy versus locoregional therapy), only randomized clinical trials were included; in the second step of the meta-analysis (GC versus MVAC), only retrospective studies were included because no randomized clinical trials were available. Authors were contacted to obtain any missing information. If the authors did not respond to our repeated requests, the missing information was excluded from related analyses. If the same study was presented more than once, we selected the most recent article with updated information.
Data Extraction
We extracted the following information from each published article: author, year of publication, country of origin, sample size, NCT regimens given, type of locoregional therapy, and follow-up period. We used odds ratios (ORs) and adjusted Cox proportional HRs for the quantitative analysis. If adjusted HRs were not available and the corresponding authors did not respond to our requests, the crude HRs were used. When both adjusted and crude HR data were not available but appropriate summary statistics or Kaplan-Meier curves were provided, we calculated HRs and 95% CIs as relevant effect measures using published methods [10].
Statistical Analysis
We performed a meta-analysis to estimate clinical outcomes, including NCT response rates and OS in control and study groups. We assessed the between-study heterogeneity by using the Cochran Q test with a significance level of p < .05. We performed initial analyses with a fixed-effect model, and confirmatory analyses were performed with a random-effect model if there was significant heterogeneity. We used inverted funnel plots and the Egger’s test to assess the possibility of publication bias. All p values were two-sided, and all analyses were performed using the Stata software (StataCorp, College Station, TX, http://www.stata.com) and Review Manager version 5.3 (Cochrane, Oxford, U.K.).
Results
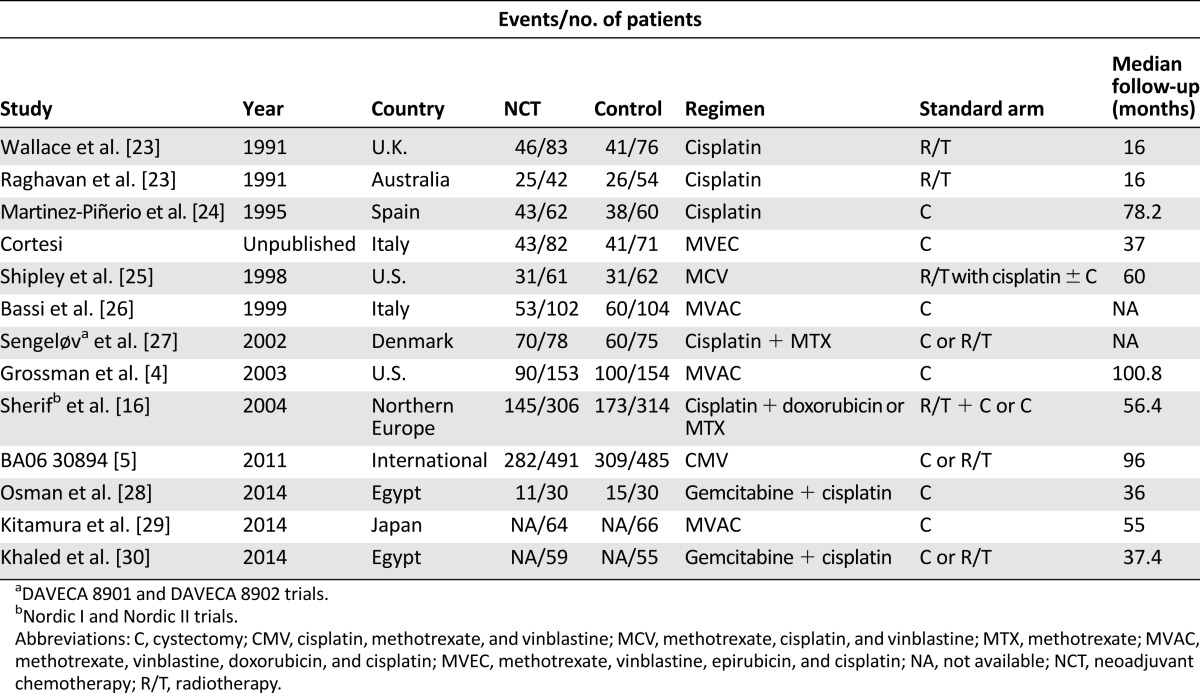
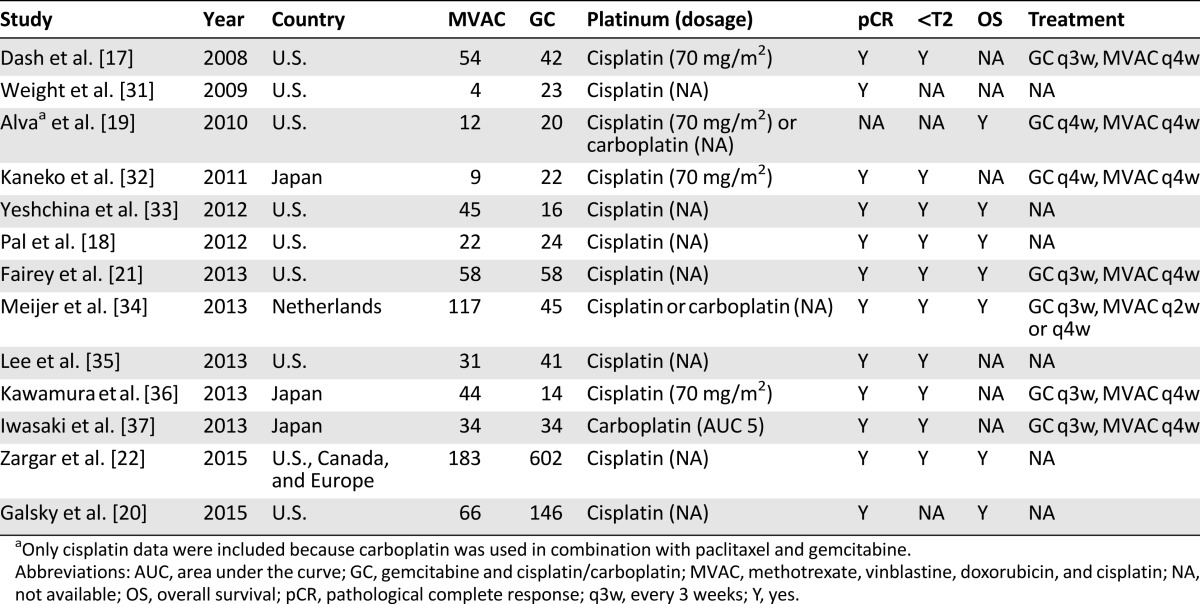
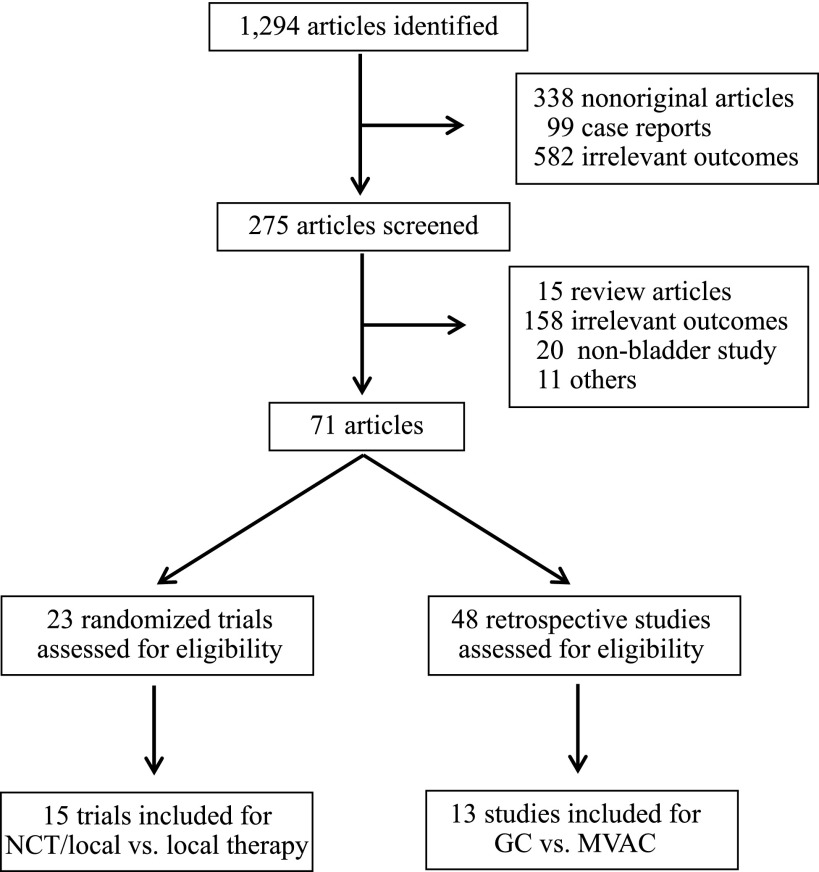
For the first step of the meta-analysis (NCT plus locoregional therapy versus locoregional therapy), 19 reports met the inclusion criteria, encompassing 15 randomized trials, all of which were cisplatin based (Table 1). Because some of the trials have been reported more than once, the data contained in the last report were used for this analysis. For example, the SWOG 8710 trial was first reported in 2001 as an abstract [11] and then was published in 2003 [4]. In addition, the BA06 30894 trial was first reported in 1999 [12] and then was updated in 2011 [5]. Further, the Nordic I and II trials were first reported in 1993 (Nordic I) [13], 1996 (Nordic I) [14], and 2002 (Nordic II) [15], respectively, and they were last reported in 2004 as a joint, updated analysis [16]. Although the study by Cortesi et al. was not published, it was included in previous meta-analyses [6, 7] and therefore was included in our analysis. For the second step of the meta-analysis, a total of 13 reports met the inclusion criteria. Notably, part of the patient information contained in the studies by Dash et al. [17], Pal et al. [18], and Alva et al. [19] were later incorporated into a larger investigation by Galsky et al. [20], whereas part of the patient information contained in the study by Fairey et al. [21] was later incorporated into the investigation by Zargar et al. [22]. We included all 13 studies in our analysis because some clinical information, such as tumor downstaging, was available in only small studies, but we also repeated our analysis excluding studies with redundant information. As a result, the final data pool consisted of 13 studies, including 1,766 bladder cancer patients who received either standard or dose-dense MVAC or GC as NCT regimens (Table 2). The flowchart of study selection is shown in Figure 1.
Table 1.
Characteristics of included randomized clinical trials
Table 2.
Characteristics of included retrospective studies
Figure 1.
Flowchart for article selection. Only randomized trials were included to compare NCT plus locoregional therapy versus locoregional therapy, whereas retrospective studies were included to compare GC versus MVAC because no randomized trials were available.
Abbreviations: GC, gemcitabine and cisplatin/carboplatin; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; NCT, neoadjuvant chemotherapy.
Neoadjuvant/Locoregional Versus Locoregional Alone
Overall Survival
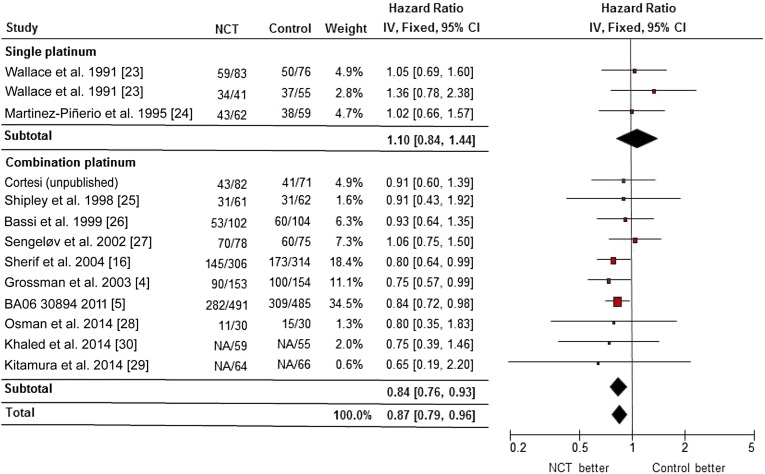
Fifteen randomized trials including 3,285 patients were eligible for this analysis. Consistent with previous meta-analysis, cisplatin-based NCT was associated with a significant OS benefit, compared with locoregional therapy alone (HR, 0.87; 95% CI, 0.79–0.96; p = .004; p = .83 for heterogeneity, I2 = 0%) (Fig. 2). NCT with cisplatin alone did not appear to provide a survival benefit (HR, 1.10; 95% CI, 0.84–1.44; p = .48; p = .70 for heterogeneity, I2 = 0%), although cisplatin-based combination NCT was associated with a significantly prolonged survival (HR, 0.84; 95% CI, 0.76–0.93; p < .001; p = .95 for heterogeneity, I2 = 0%). Notably, the majority of studies included primarily urothelial cancer patients, although the study by Khaled et al. [30] included 50% squamous cell carcinoma patients. However, our results were not substantially changed by including or excluding the study by Khaled et al. (data not shown).
Figure 2.
Forest plot of overall survival in comparison of cisplatin-based neoadjuvant chemotherapy plus locoregional therapy versus locoregional therapy alone by randomized clinical trials.
Abbreviations: CI, confidence interval; IV, inverse variance; NCT, neoadjuvant chemotherapy.
In subgroup analysis, modern NCT regimens of GC or MVAC-like (MVAC or CMV) chemotherapy were associated with a survival benefit (HR, 0.82; 95% CI, 0.74–0.91; p < .001; p = .99 for heterogeneity, I2 = 0%) and an absolute increase in 5-year survival of 8% (45%–53%), equivalent to a number needed to treat of 12.5. If only patients with cystectomy were considered, a survival benefit associated with cisplatin-based combination NCT remained (HR, 0.81; 95% CI, 0.70–0.93; p = .003; p = .99 for heterogeneity, I2 = 0%). There was no evidence of statistical heterogeneity (I2 = 0%) between the trial results. No publication bias was detected by the funnel plot or the Egger’s test in subgroup analysis (data not shown).
In subgroup analysis, modern NCT regimens of GC or MVAC-like (MVAC or CMV) chemotherapy were associated with a survival benefit and an absolute increase in 5-year survival of 8% (45%–53%), equivalent to a number needed to treat of 12.5.
GC Versus MVAC
Treatment Response
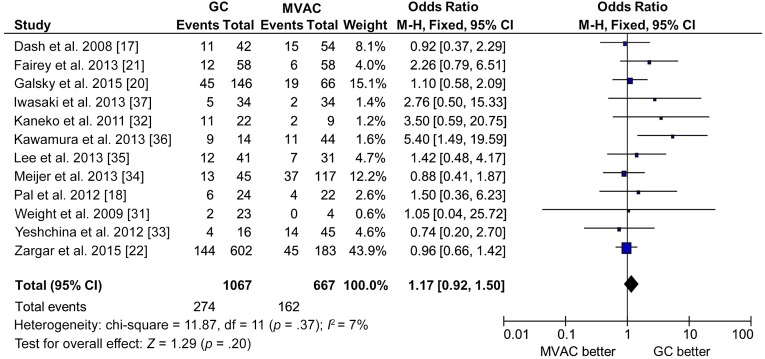
For pathological complete response (pCR), 12 studies of 1,734 patients were eligible for this analysis, including 1,067 patients of GC and 667 patients of MVAC. Overall, the pCR of GC was 25.7%, whereas the pCR of MVAC was 24.3%. There was no statistically significant difference in pCR between GC and MVAC (GC vs. MVAC: OR, 1.17; 95% CI, 0.92–1.50; p = .37 for heterogeneity, I2 = 7%) (Fig. 3). For pathological downstaging response (T < 2), 10 studies of 1,495 patients were eligible for analysis, including 898 patients of GC and 597 patients of MVAC. Again, there was no significant difference between GC and MVAC (OR, 1.07; 95% CI, 0.85–1.34; p = .19 for heterogeneity, I2 = 28%).
Figure 3.
Forest plot of pathological complete response in comparison of GC versus MVAC by retrospective studies.
Abbreviations: CI, confidence interval; GC, gemcitabine and cisplatin/carboplatin; M-H, Mantel-Haenszel method; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; NCT, neoadjuvant chemotherapy.
To control potentially inferior efficacy of carboplatin, we excluded carboplatin data (study by Iwasaki et al. [37] and carboplatin data contained in the study by Meijier et al. [34]) from our analysis. Our conclusion was not substantially changed (data not shown). To remove redundant information due to partial overlapping patient information between small and later larger studies, we excluded the studies by Dash et al. [17], Pal et al. [18], and Fairey et al. [21] from analysis. The results were not significantly changed for pCR (OR, 1.14; 95% CI, 0.87–1.49; p = .27 for heterogeneity, I2 = 19%) or pathological downstaging response (OR, 1.02; 95% CI, 0.80–1.31; p = .15 for heterogeneity, I2 = 34%). No publication bias was detected by either the funnel plot or the Egger’s test (data not shown).
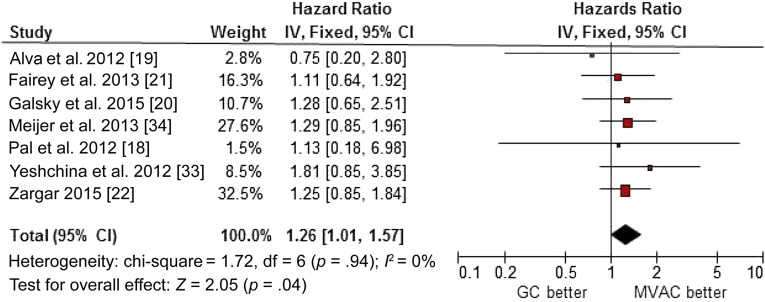
Overall Survival
Seven studies, including 1,414 patients, were eligible for this analysis. Compared with MVAC, GC was associated with a clinically and statistically significant increase in the hazard of death (HR, 1.26; 95% CI, 1.01–1.57; p = .94 for heterogeneity, I2 = 0%) (Fig. 4). After excluding carboplatin data in the study by Meijier et al. [34] and the studies by Pal et al. [18], Alva et al. [19], and Fairey et al. [21] due to redundant patient information, gemcitabine plus cisplatin remained inferior relative to MVAC in OS (HR, 1.31; 95% CI, 0.99–1.74; p = .84 for heterogeneity, I2 = 0%), but the difference was no longer statistically significant. No publication bias was detected by either the funnel plot or the Egger’s test (data not shown).
Figure 4.
Forest plot of overall survival in comparison of GC versus MVAC by retrospective studies.
Abbreviations: CI, confidence interval; GC, gemcitabine and cisplatin/carboplatin; IV, inverse variance; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin.
Discussion
To our knowledge, this is the largest quantitative analysis to examine the survival benefit of NCT in MIBC and the first quantitative analysis to compare clinical outcomes of GC with MVAC in the neoadjuvant setting. In this comprehensive two-step meta-analysis of 15 randomized clinical trials and 13 retrospective studies, we confirmed a significant survival benefit associated with platinum-based combination neoadjuvant chemotherapy. We further provided evidence that bladder cancer patients who received MVAC might have better survival outcomes, compared with those who received a GC regimen in the neoadjuvant setting.
Platinum-based combination neoadjuvant chemotherapy followed by definitive locoregional therapy, such as radical cystectomy, should be the mainstay of treatment for MIBC. Although several randomized trials and a meta-analysis in 2005 have demonstrated a clear improvement in OS, NCT remains vastly underused in the oncology community [38]. One concern for NCT modality has been the delay in curative-intent surgery while delivering chemotherapy. Two recent studies suggested that NCT can be safely administered in a short time using a dose-dense schedule, with less toxicity and at least comparable efficacy compared with conventional NCT [39, 40]. In addition, patients who receive chemotherapy before surgery are more likely to receive sufficient doses with better tolerance.
Platinum-based combination neoadjuvant chemotherapy followed by definitive locoregional therapy, such as radical cystectomy, should be the mainstay of treatment for MIBC.
In this updated meta-analysis with a larger patient population, we confirmed the conclusion of previous meta-analyses and provided a more precise estimate of the effect of NCT in MIBC. Compared with the 2005 meta-analysis, we incorporated four additional randomized trials and used updated results from three large randomized trials (Nordic I, Nordic II, and BA06 30894), consisting of information for 427 new patients and updated information for 1,596 patients. Our data showed that cisplatin-based combination NCT is associated with a 16% reduction in overall death risk, compared with locoregional therapy alone. If only GC or MVAC-like chemotherapy regimens are considered, NCT is associated with an even better survival benefit (HR, 0.82; 95% CI, 0.74–0.91) and an absolute survival benefit of 8% at 5 years (a number needed to treat of 12.5). Our results confirm the compelling argument for an increased adoption of NCT as standard care for MIBC patients.
One important question remains unanswered: What is the best NCT regimen? Traditionally, MVAC or CMV is the regimen of choice. The regimen of GC has gained favor recently because it is easy to administer and has a less toxic profile. Both cisplatin and carboplatin have been used in the past, although a randomized phase II study showed carboplatin to be inferior to cisplatin in first-line treatment of metastatic transitional cell carcinoma of the urothelium [41]. Although the data were descriptive in nature without statistical comparisons and the trial was not designed with sufficient power to detect significant differences between arms in terms of efficacy, cisplatin was used preferentially over carboplatin in the neoadjuvant setting based on extrapolation of the previously mentioned data. Nevertheless, there is no level 1 evidence to support GC in the neoadjuvant setting, and two randomized trials published in 2014, using GC as an NCT regimen, are underpowered to detect statistical difference. Instead, results from a randomized phase III trial in metastatic bladder cancer have been used to justify the routine use of GC in the neoadjuvant setting [9]. In that trial, GC was associated with similar efficacy compared with standard MVAC. We believe that extrapolation of these results to the neoadjuvant setting is potentially flawed, because these cohorts are substantially different with regard to performance status and, potentially, tumor biology. In this study, we attempted to perform an “unbiased” systematic review and meta-analysis with regard to platinum-based NCT in MIBC.
Since 2008, in the absence of definitive prospective data, a series of retrospective studies, including two international multicenter investigations, were published to compare GC versus MVAC in the neoadjuvant setting [20, 22]. Those studies did not find a difference between GC and MVAC in clinical outcomes, but most of them showed a nonsignificant increased death risk in the GC group. Therefore, we performed a meta-analysis with a relatively large sample size to increase the statistical power. Consistent with previous studies, there was no significant difference in the odds of achieving a pCR or pathological downstaging with GC versus MVAC. We found a significantly better OS associated with MVAC, which was not observed in previous individual studies. The average pCR or tumor-downstaging response of a GC regimen in our patient population was similar to MVAC and was comparable to the response rate of a recent pooled analysis of NCT in MIBC using a GC regimen (pCR: 25.7 vs. 25.6%; tumor downstaging: 44.8% vs. 46.5%) [42] and, therefore, should not cause inferior survival of a GC patient group. A repeated analysis by excluding carboplatin and smaller studies to avoid duplicate patients showed a marginally significant death risk in the gemcitabine plus cisplatin group versus the MVAC group (HR, 1.31; 95% CI, 0.99–1.74). The inconsistency between pCR and survival outcomes found in our study was different from previous reports suggesting that pCR or pathologic downstaging may be a surrogate marker for OS after NCT in bladder cancer [43, 44]. We acknowledge that the observed difference could be due to a number of factors independent of the treatment, such as dose intensity and density, duration of therapy, as well as duration and quality of clinical follow-up data, which cannot be adjusted by data extracted from published literatures. Based on our communications with other principal investigators, it appeared that a large proportion of patients in our studies received dose-dense MVAC, whereas fewer patients received the GC regimen in a dose-dense manner. We also realized that some important variables, such as patient age and performance status, which are associated with inherent selection bias in GC versus MVAC decisions, were not adjusted in our analyses. We were unable to perform any further investigation in this regard because most studies did not provide sufficiently detailed information, and an attempt at data collection for individual treatment protocols was not successful. However, the findings could be true because objective response and OS are highly correlated but may not be entirely consistent with each other. The discrepancy between the pCR rate and OS in our analysis may suggest a similar near-term efficacy between the two types of regimens as measured by response rate and a possible long-term benefit associated with MVAC. Regardless, the differences in observed survival are hypotheses generating and are worthy of further investigation in a prospective setting.
Despite our efforts to perform an accurate and comprehensive analysis, several limitations of the current meta-analysis need to be addressed. First, our analysis was based mainly on data extracted directly from the published literature. We were unable to perform further in-depth analysis, such as a stratified analysis or Kaplan-Meier survival curve, because we did not have access to individual patient information. Second, the included studies differed in study designs, such as patient selection, chemotherapeutic protocol, and follow-up time. Conclusions derived from this paper are limited because pooling retrospective studies that used markedly different approaches may result in biased analysis results. However, the patient population in these different studies seemed to be relatively homogeneous because there was no significant heterogeneity between studies, as reflected in our analysis. Third, in the meta-analysis of GC versus MVAC, some patients included in the original small studies were later incorporated into larger studies. We were unable to identify the overlapping patients owing to a lack of individual data. Although such patients constituted a small proportion of the whole patient population (<10%), there may be some redundant information in related analysis when both the small and large studies were included. However, our final results were not changed by excluding the smaller studies from the quantitative analysis.
Conclusion
Our meta-analysis demonstrated that NCT should be the standard of care in MIBC. In the neoadjuvant setting, GC and MVAC had similar treatment response rates but might be associated with different survival outcomes. However, this conclusion is subject to the limitations of retrospective meta-analysis and requires confirmation from future prospective studies with large sample sizes and better study designs.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Ming Yin, Joseph J. Drabick
Collection and/or assembly of data: Ming Yin, Richard P. Meijer, Simon Horenblas, Joseph J. Drabick
Data analysis and interpretation: Ming Yin, Monika Joshi, Richard P. Meijer, Simon Horenblas, Joseph J. Drabick
Manuscript writing: Ming Yin, Monika Joshi, Richard P. Meijer, Michael Glantz, Sheldon Holder, Harold A. Harvey, Matthew Kaag, Elisabeth E. Fransen van de Putte, Simon Horenblas, Joseph J. Drabick
Final approval of manuscript: Ming Yin, Monika Joshi, Richard P. Meijer, Michael Glantz, Sheldon Holder, Harold A. Harvey, Matthew Kaag, Elisabeth E. Fransen van de Putte, Simon Horenblas, Joseph J. Drabick
Disclosures
Monika Joshi: Bayer (C/A); Joseph J. Drabick: Merck (C/A). The other authors indicated no financial disclosures.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Footnotes
Editor’s Note: See the related commentary, “Systemic Therapy for Invasive Bladder Cancer: The Value Proposition” by Derek Raghavan on page 659 of this issue.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chavan S, Bray F, Lortet-Tieulent J, et al. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66:59–73. doi: 10.1016/j.eururo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 5.International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial J Clin Oncol 2011292171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Advanced Bladder Cancer Meta-analysis Collaboration Neoadjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis. Lancet. 2003;361:1927–1934. doi: 10.1016/s0140-6736(03)13580-5. [DOI] [PubMed] [Google Scholar]
- 7.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205; discussion 205–206. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: A systematic review and meta-analysis. J Urol. 2004;171:561–569. doi: 10.1097/01.ju.0000090967.08622.33. [DOI] [PubMed] [Google Scholar]
- 9.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 10.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natale RB, Grossman HB, Blumenstein B, et al. SWOG 8710 (int-0080): Randomized phase III trial of neoadjuvant MVAC + cystectomy versus cystectomy alone in patients with locally advanced bladder cancer. Proc Am Soc Clin Oncol. 2001;20:2a. [Abstract 3] [PubMed] [Google Scholar]
- 12.Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: A randomised controlled trial Lancet 1999354533–540. [PubMed] [Google Scholar]
- 13.Rintala E, Hannisdahl E, Fosså SD, et al. Neoadjuvant chemotherapy in bladder cancer: A randomized study. Nordic Cystectomy Trial I. Scand J Urol Nephrol. 1993;27:355–362. doi: 10.3109/00365599309180447. [DOI] [PubMed] [Google Scholar]
- 14.Malmström PU, Rintala E, Wahlqvist R, et al. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial I. J Urol. 1996;155:1903–1906. [PubMed] [Google Scholar]
- 15.Sherif A, Rintala E, Mestad O, et al. Neoadjuvant cisplatin-methotrexate chemotherapy for invasive bladder cancer—Nordic cystectomy trial 2. Scand J Urol Nephrol. 2002;36:419–425. doi: 10.1080/003655902762467567. [DOI] [PubMed] [Google Scholar]
- 16.Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: A combined analysis of two Nordic studies. Eur Urol. 2004;45:297–303. doi: 10.1016/j.eururo.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Dash A, Pettus JA, 4th, Herr HW, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer. 2008;113:2471–2477. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal SK, Ruel NH, Wilson TG, et al. Retrospective analysis of clinical outcomes with neoadjuvant cisplatin-based regimens for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2012;10:246–250. doi: 10.1016/j.clgc.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alva AS, Tallman CT, He C, et al. Efficient delivery of radical cystectomy after neoadjuvant chemotherapy for muscle-invasive bladder cancer: A multidisciplinary approach. Cancer. 2012;118:44–53. doi: 10.1002/cncr.26240. [DOI] [PubMed] [Google Scholar]
- 20.Galsky MD, Pal SK, Chowdhury S, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–2593. doi: 10.1002/cncr.29387. [DOI] [PubMed] [Google Scholar]
- 21.Fairey AS, Daneshmand S, Quinn D, et al. Neoadjuvant chemotherapy with gemcitabine/cisplatin vs. methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive urothelial carcinoma of the bladder: A retrospective analysis from the University of Southern California. Urol Oncol. 2013;31:1737–1743. doi: 10.1016/j.urolonc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Zargar H, Espiritu PN, Fairey AS, et al. Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2015;67:241–249. doi: 10.1016/j.eururo.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace DM, Raghavan D, Kelly KA, et al. Neo-adjuvant (pre-emptive) cisplatin therapy in invasive transitional cell carcinoma of the bladder. Br J Urol. 1991;67:608–615. doi: 10.1111/j.1464-410x.1991.tb15225.x. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Piñeiro JA, Gonzalez Martin M, Arocena F, et al. Neoadjuvant cisplatin chemotherapy before radical cystectomy in invasive transitional cell carcinoma of the bladder: A prospective randomized phase III study. J Urol. 1995;153:964–973. [PubMed] [Google Scholar]
- 25.Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576–3583. doi: 10.1200/JCO.1998.16.11.3576. [DOI] [PubMed] [Google Scholar]
- 26.Bassi P, Pappagallo GL, Sperandio P. Neoadjuvant mvac chemotherapy of invasive bladder cancer: Results of a multicenter phase III trial. J Urol. 1999;161:264A. [Google Scholar]
- 27.Sengeløv L, von der Maase H, Lundbeck F, et al. Neoadjuvant chemotherapy with cisplatin and methotrexate in patients with muscle-invasive bladder tumours. Acta Oncol. 2002;41:447–456. doi: 10.1080/028418602320405041. [DOI] [PubMed] [Google Scholar]
- 28.Osman MA, Gabr AM, Elkady MS. Neoadjuvant chemotherapy versus cystectomy in management of stages II, and III urinary bladder cancer Archivio Italiano di Urologia, Andrologia 201486278–283. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura H, Tsukamoto T, Shibata T, et al. Randomised phase III study of neoadjuvant chemotherapy with methotrexate, doxorubicin, vinblastine and cisplatin followed by radical cystectomy compared with radical cystectomy alone for muscle-invasive bladder cancer: Japan clinical oncology group study jcog0209. Ann Oncol. 2014;25:1192–1198. doi: 10.1093/annonc/mdu126. [DOI] [PubMed] [Google Scholar]
- 30.Khaled HM, Shafik HE, Zabhloul MS, et al. Gemcitabine and cisplatin as neoadjuvant chemotherapy for invasive transitional and squamous cell carcinoma of the bladder: Effect on survival and bladder preservation. Clin Genitourin Cancer. 2014;12:e233–e240. doi: 10.1016/j.clgc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Weight CJ, Garcia JA, Hansel DE, et al. Lack of pathologic down-staging with neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder: A contemporary series. Cancer. 2009;115:792–799. doi: 10.1002/cncr.24106. [DOI] [PubMed] [Google Scholar]
- 32.Kaneko G, Kikuchi E, Matsumoto K, et al. Neoadjuvant gemcitabine plus cisplatin for muscle-invasive bladder cancer. Jpn J Clin Oncol. 2011;41:908–914. doi: 10.1093/jjco/hyr068. [DOI] [PubMed] [Google Scholar]
- 33.Yeshchina O, Badalato GM, Wosnitzer MS, et al. Relative efficacy of perioperative gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. 2012;79:384–390. doi: 10.1016/j.urology.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 34.Meijer RP, Nieuwenhuijzen JA, Meinhardt W, et al. Response to induction chemotherapy and surgery in non-organ confined bladder cancer: A single institution experience. Eur J Surg Oncol. 2013;39:365–371. doi: 10.1016/j.ejso.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Lee FC, Harris W, Cheng HH, et al. Pathologic response rates of gemcitabine/cisplatin versus methotrexate/vinblastine/adriamycin/cisplatin neoadjuvant chemotherapy for muscle invasive urothelial bladder cancer. Adv Urol. 2013;2013:317190. doi: 10.1155/2013/317190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawamura N, Matsushita M, Okada T, et al. Relative efficacy of neoadjuvant gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management for muscle-invasive bladder cancer [in Japanese] Hinyokika Kiyo. 2013;59:277–281. [PubMed] [Google Scholar]
- 37.Iwasaki K, Obara W, Kato Y, et al. Neoadjuvant gemcitabine plus carboplatin for locally advanced bladder cancer. Jpn J Clin Oncol. 2013;43:193–199. doi: 10.1093/jjco/hys213. [DOI] [PubMed] [Google Scholar]
- 38.Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: A sign of changing tides. Eur Urol. 2015;67:165–170. doi: 10.1016/j.eururo.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: Pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–1894. doi: 10.1200/JCO.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–1901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dogliotti L, Cartenì G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 42.Yuh BE, Ruel N, Wilson TG, et al. Pooled analysis of clinical outcomes with neoadjuvant cisplatin and gemcitabine chemotherapy for muscle invasive bladder cancer. J Urol. 2013;189:1682–1686. doi: 10.1016/j.juro.2012.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: A meta-analysis. Eur Urol. 2014;65:350–357. doi: 10.1016/j.eururo.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 44.Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61:1229–1238. doi: 10.1016/j.eururo.2011.12.010. [DOI] [PubMed] [Google Scholar]