This population-based study found that compared with patients with metastatic cancer who did not receive palliative chemotherapy, those who did also received more aggressive end-of-life care and less hospice care. More than one emergency room visit, more than one intensive care unit admission, and endotracheal intubation during end-of-life care were significantly more common in patients receiving palliative chemotherapy.
Keywords: End-of-life care, Palliative chemotherapy, Aggressiveness of cancer treatment
Abstract
Introduction.
Although palliative chemotherapy during end-of-life care is used for relief of symptoms in patients with metastatic cancer, chemotherapy may lead to more aggressive end-of-life care and less use of hospice service. This is a population-based study of the association between palliative chemotherapy and aggressiveness of end-of-life care.
Patients and Methods.
Using the National Health Insurance Research Database of Taiwan, we identified 49,920 patients with metastatic cancer who underwent palliative chemotherapy from January 1, 2009, to December 31, 2011. Patients who received chemotherapy 2–6 months before death were included. Aggressiveness of end-of-life care was examined by previously reported indicators. Cardiopulmonary resuscitation and endotracheal tube intubation were included as indicators of aggressive end-of-life care. The association between palliative chemotherapy and hospice care was studied.
Results.
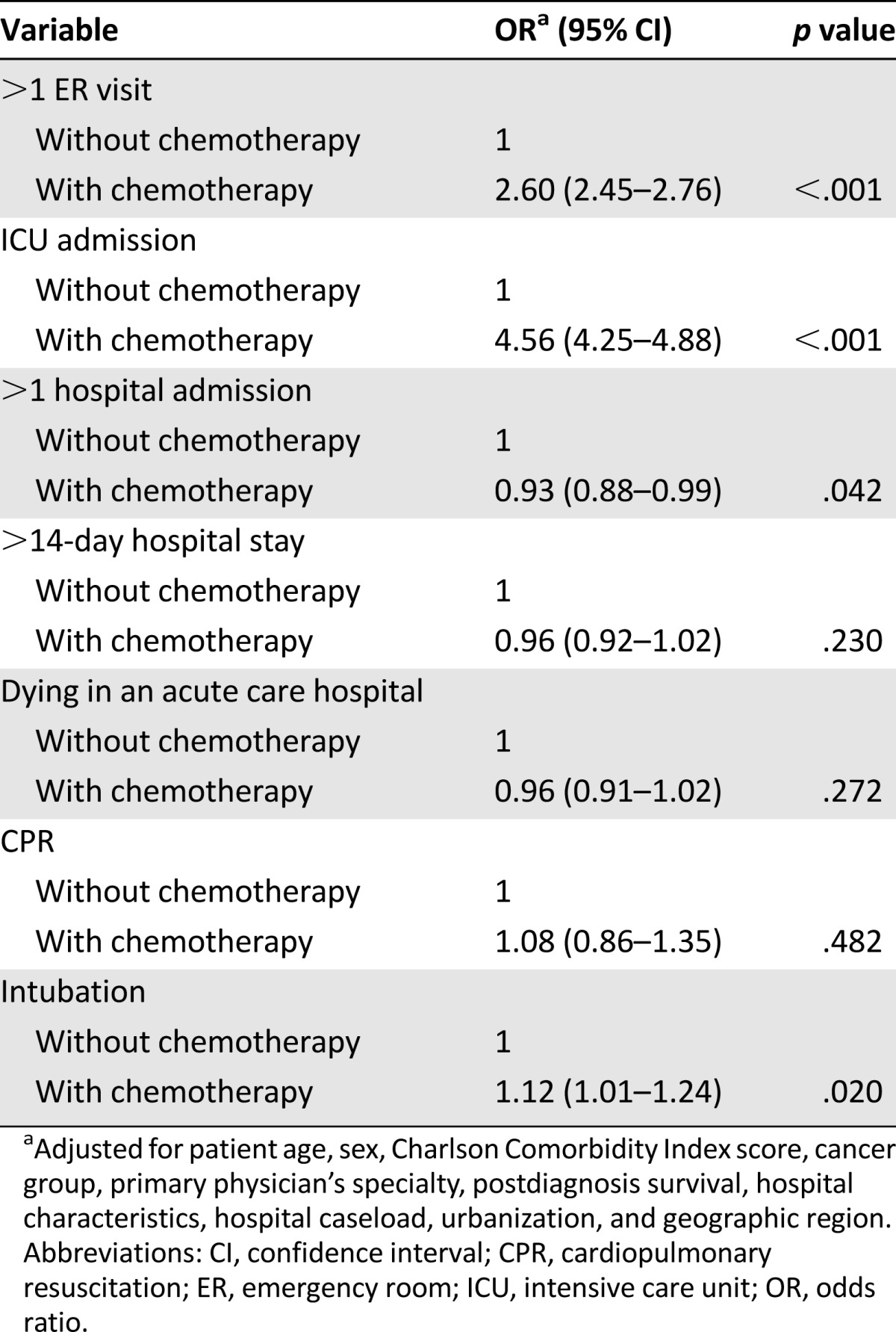
Palliative chemotherapy was associated with more aggressive treatment. After adjustment for patient age, sex, Charlson Comorbidity Index score, cancer group, primary physician’s specialty, postdiagnosis survival, hospital characteristics, hospital caseload, urbanization, and geographic regions, more than one emergency room visit (p < .001), more than one intensive care unit admission (p < .001), and endotracheal intubation (p = .02) during end-of-life care were significantly more common in patients receiving palliative chemotherapy. Patients who did not receive palliative chemotherapy received more hospice care in the last 6 months of life (p < .001).
Conclusion.
Although the decision to initiate palliative chemotherapy was made several months before death, this study showed that palliative chemotherapy was associated with more aggressive end-of-life care, including more emergency room visits and intensive care unit admissions, and endotracheal intubation. The patients who received palliative chemotherapy received less hospice service toward the end of life.
Implications for Practice:
Palliative chemotherapy is used for patients with incurable cancer toward the end of life (EOL). Aggressiveness of EOL care and hospice care are related to the quality of life of these patients. This study of data from the Taiwanese National Health Insurance Research Database found that palliative chemotherapy led to more aggressive EOL care and less hospice care. There is a need to provide patients with terminal cancer access to care information that best meets their needs, especially those patients who receive palliative chemotherapy.
Introduction
Cancer leads to 25%–30% of deaths worldwide [1, 2], including Taiwan [3]. Because of improvements in multidisciplinary and antineoplastic treatment, the aggressiveness of cancer treatment, including that of patients with noncurable cancer, has increased. According to the American Cancer Society, hospice care should be considered for patients whose life expectancy is 6 months or less [4]. High-quality end-of-life (EOL) care includes decision making, survival, symptom management, psychosocial support, and hospice care [5]. Although palliative chemotherapy near the EOL is used for relief of symptoms [6, 7], palliative chemotherapy may lead to poorer quality of life near death compared with palliative treatment [8].
Earle et al. [9] reported the indicators of aggressive end-of-life care, including chemotherapy within 14 days of death. Emanuel et al. [10] reported that between 20% and 50% of patients receive chemotherapy within 30 days of death, even if it is not curative. Discontinuing EOL chemotherapy is one of the “top five” practices that could reduce medical costs and improve patients’ care [11]. Greer et al. [12] reported that patients with concurrent palliative and oncologic care ceased intravenous chemotherapy 2 months earlier than did those without palliative care.
Patients and physicians face the difficult decision to stop chemotherapy near the end of life and patients have been reported to receive chemotherapy even 6 days before death [13]. Wright et al. [14] confirmed that palliative chemotherapy is associated with increased aggressiveness of treatment, including cardiopulmonary resuscitation, mechanical ventilation, and dying in an intensive care unit.
There is limited knowledge regarding the use of palliative chemotherapy in the months leading up to patient death and its association with more aggressive treatment. In this population-based study, we determined whether cancer patients who received palliative chemotherapy 6 months before death were more likely to receive aggressive treatment, as reported by Earle et al. [9], compared with patients who did not receive chemotherapy.
Patients and Methods
Ethics Statement
This study was reviewed and approved by the institutional review board of Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan. All personal identifying information was removed from the dataset before analysis and the requirements for written informed consent were waived.
Database
Since 1995, the National Health Insurance (NHI) program has been the universal coverage and single-payer system in Taiwan. Now the compulsory social insurance program covers approximately 99% of the residents of Taiwan and has contracts with 97% of medical providers [15, 16]. The data for this study were collected from Taiwan’s National Health Insurance Research Database (NHIRD) for the years 2009 to 2011. We designed the study period at patients’ end of life, which was defined as the last 6 months of life [4, 17]. We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for both cancer and metastasis (196.×, 197.×, 198.×, and 199.0) to compile our study cohort, which consisted of Taiwan’s decedents with metastatic cancer 6 months before death.
Diagnosis status was divided into three subgroups of cancers that were homogeneous in terms of survival and disease course [18–20]. Patients with nonmetastatic cancer and hematologic malignancies were excluded from this study. The three cancer subgroups were metastatic germ cell tumors and prostate cancer; metastatic lung, liver, and pancreatic cancer; and all other metastatic cancers. Patients with hematologic malignancies were excluded because it was previously reported that these patients tended to receive more aggressive EOL care [21].
Measurement
The key independent variable for the study was patients with metastatic cancer receiving at least 1 round of chemotherapy [14] during the 2–6 months before death. The key dependent variable of interest, aggressive EOL care, was hypothesized to be associated with palliative chemotherapy toward the end of life. The indicators of aggressive EOL care were adapted from Earle et al. [9, 22]. Palliative chemotherapy was excluded from indicators because we considered palliative chemotherapy an independent factor for aggressive EOL care. Because neutropenia was possibly related to chemotherapy, we excluded patients with acquired neutropenia (ICD-9-CM codes 288.00, 288.03, and 288.09) in the last month of life. The aggressive EOL care indicators in the last month of life were more than 1 emergency room (ER) visit, more than 1 hospital admission, more than 14 days of hospitalization, an intensive care unit (ICU) admission, or in-hospital death. We also drew on evidence supporting the futility of life-sustaining treatment for EOL care by including cardiopulmonary resuscitation and endotracheal tube intubation [23]. Data on these indicators were collected from the NHI database of in- or out-claims for the last months of life.
We also studied the association between palliative chemotherapy and hospice care. Hospice care was identified by outpatient and inpatient claims with special case type code “65” and payment type code “A.” The proxy of hospice care was at least one hospitalization for hospice care in EOL or at least three visits for outpatient hospice service. The dependent variables were hospice care in the last 6 months, hospice care in the 2–6 months before death, and hospice care in the last 1 month. The use of hospice service was studied by the following listed variables: less than 7 days of inpatient hospice service, the mean days of hospitalization for hospice, fewer than 3 visits of outpatient hospice service, and mean visits of outpatient hospice service.
Statistical Analysis
All statistical analyses were performed using SPSS version 15 (SPSS for Windows, Version 15.0, released 2006. SPSS Inc., Chicago, IL). Univariate associations were evaluated by Pearson’s chi-square test. The impact of each explanatory variable on the aggressiveness of EOL care was examined by hierarchical linear regression analysis using a random-intercept model, which accounts for patients clustering within hospitals and the continuous nature of the composite score for EOL care. Multilevel logistic regression was used to explore the association between palliative chemotherapy and the indicators of aggressive EOL care.
Results
Demographic Data and Clinical Characteristics
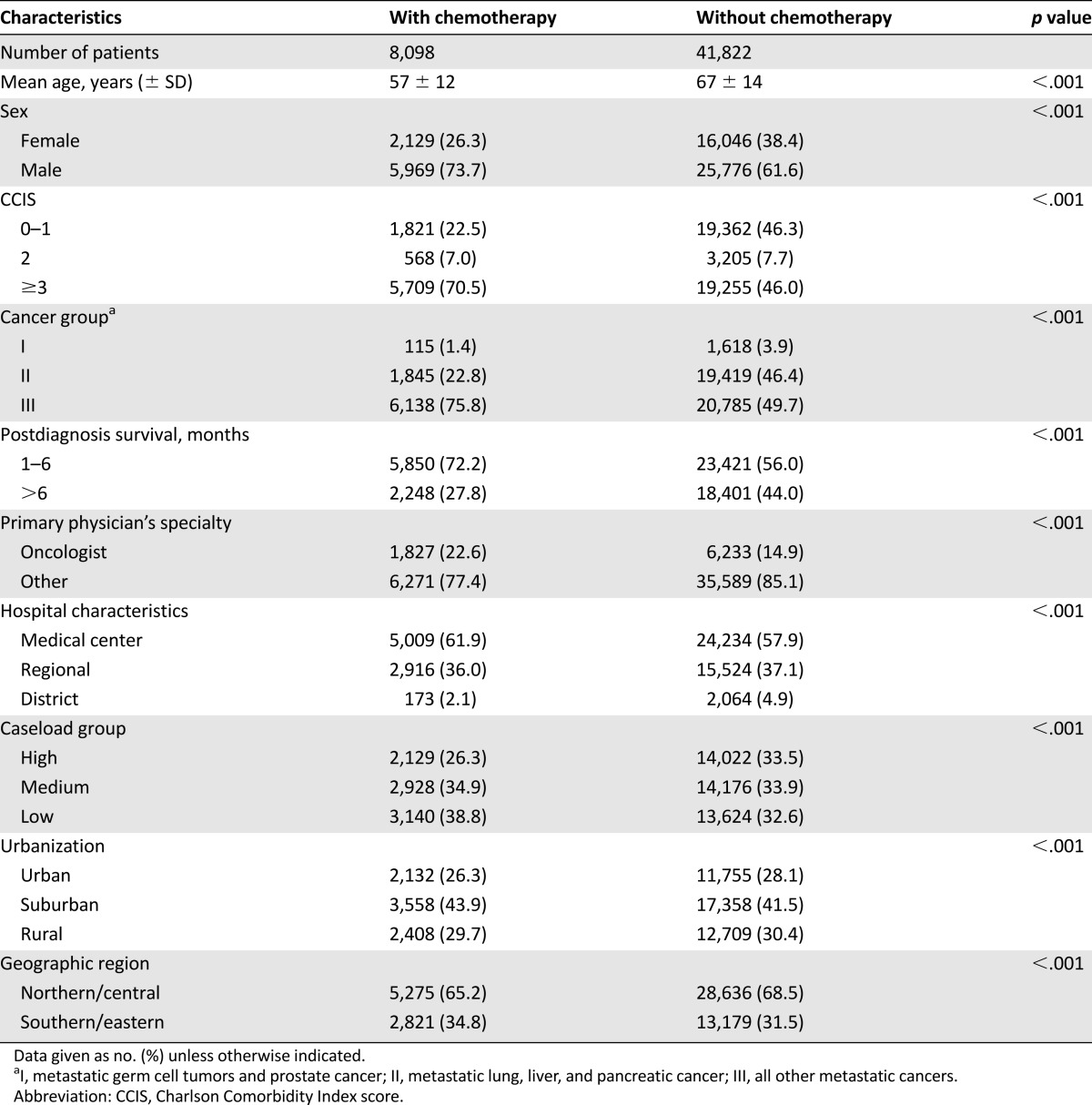
A total of 49,920 decedents who had metastatic cancer were included in this study. The baseline characteristics of the decedents with metastatic cancer are shown in Table 1. There were 8,098 patients who received palliative chemotherapy; 41,822 patients did not receive palliative chemotherapy. Younger decedents were more likely to receive palliative chemotherapy than older decedents (mean ± SD: 57 ± 12 years vs. 67 ± 14 years). Of the patients who did not receive palliative chemotherapy, 44% had a survival time of longer than 6 months.
Table 1.
Baseline characteristics (N = 49,920)
Univariate Survival Analysis
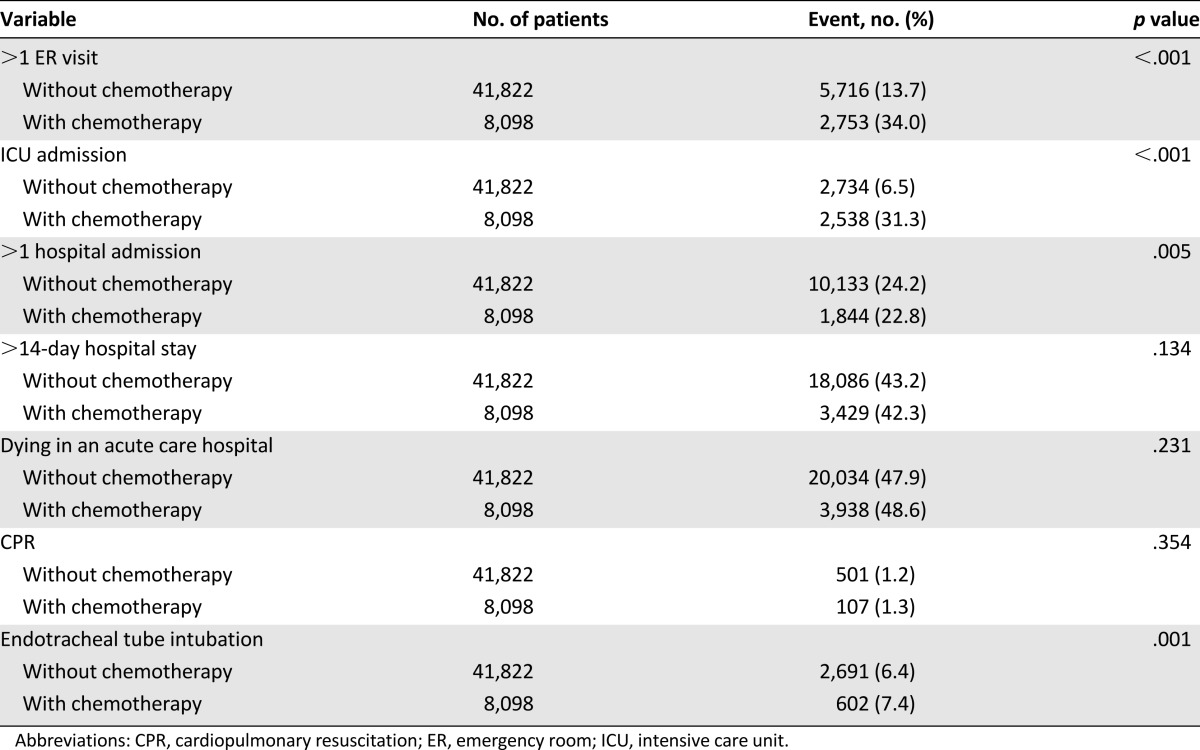
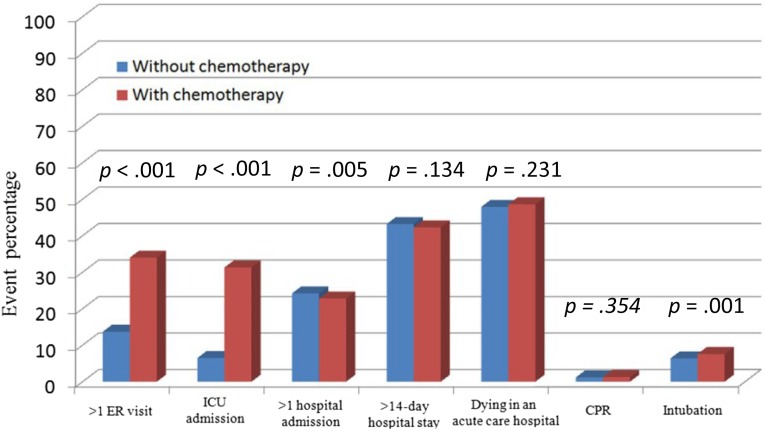
Table 2 shows that ER visits (p < .001), ICU admissions (p < .001), more than one hospital admission (p = .005), and endotracheal tube intubation (p = .001) were significantly different in the patients who received palliative chemotherapy than those who did not. With the exception of patients who had more than one hospital admission, other indicators showed that palliative chemotherapy led to more aggressive EOL care. Figure 1 shows the distribution of each indicator for the aggressiveness of EOL care. There were no significant differences between the two groups in terms of patients who were hospitalized for more than 14 days, patients who died in an acute care hospital, and patients requiring cardiopulmonary resuscitation.
Table 2.
Associations between palliative chemotherapy and indicators of aggressive care
Figure 1.
The impact of palliative chemotherapy and aggressiveness of end-of-life care.
Abbreviations: CPR, cardiopulmonary resuscitation; ER, emergency room; ICU, intensive care unit.
Multilevel Logistic Regression Model
When adjusted for age, sex, Charlson Comorbidity Index score, cancer group, primary physician’s specialty, postdiagnosis survival, hospital characteristics, hospital caseload, urbanization, and geographic regions, the following characteristics were significantly different in patients who received palliative chemotherapy than patients who did not receive palliative chemotherapy: more than one ER visit (p < .001), ICU admission (p < .001) during EOL care, and endotracheal tube intubation (p = .02) (Table 3). The number of hospital admissions of patients who did not receive palliative chemotherapy was statistically significantly higher than that of patients who received palliative chemotherapy (p = .042).
Table 3.
Multilevel logistic regression of palliative chemotherapy and indicators of aggressive end-of-life care
Palliative Chemotherapy and Hospice Care
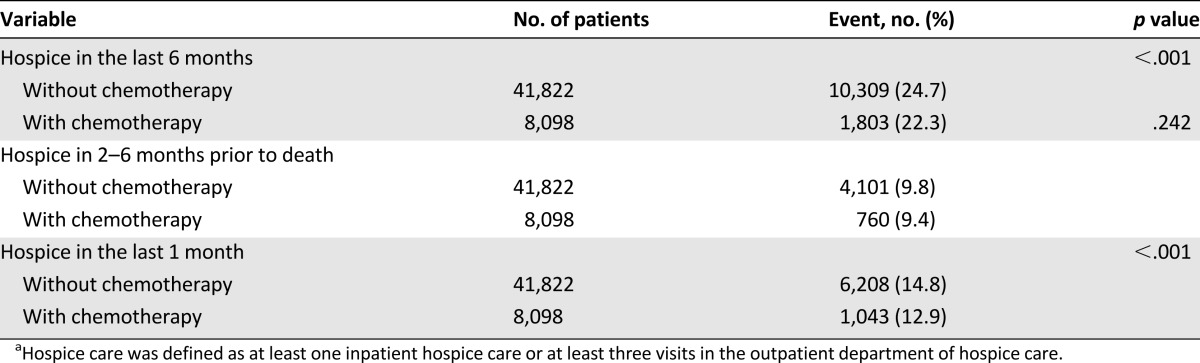
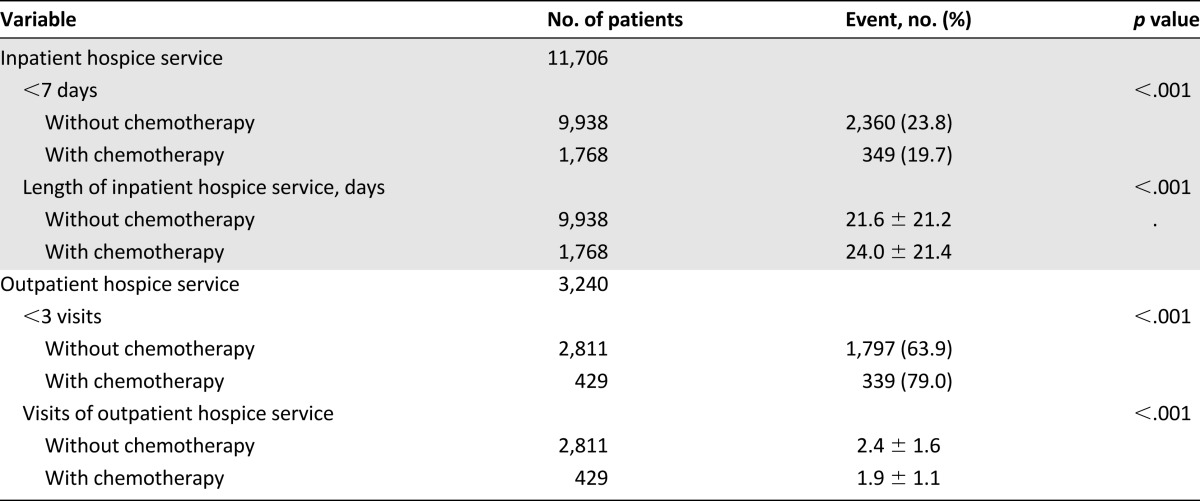
We studied the association between chemotherapy and hospice care (Table 4). Patients without palliative chemotherapy received more hospice care in the last 6 months of life (p < .001), especially in the last 1 month of life (p < .001), than those who received palliative chemotherapy. Table 5 presents the findings of our analysis of the use of hospice service in EOL care. The patients without palliative chemotherapy received less inpatient hospice service. Of the patients who underwent hospice service, 23.8% received less than 7 days of inpatient hospice service in the group without palliative chemotherapy and 19.7% in the chemotherapy group (p < .001). The mean number of days of inpatient hospice service was fewer in the patients without chemotherapy. The patients with chemotherapy had significantly less use of outpatient hospice service (79.0% had fewer than 3 visits; p < .001).
Table 4.
Associations between palliative chemotherapy and receiving hospice carea
Table 5.
Associations between palliative chemotherapy and use of hospice service
Discussion
Main Findings
In this national database study of cancer deaths in Taiwan from 2009 to 2011, we found that palliative chemotherapy was an indicator of more aggressive EOL care. Patient who received palliative chemotherapy had more frequent ER visits and ICU admissions, and underwent more endotracheal tube intubations, even though the decision to administer chemotherapy was made several months before death. Fewer hospitalizations occurred in patients who received palliative chemotherapy; after adjustment, there was still a statistically significant difference (p = .042). The patients with palliative chemotherapy had less use of hospice service at EOL, especially the outpatient hospice service.
Strengths
The strengths of this study include a nationwide, population-based, cross-sectional design with nearly complete follow-up information regarding access to health care institutes among the entire study population (99%). This uniformly organized, single-payer health insurance system minimized recall bias and selection bias. The diagnosis and quality of cancer information was confirmed based on the NHI Catastrophic Illness Database. Biopsy specimens and histologic verifications are required before malignancy can be diagnosed definitively as a catastrophic illness and treatment can be initiated so that the subjects are exempt from copayments for treatment. In addition, the data set was routinely monitored for diagnostic accuracy by the National Health Insurance Bureau of Taiwan [24].
Comparison With Other Studies
It is an international human right to have quality palliative EOL care [25, 26]. Good deaths are characterized by comfort without pain, with dignity and respect, and closeness to family members and caring persons [27]. Although benefits of palliative treatment of patients with incurable cancer have been reported [28, 29], palliative chemotherapy in the last months of life led to more aggressive EOL care, such as being excluded from hospice [30] and dying in the ICU [14].
Patient expectations of palliative therapy were high, and patients were willing to accept the effects of toxicity for modest benefit [31]. In a previous study, patients chose to receive chemotherapy if it could extend life by 4–5 months [32, 33], although most patients were not given enough information about the benefits of palliative chemotherapy [34]. Another study found that more than half of patients chose chemotherapy for even a 1-week prolongation of their life [14].
Treatment preferences of patients with advanced cancer have been reported to be related to the aggressiveness of EOL care [35, 36]. EOL conversations with physicians resulted in significantly lower health care costs in the final week of life and led to better quality of death [37]. It is still a challenge many oncologists face when discussing with patients and their caregivers the option of stopping chemotherapy [38, 39]. Being hesitant to stop futile palliative chemotherapy may lead to aggressive EOL care and life-sustaining treatment. Although discussion of EOL care has been shown to be associated with less aggressive EOL care [40], we still face difficult decisions about chemotherapy near the EOL among patients [13] and physicians. Physicians consistently overestimated prognosis by at least 30% [41]; their estimate of survival could be divided by 3.5 for actual survival [42]. In a previous study, fewer than one third of adult Taiwanese patients with terminal cancer and approximately half of their families reported that health care personnel had informed them about the prognosis and discussed the goals of future care [43]. The decision to begin palliative chemotherapy should not end the decision-making process, especially when that decision was made several months earlier [14, 44].
Limitations
There were several limitations in this study. The decision to administer palliative chemotherapy was made several months before death, but the process involved in decision-making was not shown in this study. A clear description of reasons of hospital admission, ER visits, and other definite reasons for aggressive treatment was not available from our database. Because of different biological characteristics and improvements in target therapy, palliative chemotherapy may be beneficial for select patients. Different cancer types may lead to different degrees of aggressiveness of treatment options. Physicians’ religious values, patients’ acceptance of illness, and illness severity were not included in this study, although these factors affect decision making during the last days of life.
Another limitation was our lack of access to detailed information regarding the patients’ wills, the caregivers’ wills, and completed living wills and do-not-resuscitate orders. Quality of life and symptom control of each patient were limitations of the NHIRD. The patients with palliative chemotherapy had fewer hospital admissions in EOL care, which was the only indicator of less aggressiveness.
Conclusion
Despite these limitations, the results of this study are informative. They showed that palliative chemotherapy was associated with more aggressive EOL care, including more ER visits, ICU admissions, and endotracheal tube intubations. The patients with palliative chemotherapy received significantly less hospice service in EOL. There is a need to provide patients with terminal cancer access to care information that best meets their needs, especially those patients who receive palliative chemotherapy. Although the decision to initiate palliative chemotherapy was made several months before death, more aggressive EOL care and endotracheal intubation are performed in patients who receive palliative chemotherapy.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This study was based in part on data from the National Health Insurance Research Database, as provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
Author Contributions
Conception/Design: Chin-Chia Wu, Chun-Ming Chang, Chih-Yuan Huang, Ching-Chih Lee
Provision of study material or patients: Ta-Wen Hsu, Ching-Chih Lee
Collection and/or assembly of data: Ta-Wen Hsu, Chun-Ming Chang, Chih-Yuan Huang
Data analysis and interpretation: Chin-Chia Wu, Chun-Ming Chang, Cheng-Hung Lee, Ching-Chih Lee
Manuscript writing: Chin-Chia Wu, Ta-Wen Hsu, Ching-Chih Lee
Final approval of manuscript: Chin-Chia Wu, Cheng-Hung Lee, Chih-Yuan Huang, Ching-Chih Lee
Disclosures
The authors indicated no financial relationships.
References
- 1.Brustugun OT, Møller B, Helland A. Years of life lost as a measure of cancer burden on a national level. Br J Cancer. 2014;111:1014–1020. doi: 10.1038/bjc.2014.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587–1591. doi: 10.1200/JCO.2010.31.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Health Promotion Administration, Ministry of Health and Welfare. Cancer prevention and control. Available at http://www.Hpa.Gov.Tw/english/classprint.Aspx?No=201401280006. Accessed January 14, 2015.
- 4.American Cancer Society. Nearing the end of life. Available at http://www.cancer.org/treatment/nearingtheendoflife/nearingtheendoflife/nearing-the-end-of-life-intro. Accessed December 28, 2015.
- 5. Foley KM, Gelband H, eds. Institute of Medicine: Improving palliative care for cancer. Washington, DC: National Academy Press, 2001:1. [Google Scholar]
- 6.Cunningham D, Pyrhönen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352:1413–1418. doi: 10.1016/S0140-6736(98)02309-5. [DOI] [PubMed] [Google Scholar]
- 7.Doyle C, Crump M, Pintilie M, et al. Does palliative chemotherapy palliate? Evaluation of expectations, outcomes, and costs in women receiving chemotherapy for advanced ovarian cancer. J Clin Oncol. 2001;19:1266–1274. doi: 10.1200/JCO.2001.19.5.1266. [DOI] [PubMed] [Google Scholar]
- 8.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778–784. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 10.Emanuel EJ, Young-Xu Y, Levinsky NG, et al. Chemotherapy use among Medicare beneficiaries at the end of life. Ann Intern Med. 2003;138:639–643. doi: 10.7326/0003-4819-138-8-200304150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 12.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30:394–400. doi: 10.1200/JCO.2011.35.7996. [DOI] [PubMed] [Google Scholar]
- 13.Harrington SE, Smith TJ. The role of chemotherapy at the end of life: “When is enough, enough?”. JAMA. 2008;299:2667–2678. doi: 10.1001/jama.299.22.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright AA, Zhang B, Keating NL, et al. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: Prospective cohort study. BMJ. 2014;348:g1219. doi: 10.1136/bmj.g1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachel Lu JF, Chiang TL. Evolution of Taiwan’s health care system. Health Econ Policy Law. 2011;6:85–107. doi: 10.1017/S1744133109990351. [DOI] [PubMed] [Google Scholar]
- 16.Chiang TL. Taiwan’s 1995 health care reform. Health Policy. 1997;39:225–239. doi: 10.1016/s0168-8510(96)00877-9. [DOI] [PubMed] [Google Scholar]
- 17.Chastek B, Harley C, Kallich J, et al. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8:75s–80s. doi: 10.1200/JOP.2011.000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CM, Wu CC, Yin WY, et al. Low socioeconomic status is associated with more aggressive end-of-life care for working-age terminal cancer patients. The Oncologist. 2014;19:1241–1248. doi: 10.1634/theoncologist.2014-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanvetyanon T, Leighton JC. Life-sustaining treatments in patients who died of chronic congestive heart failure compared with metastatic cancer. Crit Care Med. 2003;31:60–64. doi: 10.1097/00003246-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Campbell DE, Lynn J, Louis TA, et al. Medicare program expenditures associated with hospice use. Ann Intern Med. 2004;140:269–277. doi: 10.7326/0003-4819-140-4-200402170-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hui D, Didwaniya N, Vidal M, et al. Quality of end-of-life care in patients with hematologic malignancies: A retrospective cohort study. Cancer. 2014;120:1572–1578. doi: 10.1002/cncr.28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 23.Tang ST, Wu SC, Hung YN, et al. Trends in quality of end-of-life care for Taiwanese cancer patients who died in 2000-2006. Ann Oncol. 2009;20:343–348. doi: 10.1093/annonc/mdn602. [DOI] [PubMed] [Google Scholar]
- 24.National Health Insurance Administration, Ministry of Health and Welfare. Care for special groups: patients with catastrophic illnesses. 2015. Available at http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=1100&WD_ID=1100&webdata_id=3180. Accessed April 4, 2016.
- 25.Brennan F. Palliative care as an international human right. J Pain Symptom Manage. 2007;33:494–499. doi: 10.1016/j.jpainsymman.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Radbruch L, Payne S, de Lima L, et al. The Lisbon Challenge: Acknowledging palliative care as a human right. J Palliat Med. 2013;16:301–304. doi: 10.1089/jpm.2012.0394. [DOI] [PubMed] [Google Scholar]
- 27.Ruland CM, Moore SM. Theory construction based on standards of care: A proposed theory of the peaceful end of life. Nurs Outlook. 1998;46:169–175. doi: 10.1016/s0029-6554(98)90069-0. [DOI] [PubMed] [Google Scholar]
- 28.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 29.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 30.Browner I, Carducci MA. Palliative chemotherapy: Historical perspective, applications, and controversies. Semin Oncol. 2005;32:145–155. doi: 10.1053/j.seminoncol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Patnaik A, Doyle C, Oza AM. Palliative therapy in advanced ovarian cancer: Balancing patient expectations, quality of life and cost. Anticancer Drugs. 1998;9:869–878. doi: 10.1097/00001813-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Donovan KA, Greene PG, Shuster JL, et al. Treatment preferences in recurrent ovarian cancer. Gynecol Oncol. 2002;86:200–211. doi: 10.1006/gyno.2002.6748. [DOI] [PubMed] [Google Scholar]
- 33.Silvestri G, Pritchard R, Welch HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: Descriptive study based on scripted interviews. BMJ. 1998;317:771–775. doi: 10.1136/bmj.317.7161.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Audrey S, Abel J, Blazeby JM, et al. What oncologists tell patients about survival benefits of palliative chemotherapy and implications for informed consent: Qualitative study. BMJ. 2008;337:a752. doi: 10.1136/bmj.a752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright AA, Mack JW, Kritek PA, et al. Influence of patients’ preferences and treatment site on cancer patients’ end-of-life care. Cancer. 2010;116:4656–4663. doi: 10.1002/cncr.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med. 2009;169:480–488. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The AM, Hak T, Koëter G, et al. Collusion in doctor-patient communication about imminent death: An ethnographic study. West J Med. 2001;174:247–253. doi: 10.1136/ewjm.174.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavalli-Björkman N, Glimelius B, Strang P. Equal cancer treatment regardless of education level and family support? A qualitative study of oncologists’ decision-making. BMJ Open. 2012;2:e001248. doi: 10.1136/bmjopen-2012-001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion characteristics and care received near death: A prospective cohort study. J Clin Oncol. 2012;30:4387–4395. doi: 10.1200/JCO.2012.43.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040–4049. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]
- 43.Tang ST, Liu TW, Lai MS, et al. Congruence of knowledge, experiences, and preferences for disclosure of diagnosis and prognosis between terminally-ill cancer patients and their family caregivers in Taiwan. Cancer Invest. 2006;24:360–366. doi: 10.1080/07357900600705284. [DOI] [PubMed] [Google Scholar]
- 44.Rabow MW. Chemotherapy near the end of life. BMJ. 2014;348:g1529. doi: 10.1136/bmj.g1529. [DOI] [PubMed] [Google Scholar]