Screening echocardiograms (ECHOs) and radionuclide ventriculograms (RVGs) are often used to evaluate cardiac function before anthracycline- or trastuzumab-based therapy for breast cancer patients. A study of 600 eligible patients with early-stage breast cancer found that baseline ECHO/RVG in patients without prior cardiac history rarely yields an abnormality that prompts change in planned anthracycline and/or trastuzumab-based treatment.
Keywords: Cardiotoxicity, Cardiac function, Screening, Chemotherapy
Abstract
Background.
Cardiotoxicity can be a complication of anthracycline- or trastuzumab-based therapy for breast cancer patients. Screening echocardiograms (ECHOs) and radionuclide ventriculograms (RVGs) are often performed before administration of these agents to evaluate cardiac function. Limited evidence for the clinical utility of these screening tests is available.
Methods.
Early-stage breast cancer patients diagnosed from 2006 to 2011 (n = 1,067) with baseline ECHOs/RVGs were identified in a single institution prospective registry. Medical record review was performed to obtain pre- and post-ECHO/RVG treatment plans, baseline ECHO/RVG results, cardiac risk factors, and cardiac events. Patients with cardiac history were excluded. ECHO/RVG abnormalities were defined as ejection fraction (EF) <55%, valvular disease, left ventricular hypertrophy, and diastolic dysfunction. Cardiac events were defined as heart failure, myocardial infarction, arrhythmia, valvular disease, or angina during or after chemotherapy.
Results.
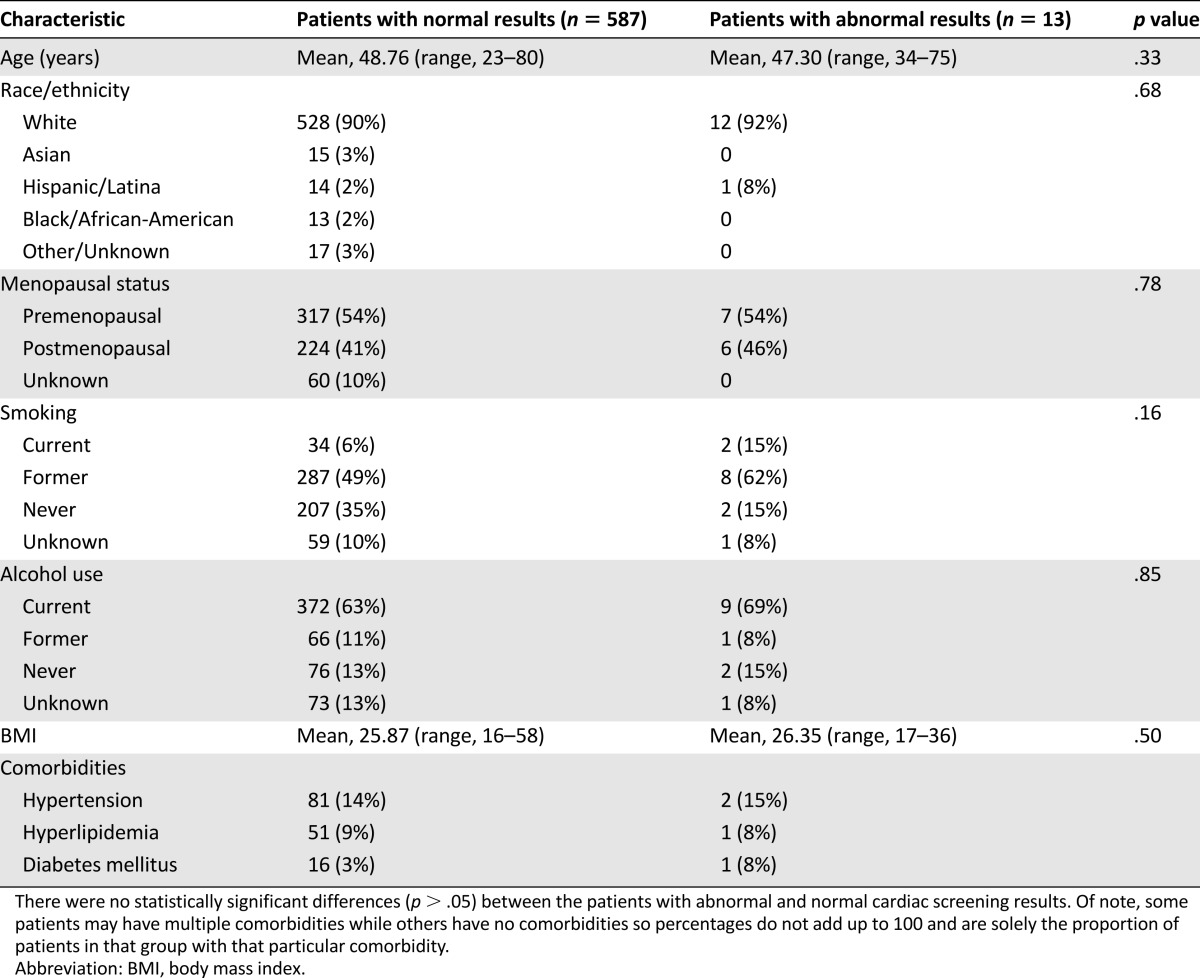
Among 600 eligible patients, abnormal ECHO/RVG results were observed in 13 (2.2%, 1.2%–3.7%), including 9 with baseline EF <55%. There were no detected changes in treatment plans, although more frequent cardiac monitoring was recommended for 2 patients. There were no significant differences in age, race, menopausal status, smoking history, alcohol use, body mass index, or medical comorbidities between patients with abnormal and normal results. In follow-up (mean, 4.0 years; range, 0–8.3), 15 patients developed cardiac events (none of whom had had abnormal baseline ECHOs/RVGs).
Conclusion.
Baseline ECHO/RVG in patients without prior cardiac history rarely yields an abnormality that prompts change in planned anthracycline- and/or trastuzumab-based treatment. Moreover, few cardiac events developed in this screened population in follow-up.
Implications for Practice:
Baseline cardiac function screening with echocardiograms or radionuclide ventriculograms is frequently performed before administration of anthracycline- or trastuzumab-based chemotherapy in breast cancer patients due to the relatively low cost and risk to patients and the concern for potential cardiotoxicity. However, at a population level, these tests can take up time and can add up to significant costs for both patients and the health care system. This study finds that in patients with no history of cardiac disease, baseline cardiac function screening rarely identifies abnormalities that change treatment plans. Moreover, few cardiac events develop in an average of 4 years of follow-up, including none in patients with abnormal baseline cardiac function screening results. This suggests that baseline cardiac function screening may have limited utility in chemotherapy planning in young breast cancer patients with no history of cardiac disease.
Abstract
摘要
背景. 心脏毒性是以蒽环类或曲妥珠单抗为基础的乳腺癌治疗方案的潜在并发症。超声心动图 (ECHO) 筛查和放射性核素心室造影 (RVG) 检查常用于在给予上述治疗前评价心功能。但这些筛查检验在临床中用途的证据仍然很有限。
方法. 从一家单中心的前瞻性登记系统里识别出2006∼2011年期间诊断为早期乳腺癌且有基线ECHO/RVG结果的患者 (n=1 067)。回顾病史以获取ECHO/RVG前后的治疗方案、基线ECHO/RVG结果、心脏危险因素和心脏事件。排除有心脏病史的患者。ECHO/RVG异常定义为射血分数 (EF) < 55%、瓣膜病变、左心室肥厚和舒张功能障碍。心脏事件定义为心力衰竭、心肌梗死、心律不齐、瓣膜病变, 或者化疗期间或化疗后心绞痛。
结果. 600例符合标准的患者中, 13例ECHO/RVG结果异常 (2.2%, 1.2%∼3.7%), 其中9例基线EF < 55%。无一例患者的治疗计划发生改变, 但2例患者被建议增加心脏监测的频率。正常结果和异常结果患者的年龄、人种、绝经状态、吸烟史、酒精摄入、体重指数和合并症情况均无差异。15例患者在随访期间 (平均4.0年, 范围: 0∼8.3) 发生心脏事件 (其中无一例基线ECHO/RVG结果异常)。
结论. 在无心脏病史的患者中, 基线ECHO/RVG很少得到导致以蒽环类和 (或) 曲妥珠单抗为基础的治疗计划改变的异常结果。此外, 这一筛查人群在随访期间较少发生心脏事件。The Oncologist 2016;21:666–670
对临床实践的提示: 乳腺癌患者在开始以蒽环类或曲妥珠单抗为基础的化疗前, 常常采用超声心电图或放射性核素心室造影进行基线心功能筛查, 因为这些检查费用相对较低, 另一方面也是考虑到治疗的潜在心脏毒性。但是在人群水平上, 这些检查可能耗费时间并且显著增加患者和医疗系统的支付成本。本研究发现在无心脏病史的患者中, 心功能筛查很少发现可导致治疗计划改变的异常结果。此外, 在平均 4 年的随访期间, 很少发生心脏事件, 且无一例基线心功能筛查结果异常的患者发生心脏事件。这提示在无心脏病史的年轻乳腺癌患者的化疗计划中, 基线心功能筛查用途有限。
Introduction
Anthracyclines and trastuzumab are two very effective systemic therapy agents widely used to treat breast cancer. However, they are associated with a risk of adverse cardiovascular complications, including cardiac dysfunction and heart failure [1–6]. Baseline cardiac screening may help avoid cardiotoxicity by detecting pre-existing cardiac conditions, resulting in more frequent cardiac monitoring, administration of cardioprotective medications, and/or modification of planned cancer therapy. Therefore, the American College of Cardiology (ACC), American Heart Association (AHA), and American Society of Nuclear Cardiology suggest that patients be screened for pre-existing cardiac conditions before potentially cardiotoxic chemotherapy [7].
Echocardiograms (ECHOs) and radionuclide ventriculograms (RVGs; also known as multigated acquisition scans) both provide a quantitative measure of cardiac function, the left ventricular ejection fraction (LVEF). Because of the noninvasive nature and broad availability of these exams, they are widely used for cardiac screening before chemotherapy [3]. Studies have estimated that between 60% and 80% of patients receive baseline cardiac evaluation with RVGs before chemotherapy, suggesting variation in implementation of the ACC and AHA recommendation [8, 9].
Limited prior research suggests that ECHOs and RVGs infrequently reveal clinically significant results that affect oncology treatment plans in this population [8–11]. In one study of 198 adults, RVGs were conducted in 80% of breast cancer patients undergoing adjuvant chemotherapy, with only 2.5% of RVGs indicating cardiac abnormalities, all in smokers [9]. Baseline ECHO results and potential effects of screening on treatment planning in patients undergoing trastuzumab therapy have not yet been studied. In addition, a significant portion of patients may have neither cardiac symptoms nor underlying cardiac disease; the utility of baseline cardiac function testing in these patients is unclear. In an effort to understand the utility of baseline cardiac function testing, we conducted a study of women who underwent baseline cardiac screening before adjuvant chemotherapy for breast cancer at Dana-Farber Cancer Institute (DFCI) from 2006 to 2011 to determine how often abnormal ECHO or RVG results changed initial treatment plans and whether abnormal screening tests were associated with cardiac events during and after chemotherapy.
Materials and Methods
We conducted a retrospective analysis of a prospectively collected cohort of newly diagnosed early-stage (stages I–III) female breast cancer patients treated at DFCI from 2006 to 2011. A total of 2,871 early-stage patients with newly diagnosed breast cancer were identified from the registry. Of these patients, 1,804 did not receive any cardiac screening, for reasons including that they were not planning to receive cardiotoxic or any chemotherapy or that the likelihood of baseline cardiac dysfunction was felt to be very low by the provider for any number of reasons such as age, no medical comorbidities, no alcohol or tobacco use, etc. We then reviewed medical record documentation to determine patients’ precardiac screening treatment recommendations, cardiac screening results, and postcardiac screening treatment recommendations, as well as treatment received and subsequent cardiac events. Among the remaining 1,067 patients with cardiac screening, 294 had electrocardiograms (EKGs) but no available ECHO or RVG, so they were excluded from our analysis. Patients were further excluded from our analysis for the following reasons: (a) diagnosis outside of timeframe (n = 2); (b) previous history of chemotherapy (n = 9); (c) previous history of radiation therapy (n = 15); (d) known cardiac history, defined as diagnosis of myocardial infarction, cardiomyopathy, or congestive heart failure (CHF) in institutional registry or medical record review (n = 32), because history of chemotherapy, radiation therapy, and cardiac disease are indications for cardiac function assessment and no longer for screening; (e) history of prior cardiac imaging with available results, because these results can influence treatment planning (n = 78); (f) no chemotherapy ever indicated in initial treatment discussions, so cardiac function screening would not be performed (n = 18); and (g) no ECHO/RVG results available in our medical record system (n = 26). Of note, 7 patients met multiple exclusion criteria. A total of 600 patients met inclusion criteria and were included in this analysis. Medical comorbidities at baseline associated with risk of cardiac disease, such as hypertension, diabetes, and hyperlipidemia, were also extracted from patients’ medication lists at the time of diagnosis from the medical record. Other risk factors for cardiac disease, such as age, race, menopausal status, smoking history, alcohol use, and body mass index (BMI), were obtained from the institutional registry. Categories for race were determined by the institutional registry, and patients self-classified their race.
Abnormal cardiac screening results were defined in two ways. The primary measure was LVEF, with normal LVEF values defined as 55% or greater and abnormal values defined as <55%. For ECHOs, secondary measures included additional abnormalities: valvular disease (defined as moderate tricuspid, pulmonic, mitral, or aortic regurgitation or stenosis), evidence of left ventricular hypertrophy, and diastolic dysfunction (impaired left ventricular relaxation).
We categorized the impact of abnormal values on treatment plans as follows: no change and no increased monitoring, no change but recommendation for closer monitoring, or change. “No change but recommendation for closer monitoring” included more frequent ECHOs or RVGs, cardiac management with medications, and/or coordination of care with a cardiologist. “Change in treatment plans” included recommendations for no chemotherapy or less cardiotoxic chemotherapy. Descriptive statistical analyses were performed to compare patients with abnormal results to patients with normal results to identify any potential predictors of abnormal results. Differences in demographics between these two groups were evaluated by using Fisher exact tests for categorical factors and Wilcoxon rank-sum tests for continuous factors.
We defined a cardiac event in follow-up as symptomatic heart failure, myocardial infarction, arrhythmia, valvular disease (as defined earlier), or coronary artery disease and correlated it to cardiac imaging including RVGs, ECHOs, and EKGs when available. The length of follow-up is defined as the start date of chemotherapy to the date of the last clinic visit or the date of diagnosis of recurrence or metastasis. We excluded follow-up data after recurrence or metastasis because patients often underwent additional chemotherapy. This study was approved by the DFCI institutional review board, and all patients had previously signed informed consent forms to participate in the institutional registry and medical record review studies.
Results
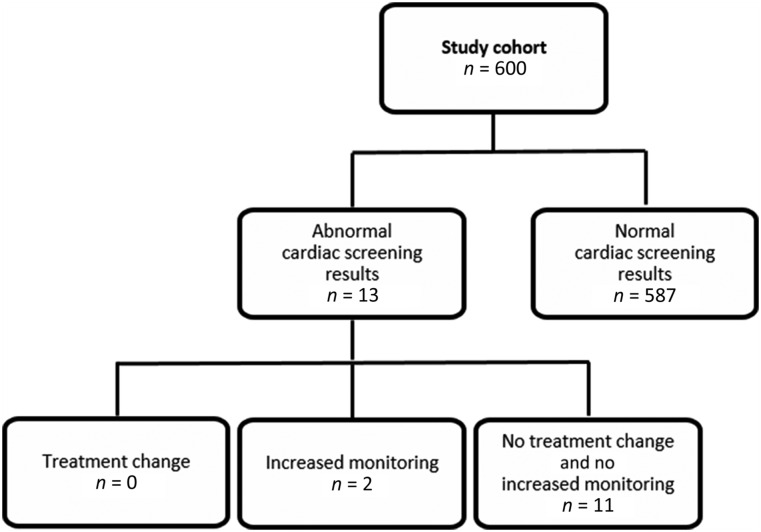
For the original 1,067 patients with baseline ECHOs, RVGs, or EKGs identified in the registry, the median age was 56 years (range, 25–91 years) (see Fig. 1 for study cohort flow diagram). Among the 600 patients included in the analytic sample, the median age was 48 years (range, 23–80 years). Prior to cardiac screening, 79% of treatment plans included anthracycline, 34% included trastuzumab, and 16% included both. ECHOs were performed in 74 of the 600 patients (12.3%) and RVGs in the remaining 526, as per institutional conventions during the study period. Abnormal ECHO and RVG results were observed in 13 of the 600 patients (2.2%, 95% confidence interval: 1.2%–3.7%), including 10 with baseline LVEF <55%, with the lowest at 50% (Fig. 2). The other 3 patients had atrial or ventricular size or thickness abnormalities on ECHO. Of these 13 patients, 4 received ECHOs, whereas the other 9 received RVGs.
Figure 1.
Study cohort. A total of 2,871 patients with newly diagnosed early-stage breast cancer were identified from the DFCI Institutional Registry. Of these patients, 1,804 had no cardiac screening performed, and 294 had only EKGs. Of the remaining 773 patients, 173 patients did not meet eligibility criteria for the reasons listed. A total of 7 patients met multiple exclusion criteria.
Abbreviation: DFCI, Dana-Farber Cancer Institute.
Figure 2.
Impact of cardiac function screening on treatment decision-making. Abnormal results were observed in 13 (2.17%, 1.2%–3.7%) of 600 patients. Of these abnormal results, none led to changes in treatment decisions (e.g., switching to a less cardiotoxic agent), although 2 patients (15.4%, 1.9%–45.5%) received increased monitoring of cardiac function throughout and after treatment.
No changes in treatment plan, such as switching to a less cardiotoxic agent, were made for any of the 13 patients. However, 2 patients were recommended to and received more frequent cardiac function assessment via RVG or ECHO and close follow-up with their provider to evaluate for symptoms of cardiac dysfunction. These 2 patients were relatively young (ages 39 and 49), had never smoked, were light alcohol drinkers (<1 drink per week), and were postmenopausal due to hysterectomy and oophorectomy. One had a BMI of 18.0, whereas the other had a BMI of 35.6, which were the extremes of the patients with abnormal cardiac screening results. They developed no cardiac events in follow-up.
Analysis of basic demographics between patients with abnormal versus normal cardiac screening results showed no statistically significant differences (p > .05) in age, race, menopausal status, smoking history, alcohol use, BMI, or medical comorbidities, including hypertension, diabetes, and hyperlipidemia (Table 1). Given the infrequency of abnormal results, further statistical analysis to determine potential predictors of abnormal results was not pursued.
Table 1.
Cohort characteristics
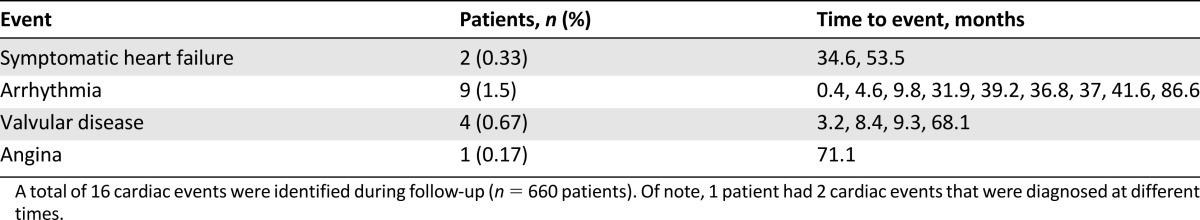
All 600 patients were followed for cardiac events with a median follow-up of 4.0 years (range, 0–8.3 years). A total of 66 patients (11%) developed recurrent or metastatic disease. A total of 15 patients (2.5%) were found to have cardiac events (Table 2). These included two cases of symptomatic heart failure, nine cases of new arrhythmias such as supraventricular tachycardia and atrial fibrillation, four cases of valvular disease, and one case of angina with coronary artery disease on catheterization. One patient developed heart failure followed by tricuspid regurgitation. As described previously, the infrequency of abnormal baseline cardiac findings precluded further analysis to identify predictors of cardiac events. Of note, none of the patients with abnormal baseline cardiac results developed cardiac events.
Table 2.
Cardiac events observed in follow-up
Discussion
Baseline cardiac screening is commonly performed to assess for pre-existing cardiac dysfunction in women planning for chemotherapy or trastuzumab therapy. However, detection of abnormalities is infrequent and has little effect on treatment recommendations or subsequent cardiac events in a population of breast cancer survivors. The results of our analysis call into question the utility of baseline pretreatment cardiac evaluation in women without cardiac history for whom systemic chemotherapy with potentially cardiotoxic agents is planned.
The results of our analysis confirm and expand upon prior research in this area. Sabel et al. conducted a retrospective study of 296 patients with breast cancer, 62 of whom had prechemotherapy RVGs, and showed a 6.5% rate of low LVEF (between 49% and 51%; low LVEF defined as ≤51%) [8]. No cases were identified in which women were not treated with anthracycline-based chemotherapy due to abnormal baseline RVGs. The authors concluded that prechemotherapy RVGs rarely screened out women considering anthracycline-based chemotherapy. Similarly, Conrad et al. conducted a retrospective study of 197 patients with diffuse large B-cell lymphoma considering anthracycline-based chemotherapy, with 117 undergoing baseline LVEF assessment [10]. Of these 117 patients, median age was 69 years, and 50% were male. Asymptomatic left ventricular dysfunction was found in 4 patients (4%) with LVEF ranging from 41% to 48%. No modifications to treatment strategies were made because of LVEF findings. Additionally, Jeyakumar et al. further confirmed in a population-based study of 238 breast cancer patients that, of the 198 patients who underwent baseline RVG screening, five patients (2.5%) had abnormal LVEF, ranging from 35% to 47% [9]. These findings resulted in change to nonanthracycline-based chemotherapy in 3 patients and no chemotherapy in 1 patient. In 2014, Bryant et al. studied 119 patients diagnosed with acute myeloid leukemia, with 71 undergoing baseline LVEF measurements with ECHO or RVG [11]. The average age in this cohort was 61 years with 62% male patients. Only 4 patients (5.6%) had abnormal values, with none that changed management. With regard to cardiac events in follow-up, Romond et al. found that in 7 years of follow-up in a randomized trial, 4.0% of patients developed cardiac events after receiving anthracyclines and trastuzumab, compared with 1.3% in patients receiving anthracyclines only [12]. Risk factors associated with development of CHF included baseline LVEF 50%–54%, age (2.3% in <50 years, 5.5% in 50–59 years, and 6.1% in ≥60 years), and antihypertensive medications at baseline [12].
Our analysis represents the largest study to assess the utility of baseline cardiac function evaluation in systemic treatment planning for breast cancer patients considering potentially cardiotoxic systemic therapy and is the first to evaluate this among patients planned to receive trastuzumab therapy. Furthermore, our study is the first to show the low risk of subsequent cardiac events in follow-up and the lack of association between prior abnormal cardiac screening and subsequent cardiac events, adding new evidence for reconsidering the routine practice of baseline cardiac function screening in this setting.
Our findings should be interpreted in consideration of several potential limitations. Although most of our study data were prospectively collected, our analysis was retrospective and may be subject to biases common to retrospective studies. Subgroup analysis to determine whether a difference exists in threshold to treat with anthracyclines versus trastuzumab could not be performed. Trastuzumab was discussed with many patients at the initial clinic visit because final pathology results were not yet available. However, at the postcardiac screening visit, when HER2 status was known, HER2-negative patients no longer required trastuzumab; as a result, further conversation about this agent did not occur, and this was not considered a change in therapy plan based on the baseline cardiac screening. Furthermore, our final study cohort was relatively young, with a mean age of 48 years, which may limit the generalizability of our findings to older patient populations, even though our study population included patients as old as 80 years. As noted in our results, the average age of our entire cohort was 57, which decreased to 48 after patients were excluded. Thus, patients excluded from analysis because of prior cardiac history, cardiac imaging, chemotherapy, or radiation therapy were older on average (mean age of the 467 excluded patients was 68.5 years, with ages ranging from 25 to 91 years). It is also worth noting that the majority of the patients seen at our institution did not have baseline cardiac screening, and only 773 of 2,871 (or 26.9%) underwent baseline ECHO/RVG testing during this time period. Some patients may have had such an early-stage cancer that chemotherapy was not warranted, and some would have been deemed to be very low risk of baseline cardiac dysfunction, so baseline ECHO/RVG testing was never performed. Moreover, some patients were enrolled in clinical trials that required baseline cardiac assessment, which may not otherwise have been performed. In addition, although our analysis provides the first evaluation of how the patients fared from a cardiac standpoint after cancer treatment, time to last follow-up was limited for some, and identification of cardiac event relied on single-institution medical record review. Finally, the majority of screening cardiac evaluations performed were RVGs because of institutional convention at the time, and results may have been different had imaging been predominantly ECHOs.
Conclusion
The results of this study suggest that baseline cardiac function screening has limited impact in clinical decision-making in young breast cancer patients without pre-existing cardiac history or other prior exposures considering anthracycline-based and/or trastuzumab chemotherapy. Thus, thorough clinical assessment for cardiac history, symptoms, risk factors, and physical exam should be performed in all patients. However, considering the low rates of cardiac events in follow-up, omitting baseline screening in young patients (<50 years) with an otherwise negative cardiac history may be prudent in the future [12]. Baseline screening can be considered on a case-by-case basis in patients with cardiac risk factors, such as hypertension, diabetes, hyperlipidemia, high BMI, alcohol, or tobacco use, although these factors were not associated with abnormal cardiac screening results in our study. However, further evaluation of the utility of baseline testing during follow-up care, and particularly in older breast cancer patients who are generally at greater risk of cardiac problems, is warranted.
Acknowledgments
This research was supported by the Pink Agenda, LIVESTRONG Foundation, and Susan G. Komen Foundation. These funding organizations had no involvement in the design and conduct of the study. A portion of these data was previously presented as a poster at the 2014 American Society of Clinical Oncology annual meeting, Chicago, IL.
Author Contributions
Conception/Design: Sandy R. Truong, William T. Barry, Javid J. Moslehi, Erica L. Mayer, Ann H. Partridge
Provision of study material or patients: Ann H. Partridge
Collection and/or assembly of data: Sandy R. Truong, Emily L. Baker, Ann H. Partridge
Data analysis and interpretation: Sandy R. Truong, William T. Barry, Javid J. Moslehi, Erica L. Mayer, Ann H. Partridge
Manuscript writing: Sandy R. Truong, William T. Barry, Javid J. Moslehi, Emily L. Baker, Erica L. Mayer, Ann H. Partridge
Final approval of manuscript: Sandy R. Truong, William T. Barry, Javid J. Moslehi, Emily L. Baker, Erica L. Mayer, Ann H. Partridge
Disclosures
Erica L. Mayer: Pfizer, Myriad, Eisai (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Keefe DL. Trastuzumab-associated cardiotoxicity. Cancer. 2002;95:1592–1600. doi: 10.1002/cncr.10854. [DOI] [PubMed] [Google Scholar]
- 2.Ky B, Vejpongsa P, Yeh ET, et al. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113:754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenihan DJ, Cardinale DM. Late cardiac effects of cancer treatment. J Clin Oncol. 2012;30:3657–3664. doi: 10.1200/JCO.2012.45.2938. [DOI] [PubMed] [Google Scholar]
- 4.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: Review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 6.Groarke J, Tong D, Khambhati J, et al. Breast cancer therapies and cardiomyopathy. Med Clin North Am. 2012;96:1001–1019. doi: 10.1016/j.mcna.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klocke FJ, Baird MG, Lorell BH, et al. ACC/AHA/ASNC Guidelines for the clinical use of cardiac radionuclide imaging—Executive summary: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) Circulation. 2003;108:1404–1418. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 8.Sabel MS, Levine EG, Hurd T, et al. Is MUGA scan necessary in patients with low-risk breast cancer before doxorubicin-based adjuvant therapy? Multiple gated acquisition. Am J Clin Oncol. 2001;24:425–428. doi: 10.1097/00000421-200108000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Jeyakumar A, DiPenta J, Snow S, et al. Routine cardiac evaluation in patients with early-stage breast cancer before adjuvant chemotherapy. Clin Breast Cancer. 2012;12:4–9. doi: 10.1016/j.clbc.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Conrad AL, Gundrum JD, McHugh VL, et al. Utility of routine left ventricular ejection fraction measurement before anthracycline-based chemotherapy in patients with diffuse large B-cell lymphoma. J Oncol Pract. 2012;8:336–340. doi: 10.1200/JOP.2012.000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant A, Sheppard D, Sabloff M, et al. A single-institution analysis of the utility of pre-induction ejection fraction measurement in patients newly diagnosed with acute myeloid leukemia. Leuk Lymphoma. 2015;56:135–140. doi: 10.3109/10428194.2014.883072. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]