The carcinoid syndrome can affect the quality of life of patients with neuroendocrine tumors. Refractoriness to somatostatin analogs (SSAs) used to treat this syndrome can occur relatively quickly. Phase III trial results suggest telotristat etiprate, a serotonin inhibitor, might represent a new target for treating SSA-resistant carcinoid syndrome. Research is needed to verify initial safety data and determine impact on survival, quality of life, and long-term efficacy outcomes.
Keywords: Neuroendocrine tumors, Malignant carcinoid syndrome, Carcinoid tumors, Serotonin, Somatostatin analogs, Telotristat etiprate
Abstract
The carcinoid syndrome represents a set of signs and symptoms associated with neuroendocrine tumors (NETs) that occur primarily when metastases are developed in the liver, resulting in the worsening of quality of life. Serotonin plays a central role in the physiology of carcinoid syndrome by promoting intestinal motility. Somatostatin analogs (SSAs) have widely demonstrated their efficacy as symptomatic relievers of carcinoid syndrome, but this control is ephemeral, being reduced by approximately 50% within the first year. The exact mechanisms of resistance to SSAs are not fully understood, but it is believed that serotonin might be involved. Patients with carcinoid syndrome present with a significant increase in serotonin plasma levels and, consequently, in the soluble urinary metabolite 5-hydroxyindole acetic acid. Telotristat etiprate is a potent inhibitor of tryptophan hydroxylase, a rate-limiting enzyme in the synthesis of serotonin, that has demonstrated in the phase III TELESTAR clinical trial a significant improvement in the control of bowel movements in patients with NETs who have carcinoid syndrome and who have progressed to an SSA. Based on these results, telotristat etiprate has emerged as a potential new option in the treatment algorithm of symptomatic control of functioning NETs. However, some issues need to be clarified, such as the safety profile of the drug outside clinical trials, the benefit in quality of life, and the possible impact on tumor growth, as well as its role within sequencing or combination treatment strategies with pre-existing drugs effective in NET treatment.
Implications for Practice:
This article reviews the literature about carcinoid syndrome, which affects patients diagnosed with neuroendocrine tumors. Few articles have been published about this syndrome and its pathophysiology. Somatostatin analogs provide symptomatic relief; however, patients may become refractory to this strategy, usually within the first year of treatment. In this context, as an agent with an innovative mechanism of action, telotristat etiprate has demonstrated activity in a phase III trial, and findings may offer a path to an improve quality of life and prolonged survival for certain patients.
Introduction
Neuroendocrine tumors (NETs) represent a complex and heterogeneous group of malignancies arising from the diffuse endocrine cells and other cells derived from the neural crest. According to the Surveillance, Epidemiology, and End Results program, the incidence of NETs has increased from 1.09 cases per 100,000 inhabitants in 1973 to 5.25 cases per 100,000 inhabitants in 2004 [1–3]. Locally advanced or metastatic disease is observed in more than one third of patients at the time of diagnosis. These tumors are characterized by a wide variability in clinical syndromes at presentation, depending on hormonal and vasoactive peptide release.
One of the most important peptides related to the development of carcinoid syndrome is serotonin. Depending on the origin of the primary tumor, approximately 8%–35% of patients with NETs develop carcinoid syndrome and suffer mainly from diarrhea and flushing episodes. Other clinical signs and symptoms are hypertension in pheochromocytomas, hyperglycemia in insulinomas, or adrenocorticotropic hormone-producing tumors or watery diarrhea in Verner-Morrison syndrome, among others [4–6]. Therefore, the inhibition of the peripheral synthesis of serotonin represents a promising target in the field of symptom control in patients with functioning NETs.
Materials and Methods
A systematic search of PubMed databases and abstracts from the European Society for Medical Oncology up to September 2015 was performed. The search terms considered were “telotristat etiprate,” “serotonin,” “carcinoid syndrome,” “resistance to somatostatin analogs,” and “serotonin receptors” for the identification of relevant and English-language studies on the physiopathology and management of carcinoid syndrome. In addition, ClinicalTrials.gov and cancer-focused congresses were searched to identify relevant studies evaluating therapies in carcinoid syndrome management.
Results
Carcinoid Syndrome
Although carcinoid tumors were first reported at the end of the 19th century, carcinoid syndrome was described in 1954 by Thorson et al. when they identified a series of signs and symptoms consisting of heart valve disease, peripheral vasomotor symptoms, and bronchoconstriction in a patient diagnosed with a metastatic intestinal NET [7]. From a pathophysiologic point of view, the tumor secretion of various substances into systemic circulation, including serotonin as the most predominant released vasoactive peptide, and also histamine, prostaglandins, tachykinins, and kallikrein, causes the carcinoid syndrome [8].
Carcinoid syndrome has been reported to be present in 8%–35% of patients with NETs and is characterized by a wide range of symptoms, such as flushing episodes, diarrhea, abdominal pain, intermittent bronchial wheezing, telangiectasia, heart valve disease, mesenteric fibrosis, and pellagra [5, 9–11]. Carcinoid crisis is considered an immediate life-threatening complication of carcinoid syndrome due to a massive release of vasoactive peptides from the tumor. This crisis can occur under conditions of stress, anesthesia, chemotherapy, or surgery [12].
Serotonin seems to play a central role in the development of the acute and chronic manifestations by acting directly on the cell membrane receptors of the enteric neurons or by enhancing peristalsis and secretory reflexes [2, 13, 14]. Indeed, serotonin is also involved in the development of secondary heart valve disease and mesenteric fibrosis because it may stimulate fibroblast growth and proliferation and synthesis of extracellular matrix (fibrogenesis) [15, 16].
Serotonin seems to play a central role in the development of the acute and chronic manifestations by acting directly on the cell membrane receptors of the enteric neurons or by enhancing peristalsis and secretory reflexes.
Serotonin Synthesis and Physiology
Serotonin is a neurotransmitter that mainly acts at the gastrointestinal tract and central nervous system (CNS) level [17]. Approximately 95% of total serotonin in the human body is released within the gastrointestinal tract: approximately 10% by the enteric neurons and 90% by the enterochromaffin cells. The remaining 5% exerts its action in the brain, crossing the blood-brain barrier [18].
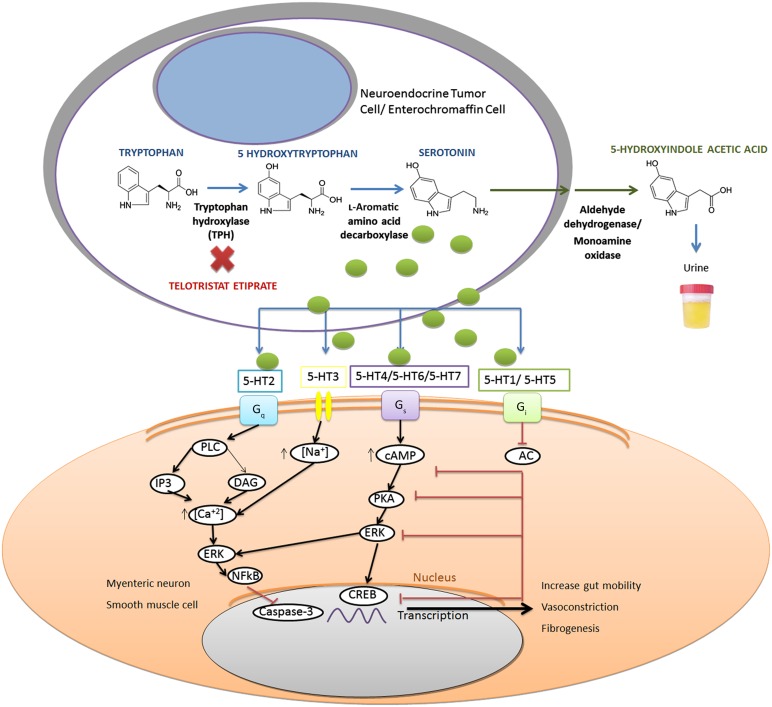
The synthesis of serotonin has its substrate in the essential amino acid tryptophan and needs two enzymatic steps. The first step is mediated by tryptophan hydroxylase (TPH). This enzyme is essential because it is considered the rate-limiting step of the systemic and neuronal synthesis of serotonin. TPH has two isoforms: TPH2 (located only in the brain) and TPH1 (located in the peripheral tissues with no presence in the CNS and encoded by a different gene) [18, 19]. l-Amino acid decarboxylase is the catalyzed enzyme involved in the second step of serotonin synthesis [20, 21] (Fig. 1). Once the serotonin is produced, it accumulates in vacuoles in the enterochromaffin cells until the cell releases the serotonin to the bloodstream or to the surrounding enteric tissue.
Figure 1.
Illustration of biosynthesis of serotonin (blue arrows), the inhibitor action of telotristat etiprate on TPH (red X), conversion of serotonin to 5-hydroxyindole acetic acid and urinary excretion (green arrows), and activating and inhibitory pathways [20, 24–28].
Abbreviations: 5-HT1-7, serotonin receptors 1 to 7; AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; CREB, cyclic AMP response element binding proteins; DAG, diacylglycerol; ERK, extracellular signal-regulated kinase; Gq (Gs, Gi), G-coupling proteins; IP3, inositol triphosphate; NFkB, nuclear factor κ-light-chain-enhancer of activated B cells; PKA, cyclic AMP-dependent protein kinase A; PLC, phospholipase C; TPH, tryptophan hydroxylase.
There, serotonin binds to serotonin-receptors that consist of seven types (5-HT1 to 5-HT7) and several subtypes, each of which is from a different location in the body and has different functions. All the serotonin receptors are G-coupled-protein receptors except 5-HT3, which is an ion channel receptor. Three main types of primary coupling to G protein receptors have been described. The 5-HT1 and 5-HT5 receptors activate Gi/Go proteins, the 5-HT2 receptors activate Gq proteins, and the 5-HT4, 5-HT6, and 5-HT7 activate Gs proteins [22–24]. (Fig. 1)
The activity performed by these G-coupling proteins is different depending on the activated receptor subtype. Gq and Gs proteins activate different intracellular pathways that lead to the increase of transcriptional mechanisms in the nucleus. Gi/Go proteins exhibit inhibitory activity, lowering intracellular cyclic adenosine monophosphate levels and repressing transcriptional factors [24–26].
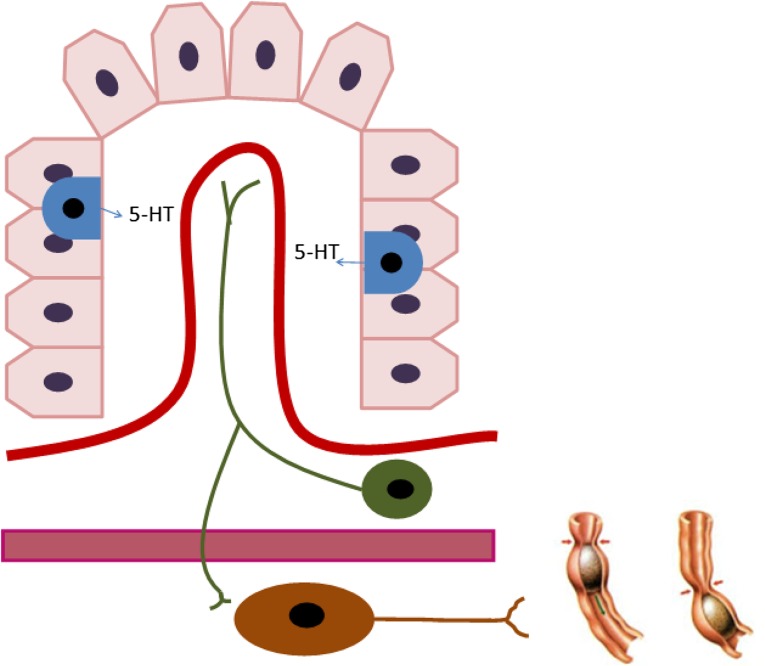
The receptors are located at different sites. Some, such as 5-HT5, 5-HT6, and 5-HT7, have a single central location. The remaining receptors are spread throughout peripheral locations in enteric neurons, enterochromaffin cells, hepatocytes, and smooth-muscle cells. In fact, 5-HT3 and 5-HT4 receptors are crucial in the regulation of intestinal motility and the peripheral peristaltic reflex. This action is performed by the release of serotonin by enterochromaffin cells to intrinsic and extrinsic efferent neurons [20, 23, 29–31] (Fig. 2).
Figure 2.
Intestinal crypt that shows secretion of serotonin by enterochromaffin cells (blue) into general circulation and efferent neurons (green). Finally, serotonin reaches myenteric neurons, resulting in increased gut motility (brown).
Abbreviation: 5-HT, 5-hydroxytryptamine.
Serotonin is metabolized in the tissues by the activity of the monoamine oxidase enzyme. In the intestine, as in the CNS, there is a reuptake serotonin system to avoid its prolonged effect in the synapse [36]. In peripheral circulation, serotonin is metabolized in the liver and kidney by the monoamine oxidase and aldehyde dehydrogenase enzymes that convert serotonin to 5-hydroxyindole acetic acid (5-HIAA), which is eliminated by the urine [20].
Resistance to Somatostatin Analogs
Somatostatin analogs (SSAs) represent the mainstay in the treatment of NETs, based on the results obtained from more than 20 years of research on the improvement of the symptoms of the carcinoid syndrome [37]. Antiproliferative effects were also demonstrated in phase III trials such as the PROMID (placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors) and CLARINET trials by using octreotide long-acting formulation (LAR) and lanreotide autogel, respectively [38, 39].
SSAs initiate their function by acting on a G-protein-coupled somatostatin receptor (SSTR) family of five members. The most commonly expressed SSTR in gastrointestinal NETs (90%) and pancreatic NETs (80%) is SSTR2, to which SSAs bind with higher affinity [40, 41].
SSAs have demonstrated efficacy in symptomatic relief of both diarrhea and flushing episodes associated with carcinoid syndrome. This effect was demonstrated in a randomized study comparing subcutaneous short-acting octreotide versus octreotide LAR.
Signaling is transmitted directly from a G-coupled SSTR activation to interrupt the cell functions or indirectly by inducing apoptosis with p53 and BAX upregulation, inhibiting proliferation regulated by tyrosine kinases and activating SHP1 and SHP2 involved in cycle arrest or modifying other SSTR-independent antiproliferative pathways, such as inhibition of insulin growth factor 1, which reduces tumor gene transcription via RAS. Derived from this indirect effect, SSAs also are able to inhibit angiogenesis by the MAPK/ERK1-2 pathway and it is also postulated that this might modulate immune and inflammatory reactions [32, 42, 43].
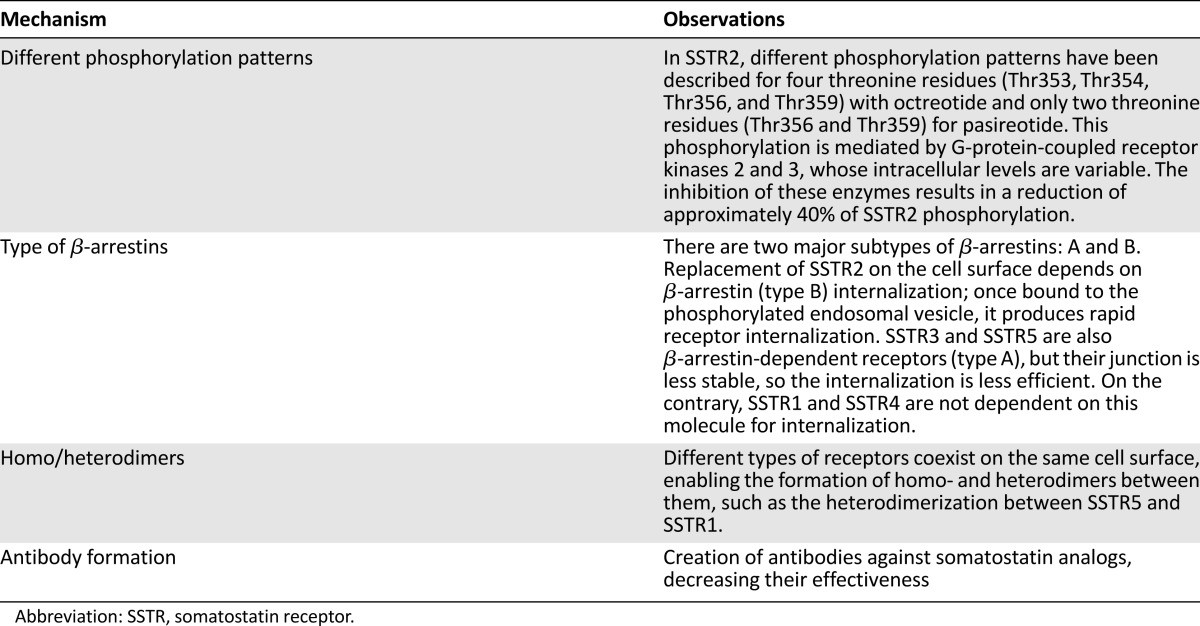
SSAs have demonstrated efficacy in symptomatic relief of diarrhea and flushing episodes associated with carcinoid syndrome. This effect was demonstrated in a randomized study comparing subcutaneous short-acting octreotide versus octreotide LAR. Symptom control, in terms of stool numbers and flushing frequency, were reduced about 50% and 80%, respectively, from baseline to week 24 in all patients treated [37]. Moreover, symptom control disappears in about half of patients during the first year of treatment with SSAs. However, this symptomatic and proliferative control does not last forever and some mechanisms of resistance to treatment have been described, although the exact one is not yet known [32–35, 44–48] (Table 1).
Table 1.
All these mechanisms, therefore, can modify the three-dimensional configuration of the receptor and may cause alteration in the binding process of SSA to SSTR, changing the receptor regulation at the cell surface and increasing its internalization and desensitization with respect to the number of SSTRs at the cell surface, causing modification of the ratio expression between different cell surface receptors, altering the downstream signaling triggered by the receptor, causing up- or downregulation of the pre-existing intracellular pathways, and even creating new intracellular signaling pathways by the formation of homo- and heterodimers between different receptor subtypes or other receptor superfamily [33–35, 44–50].
Telotristat Etiprate
The inhibition of serotonin synthesis with parachlorophenylalanine was initially tested in patients with carcinoid syndrome, and demonstrated efficacy in reducing the levels of serotonin and diarrhea frequency, but central side effects with behavioral changes and depression halted further development of this molecule in this context [51].
Several other drugs have been studied for the treatment of carcinoid syndrome, but none has reached a phase III clinical trial until telotristat etiprate. Telotristat etiprate (formerly, LX1606) is a new compound that inhibits TPH, the rate-limiting step in the synthesis of serotonin. Telotristat etiprate is a prodrug that is converted to its active drug by the esterase activity. Telotristat etiprate has a 0.028-μM in vitro power on purified human enzyme TPH2; therefore, it is a potent inhibitor of TPH, both in vitro and in vivo. As a result of the TPH inhibition, there is a reduction in the peripheral, but not CNS, serotonin levels. In one report, TPH knockout mice had a 94% reduction in serotonin levels [52]. LX1031, an initial compound that also inhibits TPH, reduced the serotonin concentration in the small intestine and peripheral blood by approximately 90%, and decreased inflammation and the number of stools in patients with nonconstipating irritable bowel syndrome. Telotristat etiprate does not distinguish between TPH1 and TPH2 but is not able to cross the blood-brain barrier; therefore, it would not reach the CNS at a high enough concentration to inhibit the TPH2 enzyme. Consequently, it does not diminish central serotonin levels, thus avoiding the side effects that it would entail otherwise [27, 28, 52–55].
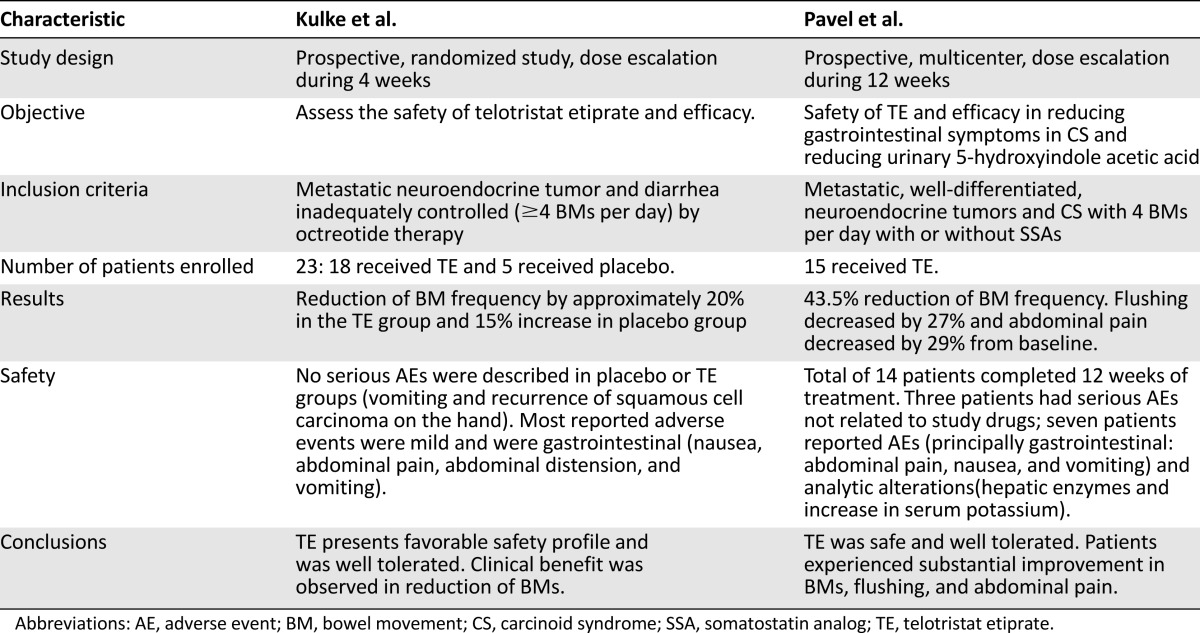
Based on the efficacy demonstrated in preclinical research, two phase II trials were performed. Kulke et al. [56] conducted a randomized study of 23 patients who received telotristat etiprate during 4 weeks (Table 2). Eighteen patients with NETs and 5 healthy volunteers received the treatment at dose escalation (range: 100–500 mg) and 5 patients received placebo. Five of 18 patients (27.7%) in the experimental treatment group achieved at least a 30% reduction in bowel movements (BMs) for more than 2 weeks compared with no reduction in the placebo group. Twelve of 16 patients (75%) receiving telotristat etiprate had at least some reduction in BMs for more than 2 weeks compared with only 1 patient in the control group. Nine of 16 (56.2%) patients in the telotristat etiprate group and none in the placebo group achieved a 50% or more reduction in, or even normalization of, the levels of urinary 5-HIAA. Tolerability of the drug was acceptable and the main adverse effects were gastrointestinal [56].
Table 2.
Pavel et al. [57] studied the administration of telotristat etiprate in 15 patients (Table 2). All patients experienced some reduction in BMs during the treatment period and 6 patients (40%) had at least a 50% decrease from baseline, from 5.9 BMs per day to an average of 2.6 BMs per day at the end of the 12 weeks of treatment. Urinary 5-HIAA levels progressively decreased during treatment to a reduction of 74.2% compared with baseline average. Telotristat etiprate was well tolerated; gastrointestinal disorders were the most common side effect. There were no reports of depression as an adverse event, which is consistent with telotristat etiprate being unable to cross the blood-brain barrier. The investigators reported one episode of increased γ-glutamyltransferase levels (Common Terminology Criteria for Adverse Events grade 3) that was not considered to be related to the study drug [57].
The results of the phase III pivotal study of telotristat etiprate have been presented. The TELESTAR (Telotristat Etiprate for Somatostatin Analogue Not Adequately Controlled Carcinoid Syndrome) trial is a randomized, double-blind study with 3 treatment arms: placebo, telotristat etiprate at a dose of 250 mg, and telotristat etiprate at a dose of 500 mg 3 times daily (45 patients in each arm) for 12 weeks. The primary end point was the reduction in the number of daily BMs compared with baseline. Patients included in this trial were previously diagnosed with a well-differentiated metastatic NET and had documented carcinoid syndrome, experiencing at least four BMs per day despite a stable dose of SSA therapy.
Telotristat etiprate at the 250- and 500-mg doses reduced the number of BMs from baseline by 29% and 35%, respectively. In the placebo group, there was also a 17% reduction in the number of BMs from baseline. Duration of response was defined as at least a 30% reduction in BM frequency during at least 50% of the time on study. Duration of response was 44% and 42% in the 250- and 500-mg groups, respectively, which was significantly different from that seen in the placebo group (p = .011 and p = .020, respectively). Moreover, telotristat etiprate reduced the concentration of urinary 5-HIAA by 40 mg per 24 hours in the 250-mg group and 57 mg per 24 hours in the 500-mg group; these concentrations were also significantly different than that seen in the placebo group (p = .001).
Telotristat etiprate was safe and well tolerated. Grade 1 and 2 depression was described in three patients (6.7%) in the placebo group, eight patients (17.7%) in the 500-mg dose group, and two patients (4.4%) in the 250-mg dose group. Treatment-related depression did not result in any telotristat etiprate discontinuation, and all cases of grade 1 and 2 depression resolved on the study. The most frequent adverse events were gastrointestinal, with nausea being described in 19 treated patients [58].
There are several unknowns about telotristat. We do not know the role of telotristat on tumor growth. Studies have not included as a primary or secondary end point the reduction in tumor lesions, and even if it is plausible to consider that the inhibition of serotonin synthesis can impact tumor growth, we need further research and clinical trials designed in that regard.
We also do not know the safety profile of telotristat in routine clinical practice. In TELESTAR trial, the incidence of grade 1 and 2 depression was similar in the placebo group and the telotristat etiprate 250-mg group, and it was slightly higher in the telotristat etiprate 500-mg group. All instances were resolved during the study without requiring the withdrawal of the study drug. Moreover, no severe grade of depression was reported. Indeed, telotristat etiprate does not seem to cross the blood-brain barrier, so it would not have depressant effects on the central nervous system. For a better assessment of the long-term safety profile of telotristat, we need to wait for the results coming from the TELEPATH (Telotristat Etiprate—Expanded Treatment for Patients with Carcinoid Syndrome Symptoms) trial [59]. Also, the activity in a broader population would be evaluated in the TELECAST (Telotristat Etiprate for Carcinoid Syndrome Therapy) trial [60].
According to the inclusion criteria of the phase III TELESTAR trial, treatment with telotristat etiprate would be started in those patients who do not reach adequate symptom control related to carcinoid syndrome with the maximum tolerated dose of SSA. Moreover, the optimal dose of telotristat etiprate is not clearly known. It seems that 250 mg obtains a significant response with a better safety profile than the 500-mg dose, but this issue was not an endpoint of the TELESTAR trial.
A deeper knowledge of telotristat etiprate activity in terms of duration of the responses, improvement of quality of life associated with the treatment, and the prevention rate of the development of mesenteric fibrosis and cardiac valve disease are lacking. Last, new combination strategies of telotristat with other active agents in the field of NETs, like everolimus, pasireotide, sunitinib, or chemotherapy, might become synergistic and prospective clinical trials have not yet started [61].
Conclusion
The carcinoid syndrome represents a group of signs and symptoms that dramatically interferes with the quality of life of a considerable group of patients with NETs. The SSAs were the first agents that demonstrated benefit in controlling this syndrome. Resistance mechanisms, although not fully known, are involved in carcinoid syndrome refractoriness to SSAs; therefore, new treatment approaches are required.
Telotristat etiprate is a potent inhibitor of the peripheral synthesis of serotonin that blocks the rate-limiting step involving the TPH enzyme. TELESTAR is a phase III study that has demonstrated that telotristat might control the number of BMs related to carcinoid syndrome. Serotonin inhibitors represent a promising new targeting approach for the treatment of patients with carcinoid syndrome resistant to SSAs. Improvement in patients’ quality of life is expected because of symptom control; however, impact on survival is yet to be defined. Therefore, further research is required to verify the initial results of the safety and impact of telotristat etiprate on survival and quality of life, as well as long-term efficacy outcomes.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Javier Molina-Cerrillo, Teresa Alonso-Gordoa, Enrique Grande
Provision of study material or patients: Javier Molina-Cerrillo
Collection and/or assembly of data: Javier Molina-Cerrillo, Olga Martínez-Sáez
Data analysis and interpretation: Javier Molina-Cerrillo, Teresa Alonso-Gordoa, Olga Martínez-Sáez
Manuscript writing: Javier Molina-Cerrillo, Teresa Alonso-Gordoa
Final approval of manuscript: Javier Molina-Cerrillo, Teresa Alonso-Gordoa, Enrique Grande
Disclosure
Enrique Grande: Ipsen, Lexicon (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Díez JJ, Grande E, Alonso T, et al. [Multidisciplinary approach in the diagnosis and therapy of patients with endocrine tumors] Med Clin (Barc) 2015;145:36–41. doi: 10.1016/j.medcli.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: A SEER analysis. J Cancer. 2012;3:292–302. doi: 10.7150/jca.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604–1620. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
- 5.Boutzios G, Kaltsas G. Clinical syndromes related to gastrointestinal neuroendocrine neoplasms. Front Horm Res. 2015;44:40–57. doi: 10.1159/000382053. [DOI] [PubMed] [Google Scholar]
- 6.Joynt KE, Moslehi JJ, Baughman KL. Paragangliomas: Etiology, presentation, and management. Cardiol Rev. 2009;17:159–164. doi: 10.1097/CRD.0b013e3181a6de40. [DOI] [PubMed] [Google Scholar]
- 7.Thorson A, Biorck G, Bjorkman G, et al. Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J. 1954;47:795–817. doi: 10.1016/0002-8703(54)90152-0. [DOI] [PubMed] [Google Scholar]
- 8.Modlin IM, Kidd M, Latich I, et al. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Pape UF, Berndt U, Müller-Nordhorn J, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2008;15:1083–1097. doi: 10.1677/ERC-08-0017. [DOI] [PubMed] [Google Scholar]
- 10.Patel C, Mathur M, Escarcega RO, et al. Carcinoid heart disease: Current understanding and future directions. Am Heart J. 2014;167:789–795. doi: 10.1016/j.ahj.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Oberg K, Akerström G, Rindi G, et al. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v223–v227. doi: 10.1093/annonc/mdq192. [DOI] [PubMed] [Google Scholar]
- 12.Schnirer II, Yao JC, Ajani JA. Carcinoid--a comprehensive review. Acta Oncol. 2003;42:672–692. doi: 10.1080/02841860310010547. [DOI] [PubMed] [Google Scholar]
- 13.Carter Y, Jaskula-Sztul R, Chen H, et al. Signaling pathways as specific pharmacologic targets for neuroendocrine tumor therapy: RET, PI3K, MEK, growth factors, and Notch. Neuroendocrinology. 2013;97:57–66. doi: 10.1159/000335136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von der Ohe MR, Camilleri M, Kvols LK, et al. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 15.Connolly HM, Pellikka PA. Carcinoid heart disease. Curr Cardiol Rep. 2006;8:96–101. doi: 10.1007/s11886-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Cardiac valvulopathy associated with exposure to fenfluramine or dexfenfluramine: U.S. Department of Health and Human Services interim public health recommendations, November 1997. MMWR Morb Mortal Wkly Rep. 1997;46:1061–1066. [PubMed] [Google Scholar]
- 17.Hamlin K, Fischer F. The synthesis of 5-hydroxytryptamine. J Am Chem Soc. 1951;73:5007–5008. [Google Scholar]
- 18.Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 19.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 20.Lesurtel M, Soll C, Graf R, et al. Role of serotonin in the hepato-gastroIntestinal tract: An old molecule for new perspectives. Cell Mol Life Sci. 2008;65:940–952. doi: 10.1007/s00018-007-7377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyce GM. Origin and metabolism of serotonin. J Cardiovasc Pharmacol. 1990;16(suppl 3):S1–S7. [PubMed] [Google Scholar]
- 22.Matsumoto-Miyai K, Yoshizumi M, Kawatani M. Regulatory effects of 5-hydroxytryptamine receptors on voiding function. Adv Ther. 2015;32(suppl 1):3–15. doi: 10.1007/s12325-015-0240-2. [DOI] [PubMed] [Google Scholar]
- 23.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 24.Chilmonczyk Z, Bojarski AJ, Pilc A, et al. Functional selectivity and antidepressant activity of serotonin 1A receptor ligands. Int J Mol Sci. 2015;16:18474–18506. doi: 10.3390/ijms160818474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbas D, DesGroseillers L, Castellucci VF, et al. Multiple serotonergic mechanisms contributing to sensitization in aplysia: Evidence of diverse serotonin receptor subtypes. Learn Mem. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raymond JR, Mukhin YV, Gelasco A, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Yang Q, Sun W, et al. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 28.Gutknecht L, Kriegebaum C, Waider J, et al. Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: Convergent data from Tph2 knockout mice. Eur Neuropsychopharmacol. 2009;19:266–282. doi: 10.1016/j.euroneuro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- 30.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am J Physiol. 1996;270:G778–G782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- 31.Pascual D, Alsasua A, Goicoechea C, et al. The involvement of 5-HT3 and 5-HT4 receptors in two models of gastrointestinal transit in mice. Neurosci Lett. 2002;326:163–166. doi: 10.1016/s0304-3940(02)00251-3. [DOI] [PubMed] [Google Scholar]
- 32.Alonso-Gordoa T, Capdevila J, Grande E. GEP-NETs update: Biotherapy for neuroendocrine tumours. Eur J Endocrinol. 2015;172:R31–R46. doi: 10.1530/EJE-14-0354. [DOI] [PubMed] [Google Scholar]
- 33.Pöll F, Lehmann D, Illing S, et al. Pasireotide and octreotide stimulate distinct patterns of sst2A somatostatin receptor phosphorylation. Mol Endocrinol. 2010;24:436–446. doi: 10.1210/me.2009-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulipano G, Stumm R, Pfeiffer M, et al. Differential beta-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–21382. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Li W, Kim HJ, et al. Characterization of somatostatin receptor expression in human pancreatic cancer using real-time RT-PCR. J Surg Res. 2004;119:130–137. doi: 10.1016/j.jss.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin J, Ajani J, Schirmer W, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol. 1999;17:600–606. doi: 10.1200/JCO.1999.17.2.600. [DOI] [PubMed] [Google Scholar]
- 38.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 39.Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:1556–1557. doi: 10.1056/NEJMc1409757. [DOI] [PubMed] [Google Scholar]
- 40.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 41.Modlin IM, Latich I, Kidd M, et al. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006;4:526–547. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72:244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 43.Butturini G, Bettini R, Missiaglia E, et al. Predictive factors of efficacy of the somatostatin analogue octreotide as first line therapy for advanced pancreatic endocrine carcinoma. Endocr Relat Cancer. 2006;13:1213–1221. doi: 10.1677/erc.1.01200. [DOI] [PubMed] [Google Scholar]
- 44.Schmid HA. Pasireotide (SOM230): Development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74. doi: 10.1016/j.mce.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Welin SV, Janson ET, Sundin A, et al. High-dose treatment with a long-acting somatostatin analogue in patients with advanced midgut carcinoid tumours. Eur J Endocrinol. 2004;151:107–112. doi: 10.1530/eje.0.1510107. [DOI] [PubMed] [Google Scholar]
- 46.Kvols LK, Oberg KE, O’Dorisio TM, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: Results from a phase II study. Endocr Relat Cancer. 2012;19:657–666. doi: 10.1530/ERC-11-0367. [DOI] [PubMed] [Google Scholar]
- 47.Öberg K. Neuroendocrine tumors of the gastrointestinal tract: Recent advances in molecular genetics, diagnosis, and treatment. Curr Opin Oncol. 2005;17:386–391. doi: 10.1097/01.cco.0000167739.56948.a9. [DOI] [PubMed] [Google Scholar]
- 48.Raderer M, Kurtaran A, Scheithauer W, et al. Different response to the long-acting somatostatin analogues lanreotide and octreotide in a patient with a malignant carcinoid. Oncology. 2001;60:141–145. doi: 10.1159/000055311. [DOI] [PubMed] [Google Scholar]
- 49.Chugani DC. α-Methyl-L-tryptophan: Mechanisms for tracer localization of epileptogenic brain regions. Biomarkers Med. 2011;5:567–575. doi: 10.2217/bmm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asnacios A, Courbon F, Rochaix P, et al. Indium-111-pentetreotide scintigraphy and somatostatin receptor subtype 2 expression: New prognostic factors for malignant well-differentiated endocrine tumors. J Clin Oncol. 2008;26:963–970. doi: 10.1200/JCO.2007.12.7431. [DOI] [PubMed] [Google Scholar]
- 51.Engelman K, Lovenberg W, Sjoerdsma A. Inhibition of serotonin synthesis by para-chlorophenylalanine in patients with the carcinoid syndrome. N Engl J Med. 1967;277:1103–1108. doi: 10.1056/NEJM196711232772101. [DOI] [PubMed] [Google Scholar]
- 52.Margolis KG, Stevanovic K, Li Z, et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63:928–937. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown PM, Drossman DA, Wood AJ, et al. The tryptophan hydroxylase inhibitor LX1031 shows clinical benefit in patients with nonconstipating irritable bowel syndrome. Gastroenterology. 2011;141:507–516. doi: 10.1053/j.gastro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin H, Cianchetta G, Devasagayaraj A, et al. Substituted 3-(4-(1,3,5-triazin-2-yl)-phenyl)-2-aminopropanoic acids as novel tryptophan hydroxylase inhibitors. Bioorg Med Chem Lett. 2009;19:5229–5232. doi: 10.1016/j.bmcl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Kim JJ, Wang H, Terc JD, et al. Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2015;309:G455–G465. doi: 10.1152/ajpgi.00299.2014. [DOI] [PubMed] [Google Scholar]
- 56.Kulke MH, O’Dorisio T, Phan A, et al. Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer. 2014;21:705–714. doi: 10.1530/ERC-14-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavel M, Hörsch D, Caplin M, et al. Telotristat etiprate for carcinoid syndrome: a single-arm, multicenter trial. J Clin Endocrinol Metab. 2015;100:1511–1519. doi: 10.1210/jc.2014-2247. [DOI] [PubMed] [Google Scholar]
- 58.ClinicalTrials.gov. NCT01677910 TELESTAR (Telotristat Etiprate for Somatostatin Analogue Not Adequately Controlled Carcinoid Syndrome). Available at https://clinicaltrials.gov/ct2/show/study/NCT01677910?term=telestar&rank=1. Accesed at 01/04/2016. [Google Scholar]
- 59.ClinicalTrials.gov. Telotristat etiprate - expanded treatment for patients with carcinoid syndrome symptoms (TELEPATH). NCT02026063. Available at https://clinicaltrials.gov/ct2/show/NCT02026063. Accessed January 4, 2016.
- 60.ClinicalTrials.gov. Telotristat etiprate for carcinoid syndrome therapy (TELECAST). NCT02063659. Available at https://clinicaltrials.gov/ct2/show/NCT02063659?term=NCT02063659&rank=1. Accessed January 4, 2016.
- 61.Wolin EM, Jarzab B, Eriksson B, et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075–5086. doi: 10.2147/DDDT.S84177. [DOI] [PMC free article] [PubMed] [Google Scholar]