Analysis of data from 29 neuroendocrine tumor patients receiving the combination of capecitabine and temozolomide found that, although adverse reactions were experienced, this regimen had activity and most patients seemed to tolerate this combination well.
Keywords: Neuroendocrine tumors, Chemotherapy, Capecitabine, Temozolomide
Abstract
Background.
Neuroendocrine tumors (NETs) are commonly treated with multimodality therapy. The combination of capecitabine and temozolomide (CAPTEM) has been suggested as a treatment option for patients with metastatic NETs. We present our experience with CAPTEM.
Methods.
Data on NET patients who were placed on CAPTEM and received at least one cycle were obtained from a Velos eResearch database. Response rate was calculated by RECIST 1.1. Overall survival and progression-free survival (PFS) were calculated by the Kaplan-Meier survival method.
Results.
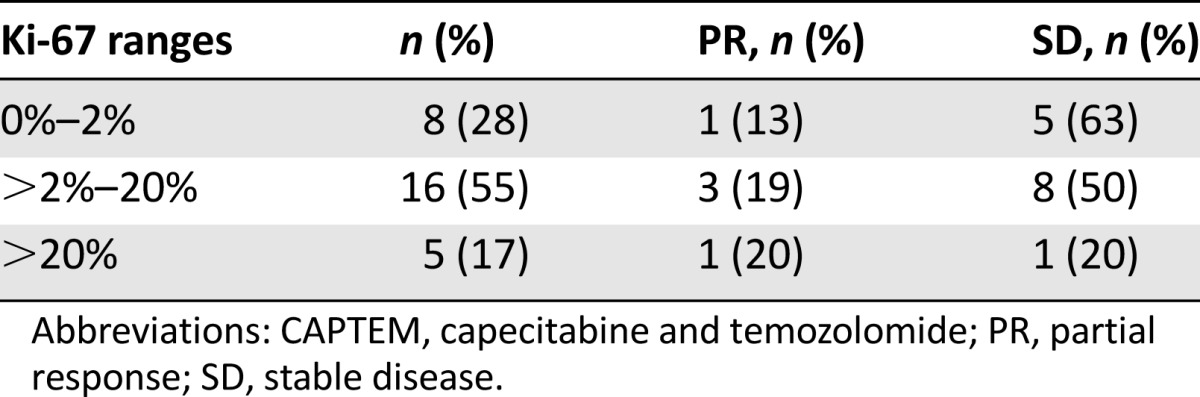
A total of 29 patients (17 male and 12 female) were included. Median age at CAPTEM initiation was 58 years (range: 26–77). Primary tumors included 9 small bowel (31%), 15 pancreas (52%), 3 lung (10%), and 2 rectum (7%). Median number of CAPTEM cycles was 8 (range: 1–55). Partial response occurred in 5 patients (5 of 29, 17%); 14 patients (14 of 29, 48%) had stable disease, and 10 patients (10 of 29, 34%) had progressive disease. A total of 3 (20%) and 5 (33%) pancreatic NETs experienced partial response and stable disease, respectively. A total of 2 (14%) and 9 (64%) nonpancreatic NETs experienced partial response and stable disease, respectively. Partial response was noted in 1 patient (13%) and stable disease in 5 patients (63%) with Ki-67 values of less than 2%. In patients with Ki-67 values of 2%–20%, partial response was noted in 3 (19%) and stable disease in 8 (50%). Partial response and stable disease were noted in 1 patient each (20%) with Ki-67 values greater than 20%. Median PFS was 12 months. Adverse reactions caused dose reductions in 24% of patients.
Conclusion.
Although adverse reactions were experienced, most patients tolerated this regimen. CAPTEM should be considered as a reasonable treatment option for metastatic NET patients.
Implications for Practice:
The role of chemotherapy in neuroendocrine tumors has evolved in recent years. The results of this study suggest that the combination of capecitabine and temozolomide provides an adequate treatment option and may prolong survival in patients with a wide variety of metastatic neuroendocrine tumors. Although prospective data are needed, this research adds to the abundance of retrospective experience with this combination that appears to show that capecitabine and temozolomide could potentially be an option for patients with advanced neuroendocrine tumors who have progressed on standard treatment.
Introduction
Neuroendocrine tumors (NETs) are uncommon malignancies, but are becoming increasingly more recognized [1, 2]. These tumors can occur throughout the body and are generally treated with surgical resection for curative intent if localized [1–3]. In those patients who experience recurrence or who present at advanced stages, treatment options can vary based on extent of disease, biochemical evaluation, the presence of octreotide or 131I-metaiodobenzylguanidine (MIBG) uptake, performance status, and patient and physician preference [4].
Treatment options in patients with metastatic neuroendocrine disease can generally be divided into four different modalities: surgical, liver-directed, radionuclide, or medical. Surgical therapy includes both cytoreduction and, in some cases, multivisceral organ transplantation [3, 4]. Liver-directed therapy includes bland, chemo-, and radioembolization [4, 5]. Radionuclide therapy options in patients with neuroendocrine tumors include peptide radionuclide receptor therapy or MIBG [4, 6]. Nearly all patients will have some form of medical treatment with a somatostatin analog in addition to either targeted therapy or chemotherapy at some point in their clinical course [7–9]. All of these options are considered when a patient initially presents for evaluation.
The role of chemotherapy in NETs has evolved in recent years. The use of steptozocin-based therapies has been the mainstay of treatment for both pancreatic and nonpancreatic NETs [10, 11]. This regimen is sometimes limited by its toxicity. More recently, the oral alkylating agent temozolomide has become a treatment option and has been used as both a single agent and also in combinations, such as thalidomide/temozolomide, bevacizumab/temozolomide, and capecitabine/temozolomide (CAPTEM) [12–16].
The CAPTEM regimen has been shown to have significant activity in pancreatic NETs. In a retrospective analysis of 30 pancreatic NET patients, Strosberg et al. demonstrated a 70% response rate and a median progression-free survival (PFS) of 18 months [17]. A similar retrospective analysis that included both pancreatic and nonpancreatic NETs with PFS of the entire cohort reported at 4.7 months showed pancreatic NETs having a PFS of 4.9 months versus 2.8 months for nonpancreatic NETs [18]. Most recently, a prospective phase II study looking at CAPTEM in both pancreatic and nonpancreatic NETs has closed. An interim analysis of 28 patients has shown an overall response rate of 43% and rate of stable disease of 54% [19]. The final results of this study are pending. This work will describe our findings with the use of CAPTEM in both pancreatic and nonpancreatic NETs.
Methods
Our institution (Louisiana State University/Ochsner Neuroendocrine Tumor Program) is a tertiary referral center that offers multidisciplinary care for patients with all types of NETs. Patients presenting to our clinic are entered into a secure, online Velos eResearch database (Velos, Inc., Fremont, CA, http://velos.com) for rapid identification for inclusion into analyses. This analysis included all NET patients that had at least one cycle of CAPTEM from November 2010 to June 2015. All patients had a histologically confirmed metastatic NET and were not candidates for surgical resection. Patients were included if they had measurable disease based on computed tomography (CT) or magnetic resonance imaging (MRI).
Dosing Regimen
The dosing of CAPTEM was as follows: capecitabine 750 mg/m2 by mouth twice daily on days 1–14 and temozolomide 200 mg/m2 by mouth once daily on days 10–14. Each cycle was 28 days long. Patients received monthly complete blood counts and a comprehensive metabolic panel. Each patient remained on this regimen for three cycles before repeat CT or MRI to assess disease response to treatment.
Patient Demographics
Baseline demographics, tumor characteristics, prior oncologic treatment, and Ki-67 proliferative indices were recorded for each patient. Side effect profile was extracted from patient records and categorized according to the Clavien-Dindo grading classification (grades I–IV). Radiographic response was determined by using RECIST 1.1. Patients who had incomplete records or were lost to follow-up were excluded from analysis. This study obtained appropriate dual institutional review board (IRB) approval from the Louisiana State University Health Sciences IRB and the Ochsner Clinical Foundation IRB.
Statistical Analysis
Rate of response (RR), progression-free survival (time from first administration of CAPTEM to documented disease progression, administration of alternative treatment, or death), and overall survival (OS) were used as endpoints of this study. PFS and OS were calculated by the Kaplan-Meier survival method. Overall survival rate 2 years from initiation of treatment was also calculated. Statistical analyses were performed by using MedCalc for Windows (Version 15.6.1; MedCalc Software, Ostend, Belgium, https://www.medcalc.org).
Results
We included 29 (17 male and 12 female) patients that were diagnosed with NETs and treated with a minimum of one cycle of CAPTEM. Median patient age at initiation of treatment was 58 years (range: 26–77). A total of 15 patients (52%) had pancreatic NETs, 9 patients (31%) had small bowel NETs, 3 patients (10%) had lung NETs, and 2 patients (7%) had rectal NETs. The majority of metastatic disease was located in the liver and lymph nodes (54% and 21%, respectively). A total of 17% of patients (6 of 29) had bone metastasis, and 8% (3 of 29) had metastasis to the lung. In 19 patients (66%), the tumors were well differentiated, in 2 (7%) they were moderately differentiated, and in 2 others (7%) theywere poorly differentiated. Tumor differentiation of 6 (21%) patients was unknown.
All patients had Ki-67 immunohistochemical stains performed on their biopsied tumors. Of these patients, 8 (28%) had Ki-67 values that were less than 2%, 16 (55%) had Ki-67 values from 2% to 20%, and 5 (17%) had Ki-67 values greater than 20%. Radiographic responses based on Ki-67 ranges are shown in Table 1.
Table 1.
Radiographic responses to CAPTEM treatment sorted by Ki-67 proliferative index ranges
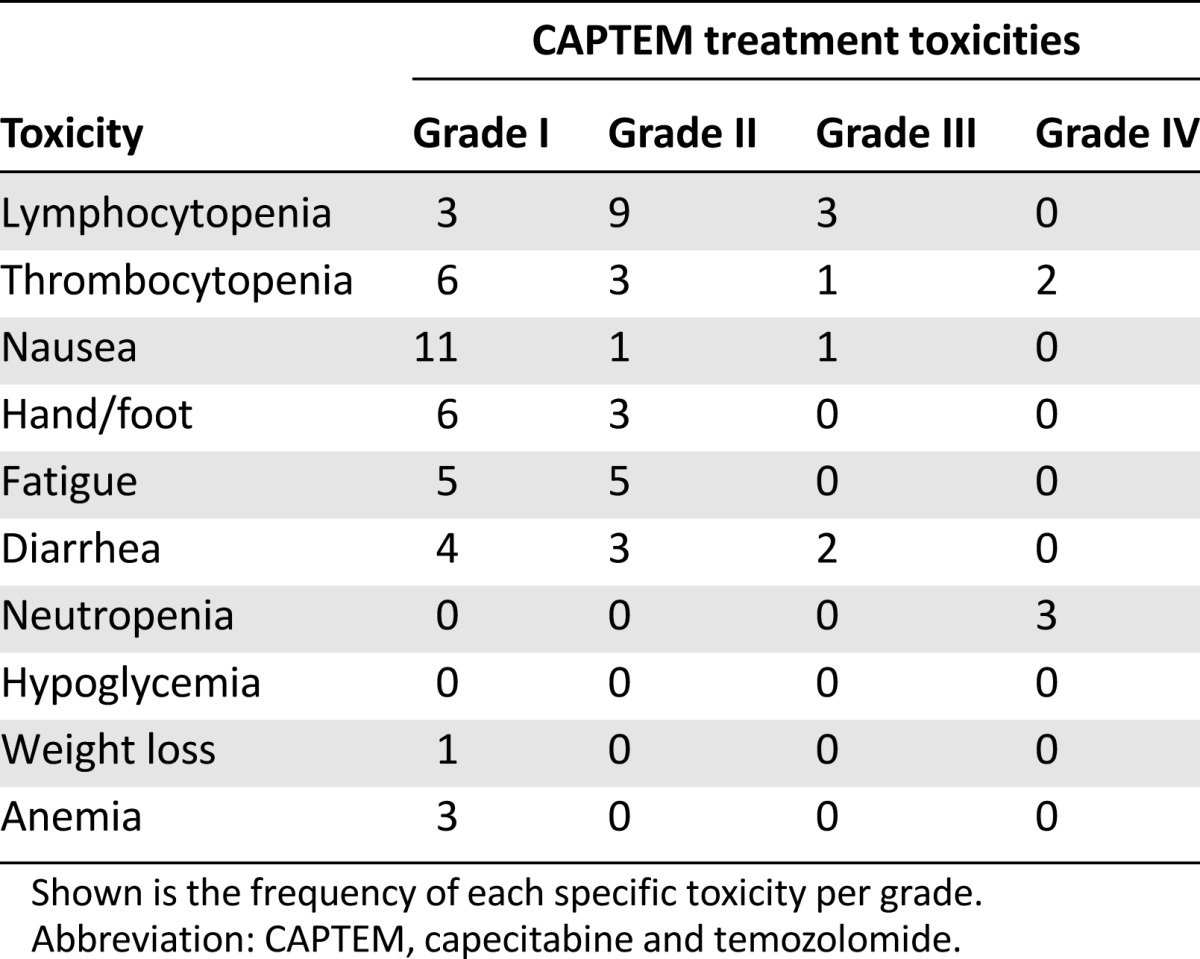
Three patients (10%) discontinued treatment because of adverse reactions. There were 5 grade IV toxicities, 7 grade III toxicities, 24 grade II toxicities, and 39 grade I toxicities. A total of 7 patients (24%) required a dose reduction owing to drug toxicity. Details of specific toxicities are listed in Table 2.
Table 2.
CAPTEM treatment toxicities stratified by Clavien-Dindo grades I–IV
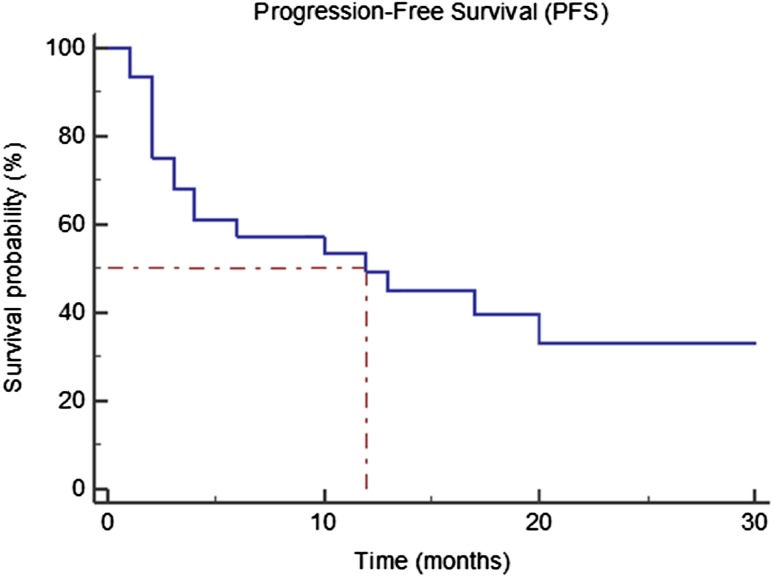
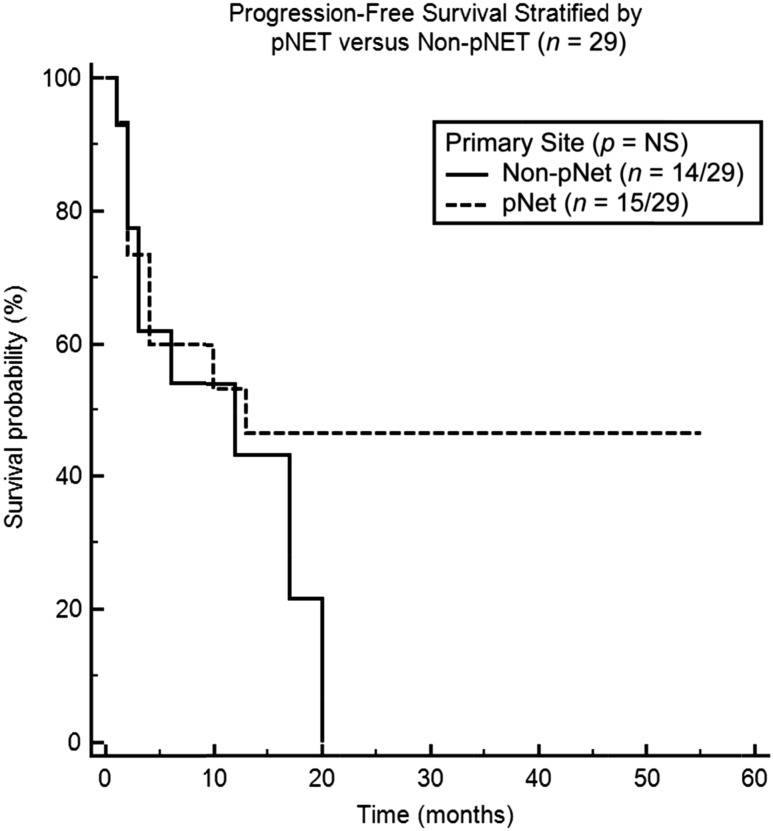
Four patients (14%) died during this study, all because of progressive disease. The median survival for the entire cohort was not reached. However, the Kaplan-Meier 2-year survival rate is 97%, estimated from the time of initial treatment with CAPTEM. The median PFS was estimated to be 12 months (95% confidence interval [CI]: 4–20 months) from initiation of CAPTEM, as shown in Figure 1. PFS stratified by pancreatic versus nonpancreatic NETs was not statistically significant (p > .05; Fig. 2). Median PFS for pancreatic NETs versus nonpancreatic NETs was 12 and 13 months, respectively.
Figure 1.
Progression-free survival (PFS) from the date of initiation of capecitabine and temozolomide treatment for the entire cohort. Median PFS for the entire cohort was 12 months.
Figure 2.
Progression-free survival (PFS) stratified by nonpancreatic versus pancreatic neuroendocrine tumors (NETs). Median PFS was 12 and 13 months for nonpancreatic NETs and pancreatic NETs, respectively.
Abbreviations: NS, not significant; pNET, pancreatic neuroendocrine tumor.
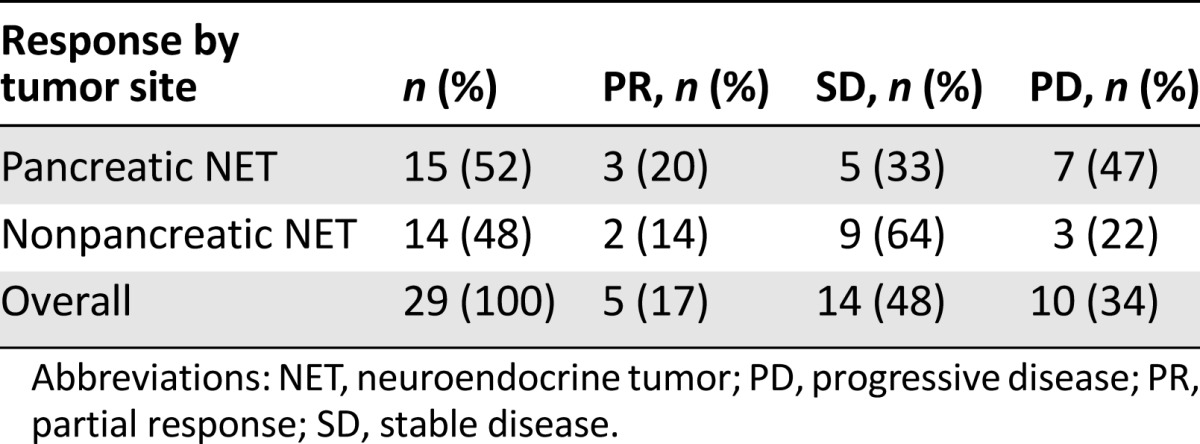
The median number of cycles for the entire cohort was 8, ranging from 1 to 60 cycles. Rate of response was calculated based on the patients’ imaging studies during their follow-up visit via RECIST 1.1. A total of 5 patients (17%) were considered to have partial response, 14 patients (48%) had stable disease, and 10 patients (34%) were noted to have progressive disease, as shown in Table 3. A total of 3 (20%) and 5 (33%) pancreatic NETs experienced partial response and stable disease, respectively. A total of 2 (14%) and 9 (64%) nonpancreatic NETs experienced partial response and stable disease, respectively.
Table 3.
Differences in primary tumor site response rates
Discussion
NETs are rare neoplasms that are becoming increasingly more recognized [1, 2]. This increase in recognition stems partly because of an improvement in diagnostic technologies, but also an amplified awareness of this rare malignancy. The current dogma of treatment with curative intent for NETs is surgical resection, but not all patients qualify as surgical candidates because of late stage at presentation or large tumor burden where resection is not feasible [1–3]. With these patients, options for disease management can be limited based on symptoms, biochemical markers, radiographic imaging, and treatment preference of the patient or physician.
We, and others, have shown that CAPTEM can be an adequate option for treatment of patients with metastatic NETs when surgery is no longer indicated. In our study, 17% of patients experienced a partial response and 48% had stability of disease, with a median PFS of 12 months. This benefit was seen across a broad group of primary tumor sites including pancreatic, rectal, small bowel, and lung. Partial responses were also seen in the subset of pancreatic and nonpancreatic NETs. However, these are small numbers, and these groups had a proportion of patients who still derived benefit.
This regimen was generally well-tolerated, with the median duration of CAPTEM treatment being eight cycles. The toxicities (shown in Table 1) that our patients experienced while on the CAPTEM regimen are not uncommon and have been described previously [17, 18, 20]. Although the majority of these complications were considered minor (Clavien-Dindo grades I and II), major complications (Clavien-Dindo grades III and IV) were experienced. The majority of patents were able to proceed without dose modification. However, 24% of our cohort did require dose adjustment because of toxicity.
Other retrospective studies have been performed using CAPTEM in patients with NETs. Strosberg et al. reported a series of 30 pancreatic NET patients who achieved median PFS of 18 months and response rate of 70% [17]. These patients were chemotherapy naïve, but 23% had received hepatic cytoreduction. In our cohort, patients with pancreatic NETs achieved a median PFS of 13 months and had a response rate of 20% and stable disease seen in 33%. This discrepancy is likely due to the fact that our cohort had a much more heavily pretreated population, with 76% of the entire cohort of patients having prior cytoreduction, targeted therapy, radionuclide therapy, liver-directed or chemotherapy, or a combination of these modalities. A similar retrospective study had shown first line CAPTEM in pancreatic NETS demonstrating a median PFS of 15.9 months versus 3.1 months when used in subsequent lines of treatment [18]. Dosing was the same in these studies.
Ki-67 proliferative index was measured in our patients, with the majority (16 of 29; 55%) having a Ki-67 from 2% to 20%. A total of 19% of these patients experienced a partial response, and 63% had stability of disease on the CAPTEM regimen. It is also interesting to note that 13% and 63% of patients experienced partial response and stable disease, respectively, with Ki-67 values less than 2%. Although only 5 patients with Ki-67 greater than 20% were included in our analysis, 20% of those had a partial response and 20% had stability of disease. This indicates that CAPTEM may be beneficial in high-grade tumors.
There is a wide variation in the clinical course and outcomes of patients with Ki-67 values greater than 20%. Some investigators suggest that a cutoff of 55% be used to determine whether patients should receive platinum-based chemotherapy [21]. In our high-grade cohort, the range of Ki-67 values was 30%–75%.
Our study is limited because of its retrospective design and small sample size. However, this report adds to other retrospective studies with similarly encouraging results in an understudied population of uncommon tumors. Another limitation of our study is the fact that the Louisiana State University/Ochsner Neuroendocrine Clinic is a tertiary referral center where patients are generally followed by referring oncologists and seen in our center at 3- to 6-month intervals. Nevertheless, we were still able to capture the majority of treatment-related adverse events and outcomes. In addition, patients who were lost to follow-up or did not have adequate records were excluded from this study.
A prospective phase II study examining the use of CAPTEM in a wide range of NETs has been closed to accrual. Final results of this study have yet to be published. However, an interim analysis has been presented with very encouraging results [19]. In this analysis, 28 of a planned 38 patients (including typical and atypical carcinoid, pituitary, pancreatic NET, and medullary thyroid tumors) had an overall RR of 43%, with stability of disease seen in 54%. The median PFS was greater than 22.2 months and ongoing OS greater than 29.1 months. The dose of the capecitabine was the same as that used in our study. However, the temozolomide portion was dosed twice daily. It is not known whether dosing daily or twice daily changes the efficacy of CAPTEM. We eagerly await final results of this trial.
Conclusion
Many questions remain concerning the medical management of metastatic NETs, such as the timing of initiation of treatment, ideal sequencing of treatments, equality of treatment of different types of NETs, the ideal use the Ki-67 index, and the benefits of novel therapeutic agents (i.e., immunotherapy). The results of our study suggest that CAPTEM provides an adequate treatment option and may prolong survival in patients with a wide variety of metastatic NETs. Although prospective data are needed, there is an abundance of retrospective experience in this combination that appears to show that CAPTEM could potentially be an option for patients with advanced NETs who have progressed on standard treatment.
Footnotes
For Further Reading: James C. Yao, Diane Reidy Lagunes, Matthew H. Kulke. Targeted Therapies in Neuroendocrine Tumors (NET): Clinical Trial Challenges and Lessons Learned. The Oncologist 2013;18:525–532.
Implications for Practice: With the approval of two new drugs, everolimus and sunitinib, for the treatment of patients with well-differentiated pancreatic neuroendocrine tumors, we are witnessing a shift from case series and single-arm studies toward prospective, randomized controlled clinical trials and evidence-based therapy in the neuroendocrine tumor field. However, the clinical development of these agents highlights the potential challenges awaiting other new drugs in this area. Focusing on the strengths, weaknesses, and limitations inherent in trial design can help identify pitfalls and potentially hasten the approval of drugs successfully developed to treat patients with neuroendocrine tumors.
Author Contributions
Conception/Design: Robert A. Ramirez, Aman Chauhan, J. Philip Boudreaux, Yi-Zarn Wang, Eugene A. Woltering
Provision of study material or patients: Robert A. Ramirez, Aman Chauhan, J. Philip Boudreaux, Yi-Zarn Wang, Eugene A. Woltering
Collection and/or assembly of data: Robert A. Ramirez, David T. Beyer, Aman Chauhan, J. Philip Boudreaux, Yi-Zarn Wang, Eugene A. Woltering
Data analysis and interpretation: Robert A. Ramirez, David T. Beyer
Manuscript writing: Robert A. Ramirez, David T. Beyer, Aman Chauhan
Final approval of manuscript: Robert A. Ramirez, David T. Beyer, Aman Chauhan, J. Philip Boudreaux, Yi-Zarn Wang, Eugene A. Woltering
Disclosures
Robert A. Ramirez: Novartis (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yalcin S, Öberg K. Neuroendocrine Tumours. Berlin, Germany: Springer; 2015. [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Boudreaux JP. Surgery for gastroenteropancreatic neuroendocrine tumors (GEPNETS) Endocrinol Metab Clin North Am. 2011;40:163–171, ix. doi: 10.1016/j.ecl.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25:458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 5.O’Toole D, Ruszniewski P. Chemoembolization and other ablative therapies for liver metastases of gastrointestinal endocrine tumours. Best Pract Res Clin Gastroenterol. 2005;19:585–594. doi: 10.1016/j.bpg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 7.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 8.Öberg K, Norheim I, Theodorsson E. Treatment of malignant midgut carcinoid tumours with a long-acting somatostatin analogue octreotide. Acta Oncol. 1991;30:503–507. doi: 10.3109/02841869109092409. [DOI] [PubMed] [Google Scholar]
- 9.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 10.Turner NC, Strauss SJ, Sarker D, et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br J Cancer. 2010;102:1106–1112. doi: 10.1038/sj.bjc.6605618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalique S, Meyer T. Streptozocin-based chemotherapy: Still a standard of care for neuroendocrine tumours? In: Raymond E, Faivre S, Ruszniewski P, editors. Management of Neuroendocrine Tumors of the Pancreas and Digestive Tract. Paris, France: Springer; 2014. pp. 65–75. [Google Scholar]
- 12.Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 13.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 14.Chan JA, Stuart K, Earle CC, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30:2963–2968. doi: 10.1200/JCO.2011.40.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine RL, Fogelman DR, Schreibman SM. Effective treatment of neuroendocrine tumors with temozolomide and capecitabine. J Clin Oncol. 2005;23(suppl):4216. [Google Scholar]
- 16.Lyons JM, 3rd, Abergel J, Thomson JL, et al. In vitro chemoresistance testing in well-differentiated carcinoid tumors. Ann Surg Oncol. 2009;16:649–655. doi: 10.1245/s10434-008-0261-z. [DOI] [PubMed] [Google Scholar]
- 17.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peixoto RD, Noonan KL, Pavlovich P, et al. Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non-PNETs. J Gastrointest Oncol. 2014;5:247–252. doi: 10.3978/j.issn.2078-6891.2014.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine RL, Gulati AP, Tsushima D, et al. Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol. 2014;32(suppl):179. [Google Scholar]
- 20.Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71:663–670. doi: 10.1007/s00280-012-2055-z. [DOI] [PubMed] [Google Scholar]
- 21.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]