This review evaluates and summarizes the most relevant medication screening tools for older patients with cancer and discusses key differences.
Keywords: Geriatrics, Oncology, Polypharmacy, Potentially inappropriate medications
Abstract
Inappropriate medication use and polypharmacy are extremely common among older adults. Numerous studies have discussed the importance of a comprehensive medication assessment in the general geriatric population. However, only a handful of studies have evaluated inappropriate medication use in the geriatric oncology patient. Almost a dozen medication screening tools exist for the older adult. Each available tool has the potential to improve aspects of the care of older cancer patients, but no single tool has been developed for this population. We extensively reviewed the literature (MEDLINE, PubMed) to evaluate and summarize the most relevant medication screening tools for older patients with cancer. Findings of this review support the use of several screening tools concurrently for the elderly patient with cancer. A deprescribing tool should be developed and included in a comprehensive geriatric oncology assessment. Finally, prospective studies are needed to evaluate such a tool to determine its feasibility and impact in older patients with cancer.
Implications for Practice:
The prevalence of polypharmacy increases with advancing age. Older adults are more susceptible to adverse effects of medications. “Prescribing cascades” are common, whereas “deprescribing” remains uncommon; thus, older patients tend to accumulate medications over time. Older patients with cancer are at high risk for adverse drug events, in part because of the complexity and intensity of cancer treatment. Additionally, a cancer diagnosis often alters assessments of life expectancy, clinical status, and competing risk. Screening for polypharmacy and potentially inappropriate medications could reduce the risk for adverse drug events, enhance quality of life, and reduce health care spending for older cancer patients.
Introduction
Inappropriate medication use is extremely common among older adults, with nearly 100,000 annual emergency department visits in the United States attributable to adverse drug events [1]. Numerous studies have discussed the importance of a comprehensive medication assessment in the general geriatric population [1–4]. However, only a handful of studies have evaluated inappropriate medication use in the geriatric oncology patient, and most are retrospective. Older adults with cancer require additional considerations compared with the general community-dwelling geriatric population. Older patients with cancer are more frail and have a higher comorbidity burden than those without a cancer diagnosis [5]. Additionally, both cancer and its treatments often require multiple supportive care medications, which may increase the risk for adverse effects for the older adult. Polypharmacy (PP) is a term with an evolving definition; its definition has been reviewed extensively elsewhere but is commonly described as five or more medications [2–8]. One study found that 80% of older patients with a recent diagnosis of cancer took more than five medications [6].
For the older patient with cancer, the definition of PP cannot simply denote quantity but must include consideration of appropriateness. For example, benzodiazepines and anticholinergic agents are consistently listed as potentially inappropriate medications (PIMs) on medication screening tools, but for the older cancer patient with chemotherapy-induced nausea and vomiting, these medication classes may be necessary. Moreover, oncology-specific drug-drug interactions, drug-nutrient interactions, complementary and alternative medicine (CAM), medication underuse, and adherence all need to be considered. The available medication screening tools each have advantages and disadvantages, and their applicability to an individual with cancer has not been well-elucidated.
Evaluating PP and PIMs can be overwhelming for the clinician. Integration of clinical pharmacy services into a multidisciplinary team improves outcomes in older adults with cancer [8–10]. Likewise, high rates of PP in this population have consistently been associated with impaired physical function, cognitive function, and falls; the effects of deprescribing and reducing PP to improve these outcomes have not been described extensively in the literature [6]. Use of medications is central to the care of cancer patients, and providers should be familiar with the screening tools available and their current pitfalls. The National Institute on Aging and National Cancer Institute have recently emphasized that medication use in older adults with cancer is lacking significant research and organization; therefore, an emphasis on this topic is essential [9, 10].
Currently, almost a dozen medication screening tools exist for the older adult. Each available tool can improve aspects of the care of older cancer patients, but no single tool has been developed for this population. This review evaluates and summarizes the most relevant medication screening tools for older patients with cancer and discusses key differences.
Beers Criteria 2015
The Beers criteria were first developed in 1991 as a tool to determine potentially inappropriate prescribing of medications for elderly patients. The criteria are based on expert consensus and extensive literature review. The criteria have been updated four times, most recently in 2015. Medications are classified into three categories: PIMs in older adults, PIMs in older adults due to drug-disease or drug-syndrome interactions that may exacerbate the disease or syndrome, and PIMs to be used with caution in older adults. The most recent edition includes sections on drugs to be avoided or modified based on kidney function and a list of drug-drug interactions associated with potential harm in the elderly [7].
Advantages
The Beers criteria are the most cited and widely used screening tool for PIM use in the elderly and are endorsed by the American Geriatrics Society (AGS). They contain a comprehensive list of PIMs and are well organized and accessible. Specific recommendations and quality of evidence are provided. The authors state in their review that some recommendations may not apply to patients in certain circumstances, such as palliative and hospice care. The newest edition includes supplemental documents discussing alternative medications to those deemed inappropriate and a concise summary of how to apply the updated criteria.
Disadvantages
The Beers criteria do not discuss drug-nutrient interactions, medication underuse, CAM, over-the-counter (OTC) medications, or medication adherence. Likewise, the tool lacks clear recommendations for appropriate dosing and dosing frequency. As new drugs come onto the market, the criteria require continuous updating. Furthermore, recent studies have shown that the Beers criteria screening tool lacks specificity for the prediction of clinically relevant outcomes and that other tools, such as the Screening Tool to Alert doctors to Right Treatment (START)/Screening Tool of Older Persons’ Prescriptions (STOPP) criteria, may be more beneficial [7, 8].
Patients With Cancer
The use of the Beers criteria in the general older adult population has been described, but only a few studies exist in the older cancer population [3, 4]. A recent study by Nightingale et al. retrospectively assessed the use of the Beers criteria for patients with cancer. The Beers criteria detected the highest prevalence of PP and PIMs compared with other screening tools. Further studies are needed to assess the validity of the Beers criteria for older cancer patients [11].
Although most of the Beers criteria can be applied to elderly patients with cancer, additional considerations apply. For example, diphenhydramine is typically contraindicated in elderly patients but is a common premedication for some anticancer therapies and may be appropriate in this setting. Other medications or medication classes included in the Beers criteria that may be appropriate for patients with cancer include atypical antipsychotics, tricyclic antidepressants (TCAs), benzodiazepines, metoclopramide, and nonsteroidal anti-inflammatory drugs (NSAIDs). The benefits of these medication classes in elderly cancer patients may outweigh the risks in certain clinical situations. For example, the addition of a low-dose TCA to an elderly patient's pain regimen may be beneficial for uncontrolled chemotherapy-induced neuropathy. Likewise, use of a benzodiazepine for anticipatory nausea may be appropriate. Lorazepam should be considered the benzodiazepine of choice because of minimally affected phase II hepatic metabolism in elderly patients; other benzodiazepines that are cleared via phase I hepatic metabolism (oxidation, sulfation) should be avoided because of downregulation of phase I hepatic enzymes in the elderly and the potential for drug accumulation [12]. Finally, the use of NSAIDs in elderly patients is often considered inappropriate because of risk for gastrointestinal bleeding, but in the setting of metastatic bone pain, an NSAID should be considered the drug of choice. Other drugs or drug classes present on the Beers criteria may be appropriate and should be evaluated clinically based on patient-specific factors.
Medication Appropriateness Index
The Medication Appropriateness Index (MAI), developed in 1992 by Hanlon et al., measures appropriate prescribing based on a 3-point rating scale of a 10-item list [3, 13]. The original tool was modified by Samsa et al. to provide a single score for each medication assessed. For each item, the medication can be deemed appropriate, marginally appropriate, or inappropriate on the basis of such criteria as indication, effectiveness, dosage, directions, drug-drug interactions, drug-disease interactions, medication duplication, and cost [13].
Advantages
Through the use of the MAI, multiple elements of drug therapy can be assessed simultaneously. The MAI can be applied to any medication and takes into account practical aspects of care, such as medication administration, duration of therapy, and cost. The MAI can be applied to medications taken on an as-needed basis, OTC medications, and CAM therapies. Studies have validated the MAI in both the ambulatory and inpatient settings and documented excellent intrarater and inter-rater reliability. The MAI provides an easy, stepwise approach to determine whether a medication is appropriate and engages clinical judgment with each therapy.
Disadvantages
The MAI does not address drug allergies, adverse drug reactions, adherence, or medication underuse. The MAI is resource-intensive, requiring on average 10 minutes per medication. Often, not enough information is available to apply all 10 items to each medication. Despite good intrarater and inter-rater reliability, clinical judgment can be subjective and result in inconsistent application. The MAI has not been extensively used for evaluation of patient outcomes as compared with the Beers criteria [3, 13].
Patients With Cancer
The MAI has primarily been studied in older veterans; currently no comprehensive study has used MAI for patients with cancer [13–17]. Despite the lack of data, the MAI could be used and modified for the cancer patient because the 10-item list evaluates relevant aspects of care for a patient with cancer. For example, several Danish studies have noted an increased prevalence of PP and PIMs in cancer patients in the 6 months preceding their cancer diagnosis. After diagnosis and anticancer treatment, many of these symptoms subsided but medications were continued inappropriately [14]. The MAI is an ideal tool for serially assessing for appropriateness, specifically indication (Panel 1), and would have been beneficial for the patients in the Danish studies as a quality indicator. The ideal interval for repeating the MAI is unknown and requires further evaluation.
The MAI can be used alongside other screening tools (e.g., Beers criteria) to fine-tune what is deemed appropriate without solely relying on clinical judgment. As portrayed in the example above, this tool may be beneficial to the cancer patient and is advantageous because it stimulates clinical judgment on a case-by-case as well as a serial basis.
Start/Stopp
START is a comprehensive tool used to determine appropriateness of initial prescribing of medications, whereas STOPP evaluates existing medication regimens. Both tools use evidence-based rules to avoid commonly encountered instances of PIMs and prescribing omissions. START consists of 22 criteria organized by physiologic system (cardiovascular, central nervous system, gastrointestinal, musculoskeletal, respiratory, urogenital, and endocrine). STOPP incorporates 65 criteria that are also organized by physiologic system, with additional focus on analgesics, duplicate drug classes, and drugs that increase fall risk. These screening tools were developed by a consensus panel of 18 experts [8–18].
Advantages
The START/STOPP criteria are effective at identifying PP targets for intervention [6]. These tools have been applied in the primary care, nursing home, and inpatient settings. Similar to the MAI, START/STOPP has excellent interrater reliability [8, 18]. START/STOPP assesses drug-drug and drug-disease interactions, duplicate therapies, and therapies that increase fall risk. The AGS supports the use of START/STOPP in conjunction with the Beers criteria. Although STOPP criteria do not capture all instances of inappropriate prescribing, they target common avoidable instances of inappropriate prescribing that can be deemed relevant to clinical practice. START/STOPP also has a section for recommended therapeutic alternatives [18].
Disadvantages
Similar to the Beers criteria, START/STOPP will need continuous updating as new literature is available and additional drugs come onto the market. Both of these tools need additional validation in different clinical settings, and further studies looking at long-term patient outcomes are needed. START/STOPP does not evaluate the use of CAM, OTC therapies, or medication underuse.
Patients With Cancer
Recently, a retrospective study compared STOPP, the Beers criteria, and the Healthcare Effectiveness Data and Information Set Drugs to Avoid in the Elderly (HEDIS DAE) in patients with cancer. The study found that STOPP and the Beers criteria have significant overlap, and, in concordance with the AGS, the authors offer additional evidence supporting the use of STOPP and Beers simultaneously [9]. Additional studies have applied and discussed the use of START/STOPP in the cancer population while noting limitations and the current lack of evidence [8, 11–18].
START/STOPP is particularly relevant to the cancer population in terms of therapy duration and duplication. As discussed previously for the Beers criteria, some medications listed as inappropriate may be indicated for cancer patients. For instance, a short course of an NSAID may be necessary for bone pain, but if a patient is taking more than one NSAID, this would be considered a duplication and inappropriate per the STOPP criteria. Constipation is a major issue for older cancer patients, particularly those exposed to certain chemotherapies. Moreover, common circumstances that may exacerbate constipation noted in the STOPP criteria include the use of a calcium-channel blockers, use of opioids for more than 2 weeks without a laxative, and the use of an anticholinergic bladder antispasmodic; deprescribing of inappropriate therapies or the addition of stimulant laxatives should be considered in these situations (Panel 2). Another relevant issue discussed in STOPP is the inappropriate use of proton-pump inhibitors (PPIs) at full dose for longer than 8 weeks. The use of PPIs is common in patients with gastrointestinal cancers, and, although efficacious in management of acid reflux, long-term PPI use has many pitfalls in the patient with cancer. For instance, long-term PPI use increases the risk for hypocalcemia, hypomagnesemia, osteoporosis, fractures, and infections [19].
Overall, managing adult cancer patients requires an individualized approach, and the situation-based assessment provided by START/STOPP helps to provide aspects of appropriate patient-centered care.
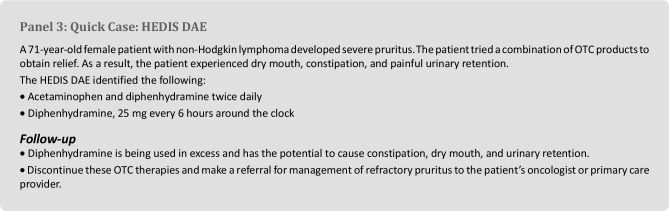
Hedis Dae
HEDIS DAE is a health care quality measure that was developed by the National Committee on Quality Assurance to monitor and evaluate prescribing patterns in older adult patients in the United States. HEDIS DAE is part of a series of clinical measure and domains used as quality indicators of health care. HEDIS measures are used by most insurance providers, including Medicare and Medicaid [11].
Overall, managing adult cancer patients requires an individualized approach, and the situation-based assessment provided by START/STOPP helps to provide aspects of appropriate patient-centered care.
Advantages
The HEDIS DAE is a concise and straightforward screening tool. The tool is separated by medication class and is organized in a similar manner as the Beers criteria. Sections of the list are comprehensive and include OTC combination products.
Disadvantages
The HEDIS DAE list is not all-inclusive, does not provide rationales for avoidance, and does not include drug-disease or drug-drug interactions. Moreover, it does not take into account underuse, adherence, or therapeutic duplication. Short-acting benzodiazepines, NSAIDs, clonidine, doxepin, doxazosin, and medications with anticholinergic effects are not listed as drugs to avoid, although these are clinically relevant medications in the elderly and should be assessed during a comprehensive medication assessment. Evidence supporting the use of the HEDIS DAE is lacking, and data derived from this tool may be limited for use in insurance claims and metrics.
Patients With Cancer
The HEDIS DAE criteria were recently compared with the Beers and STOPP criteria in cancer patients [11]. Even in cancer patients, the HEDIS DAE does not capture as many inappropriate or unnecessary medications as the Beers or STOPP criteria, but the HEDIS DAE does list inappropriate combination products that are commonly used in patients with cancer (Panel 3). On the basis of minimal evidence available in the cancer population, the HEDIS DAE may not be a valuable tool for these patients.
Improving Prescribing in the Elderly Tool
The Improving Prescribing in the Elderly Tool (IPET), also referred to as the “Canadian criteria,” is a list of 14 instances in which inappropriate prescribing may occur for an elderly patient. The tool was developed in 1997 by an expert panel in Canada and has been validated by two studies in acutely hospitalized elderly patients. IPET discusses use of medication classes, such as β-blockers, calcium-channel blockers, thiazide diuretics, TCAs, NSAIDs, and anticholinergic agents [4, 20, 21].
Advantages
IPET can be used as a quick reference for busy clinicians. Two studies prospectively evaluated the IPET tool in acutely hospitalized elderly patients; at least one inappropriate medication was identified from home medication lists in 12.5% and 22% of patients, respectively [20, 21].
Disadvantages
Similarly to the HEDIS DAE measure, the IPET is not all-inclusive and does not evaluate drug-disease or drug-drug interactions. The tool lists only 14 examples of inappropriate prescribing, and most recommendations are general and outdated. For example, the IPET recommends avoiding β-blockers in congestive heart failure (CHF), which may be appropriate for acute decompensation but not for long-term management of CHF. Additionally, 3 of 14 IPET criteria are tailored toward TCA use, resulting in duplicate recommendations. Data establishing a link between the use of IPET and the prevention of clinically relevant outcomes are lacking.
Patients With Cancer
Specific examples for the use of IPET in cancer patients are not evident on the basis of clinical studies, but IPET could be used in a similar manner as Beers or the STOPP criteria. Currently, no studies support the use of IPET in the cancer population; therefore, use cannot be recommended.
Zhan Criteria
The Zhan criteria tool is a modified version of the Beers criteria. The tool mimics the first section of the Beers criteria and includes medications that should always be avoided, medications that should rarely be used, and medications that are sometimes indicated. The criteria were originally developed to more concisely define the inappropriate medications listed on the Beers criteria. The two tools have been used concomitantly in various studies [22, 23].
Advantages
The Zhan criteria have two advantages. First, the criteria can be quickly reviewed by the clinician. Second, the criteria have been used effectively as a retrospective screening tool for medication review, specifically in population-based studies of PIMs and PP [23].
Disadvantages
The Zhan criteria tool has a low level of intrarater reliability [23]. The screening tool is not all-inclusive; it provides only information stated in the first section of the Beers criteria. As with many of the other screening tools, the Zhan criteria tool does not look at drug-drug interactions, drug-disease interactions, underuse, or CAM.
Patients With Cancer
Two studies in older adult cancer patients reference the use of the Zhan criteria. One study evaluated the effect of PIMs and PP on chemotherapy-related toxicity in older adults, specifically focusing on high-grade toxicities. While the tool helped quantitate PIMs and PP, the ability to account for clinically relevant outcomes in the study was absent [16, 23]. Given the limited data, the use of the Zhan criteria tool in older cancer patients cannot be recommended.
Assessing Care of Vulnerable Elders-3
The Assessing Care of Vulnerable Elders-3 (ACOVE-3) project defines a set of quality indicators for the medical care provided to older adults. This project was developed in collaboration with RAND Health and Pfizer in the early 1990s. This extensive document uses IF-THEN-BECAUSE statements to guide decision making for older adults who are at a high risk for death or functional decline during a 2-year period. Certain sections of the tool discuss medication use continuity or transitions of care; medication review for cognitively impaired patients; and more specific topics, such as the appropriate use of bowel regimens with opioid use. Twenty-four quality indicators guide medication use, and another 368 quality indicators guide chronic disease management [24].
Advantages
The ACOVE-3 project is evidence-based and guided by a national panel of geriatric clinician experts. The tool focuses not only on medications but also on common comorbidities in older adults. Each quality indicator is assessed by a content expert in the corresponding topic. The amount of information assessed is comprehensive and focuses on the process of care rather than adverse outcomes. This tool is designed to measure care at the population level (i.e., health system, health plan, or medical group) and can collect a large amount of data for benchmarking and quality improvement purposes.
Disadvantages
The ACOVE project has undergone three major revisions since 1999. A pitfall of the ACOVE-3 tool is the need for consistent updating. Its comprehensive nature precludes efficient evaluation of patients in clinic.
Patients With Cancer
The medication quality indicators of the ACOVE-3 tool have not been studied in patients with cancer. Nevertheless, the IF-THEN-BECAUSE method could be applied to develop a prescribing/deprescribing algorithm (Panel 4). Using an ACOVE-3 “backbone,” one could modify other existing screening tools and tailor specific recommendations to older patients with cancer.
Simply reducing the number of medications taken daily can increase appetite and decrease gastrointestinal symptoms. Stopping a medication that has no current clinical benefit can even increase a patient's quality of life and mood and add to a feeling of control over one's health.
A quick and easy algorithm using a condition or symptom-based approach may lead to medication discontinuation, decreased pill burden, decreased health care costs, and increased patient satisfaction. The same standard approach could be applied for such situations as recent falls, episodes of orthostasis, insomnia, new-onset delirium, difficulty swallowing, and other geriatric syndromes. The ACOVE-3 tool and its simple method could emphasize the need for a medication assessment in certain clinical situations.
Discussion
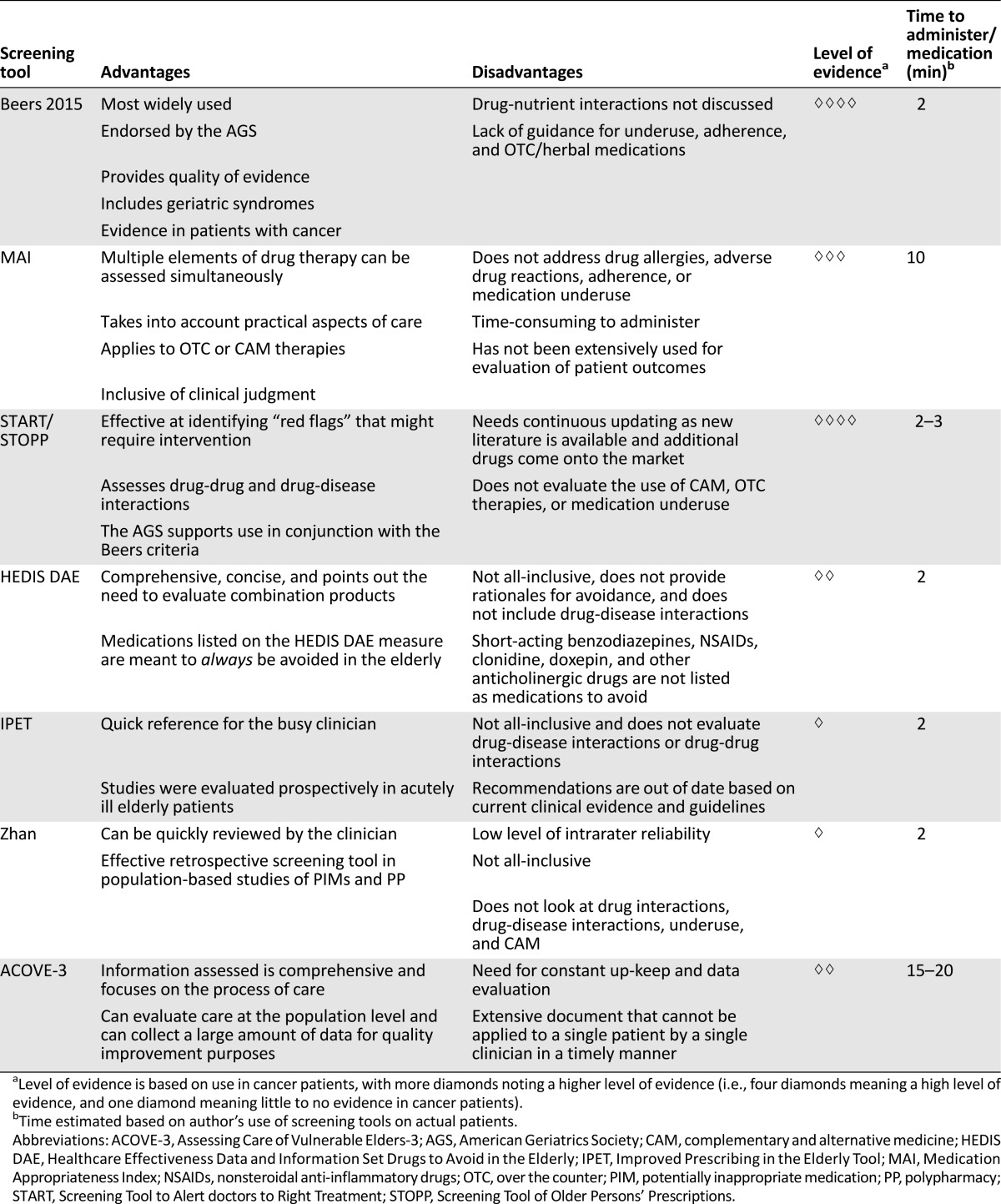
The assessment of medication appropriateness in elderly cancer patients is a complicated task. Several validated tools exist, but none have been studied extensively in the geriatric cancer population. Table 1 compares the screening tools reviewed here. Although no single tool or criterion discussed previously provides all the necessary guidance for a complete medication assessment, a compilation of certain tools and the use of clinical judgment can yield a comprehensive review. A considerable gap in knowledge currently exists regarding which tool or combination of tools is best for the patient with cancer and whether these tools affect clinical outcomes, including patient quality of life [22–29]. Likewise, no comprehensive prospective study has applied a cancer-patient specific screening tool to evaluate the effect of PP and PIMs on patient care.
Table 1.
Comparison of geriatric medication screening tools
All clinicians involved in the care of older patients with cancer should be familiar with medication assessment. Medication time-to-benefit, patient life expectancy, individual goals of care, and target of drug therapy all need to be considered when managing patients’ medications [30]. Looking beyond the typical PIMs in the elderly (anticholinergics, benzodiazepines) and using goals of care and overall benefit of the medication as deciding factors are recommended. For instance, no screening tool directly discourages the use of statins in older adults, although inappropriateness in elderly patients based on adverse effects and lack of time-to-benefit has been described [30–34]. Moreover, common symptoms reported by cancer patients, such as fatigue, decreased appetite, constipation, dyspepsia, dysgeusia, and early satiety, should each be considered drug side effects until proven otherwise. Simply reducing the number of medications taken daily can increase appetite and decrease gastrointestinal symptoms. Stopping a medication that has no current clinical benefit can even increase a patient's quality of life and mood and add to a feeling of control over one's health [30].
The pharmacist has been shown to be the most effective person to provide medication therapy management and medication reconciliation in the general population, but the ideal person to provide a medication assessment in older cancer patients is still unknown [31]. Interdisciplinary management is optimal, with teams consisting of a geriatric oncologist, oncology pharmacist, nurse, physical therapist, social worker, and nutritionist.
Conclusion
On the basis of this review and the evidence supporting certain tools in patients with cancer, the authors support the use of START/STOPP, the Beers criteria, and the MAI concurrently. The development of a standardized deprescribing algorithm that takes into account PP, PIMs, medication underuse, medication-condition matching, drug-drug interactions, CAM, patient performance status, and goals of care needs to be developed. The goal of deprescribing should be to minimize unnecessary medications and optimize adherence to medications that promote cancer treatment outcomes and/or improve quality of life. This deprescribing tool should be included in a comprehensive geriatric oncology assessment for every older patient with cancer. A possible limitation of this approach relates to restrictions on time and staffing resources in busy oncology practices. Although clinical pharmacists may be the best suited to apply and interpret a deprescribing algorithm, they are not directly available to patients in many practices. Moreover, a deprescribing algorithm is a potentially time-intensive intervention. With the advent of sophisticated electronic medical records (EMR) systems, however, it is now feasible to build and implement automated clinical decision support within some EMRs. In the future, such tools may help providers in prioritizing deprescribing and medication adjustments, and could be linked to patient education materials to enhance efficacy of the intervention. Finally, prospective studies are needed to evaluate such a tool to determine its feasibility and effect in older patients with cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception/Design: Andrew M. Whitman
Collection and/or assembly of data: Andrew M. Whitman
Manuscript writing: Andrew M. Whitman, Kathlene A. DeGregory, Amy L. Morris, Erika E. Ramsdale
Final approval of manuscript: Andrew M. Whitman, Kathlene A. DeGregory, Amy L. Morris, Erika E. Ramsdale
Disclosures
The authors indicated no financial relationships.
References
- 1.Price SD, Holman CD, Sanfilippo FM, et al. Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother. 2014;48:6–16. doi: 10.1177/1060028013504904. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi M, Scarcelli C, Niro V, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: A prospective study of 1756 patients. Drug Saf. 2008;31:545–556. doi: 10.2165/00002018-200831060-00009. [DOI] [PubMed] [Google Scholar]
- 3.Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium.”. JAMA. 2010;304:1592–1601. doi: 10.1001/jama.2010.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page RL, 2nd, Linnebur SA, Bryant LL, et al. Inappropriate prescribing in the hospitalized elderly patient: Defining the problem, evaluation tools, and possible solutions. Clin Interv Aging. 2010;5:75–87. doi: 10.2147/cia.s9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101:1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner JP, Shakib S, Singhal N, et al. Prevalence and factors associated with polypharmacy in older people with cancer. Support Care Cancer. 2014;22:1727–1734. doi: 10.1007/s00520-014-2171-x. [DOI] [PubMed] [Google Scholar]
- 7.The American Geriatrics Society 2015 Beers Criteria Update Expert Panel American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015 doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): Application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing. 2008;37:673–679. doi: 10.1093/ageing/afn197. [DOI] [PubMed] [Google Scholar]
- 9.Lichtman SM. Polypharmacy: Geriatric oncology evaluation should become mainstream. J Clin Oncol. 2015;33:1422–1423. doi: 10.1200/JCO.2014.60.3548. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171:1013–1019. doi: 10.1001/archinternmed.2011.215. [DOI] [PubMed] [Google Scholar]
- 11.Nightingale G, Hajjar E, Swartz K, et al. Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol. 2015;33:1453–1459. doi: 10.1200/JCO.2014.58.7550. [DOI] [PubMed] [Google Scholar]
- 12.Hilmer SN, Shenfield GM, Le Couteur DG. Clinical implications of changes in hepatic drug metabolism in older people. Ther Clin Risk Manag. 2005;1:151–156. doi: 10.2147/tcrm.1.2.151.62914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045–1051. doi: 10.1016/0895-4356(92)90144-c. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen TL, Herrstedt J, Friis S, et al. Polypharmacy and drug use in elderly Danish cancer patients during 1996 to 2006. J Geriatr Oncol. 2012;3:33–40. [Google Scholar]
- 15.Maggiore RJ, Gross CP, Hurria A. Polypharmacy in older adults with cancer. The Oncologist. 2010;15:507–522. doi: 10.1634/theoncologist.2009-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggiore RJ, Dale W, Gross CP, et al. Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: Effect on chemotherapy-related toxicity and hospitalization during treatment. J Am Geriatr Soc. 2014;62:1505–1512. doi: 10.1111/jgs.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosa D, Cai S, Gidmark S, et al. Potentially inappropriate medication use in veterans residing in community living centers: Have we gotten better? J Am Geriatr Soc. 2013;61:1994–1999. doi: 10.1111/jgs.12516. [DOI] [PubMed] [Google Scholar]
- 18.O’Mahony D, Gallagher P, Ryan C, et al. STOPP & START criteria: A new approach to detecting potentially appropriate prescribing in old age. Eur Geriatr Med. 2010;1:45–51. [Google Scholar]
- 19.Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci. 2011;56:931–950. doi: 10.1007/s10620-010-1560-3. [DOI] [PubMed] [Google Scholar]
- 20.O’Mahony D, Gallagher PF. Inappropriate prescribing in the older population: Need for new criteria. Age Ageing. 2008;37:138–141. doi: 10.1093/ageing/afm189. [DOI] [PubMed] [Google Scholar]
- 21.Naugler CT, Brymer C, Stolee P, et al. Development and validation of an improving prescribing in the elderly tool. Can J Clin Pharmacol. 2000;7:103–107. [PubMed] [Google Scholar]
- 22.Balducci L, Goetz-Parten D, Steinman MA. Polypharmacy and the management of the older cancer patient. Ann Oncol. 2013;24(suppl 7):vii36–vii40. doi: 10.1093/annonc/mdt266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan C, Sangl J, Bierman AS, et al. Potentially inappropriate medication use in the community-dwelling elderly: Findings from the 1996 Medical Expenditure Panel Survey. JAMA. 2001;286:2823–2829. doi: 10.1001/jama.286.22.2823. [DOI] [PubMed] [Google Scholar]
- 24.Wenger NS, Roth CP, Shekelle P. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(suppl 2):S247–S252. doi: 10.1111/j.1532-5415.2007.01328.x. [DOI] [PubMed] [Google Scholar]
- 25.Puts MTE, Costa-Lima B, Monette J, et al. Medication problems in older, newly diagnosed cancer patients in Canada: How common are they? A prospective pilot study. Drugs Aging. 2009;26:519–536. doi: 10.2165/00002512-200926060-00008. [DOI] [PubMed] [Google Scholar]
- 26.Lees J, Chan A. Polypharmacy in elderly patients with cancer: Clinical implications and management. Lancet Oncol. 2011;12:1249–1257. doi: 10.1016/S1470-2045(11)70040-7. [DOI] [PubMed] [Google Scholar]
- 27.Sokol KC, Knudsen JF, Li MM. Polypharmacy in older oncology patients and the need for an interdisciplinary approach to side-effect management. J Clin Pharm Ther. 2007;32:169–175. doi: 10.1111/j.1365-2710.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen TL, Hallas J, Land LH, et al. Comorbidity and polypharmacy in elderly cancer patients: The significance on treatment outcome and tolerance. J Geriatr Oncol. 2010;1:87–102. [Google Scholar]
- 29.National Comprehensive Cancer Network. Guidelines Version NCCN. 2.2015. Older Adult Oncology. Fort Washington, PA: National Comprehensive Cancer Network; 2015. [Google Scholar]
- 30.Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life. Arch Intern Med. 2006;166:605–609. doi: 10.1001/archinte.166.6.605. [DOI] [PubMed] [Google Scholar]
- 31.Avery M, Williams F. The importance of pharmacist providing patient education in oncology. J Pharm Pract. 2015;28:26–30. doi: 10.1177/0897190014562382. [DOI] [PubMed] [Google Scholar]
- 32.Saarelainen LK, Turner JP, Shakib S, et al. Potentially inappropriate medication use in older people with cancer: prevalence and correlates. J Geriatr Oncol. 2014;5:439–446. doi: 10.1016/j.jgo.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Kutner JS, Blatchford PJ, Taylor DH, Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern Med. 2015;175:691–700. doi: 10.1001/jamainternmed.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeBlanc TW, McNeil MJ, Kamal AH, et al. Polypharmacy in patients with advanced cancer and the role of medication discontinuation. Lancet Oncol. 2015;16:e333–e341. doi: 10.1016/S1470-2045(15)00080-7. [DOI] [PubMed] [Google Scholar]