Abstract
Cell migration depends on the dynamic formation and turnover of cell adhesions and is tightly controlled by actomyosin contractility and local Ca2+ signals. The divalent cation channel TRPM7 (Transient Receptor Potential cation channel, subfamily Melastatin, member 7) has recently received much attention as a regulator of cell adhesion, migration and (localized) Ca2+ signaling. Overexpression and knockdown of TRPM7 affects actomyosin contractility and the formation of cell adhesions such as invadosomes and focal adhesions, but the role of TRPM7-mediated Ca2+ signals herein is currently not understood. Using Total Internal Reflection Fluorescence (TIRF) Ca2+ fluorometry and a novel automated analysis routine we have addressed the role of Ca2+ in the control of invadosome dynamics in N1E-115 mouse neuroblastoma cells. We find that TRPM7 promotes the formation of highly repetitive and localized Ca2+ microdomains or “Ca2+ sparking hotspots” at the ventral plasma membrane. Ca2+ sparking appears strictly dependent on extracellular Ca2+ and is abolished by TRPM7 channel inhibitors such as waixenicin-A. TRPM7 inhibition also induces invadosome dissolution. However, invadosome formation is (functionally and spatially) dissociated from TRPM7-mediated Ca2+ sparks. Rather, our data indicate that TRPM7 affects actomyosin contractility and invadosome formation independent of Ca2+ influx.
Keywords: Adhesion, Ca2+ imaging, Ca2+ signaling, Invadosome, TIRF microscopy, TRPM7
1. Introduction
Cell migration is driven by the spatiotemporal regulation of cell adhesions and cytoskeletal contractility [1,2] and it is a prominent feature of cells throughout embryonic development as well as in adult life. Cell adhesions, such as focal complexes (FCs) and focal adhesions (FAs) link the extracellular matrix (ECM) to the actomyosin cytoskeleton and mediate traction at the leading edge of migrating cells, whereas their disassembly at the trailing edge allows cell detachment and net forward movement [3]. Local actomyosin contractility is a critical determinant of cell adhesion dynamics (i.e. formation, turnover and disassembly of adhesive structures) [1,4] and is under tight control of cellular signals such as the small GTPases Rho, Rac and Cdc42, [5,6] and Ca2+. For example, it was recently proposed that localized Ca2+ signals (termed Ca2+ sparks or flickers) control lamellipodium protrusion and retraction and determine the direction of cell migration [7,8].
While much of what we know about dynamic control of cell adhesions has been learned from studies on FCs and FAs, more recently invadosome-type adhesions have become the subject of intense study. Invadosomes is a collective term that covers both podosomes in monocyte-derived cells and the closely related invadopodia in tumor cells [9]. Like FAs, these structures connect the cellular cytoskeleton to the ECM and sense mechanical forces [10], but they distinguish themselves by the ability to degrade ECM-components so as to facilitate cell invasion [9,11]. Invadosome biogenesis and turnover are modulated by cellular tension [4,12,13], mechanical cues [14,15] and receptor agonists [16–18], but the molecular mechanisms that coordinate invado-some dynamics are not well understood.
The role of (local) Ca2+ signals in controlling invadosome dynamics has remained largely understudied [19]. Invadosomes contain a variety of proteins that regulate actomyosin remodeling, many of which are Ca2+-sensitive. In addition, several Ca2+-permeable channels have been identified in invadosomes [19–21], including TRPV2 and TRPM7, two members of the Transient Receptor Potential (TRP) family of cation channels. TRP channels act as scaffolds for the assembly of large multiprotein complexes at the membrane and many of them are involved in sensory transduction [22,23]. TRPM7 (TRP subfamily Melastatin, member 7) is implicated in mechanotransduction [7,24,25] which is particularly intriguing as it is a bifunctional protein that consists of a non-selective divalent cation channel fused to an alpha-kinase [26–28]. Substrates for the TRPM7 kinase include annexin-1 [29] and myosin II isoforms [20,30]. Moreover, TRPM7 was demonstrated to regulate actomyosin contractility, cell adhesion and cell migration in a variety of cell types [7,20,31–34]. We, for example, previously demonstrated that in neuroblastoma cells (N1E-115) TRPM7 upregulation causes actomyosin relaxation, concomitant with formation of invadosomes, whereas in breast cancer cells (MDA-MB-231) knockdown of TRPM7 induced a contractile phenotype, which was accompanied by an increase in FA numbers [20,34]. Additionally, TRPM7 was shown to mediate localized Ca2+ influx in response to mechanical stress, which is thought to steer directional cell migration [7]. TRPM7 may therefore act as a mechanosensor that guides Ca2+-mediated cytoskeletal rearrangements and cell adhesions. It is, however, unclear to what extent TRPM7-mediated Ca2+-sparks drive local alterations in cell adhesion dynamics, as it has been difficult to dissociate the role of TRPM7 in ignition of Ca2+ signals from its function as a signaling scaffold and a kinase.
Given its enrichment at invadosomes and its contribution to Ca2+ spark formation, we hypothesized that TRPM7 mediates localized Ca2+ signaling events that modulate invadosome dynamics. To test this notion, we developed a sensitive assay to detect Ca2+ sparks at high spatiotemporal resolution and studied the dynamics of TRPM7-mediated invadosomes in N1E-115 cells.
2. Materials and methods
2.1. Materials and constructs
Ionomycin and bradykinin were from Calbiochem-Novabiochem (La Jolla, CA, USA). Fura Red-AM, Oregon Green 488 BAPTA-1-AM, EDTA-AM, Pluronic F-127 and Phalloidin-Alexa-586 were from Invitrogen (Eugene, OR, USA). Salts were from Merck (Darmstadt, Germany) and Dulbecco's MEM, fetal calf serum, penicillin and streptomycin were obtained from Gibco BRL-Invitrogen (Paisley, Scotland). The monoclonal vinculin antibody, Alexa488-conjugated secondary antibodies and 2-aminoethyl diphenylborinate (2-APB) were from Sigma–Aldrich (St. Louis, MO, USA). The TRPM7 antibody was from Alomone Labs (Jerusalem, Israel). Polyethylenimine (PEI, Linear, Cat# 23966-2) transfection reagent was from Polysciences Inc. (Warrington, USA). Lifeact-eGFP/dsRed and actin-mRFP were kind gifts of Dr. Michael Sixt (Institute of Science and Technology, Klosterneuburg, Austria) and prof. Dr. Rudolf E. Leube (Institute of Molecular and Cellular Anatomy, RWTH Aachen University, Aachen, Germany), respectively. Full length mouse TRPM7 cDNA, wild type (WT) cloned into LZRS-neo was previously described [20].
2.2. Isolation of waixenicin-A
Polyps of Sarcothelia edmondsoni were collected by hand at a depth of 1–3 m in Kailua Bay (Oahu, Hawaii). The freeze-dried polyps were ground by mortar and pestle and percolated exhaustively with hexane. The hexane extract was dried under vacuum and fractionated by first normal phase then reversed phase HPLC to give pure waixenicin-A. The compound identity was established by NMR (in d6-benzene and d4-methanol) and LCMS, in comparison to in-house reference data [43].
2.3. Cell culture and transfection
N1E-115 mouse neuroblastoma cells stably overexpressing TRPM7-HA and empty vector control were generated by retroviral transductions, as described elsewhere [20]. Cells were seeded on 24-mm glass-coverslips in 6-well plates in DMEM supplemented with 10% FCS (D10F) and antibiotics. Transfections were with PEI transfection reagent at 1 μg DNA per well per construct. The medium was refreshed 12–16 h after transfection.
2.4. Intracellular Ca2+ determinations
For pseudo-ratiometrical Ca2+ recordings, cells on glass coverslips were incubated for 30 min in a 200 μl volume of D10F containing Fura Red-AM (37 μM), Oregon Green 488 BAPTA-1-AM (8 μM) and Pluronic F-127 (0.1%), followed by further incubation in 2 ml HEPES-buffered saline (HBS), pH 7.3, for at least 15 min. HBS contained 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES (pH 7.3) and 10 mM glucose. Coverslips were mounted on a Leica TCS SP5 confocal microscope and recordings were made at 37 °C in HBS. Excitation of Oregon Green-488 and Fura-Red was at 488 nm and fluorescence emission was detected at 500–550 nm and at >600 nm, respectively. All Ca2+ recordings are normalized by setting the response to ionomycin to 100%.
2.5. TIRF recording of Ca2+ spark activity
Cells were seeded on glass coverslips, transfected with actin-mRFP or Lifeact-dsRed where indicated, and cultured overnight in DMEM supplemented with 10% FCS and antibiotics. Cells were loaded simultaneously with the membrane-permeable fast Ca2+ indicator Oregon Green 488 BAPTA-1-AM (8 μM, Molecular Probes) and slow divalent chelator EDTA-AM (25 μM, Molecular Probes) according to the protocol detailed in the previous paragraph. Experiments were performed at 37 °C in HBS, pH 7.3.
Ca2+ sparks were imaged using a Leica AM TIRF MC microscope with a HCX PL APO 63x, 1.47 NA oil immersion lens. Excitation was at 488 nm and detection of fluorescence emission was by a QUAD/ET filter cube (Leica). Before each experiment, automatic laser alignment was carried out and TIRF penetration depth was set to 110 nm. Data were acquired for 100 s at 10 Hz frame rate and stored on disk.
TIRF time-series were subsequently processed with a custom-made analysis routine (macro) written for ImageJ 1.42 (NIH, USA) as described in detail in the text. The ImageJ macro is listed in Appendix A. Ca2+ spark activity over time was represented in heatmaps as detailed in the main text and in the legend to the figures. Briefly, individual calcium sparks were identified in time-lapse image series by thresholding and the resulting mask images were summed to create the single-image heatmap. Sites of recurrent (or prolonged) Ca2+ spark activity are termed Ca2+ hotspots. Each individual Ca2+ hotspot thus reflects the number of times the threshold (set to discriminate Ca2+ sparks from the background signal) was passed. For the 3-D representation of the heatmap, the ImageJ plugin ‘Interactive 3D surface plot’ is used to depict Ca2+ spark density as the height of the plot (http://rsbweb.nih.gov/ij/plugins/surface-plot-3d.html). For detailed analyses automated Ca2+ spark detection was optionally fine-tuned by manual control of e.g. threshold levels.
2.6. Immunofluorescent stainings and gelatin degradation
Cells grown for 24 h on glass coverslips were fixed for 10 min at room temperature in phosphate-buffered saline (PBS) and 4% paraformaldehyde. Subsequently, cells were permeabilized with 0.1% triton X-100 in PBS. Cells were incubated with mouse anti-vinculin (1:200) or rabbit anti-TRPM7 (1:500) for minimally 60 min followed by Alexa-488-conjugated anti-mouse or anti-rabbit Ig (1:400), respectively. F-actin was visualized by Alexa-568-phalloidin (0.1 μg/100 μl). The cells were mounted in mowiol and imaged with a TCS-SP5 confocal microscope.
For matrix degradation assays, fluorescent gelatin-coated coverslips were prepared using 100 μg/ml gelatin-Oregon Green 488 (Invitrogen, Eugene, OR, USA). Poly-l-lysine coated coverslips were overlayed with a drop of gelatin-Oregon Green 488 for 30 min at room temperature, followed by cross-linking in ice-cold 0.5% gluteraldehyde. Using 5 mg/ml NaBH4, free gluteraldehyde reactive chains were quenched. Subsequently, coverslips were sterilized in 70% EtOH, washed with PBS and D10F, before N1E-115/TRPM7 cells (50,000) were seeded and incubated for 12–24 h at 37 °C and 5% CO2. Finally, cells were processed as above to visualize the F-actin-dense core of invadosomes with Alexa-568-phalloidin (0.1 μg/100 μl). Typically, in this assay, focal sites of gelatin degradation appear as black spots as a result of secretion of metalloproteases at invadosomes.
2.7. Assaying cell shape and invadosomes
To study the effect of waixenicin-A on cell adhesion and spreading, cells (50,000/well) were seeded on 6-well culture dishes in D10F containing either vehicle (methanol) or waixenicin-A and assayed by time lapse microscopy using a Zeiss Axiovert or Leica Differential Interference Contrast (DIC) microscope for 2 h at 37 °C and 5% CO2. Experiments were performed in 8-fold and cell adhesion and spreading were judged from stored data. Analysis was by detecting mean cell surface using ImageJ software.
To study the acute effects of treatment with waixenicin-A, cells were grown on glass coverslips for 24 h in D10F, mounted and transferred to a Leica TCS SP5 confocal microscope and maintained at 37 °C and 5% CO2. Cells were imaged at two-minute intervals for up to 2 h. After a baseline recording (mock-treatment; methanol) of 15–30 min, waixenicin-A was added from a concentrated stock solution. The effect of treatment on number and turnover of invadosomes was assessed in cells expressing Lifeact-eGFP by counting. The effect of extracellular Ca2+ chelation by BAPTA on invadosomes was tracked in Lifeact-eGFP-positive cells for an extended period of time (>20 h). For these experiments, baseline recording (mock treatment) was 3 h and image interval was 3 min.
To determine whether chelation of intracellular Mg2+ by EDTA-AM treatment would affect invadosome characteristics (formation, architecture, dissolution etc.), Lifeact-eGFP cells were loaded as for Ca2+ spark experiments (see above) and cells were subsequently imaged at 3 min intervals for >20 h.
2.8. Statistics
The distribution of number of Ca2+ hotspots per cell appeared skewed to the left and was therefore analyzed by the non-parametric Mann–Whitney U-test. Continuous data that did approximate a Gaussian distribution were analyzed by the appropriate Student's t-test. Unpaired and paired categorical data were analyzed by the Fischer's exact test and the McNemar's test, respectively.
3. Results
3.1. TRPM7 and invadosomes
TRPM7 is expressed in most, if not all, cell types [26,27,35]. To directly test for a role of TRPM7 in the generation of Ca2+ sparks and their contribution to invadosome formation, we used a previously characterized variant of mouse N1E-115 neuroblastoma cells transduced with HA-tagged mouse TRPM7 to stably express TRPM7 up to ~3-fold of endogenous levels [20,36]. The vast majority of these N1E-115/TRPM7 cells contain invadosomes at the ventral plasma membrane (‘central invadosomes’), underneath the nucleus (Fig. S1A, left panel). In a subset of the cells, we also observed prominent and very distinct invadosomes at the cell periphery (‘peripheral invadosomes’) that typically are larger in size than the central ones ([20] and Fig. S1A, right panel). Both types of invadosomes in N1E-115/TRPM7 cells are proteolytically active, as evidenced by the focal degradation of fluorescently labeled gelatin (Fig. S1B). As reported earlier, TRPM7 localizes to peripheral invadosomes ([20] and Fig. S1C and D), where it may affect invadosome formation, turnover or function, although this notion requires further evidence.
3.2. Ca2+ spark visualization
Local fast Ca2+ alterations such as Ca2+ sparks (for terminology see [37]) are usually hard to detect because they typically have a minor amplitude. Moreover, both free Ca2+ and dye-bound Ca2+ rapidly diffuse through the cytosol, quickly smoothing out any local elevations in free Ca2+. One established method to detect Ca2+ sparks is to load cells with both a fast (binding rate of a few ms; [38]), low-affinity BAPTA-based Ca2+ indicator and with an excess of the slow (binding rate ~1 s), high-affinity Ca2+ chelator EGTA [39–41]. Thus, local high Ca2+ concentrations at entry sites rapidly light up the indicator, whereas spreading of the signal beyond a few micrometers is prevented by the slowly acting EGTA buffer. Furthermore, we used Total Internal Reflection Fluorescence (TIRF) microscopy to isolate local Ca2+ signals in the basal membrane from the bulk cytosolic signal at distances larger than ~200 nm from the coverslip.
We initially used membrane permeable AM-esters of the fast, low-affinity Ca2+ indicator Fluo-5F [Kd (Ca2+) = 2.3 μM] and EGTA to load the cells, but failed to observe local or global fluctuations in fluorescence over time (data not shown). Fluo-5F, however, also failed to report the TRPM7-dependent sustained plateau phase of Ca2+ influx that typically follows the large Ca2+ peak from internal stores after stimulation with bradykinin (BK) in these cells ([36] and Fig. S2A). The sustained increases in [Ca2+]i upon TRPM7 opening are small and hence likely below the detection limit of Fluo-5F. In the next experiments we therefore used the high-affinity Ca2+ indicator Oregon Green 488 BAPTA-1-AM which we used to study TRPM7-mediated Ca2+ influx in our previous study ([36] and Fig. S2B). Again, however, only very minor fluorescence fluctuations were observed in these TIRF time-lapses.
The activity of TRPM7 channels depends strongly on [Mg2+]i [27] and lowering intracellular free Mg2+ by loading cells with the diva-lent chelator EDTA-AM augments both TRPM7 currents as detected by perforated patch clamp and Ca2+ influx as recorded by Ca2+ fluorometry in intact cells [36]. Strikingly, when we used EDTA-AM instead of EGTA-AM we readily observed Ca2+ sparks as small, localized and short-lived changes in fluorescence intensity in a large fraction of the cells (Video 1). We therefore adopted the latter conditions for the remainder of our experiments.
3.3. Automated background correction and Ca2+ spark detection
Initial results revealed that Ca2+ sparks were of low amplitude, whereas the background signal appeared non-uniform and showed slow fluctuations, due to variations in the TIRF illumination or focus (Video 1, Fig. 1A and B and Fig. S3A). This complicated detailed characterization and we therefore set out to optimize visualization and characterization by automating Ca2+ spark detection with ImageJ software.
Fig. 1.
EDTA-AM pretreatment unveils Ca2+ sparks in N1E-115/TRPM7 cells. (A) N1E-115/TRPM7 cells loaded with Oregon Green 488 BAPTA-1-AM and EDTA-AM were imaged for 100 s at 10 Hz frame rate by TIRF microscopy. (a) Left column: three examples of unprocessed images; middle column, images corrected by subtraction of a static background (i.e. the minimum intensity of the entire time-series); right column, images processed by the running background correction as detailed in the text. Note the normalization of cellular fluorescence signal and the much improved visualization of Ca2+ sparks in this column. (b) Intensity profiles taken along the dashed lines in each of the panels in a. Note that the uneven fluorescence present in the unprocessed images is effectively normalized by the running background correction (open arrows) enabling clear detection of Ca2+ sparks (closed arrows). (B) Representative Ca2+ traces for two ROIs (see C) with Ca2+ spark activity and two corresponding background (*) ROIs. Left column: in unprocessed traces Ca2+ sparks appear on a noisy background that shows local slow fluctuations (compare e.g. ROI-1 and ROI-2). High-amplitude Ca2+ sparks in ROI-1 can be easily thresholded but low-amplitude signals in ROI-2 are buried in background. The running background correction (column 3) but not the static correction (column 2) eliminates the fluctuations sufficiently to allow discrimination of low-amplitude signals (ROI-2) by setting a single detection threshold (gray dashed lines in the Ca2+ traces). Right column: zoom-in of the data in the gray boxes in the third column, showing detected Ca2+ sparks (*). (C) Left: input image; middle: 2-D heatmap of Ca2+ hotspots; right: 3-D representation of the heatmap emphasizing existence of hotspots of spark activity. Pseudocolors depict the number of frames in which [Ca2+]i exceeded the detection threshold. Note that Ca2+ hotspots are predominantly found at the cell periphery. Dashes indicate cell outlines. Scale bar = 20 μm.
First, we corrected the temporal background fluctuations by employing a running-background correction routine in which the background is updated dynamically in the time-series. A simple running-background correction in which frame i – 1 serves as the background for frame i does not suffice because it would effectively truncate all Ca2+ sparks that last longer than one frame. We therefore designed a running background correction in which the background signal for frame i is calculated from the (pixelwise) minimal value of two frames before (i – 15 and i – 5) and two after (i + 5 and i + 15) that image. The range of ±5 and ±15 frames from image i was experimentally optimized to be close enough to accurately reflect any slow fluctuations in background and yet wide enough to minimize the chance that Ca2+ sparks would be present in all four background frames. Of several background correction algorithms tried, this one cleaned up the fluorescence signal most effectively and it emphasized only those intensity changes that corresponded to true Ca2+ sparks (Fig. 1A and B). In addition, we applied a 19 × 19 square kernel smoothing filter for visualization (but not for measurement) purposes. The line profiles and Ca2+ traces of selected Ca2+ sparks in Fig. 1A and B as well as the processed time-series in Video 2 and Fig. S3C illustrate the remarkable effectiveness of our running background-correction routine.
We next automated detection of Ca2+ sparks by applying a threshold that effectively segmented out Ca2+ sparks in the smoothed, background-corrected time series, yielding a binary mask (Video 3 and Fig. S3D and E). The spatial distribution and activity of Ca2+ sparks over time was finally captured in a single ‘heatmap’ by summing all thresholded mask images of the time-series. Thus, the heatmap reflects the number and localization of Ca2+ hotspots, with a single hotspot reporting the recurrence and duration of Ca2+ sparks (i.e. the number of frames a spark is detected in the series), but not their amplitude. Interestingly, this heatmap representation clearly highlighted subcellular locations with intense Ca2+ spark activity, that we will call ‘Ca2+ hotspots’ (Fig. 1C). Often, hotspots were most prominent at the cell periphery where most reorganization of the cytoskeleton occurs. Time courses of individual Ca2+ spark activity that were determined from regions of interest (ROI) in processed image stacks revealed that Ca2+ spark duration was generally highly variable, lasting between 0.1 s and ~5 s (median ~0.4 s), whereas spark amplitude (ΔF/F) averaged 23% ± 12% (N = 54 ROIs and 146 sparks in 10 cells).
In conclusion, we worked out an image analysis routine to reliably and sensitively detect Ca2+ sparks. See Appendix A for a listing of the ImageJ macro, which will be available upon request.
3.4. Ca2+ sparks depend on Ca2+ influx and TRPM7 activity
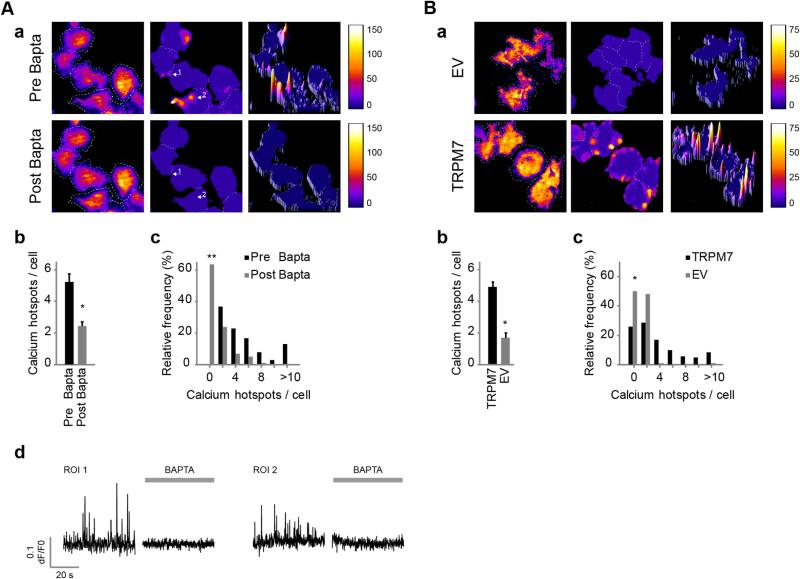
Ca2+ signals may originate from either influx through ion channels in the plasma membrane or from internal stores. We first addressed whether Ca2+ sparks in N1E-115/TRPM7 cells require a plasma membrane component. Removal of extracellular free Ca2+ by chelation with BAPTA (4–8 mM) rapidly inhibited Ca2+ sparks (Fig. 2A), evidenced by the complete silencing of a significant proportion of active cells (63.4% decrease, n = 101, ). The remaining cells showed a large decrease in number of Ca2+ hotspots per cell (53.6% ± 5.3% decrease, n = 101 cells [pre] and n = 37 cells [post], p < 0.001).
Fig. 2.
Ca2+ sparking requires Ca2+ influx and correlates with TRPM7 expression levels. (A) N1E-115/TRPM7 cells loaded with Oregon Green 488 BAPTA-1-AM and EDTA-AM were imaged before and after the application of BAPTA. (a) Left: input image; middle and right, 2-D and 3-D heatmaps, respectively. Representative heatmaps of Ca2+ spark formation over time before (top) and after (bottom) application of BAPTA are depicted. For interpretation of heatmaps, see the legend of Fig. 1C and the text. (b) Average number of Ca2+ hotspots per cell before and after application of BAPTA (n = 101 [pre] and n = 37 [post], p < 0.001 versus pre; Mann–Whitney U-test, two-tailed). (c) Frequency histogram (n = 101, versus pre, McNemar's test for paired proportions, two-tailed). (d) Ca2+ spark traces of representative Ca2+ hotspots (arrows in a) before (left) and after (right) BAPTA treatment. (B) N1E-115/TRPM7 cells were compared to their empty-vector controls with 3-fold lower TRPM7 expression. (a) Representative heatmaps of Ca2+ spark activity. (b) Mean number of Ca2+ hotspots per cell that showed Ca2+ sparking (n = 58 [N1E-115/EV] and n = 202 [N1E-115/TRPM7], p < 0.001; Mann–Whitney U-test, two-tailed). (c) Frequency distribution of sparking activity (n = 116 [N1E-115/EV], and n = 272 [N1E-115/TRPM7], p < 0.001; Fisher's exact test, two-tailed). Data in this figure represent mean ± SEM of at least 5 independent experiments. *p < 0.001 and **p < 0.0001.
To determine whether TRPM7 is the membrane channel that mediated Ca2+ sparks we initially established that its expression levels correlate with Ca2+ spark activity (Fig. 2B). Since TRPM7 knockdown via RNA interference severely affects cell viability in this cell system (as well as many others [42,43]; and data not shown), we alternatively compared N1E-115/TRPM7 cells to the empty-vector control cells (‘N1E-115/EV’) that have 2–3 times lower expression of TRPM7 [20]. Indeed, N1E-115/EV cells appeared much less active, as both the percentage of cells that display Ca2+ sparks (N1E-115/TRPM7 = 74%, n = 272; N1E-115/EV = 50%, n = 116, p < 0.001) and the number of Ca2+ hotspots per active cell (1.69 ± 0.31 hotspots per control cell, n = 58, versus 4.9 ± 0.32 hotspots per TRPM7 overexpressing cell, n = 202, p < 0.001) were strongly decreased.
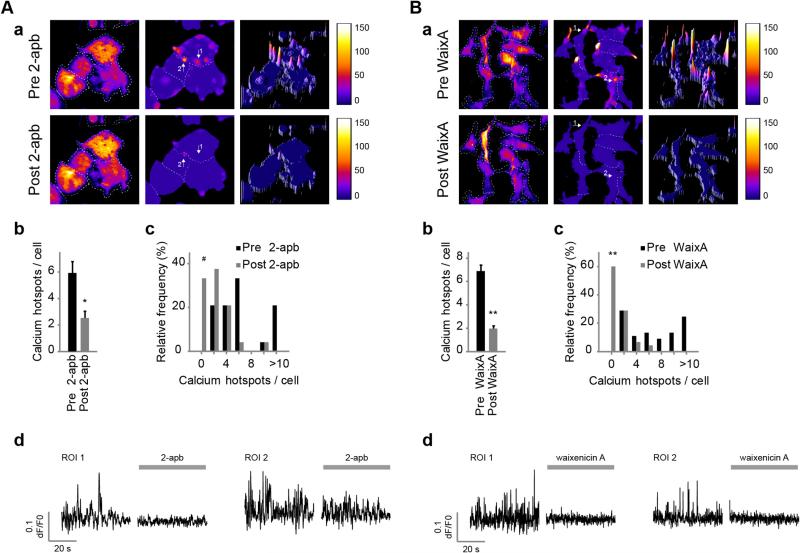
Furthermore, treatments known to affect TRPM7 permeation also affected Ca2+ sparking in N1E-115/TRPM7 cells. For example, application of 2-aminoethyl diphenylborinate (2-APB, 100 μM), which rapidly inhibits macroscopic TRPM7 Ca2+ signals ([36] and Fig. S4B), similarly abolished Ca2+ spark activity (Fig. 3 A and Video 4), decreasing Ca2+ hotspots by 57.2% ± 8.5% (n = 24 cells [pre] and n = 17 cells [post], p < 0.001) and completely preventing Ca2+ spark formation in 33.3% of previously active cells (n = 24, p = 0.013). In addition, increasing the extracellular concentration of Mg2+ to supraphysiological levels (10–20 mM) completely prevented Ca2+ spark formation in a significant proportion of previously active cells (37.2% decrease, n = 86, versus pre-MgCl2; McNemar's test for paired proportions, two-tailed) and diminished Ca2+ hotspots in the remainder of cells within several minutes post application (n = 86 [pre] and n = 65 [post], p = 0.123; Mann–Whitney U-test, two-tailed). Hence, our data indicate that Ca2+ influx through the TRPM7 pore underlies Ca2+ spark activity in N1E-115/TRPM7 cells.
Fig. 3.
Ca2+ sparks are mediated by TRPM7. Application of (A) 2-APB (100 μM) and (B) waixenicin-A (3–10 μM) inhibit TRPM7 and significantly reduce Ca2+ spark formation. (a) Heatmaps of Ca2+ sparking before (top) and after (bottom) application of inhibitors. (b) Number of Ca2+ hotspots per active cell (2-APB: n = 24 [pre] and n = 17 [post], p < 0.001; and waixenicin-A: n = 45 [pre] and n = 20 [post], ; Mann–Whitney U-test, two-tailed). (c) Frequency distribution of number of hotspots per cell before and after application of inhibitors (2-APB: n = 24, p < 0.01; and waixenicin-A: n = 45, ; McNemar's test for paired proportions, two-tailed). (d) Potent inhibition of Ca2+ spark activities at hotspots (indicated with arrows in a) following addition of 2-APB (see also Video 4) and waixenicin-A. Note that inhibition by waixenicin-A required preincubation for a few minutes. Data are mean ± SEM of at least 5 independent experiments. #p < 0.01, and **p < 0.0001.
3.5. Waixenicin-A blockage of Ca2+ spark activity
While 2-APB rapidly blocks Ca2+ influx through TRPM7, it also is known to affect other Ca2+ channels including ICRAC and IP3 receptors. We therefore turned our attention to waixenicin-A, a metabolite isolated from extracts of the soft coral S. edmondsoni, which potently and specifically inhibits TRPM7 channels [44,45]. Although it is currently not clear whether this is due to pore blockage or perhaps through other mechanism(s) that involve binding of the compound to TRPM7, waixenicin-A is, to date, considered the most specific inhibitor of this channel. In line with these reports, pre-incubation with waixenicin-A completely prevented the characteristic TRPM7-dependent sustained Ca2+-influx phase upon BK-stimulation, as illustrated by Ca2+ fluorometry experiments (Fig. S4C). Waixenicin-A treatment also strongly suppressed Ca2+ sparking (Fig. 3B), apparent from both a large drop in the fraction of cells that exhibit Ca2+ sparks (60% decrease, n = 45, ) and from the strongly decreased number of Ca2+ hotspots in the remaining active cells (71.7% ± 5.5% decrease, n = 45 cells, ). Taken together, these data clearly establish TRPM7 as the carrier of Ca2+-influx in our cells.
3.6. Ca2+ sparks do not localize to invadosomes
To address the potential contribution of TRPM7-mediated Ca2+ sparks to invadosome dynamics, we set out to track Ca2+ spark activity and invadosomes simultaneously. Invadosomes are conveniently visualized by monitoring their actin-dense core with actin markers such as mRFP-actin or Lifeact-dsRed. The very intense staining of both these markers in invadosome cores overlaps well with known invadosome markers like vinculin, Tks4 and cortactin [46,47] (Fig. S1A and data not shown). We confirmed that both markers did not affect Ca2+ spark formation (4.3 ± 0.6 and 4.6 ± 0.7 Ca2+ hotspots per cell, untransfected-control, n = 54, versus RFP-actin-transfected, n = 31, respectively, p = 0.73, Mann–Whitney parameter free test, 2-tailed; results obtained with Lifeact-dsRed were similar but not quantified in detail). We also checked that invadosome formation, numbers, architecture and dynamics were unaffected by the conditions used to visualize Ca2+ sparks, i.e. by preincubation with EDTA-AM (Fig. S5).
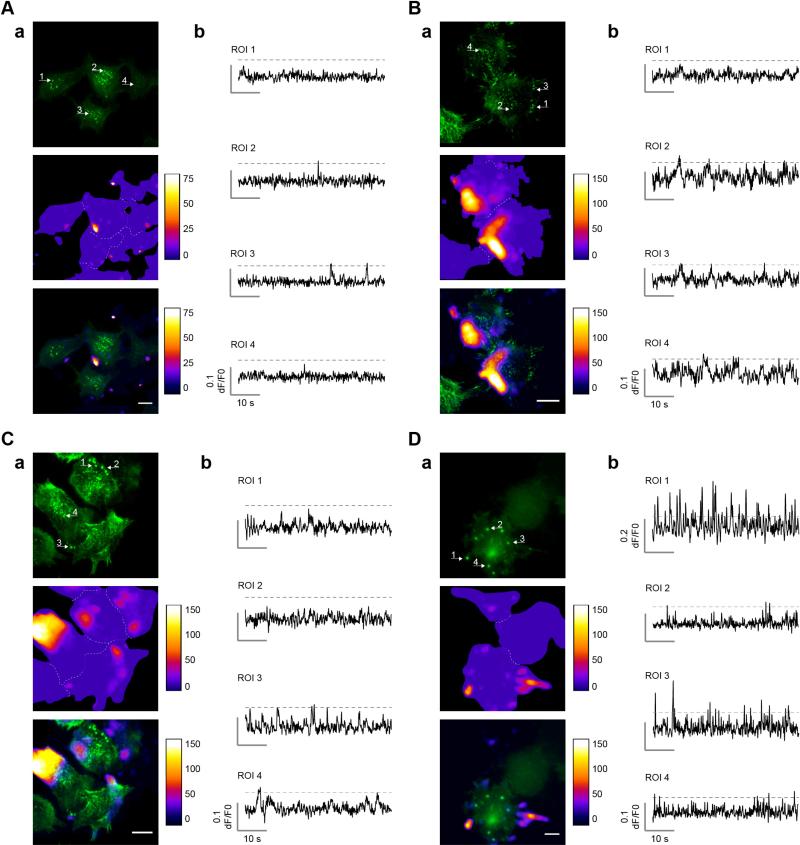
As mentioned before, in those experiments Ca2+ sparks appeared mostly absent from the cell center and were found almost exclusively at the periphery where dynamic actin remodeling takes place (see Figs. 1C, 2 and 3). TRPM7-mediated Ca2+ sparks are, therefore, unlikely to contribute considerably to formation and turnover of central invadosomes (see Fig. S1A), and indeed no evidence for significant colocalization of Ca2+ sparks with central invadosomes was found (Fig. 4A and B).
Fig. 4.
Ca2+ hotspots do not localize specifically to invadosomes. (A)–(D) Four examples demonstrating the distribution of Ca2+ hotspots and invadosomes in N1E-115/TRPM7 cells. Invadosomes are visualized as bright actin dots, labeled with RFP-actin or Lifeact-dsRed (here in green). Shown in each panel is actin (top), Ca2+ hotspots (middle) and overlay (bottom). Please note that the minimum value of the LUT for the Ca2+ hotspot image in the overlay was adjusted to improve visualization of colocalization with invadosomes. Arrows indicate location of the Ca2+ traces in (b). In (A) Ca2+ hotspots are primarily located in the periphery, away from central invadosomes. In (B) Ca2+ hotspots appear along one side of the cell and are clearly excluded from invadosomes. In (C) several neighboring peripheral invadosomes (ROI 1 + 2) do not colocalize with Ca2+ hotspots. In (D) two peripheral invadosomes overlap with highly active Ca2+ hotspots (ROI 1 + 3), whereas others are mostly silent (ROI 2 + 4). As detailed in the text, statistical analysis reveals no significant colocalization of Ca2+ hotspots with invadosomes; thus, panels A–C are representative for the vast majority of observations whereas panel D presents a single outlier where some colocalization seems to occur. Scale bar = 20 μm.
Since immuno-localization showed that TRPM7 was more apparent in peripheral invadosomes (see Fig. S1C) we expected that Ca2+ sparking would be more pronounced at those sites, but detailed analysis showed that this was not the case. In the vast majority of experiments we found no evidence for colocalization of Ca2+ hotspots with peripheral invadosomes at all (Fig. 4B–D). Occasionally we observed some overlap of Ca2+ sparks with peripheral invadosomes (Fig. 4D), but these events represented a minor proportion of the studied peripheral invadosomes. Statistical analyses revealed that ~92% of detected Ca2+ hotspots (n = 107) were outside invadosomes whereas conversely, only ~11% of studied invadosomes showed Ca2+ spark activity (n = 79). Since we calculated that the footprint of Ca2+ hotspots in a cell on average covers ~17% of the total cell surface (mean ± SEM; cell size = 1309 ± 61.3 μm2, n = 96; Ca2+ hotspot size = 43.6 ± 6.4 μm2, n = 178; Ca2+ hotspots per cell = 4.9 ± 0.32, n = 202), the probability of randomly observing at least 11% of invadosomes with Ca2+ spark activity (n = 79) is very high (P0.17(x ≥ 11%) = 0.93). Moreover, we did not observe any invadosomes that formed or disappeared at Ca2+ hotspots during, prior to or following Ca2+ spark ignition.
In summary, despite the extensive numbers of cells and invadosomes that we studied, we found no evidence in support of our hypothesis that TRPM7 serves to set up a local microenvironment of increased Ca2+ levels in invadosomes. We therefore have to reject the hypothesis that TRPM7 controls invadosome dynamics via localized Ca2+ signals.
3.7. Cytosolic Ca2+ levels and invadosomes
Apart from mediating Ca2+ sparks, TRPM7 expression has also been shown to affect basal Ca2+ levels in N1E-115 cells [20,36]. Buffering of extracellular Ca2+ by BAPTA (2–8 mM) to nanomolar levels not only inhibited Ca2+ sparking (Fig. 2A) but also reverted the increase of basal Ca2+ levels that is apparent in N1E-115/TRPM7 cells ([36] and Fig. S4A). Could such Ca2+ changes at a more global (cell-wide) scale, rather than in the invadosome microenvironment, somehow affect invadosome dynamics? We assayed invadosome dynamics by recording confocal time-lapse movies of cells expressing GFP-actin or Lifeact-GFP over prolonged times. Strikingly, addition of BAPTA (2–8 mM) failed to noticeably affect invadosome formation or turnover (n = 36 time-lapse recordings in 6 separate experiments) (Video 5). Similar results were obtained with the Ca2+ chelator EGTA, or when the imaging buffer was replaced by nominally Ca2+-free solution (data not shown). This demonstrates that like the TRPM7-mediated Ca2+ sparks, also the effects of TRPM7 expression on basal Ca2+ levels are not involved in regulating invadosome dynamics. As expected from the essential role of extracellular Ca2+ in cell adhesion [48,49], cells eventually detached when exposed to nominally free Ca2+ buffer for prolonged periods of time, i.e. 8 h. However, most of those cells that were still adherent after such treatment did contain invadosomes (n = 60 fields of cells in 6 separate experiments) (Fig. S6). This is notable because the examination time point, 8 h post BAPTA-treatment, well exceeds the average lifetime of invadosomes (up to a few hours) in these cells. The presence of invadosomes at this time point, therefore, indicates that neither local Ca2+ sparking, nor basal Ca2+ levels are dominant in the regulation of invadosomes in these cells.
3.8. Effect of waixenicin-A on cellular contractility and adhesion
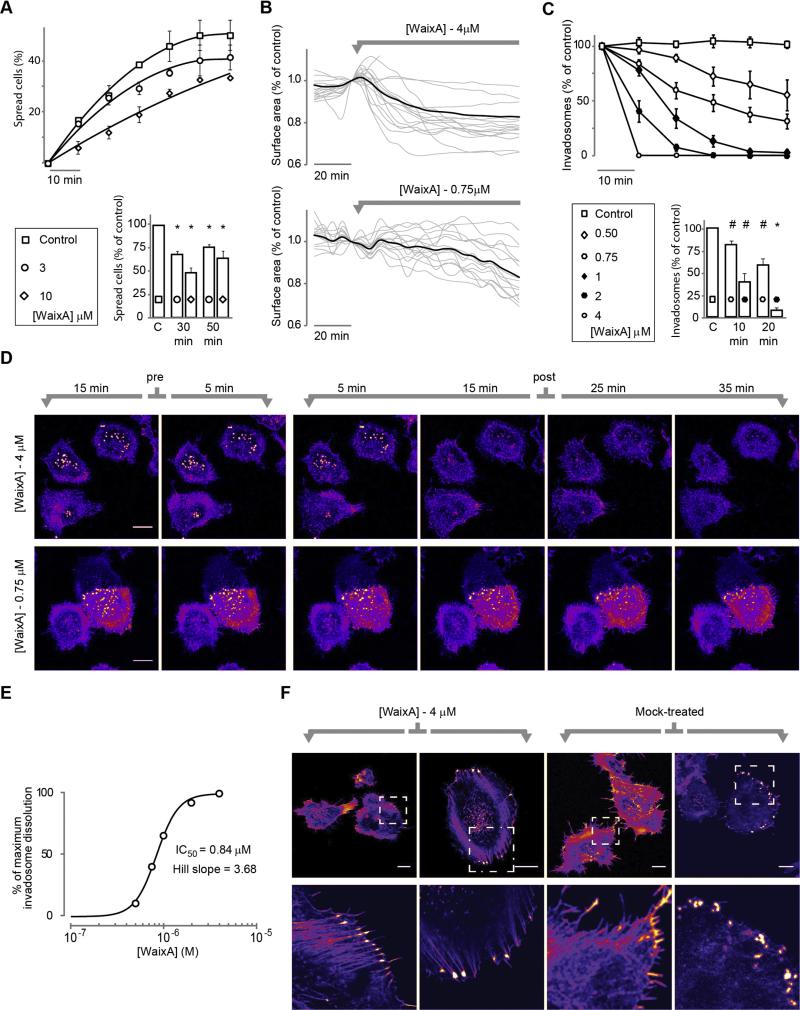
The role of TRPM7 channels in controlling cell adhesion and migration has been well established in a range of different cell types [7,20,31,32,34,50,51]. In N1E-115 cells, the most prominent effects of TRPM7 upregulation are an increase in basal Ca2+ levels and relaxation of the cytoskeleton, which leads to a spread phenotype and the formation of invadosomes [20]. However, although TRPM7 localizes to invadosomes ([20] and Fig. S1C), our experiments show that Ca2+ levels play no dominant role in determining invadosome dynamics in these cells (Fig. 4 and Fig. S6). Experiments we carried out with waixenicin-A may shed some light on this apparent contradiction. When testing the effects of waixenicin-A on cytoskeletal organization of N1E-115/TRPM7 cells, we noted that initial spreading after seeding of cells was impaired in the presence of waixenicin-A (25–50% decrease compared to control, p < 0.001; n = 5 experiments; Fig. 5A). Conversely, N1E-115/TRPM7 cells that were allowed to adhere and spread to coverslips in serum-containing DMEM showed a mild contraction response within minutes after addition of waixenicin-A, indicative of increased cellular tension (Fig. 5B). This response was concentration dependent and typically proceeded slowly at lower concentrations (10% loss of surface area for 4 μM and 0.75 μM was 25 and 70 min, respectively; Fig. 5B). Even more striking was the effect of waixenicin-A on invadosomes. Addition of high doses (≥2 μM) of waixenicin-A caused a very marked, rapid and complete dissolution of invadosomes (Fig. 5C and D) which typically was complete within 10 min, as assayed by live-cell imaging. At lower doses changes progressed more gradually with clear effects still present at doses as low as 0.5 μM (Fig. 5C and D and Video 6). The dose–response curve (taken at 20 min post addition of waixenicin-A; Fig. 5E) reveals an IC50 of 0.84 μM and appears quite steep. We therefore analyzed for cooperativity by Hill slope analysis, which revealed a Hill coefficient of 3.68, i.e. close to 4. It is therefore tempting to speculate that the steep dose–response curve reflects binding of one molecule of waixenicin-A to each of the four subunits of the TRPM7 channel, although direct evidence for this notion is lacking. In any case, the rapid disappearance of invadosomes following TRPM7 inhibition is in agreement with the notion that TRPM7-mediated loss of actomyosin contractility regulates invadosome formation and turnover [12,13]. To further test this, we assayed cells at 24–48 h post waixenicin-A treatment. Indeed, the majority of cells showed extensive formation of focal adhesions and stress fibers at these late time points (Fig. 5F), indicating increased cellular tension [52]. Hence, waixenicin-A appears to revert the morphological phenotype of TRPM7 overexpression in N1E-115. We also conclude that waixenicin-A is a promising and powerful pharmacological tool to study the effects of TRPM7 on adhesion and migration.
Fig. 5.
Waixenicin-A treatment reverts TRPM7-mediated cell spreading and actomyosin-relaxation. (A) Top: effect of waixenicin-A treatment on cell spreading upon seeding. Bottom: quantification (mean ± SEM) of initial spreading at 30 and 50 min (p < 0.001 versus control, one-sample t-test. Experiments were repeated 5 times independently, each for at least 10 fields of view per condition). (B) Waixenicin-A causes cell contraction. Cell surface area was tracked over time by image analysis before and after application of waixenicin-A (0.75 μM and 4 μM). Traces (black, average response; gray, individual responses) were normalized to the cell surface area at the frame before application. (C) Top: effect of waixenicin-A treatment (added at t = 0) on invadosome numbers. Bottom: quantification (mean ± SEM) of invadosome dissolution at 10 and 20 min after application (2 μM, n = 5 fields of view; 0.75 μM, n = 15, counting hundreds of invadosomes per data point, p < 0.005 versus control, one-sample t-test). (D) Typical examples of confocal time-series experiments that show concentration-dependent invadosome dissolution upon waixenicin-A treatment (0.75 μM and 4 μM) in N1E-115/TRPM7 cells that stably express GFP-actin (see also Video 6). (E) Dose–response curve derived from the data in (C) at 20 min after application. (F) Examples of adherent N1E-115/TRPM7 cells treated with waixenicin-A (4 μM) or vehicle for 24 h. F-actin stained with Phalloidin-Alexa568. Note the induction of focal adhesions and stress fibers upon exposure to waixenicin-A (see zoom-in). Scalebar = 20 μm. *p < 0.001 and #p < 0.005.
In summary, these data support the view that TRPM7 activity affects the tension-relaxation balance of the actomyosin cytoskeleton, which in turn modulates the formation of various adhesion structures. Strikingly, however, the effect of waixenicin-A appears independent of Ca2+ influx through the TRPM7 channel.
4. Discussion
There is ample evidence that ion channels can set up intracellular Ca2+ microdomains to locally regulate effector proteins. Synaptic transmission in neurons provides a clear example of such a model [53]. N- and P/Q-type voltage-operated Ca2+ channels in synaptic terminals generate brief and localized Ca2+ transients that activate the SNARE-complex to initiate neurotransmitter release [54–56]. In the present study we focused on the role of such Ca2+ sparks in the control of cell adhesion dynamics, in particular of invadosomes in neuroblastoma cells. Expression levels of the Ca2+-permeable divalent cation channel TRPM7 are known to affect adhesion and migration [20,31–34,50,51,57], and we have shown that the channel localizes to a ring around invadosomes ([20] and Fig. S1C and D). Such a constellation theoretically constitutes the ideal architecture to generate local Ca2+ signals, and indeed a variety of Ca2+ sensitive regulatory proteins have been identified in invadosomes [9,19–21,58]. We addressed the hypothesis that TRPM7 exerts its effect on adhesion dynamics by mediating local Ca2+ signals so as to modulate Ca2+-sensitive cytoskeletal effector proteins. TRPM7 itself may be one of the effectors because Ca2+ influx through the channel appears to be required for the association of its kinase domain with the myosin II heavy chain [20,30].
To address our hypothesis we developed a sensitive assay that greatly improves the visualization of local Ca2+ signals and reliably detects them at a high spatiotemporal resolution. Our data indicate that TRPM7 indeed plays an essential role in mediating localized Ca2+ sparks at the basal membrane of neuroblastoma cells. Ca2+ hotspots are prevalent at the periphery of the cells. However, despite the sensitivity of our analysis we only sporadically detected such Ca2+ sparks at invadosomes, and overall the spatial distribution of Ca2+ hotspots showed no enrichment in invadosomes. Thus, our study dissociates TRPM7-mediated Ca2+ sparking from invadosome dynamics and actomyosin contractility.
Remarkably, inhibition of TRPM7 with waixenicin-A (but not interfering with Ca2+ influx by chelation of extracellular Ca2+) caused rapid and complete dissolution of invadosomes and, on a longer timescale, induced a more contractile phenotype in neuroblastoma cells, as evidenced from the development of numerous FAs and stress fibers. How waixenicin-A causes this effect, in a manner unrelated to Ca2+ influx, remains to be investigated. We previously reported that phosphotransferase-activity of TRPM7's kinase domain was not required for the induction of cell spreading, as evident by using a kinase-dead mutant (D1775A), and suggested that this instead may be caused by altered signaling through elevated basal [Ca2+]i [20], but our current data show that this latter viewpoint requires revision. Yet another model was put forward by Su and colleagues, who proposed that TRPM7 modulates the actomyosin cytoskeleton and its contractility by regulating [Mg2+]i [32]. Indeed, TRPM7 channels readily carry Mg2+ ions too, but at least in our cells, waixenicin-A induced alterations in Mg2+ influx cannot be held responsible for the observed cytoskeletal effects because its chelation with 10 mM EDTA was without effect (data not shown). Alternatively, we should consider the possibility that waixenicin-A may alter interactions of TRPM7 with other proteins or may even induce endocytosis of TRPM7, thereby affecting its proposed scaffolding function. Lastly, we cannot fully exclude the possibility that waixenicin-A may have off-target effects, although a previous study reported that waixenicin-A did not affect other ion channels (including TRP members and CRAC) at concentrations well-above those employed in the current study [44]. In any case, the responses observed upon application of waixenicin-A resemble that of TRPM7 knockdown in MDA-MB-231 breast cancer cells [34] and generally are in line with the notion that TRPM7 expression levels affect cytoskeletal organization and cellular tension [20,32,34]. Cellular tension is an important determinant of tumor cell transformation and malignancy (e.g. [59,60]) and indeed, TRPM7 has recently been implicated in breast cancer metastasis [34] and high expression is associated with adverse pathological parameters [57,61].
Several recent studies have addressed the possible role of localized Ca2+ signals in the turnover of adhesion sites. Our identification of a key role for TRPM7 in the ignition of Ca2+ sparks is in line with the results of Wei and colleagues, who showed by knockdown and inhibitor studies that TRPM7-mediated Ca2+ ‘flickering’ is involved in guiding of fibroblast lamellipodia during directional migration experiments. They presented a model in which TRPM7, together with type 2 IP3 receptors, evokes Ca2+ sparks at the leading edge of migrating fibroblasts and argue that those events are involved in the regulation of traction forces that are associated with migration [7]. Whether or not the Ca2+ sparks in their study modify the dynamics of FAs, and which effector proteins are affected remains to be determined. In another recent study, TRPV2 channels were demonstrated to localize to invadosomes in macrophages [19]. This study showed that fMLP-induced Ca2+ influx through TRPV2 triggered dissolution of invadosomes through activation of the Ca2+-sensitive protein tyrosine kinase Pyk2 [19] but it did not focus on resolving local Ca2+ microdomains.
Whereas thus the existence and exact role of localized Ca2+ signals in adhesive structures in various cell types may be debated, it has become clear that invadosomes contain a number of Ca2+-sensitive protein constituents, including myosins, gelsolin, tropomyosins, calponin and vinculin. In microglial invadosomes the Ca2+ release activated channel (CRAC) Orai1 was identified [21] and inhibitor studies suggested a function in formation of these structures. Notably, this study found no evidence for a role of TRPM7 (or other TRP channels tested) in invadosome formation. In addition, the authors identified calmodulin as a prominent constituent in invadosomes. This small Ca2+ binding protein conveys Ca2+ sensitivity to its (many) protein interactors, among which are several TRP channels [62] and cytoskeletal regulators, and thus is a critical determinant of Ca2+ signaling [63,64].
In summary, functional studies providing evidence for the invadosome as a hub for localized Ca2+ signaling have been very limited, and indeed consensus on the importance of Ca2+ in general (be it localized Ca2+ influx, cytosolic Ca2+ levels or extracellular Ca2+) for invadosome dynamics has not been reached. For example, Ca2+ influx may trigger invadosome dissolution in some studies [19,65] and it was required for invadosome formation in others [21], whereas we here describe that switching to nominal [Ca2+]e does not noticeably affect de novo formation or dynamic behavior of invadosomes. In addition to the different experimental strategies employed in these studies, it may be anticipated that cell-type specific differences govern the variation in these results. As to how TRPM7 controls the actomyosin cytoskeleton and cell adhesion dynamics, either the activity of its kinase-domain [20,66] as well as Ca2+-influx [7,20,31,33] and Mg2+-influx [32,50] through its pore have been proposed. Based on the results of the present study we conclude that TRPM7 regulates invadosome dynamics by affecting actomyosin-based tension conditions in adherent cells independent of (localized) Ca2+ influx. Just how interfering with TRPM7 functioning exerts these effects remains a challenge for future research.
Supplementary Material
Acknowledgements
We thank Dr. W. Moolenaar and J. Middelbeek for critical reading of the manuscript and members of our department for discussion. Dr. L. Oomen and L. Brocks are acknowledged for help with TIRF microscopy, and Mr. D. Cagle is thanked for assistance in isolating waixeincin-A. This work was supported by Koningin Wilhelmina Fonds grants KUN2007-3733 and NKI 2010-4626 (to FNvL and KJ), by a Nederlandse Organisatie voor Wetenschappelijk Onderzoek investment grant (to KJ), and by National Institutes of Health grants P20 GM-103466-11 (to FDH) and P01 GM078195 (to AF).
Abbreviations
- 2-APB
2-aminoethyl diphenylborinate
- BK
bradykinin
- ECM
extracellular matrix
- FA
focal adhesion
- FC
focal complex
- TIRF
Total Internal Reflection Fluorescence (microscopy)
- TRPM7
Transient Receptor Potential cation channel, subfamily Melastatin, member 7
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors declare that all experiments comply with Dutch and American laws.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ceca.2013.09.003.
References
- 1.Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol. 2002;34:746–761. doi: 10.1016/s1357-2725(01)00171-6. [DOI] [PubMed] [Google Scholar]
- 2.Rottner K, Stradal TE. Actin dynamics and turnover in cell motility. Curr. Opin. Cell Biol. 2011;23:569–578. doi: 10.1016/j.ceb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 4.Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J. Cell Sci. 2004;117:223–231. doi: 10.1242/jcs.00839. [DOI] [PubMed] [Google Scholar]
- 5.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai FC, Meyer T. Ca(2+) pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr. Biol. 2012;22:837–842. doi: 10.1016/j.cub.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linder S, Wiesner C, Himmel M. Degrading devices: invadosomes in proteolytic cell invasion. Annu. Rev. Cell Dev. Biol. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- 10.Collin O, Na S, Chowdhury F, Hong M, Shin ME, Wang F, Wang N. Self-organized podosomes are dynamic mechanosensors. Curr. Biol. 2008;18:1288–1294. doi: 10.1016/j.cub.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Helden SF, Oud MM, Joosten B, Peterse N, Figdor CG, van Leeuwen FN. PGE2-mediated podosome loss in dendritic cells is dependent on actomyosin contraction downstream of the RhoA-Rho-kinase axis. J. Cell Sci. 2008;121:1096–1106. doi: 10.1242/jcs.020289. [DOI] [PubMed] [Google Scholar]
- 13.Lener T, Burgstaller G, Crimaldi L, Lach S, Gimona M. Matrix-degrading podosomes in smooth muscle cells. Eur. J. Cell Biol. 2006;85:183–189. doi: 10.1016/j.ejcb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.van den Dries K, van Helden SF, Riet JT, Diez-Ahedo R, Manzo C, Oud MM, van Leeuwen FN, Brock R, Garcia-Parajo MF, Cambi A, et al. Geometry sensing by dendritic cells dictates spatial organization and PGE(2)-induced dissolution of podosomes. Cell Mol. Life Sci. 2012;69:1889–1901. doi: 10.1007/s00018-011-0908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geblinger D, Zink C, Spencer ND, Addadi L, Geiger B. Effects of surface micro-topography on the assembly of the osteoclast resorption apparatus. J. R. Soc. Interface. 2012;9:1599–1608. doi: 10.1098/rsif.2011.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harper K, Arsenault D, Boulay-Jean S, Lauzier A, Lucien F, Dubois CM. Autotaxin promotes cancer invasion via the lysophosphatidic acid receptor 4: participation of the cyclic AMP/EPAC/Rac1 signaling pathway in invadopodia formation. Cancer Res. 2010;70:4634–4643. doi: 10.1158/0008-5472.CAN-09-3813. [DOI] [PubMed] [Google Scholar]
- 17.Rottiers P, Saltel F, Daubon T, Chaigne-Delalande B, Tridon V, Billottet C, Reuzeau E, Genot E. TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J. Cell Sci. 2009;122:4311–4318. doi: 10.1242/jcs.057448. [DOI] [PubMed] [Google Scholar]
- 18.Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil. Cytoskeleton. 2009;66:303–316. doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagasawa M, Kojima I. Translocation of calcium-permeable TRPV2 channel to the podosome: its role in the regulation of podosome assembly. Cell Calcium. 2012;51:186–193. doi: 10.1016/j.ceca.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui TA, Lively S, Vincent C, Schlichter LC. Regulation of podosome formation, microglial migration and invasion by Ca2+-signaling molecules expressed in podosomes. J. Neuroinflammation. 2012;9:250. doi: 10.1186/1742-2094-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damann N, Voets T, Nilius B. TRPs in our senses. Curr. Biol. 2008;18:R880–R889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 23.Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat. Rev. Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 24.Numata T, Shimizu T, Okada Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol. Cell Physiol. 2007;292:C460–C467. doi: 10.1152/ajpcell.00367.2006. [DOI] [PubMed] [Google Scholar]
- 25.Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006;98:245–253. doi: 10.1161/01.RES.0000200179.29375.cc. [DOI] [PubMed] [Google Scholar]
- 26.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 27.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 28.Ryazanova LV, Pavur KS, Petrov AN, Dorovkov MV, Ryazanov AG. Novel type of signaling molecules: protein kinases covalently linked with ion channels. Mol. Biol. 2001;35:271–283. [PubMed] [Google Scholar]
- 29.Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J. Biol. Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 30.Clark K, Middelbeek J, Dorovkov MV, Figdor CG, Ryazanov AG, Lasonder E, van Leeuwen FN. The alpha-kinases TRPM6 and TRPM7, but not eEF-2 kinase, phosphorylate the assembly domain of myosin IIA, IIB and IIC. FEBS Lett. 2008;582:2993–2997. doi: 10.1016/j.febslet.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J. Biol. Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su LT, Liu W, Chen HC, Gonzalez-Pagan O, Habas R, Runnels LW. TRPM7 regulates polarized cell movements. Biochem. J. 2011;434:513–521. doi: 10.1042/BJ20101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JP, Luan Y, You CX, Chen XH, Luo RC, Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca(2+) influx. Cell Calcium. 2010;47:425–432. doi: 10.1016/j.ceca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R, Wieringa B, Canisius SV, Zwart W, Wessels LF, et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res. 2012;72:4250–4261. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- 35.Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J. Recept. Signal Transduct. Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 36.Langeslag M, Clark K, Moolenaar WH, van Leeuwen FN, Jalink K. Activation of TRPM7 channels by phospholipase C-coupled receptor agonists. J. Biol. Chem. 2007;282:232–239. doi: 10.1074/jbc.M605300200. [DOI] [PubMed] [Google Scholar]
- 37.Cheng H, Lederer WJ. Calcium sparks. Physiol. Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 38.Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- 39.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J. Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song LS, Sham JS, Stern MD, Lakatta EG, Cheng H. Direct measurement of SR release flux by tracking ‘Ca2+ spikes’ in rat cardiac myocytes. J. Physiol. 1998;512(Pt 3):677–691. doi: 10.1111/j.1469-7793.1998.677bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wykes RC, Lee M, Duffy SM, Yang W, Seward EP, Bradding P. Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J. Immunol. 2007;179:4045–4052. doi: 10.4049/jimmunol.179.6.4045. [DOI] [PubMed] [Google Scholar]
- 43.Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J. Pharmacol. Sci. 2004;95:403–419. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- 44.Zierler S, Yao G, Zhang Z, Kuo WC, Porzgen P, Penner R, Horgen FD, Fleig A. Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J. Biol. Chem. 2011;286:39328–39335. doi: 10.1074/jbc.M111.264341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim BJ, Nam JH, Kwon YK, So I, Kim SJ. The role of waixenicin a as transient receptor potential melastatin 7 blocker. Basic Clin. Pharmacol. Toxicol. 2013;112:83–89. doi: 10.1111/j.1742-7843.2012.00929.x. [DOI] [PubMed] [Google Scholar]
- 46.Buschman MD, Bromann PA, Cejudo-Martin P, Wen F, Pass I, Court-neidge SA. The novel adaptor protein Tks4 (SH3PXD2B) is required for functional podosome formation. Mol. Biol. Cell. 2009;20:1302–1311. doi: 10.1091/mbc.E08-09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjaastad MD, Nelson WJ. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. Bioessays. 1997;19:47–55. doi: 10.1002/bies.950190109. [DOI] [PubMed] [Google Scholar]
- 49.Cox EA, Huttenlocher A. Regulation of integrin-mediated adhesion during cell migration. Microsc. Res. Tech. 1998;43:412–419. doi: 10.1002/(SICI)1097-0029(19981201)43:5<412::AID-JEMT7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 50.Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and magnesium in the stimulation of osteoblast proliferation and migration by platelet-derived growth factor. Am. J. Physiol. Cell Physiol. 2009;297:C360–C368. doi: 10.1152/ajpcell.00614.2008. [DOI] [PubMed] [Google Scholar]
- 51.Gao H, Chen X, Du X, Guan B, Liu Y, Zhang H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium. 2011;50:559–568. doi: 10.1016/j.ceca.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 53.Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Curr. Opin. Neurobiol. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Reid CA, Bekkers JM, Clements JD. N- and P/Q-type Ca2+ channels mediate transmitter release with a similar cooperativity at rat hippocampal autapses. J. Neurosci. 1998;18:2849–2855. doi: 10.1523/JNEUROSCI.18-08-02849.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reid CA, Bekkers JM, Clements JD. Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci. 2003;26:683–687. doi: 10.1016/j.tins.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Qian J, Noebels JL. Presynaptic Ca2+ channels and neurotransmitter release at the terminal of a mouse cortical neuron. J. Neurosci. 2001;21:3721–3728. doi: 10.1523/JNEUROSCI.21-11-03721.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rybarczyk P, Gautier M, Hague F, Dhennin-Duthille I, Chatelain D, Kerr-Conte J, Pattou F, Regimbeau JM, Sevestre H, Ouadid-Ahidouch H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer. 2012;131:E851–E861. doi: 10.1002/ijc.27487. [DOI] [PubMed] [Google Scholar]
- 58.Calle Y, Carragher NO, Thrasher AJ, Jones GE. Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J. Cell Sci. 2006;119:2375–2385. doi: 10.1242/jcs.02939. [DOI] [PubMed] [Google Scholar]
- 59.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol. Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 62.Zhu MX. Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451:105–115. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]
- 63.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 64.Haeseleer F, Imanishi Y, Sokal I, Filipek S, Palczewski K. Calcium-binding proteins: intracellular sensors from the calmodulin superfamily. Biochem. Biophys. Res. Commun. 2002;290:615–623. doi: 10.1006/bbrc.2001.6228. [DOI] [PubMed] [Google Scholar]
- 65.Miyauchi A, Hruska KA, Greenfield EM, Duncan R, Alvarez J, Barattolo R, Colucci S, Zambonin-Zallone A, Teitelbaum SL, Teti A. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J. Cell Biol. 1990;111:2543–2552. doi: 10.1083/jcb.111.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clark K, Middelbeek J, Lasonder E, Dulyaninova NG, Morrice NA, Ryazanov AG, Bresnick AR, Figdor CG, van Leeuwen FN. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J. Mol. Biol. 2008;378:790–803. doi: 10.1016/j.jmb.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.