Abstract
The arabinosyltransferases responsible for the biosynthesis of the arabinan domains of two abundant heteropolysaccharides of the cell envelope of all mycobacterial species, lipoarabinomannan and arabinogalactan, are validated drug targets. Using a cell envelope preparation from Mycobacterium smegmatis as the enzyme source and di- and tri-mannoside synthetic acceptors, we uncovered a previously undetected arabinosyltransferase activity. Thin layer chromatography, GC/MS and LC/MS/MS analyses of the major enzymatic product are consistent with the transfer of an arabinose residue to the 6 position of the terminal mannosyl residue at the non-reducing end of the acceptors. The newly identified enzymatic activity is resistant to ethambutol and could correspond to the priming arabinosyl transfer reaction that occurs during lipoarabinomannan biosynthesis.
Introduction
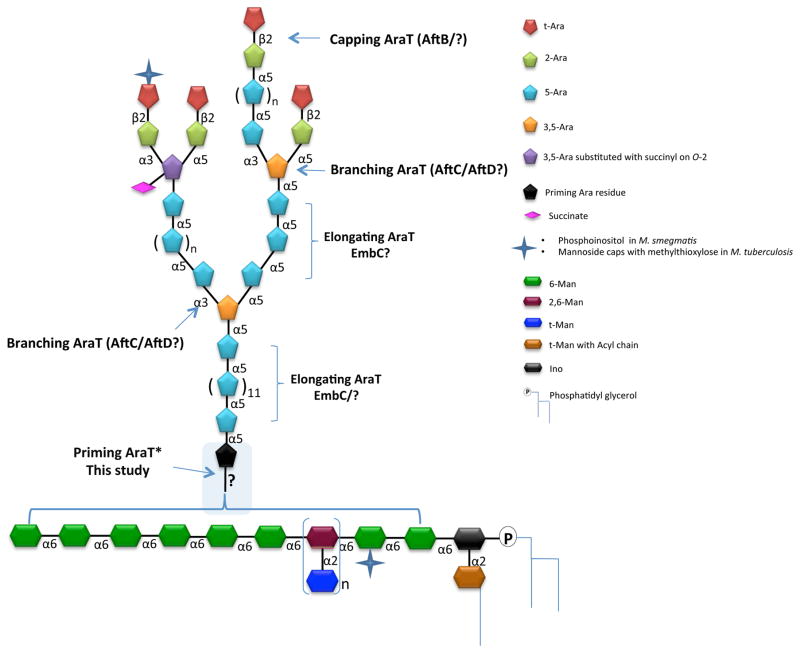
Tuberculosis is the most deadly infectious disease in the world killing 1.5 million people annually1. The continuing rise of multidrug-resistant Mycobacterium tuberculosis places a high priority on the development of new chemotherapeutics with novel modes of action. In this context, elucidating the biosynthetic pathways allowing M. tuberculosis to synthesize and assemble its complex cell envelope represents a crucial area of research. Two essential D-arabinofuranosyl-containing heteropolysaccharides, arabinogalactan (AG) and lipoarabinomannan (LAM) (Fig. 1), populate the cell envelope of all Mycobacterium species2. These complex glycoconjugates play various critical roles in the physiology of mycobacteria and their interactions with the host2. Owing to their central involvement in the elongation and branching of the arabinan domains of AG and LAM, arabinosyltransferases (AraTs) and the enzymes involved in the formation of decaprenyl-phosphoryl-β-D-arabinofuranose (DPA) - the arabinosyl donor used in all AraT-mediated transfer reactions -, are essential enzymes whose therapeutic potential has been well validated2, 3. Based on the structural organization of the arabinan domain of LAM2 (Fig. 1), at least four different linkage-specific AraTs are likely to be needed to complete its biosynthesis. To date, AftC, an α-(1→3) branching AraT4, AftD, another branching AraT5, and EmbC, a proposed α-(1→5) arabinan chain elongating enzyme6–10, are the only AraTs shown to be involved in the LAM pathway, while the priming AraT responsible for the transfer of the first arabinosyl residue onto the mannan backbone of LAM, and the capping AraT(s) responsible for the β-(1→2) linkages at the non-reducing end of the arabinan domain specific to LAM remain unknown. In the present study, a cell free assay using synthetic mannoside acceptors and Mycobacterium smegmatis membrane and envelope preparations was developed to probe the priming AraT activity specific to LAM biosynthesis.
Figure 1. Structure of LAM.
The mannan core of LM and LAM is composed on average of 20–25 α–(1→6)-linked Manp residues occasionally substituted at C-2 by single Manp units in M. smegmatis and M. tuberculosis. Single Manp substitutions occur at C-3 in M. chelonae 25. Due to the findings reported herein, we have purposely left the details of the attachment of the arabinan to the mannan very general rather than the previously believed idea that it is attached at O-2 of one of the 6-linked mannosyl residues.
RESULTS AND DISCUSSION
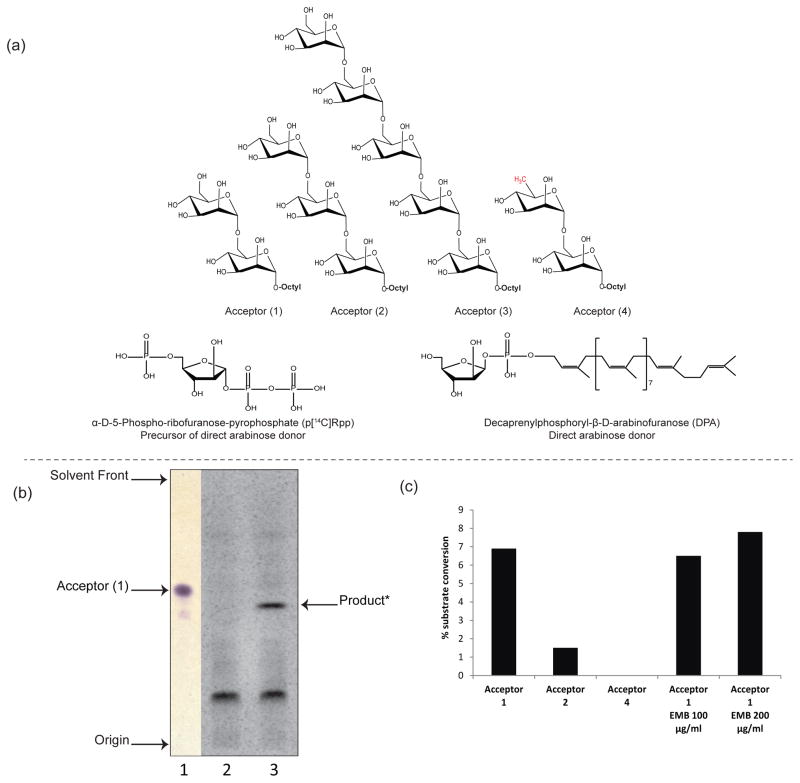
We and others have previously evaluated the effectiveness of several synthetic neoglycolipids as substrates for mycobacterial glycosyltransferase activities11, 12. Specifically, di- and trimannosides with an octyl aglycon chain (Fig. 2a) have shown acceptor capabilities with α-(1→6) mannosyltransferases in cell-free assay systems13–15. Using synthetic galactosyl acceptors, earlier studies probed the priming AraT activity of the AG pathway16, 17. As part of our continuing research towards understanding LAM biosynthesis, we sought to address the question of the priming AraT activity of the LAM pathway using a variety of synthetic mannoside acceptors (Fig. 2a, 1–4), and a membrane plus cell envelope preparation from M. smegmatis as the enzyme source. First, a radioactivity-based assay was developed to test the synthetic disaccharide Man-(1→6)-Man-(1→octyl (1) as an arabinose acceptor. DP[14C]A, generated in situ from p[14C]Rpp, served as the arabinosyl donor (Fig. 2a)5. Thin layer chromatography (TLC) analysis of the radiolabeled enzymatic products followed by autoradiography revealed the formation of a single radioactive compound migrating slower than the dimannoside acceptor, that was absent from the control reaction lacking acceptor (1) (Fig. 2b). Thus, M. smegmatis extracts contain an enzyme with AraT activity capable of transferring [14C]arabinose onto acceptor (1).
Figure 2. Evidence for the enzymatic transfer of an arabinosyl residue onto linear mannoside acceptors in M. smegmatis cell-free extracts.
(a) Structures of the synthetic mannosides acceptors bearing an octyl chain (1–4) that were used to probe the novel (1→6) AraT activity in M. smegmatis extracts; structures of pRpp and the D-arabinofuranose donor, DPA.
(b) TLC analysis of the AraT reaction product formed using acceptor (1) - Lane 1, non-radioactive dimannoside acceptor (1) visualized upon spraying with α-naphthol and heating; Lane 2, TLC autoradiogram showing the product of the reaction run in the absence of acceptor (1) (negative control); Lane 3, complete assay showing a radiolabeled product migrating with a lower Rf than acceptor (1).
(c) Percentage enzymatic conversions of substrates into their arabinose-containing products. Acceptor (1) appears as the most efficient substrate in the AraT reaction. The absence of product formation with C6-deoxy (4)-modified disaccharide analog suggests that the C-6 hydroxyl group is essential for (1→6) AraT activity. EMB at a concentration of 100–200 μg/mL does not inhibit the transfer of an arabinosyl residue onto acceptor (1). The results shown are representative of at least two independent assays performed with different enzyme preparations.
The front-line antituberculosis drug, ethambutol (EMB), is known to inhibit the EmbA, EmbB and EmbC AraTs18–20. The addition of high concentrations of EMB to the reaction mixture (100 and 200 μg mL−1; the MIC of EMB against the M. smegmatis strain used in this study is 7.5 μg mL−1), however, had no significant effect on the transfer of an arabinosyl residue to acceptor (1) (Fig. 2c).
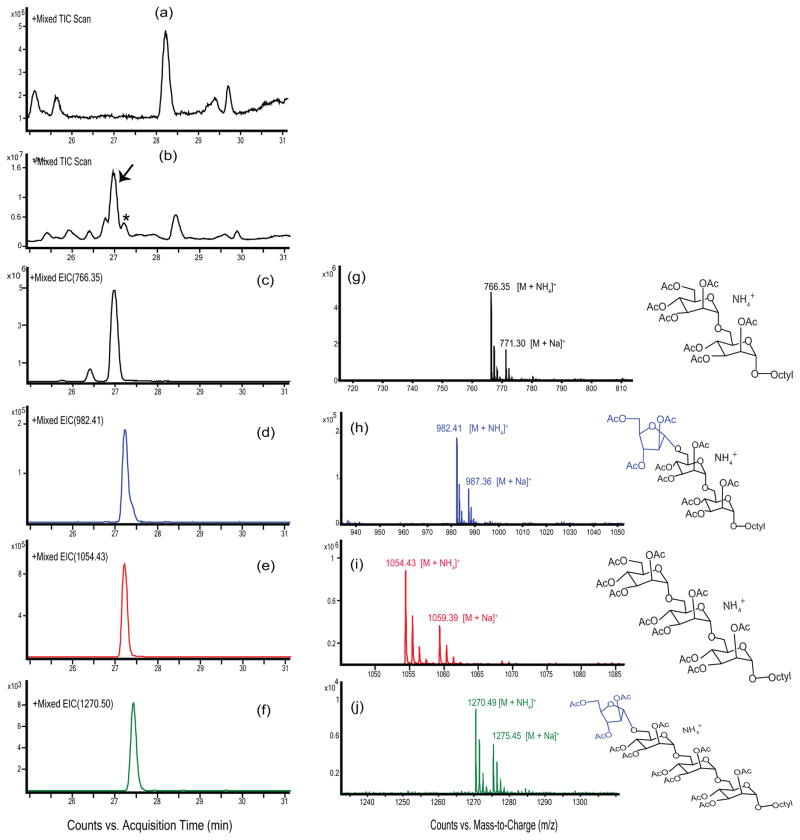
To facilitate the characterization of the major enzymatic product of the reaction, a similar AraT assay as described above was next performed using non-radioactive DPA synthesized in-house21 as the direct arabinofuranosyl donor (Fig. 2a). 1-Butanol-extracted enzymatic products were per-O-acetylated as described in the Supplementary Methods, and the products were analyzed by LC/MS with high mass resolution. Both the unreacted acceptor substrate (1) (marked with an arrow in Fig. 3b) and the major enzymatic product (marked with an asterisk in Fig. 3b) were detected by this method as peaks eluting around 27 min. These peaks were not present in the negative control reaction lacking the dimannoside acceptor (1) (Fig. 3a). The unreacted acceptor (1) and its single arabinose residue-containing enzymatic product were identified by extracted ion chromatograms (EICs) of their corresponding [M+NH4]+ ions (Fig. 3c–f); the relative amounts of the compounds can be noted by the values of the y-axis. The mass spectrum of the unreacted dimannoside acceptor (1) revealed strong pseudo-molecular ions with m/z 766.35 and 771.30 as ammonium and sodiated adducts (Fig. 3g). Most notable was the presence of mass spectrum ions m/z 982.41 [M+NH4]+ and 987.36 [M+Na]+ for the enzymatic product (Fig. 3h). The presence of these ions confirms that a single arabinosyl residue was enzymatically transferred to give the product Araf- (1-?)- Man-(1→6)-Man-(1→octyl. Interestingly, we also identified small amounts of an arabinose-containing tetrasaccharide enzymatic product at m/z 1270.49 [M + NH4]+ (Fig. 3j), suggesting that the Man-(1→6)-Man-(1→octyl acceptor was endogenously converted to the trimannoside Manp-(1→6)-Man-(1→6)-Man-(1→octyl (as shown on the EIC in Fig. 3e) which then served as an acceptor in the AraT reaction. The presence of Manp-(1→6)-Man-(1→6)-Man-(1→octyl was confirmed by the presence of m/z 1054.43 [M+NH4]+ as shown in Fig. 3i. Presumably, mannosylation of the acceptor occurred due to the presence of an endogenous mannosyltransferase and mannose donor in the reaction mixture.
Figure 3. LC/MS analysis of the AraT reaction products.
Membrane and cell envelope preparations from M. smegmatis were incubated without arabinosyl donor or mannoside acceptor, or with DPA donor and acceptor (1). Traces (a) and (b) show the total ion chromatograms of the control lacking substrates and complete reaction, respectively. A series of peaks centered around 27 min is present in the substrate containing reaction (b) but not in the control (a). Unreacted substrate (1) was identified using selected ion monitoring of the [M+NH4]+ ion at m/z 766.34 (c); the mass spectrum is shown in (g). The mono-arabinosylated product was identified using selected ion monitoring of the [M+NH4]+ ion at m/z 982.41; the mass spectrum is shown in (h). Even though no mannose donors (GDP-Man or polyprenyl-phosphomannose) were added to the reaction, the presence of endogenous mannose donor in the membrane fraction allowed for the mono-mannosylation of acceptor (1) as shown by selected ion monitoring of the [M+NH4]+ ion at m/z 1054.43. This trimannoside was also arabinosylated as shown by selected ion monitoring of the [M+NH4]+ ion at m/z 1270.49; the mass spectrum is shown in (j). In all mass spectra, the ion at 5 amu higher mass than the [M+NH4]+ ion corresponds to the [M+Na]+ ion.
Synthetic mannosides acceptors of varying chain length were next tested in the AraT assay to compare their effectiveness as acceptor substrates (see Fig. 2a, acceptors 1–3). We found that the AraT activity was predominant with dimannoside (1) compared to the trimannoside (2) (Fig. 2c), and we were not able to detect any transfer activity with the pentamannoside substrate (3) (data not shown). Stability issues with the pentamannoside acceptor may account for the latter result as we found this substrate to be degraded into di, tri, and tetra-mannosides, consistent with earlier observations14.
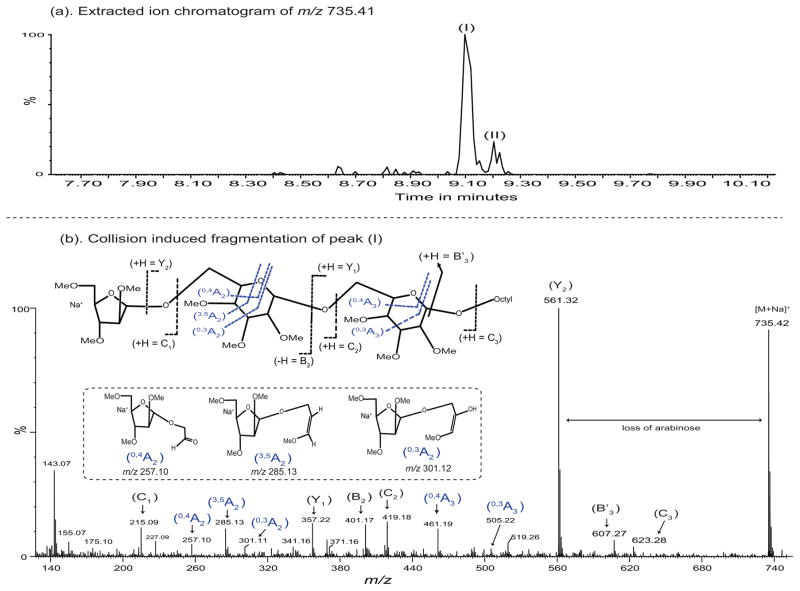
To determine the identity of the mannosyl residue modified by the arabinose on the Man-(1→6)-Man-(1→octyl acceptor, and the nature of its linkage, the enzymatic products were per-O-methylated22 and analyzed by LC/MS/MS. O-methyl derivatives of a major (peak I) and minor (peak II) Ara-Man2-octyl products were detected. The major per-O-methylated enzymatic product (peak I; Fig. 4a), with a precursor ion at m/z 735.42 [M + Na]+ was selected and fragmented by high energy (60 V) CID MS/MS (Fig. 4b). The MS/MS fragmentation pattern was consistent with a linear enzymatic product rather than a branched one (Fig. 4b). Thus, fragment ions at m/z’s 419.17 (C2), 357.22 (Y1), 401.17 (B2) and 461.19 (0,4A3) can only be formed with these m/z values from a linear trisaccharide. The lack of ions consistent with a non-reducing terminal mannosyl residue confirms the linear structure.
Figure 4. MS/MS analysis of the arabinosylated acceptor (1).
The [M+Na]+ion of the major arabinosylated product at m/z 735.42 (a) was subjected to collision-induced fragmentation resulting in the spectrum shown in (b). All ions are present in the sodiated form of otherwise neutral fragments (except possibly m/z 143 which might be the terminal arabinosyl C-1 cation minus methanol). The formation and structure of the fragments of the product where the arabinosyl residue is attached at O-6 of what was the terminal mannosyl residue is shown in (b). As discussed in the text, the MS/MS spectrum strongly suggests that the structure shown in (b) is correct and is supported by the GC/MS data shown in Supplementary Figure 1. The MS/MS spectrum fragment ions obtained by glycosidic and cross-ring fragmentation were identified according to Domon and Costello nomenclature26. The formation of B′3 ion at m/z 607.23 was identified as previously described27.
The internal cross ring cleavage of the internal mannosyl residue allowed the position on the mannosyl residue to be postulated in the same MS/MS analysis. Surprisingly, the results were consistent with the position of the arabinosyl substitution being on O-6 of the mannosyl residue, and not on O-2 as expected from earlier data from our laboratories23, which postulated that the arabinan was attached to O-2 of a mannosyl residue. This is shown by the three ions at m/z 257.13 (0,4A2), 285.11 (3,5A2) and 301.14 (0,3A2) (Fig. 4b). The (3,5A2) and (0,3A2) ions are consistent with the arabinosyl residue on O-4 or O-6; the (0,4A2) ion is consistent only with the arabinosyl residue being present on O-6 of the mannosyl residue. Although somewhat weak, an E ion at m/z 371.17 is not consistent with the arabinosyl residue being attached at O-2. We thus conclude from our LC/MS/MS analysis that the arabinosyl residue is attached to O-6 of the terminal mannosyl residue located at the non-reducing end of acceptor (1) and has the structure Araf-(1→6)-Manp-(1→6)-Manp-(1→octyl.
To confirm the structure of the major product (peak I, Fig. 4a), it was partially purified by LC using two columns in tandem. The partially purified major product was then hydrolyzed, reduced with NaBD4 and acetylated, and the resultant partially methylated partially acetylated alditols analyzed by GC/MS. Unfortunately, the major Ara-Man2-octyl product co-purified with substantial amounts of Man2-octyl substrate resulting in considerable amounts of the partially methylated partially acetylated alditols from the substrate. Also, the amounts of the desired compounds were low compared to contaminants. However, by use of selected ion chromatographs, it was clearly shown that the expected 2,3,5 tri-O-methyl 1,4-di-O-acetyl arabinitol (t-Araf) from the product, 2,3,4 tri-O-methyl 1,5,6-tri-O-acetyl mannitol (6-Manp) from both substrate and product, and 2,3,4,6 tri-O-methyl 1,5-di-O-acetyl mannitol (t-Manp) from the substrate were present (see Supplementary Figure 1). In addition, 3,4,6 tri-O-methyl 1,2,5-tri-O-acetyl mannitol, 2,4,6 tri-O-methyl 1,3,5-tri-O-acetyl mannitol, and 2,3,6 tri-O-methyl 1,4,5-tri-O-acetyl mannitol products expected from 2-, 3, and 4-linked mannose were not present as shown using selected ion chromatographs of ions specific for these compounds.
The second quantitatively minor Ara-Man2-octyl (peak II; Fig. 4a) was also analyzed by LC/MS/MS. Due to its low amounts, we were unable to obtain complete structural information on it. The MS/MS spectrum was consistent, for the most part, with the arabinosyl residue being attached to the interior mannosyl residues as shown in (see Supplementary Figure 2). Thus, it is possible that this molecule is the expected Ara-(1→2)-[Man-(1→6)]-Man-(1→octyl but the spectrum certainly does not show the linkages clearly and even shows some ambiguity in regards to the branched nature of the component.
Finally, to assess the dependence of the new AraT activity detected herein for the O-6′ hydroxyl group of the terminal mannose of acceptor (1), non-radiolabeled assays were repeated using C6-deoxygenated disaccharide as acceptor substrate (Fig. 2a, acceptors 4). This substrate was previously reported to serve as acceptor for unknown mannosyltransferase14. As expected, we did not observe any (1→6) AraT activity with this substrate (Fig. 2c).
Conclusions
The present study is the first report of an EMB-resistant mycobacterial (1→6) AraT capable of utilizing synthetic (1→6)-Manp disaccharide and trisaccharide as acceptors in the formation of arabinose-containing tri- and tetra-saccharides. The structure of the major product using the dimannoside acceptor as Araf-(1→6)-Manp-(1→6)-Manp-(1→octyl (as determined by LC/MS/MS and further supported by GC/MS results) was surprising given an earlier study by Chatterjee et al.23 which had proposed that the priming arabinose on the mannan backbone of LAM from M. tuberculosis was on the 2 position of a 2,6-linked mannosyl residue. Thus, as frequently is the case, biosynthetic data supplements pure structural data and, to investigate the reasons for this discrepancy, studies are being undertaken in our laboratory to revisit the linkage of the priming arabinosyl residue in the LAM of M. smegmatis and M. tuberculosis. Preliminary studies based on the enzymatic degradation of the truncated LAM produced by an embC knockout mutant of M. smegmatis indicate that, at least in this species, significant amounts of an arabinosyl residue attached at O-6 of a mannosyl residue are present (unpublished work in progress), consistent with the major enzymatic activity detected herein. Whether M. tuberculosis LAM and M. smegmatis LAM are different in this respect is still under investigation. The presence of a minor product which might be an arabinosyl residue attached to the 2 position of a 6-linked mannosyl product (although this structure is far from demonstrated) further complicates our understanding of LAM structure and its biosynthesis. It is possible that arabinosyl residues are found attached to mannosyl residues in two ways and, thus, we emphasize that the finding of the present work requires detailed structural investigation of LAM from both M. smegmatis and M. tuberculosis.
Another important aspect of the present work is the methodology developed to identify the priming arabinosyltransferase(s) of LAM. Overexpression of candidate genes in M. smegmatis followed by enzymatic analysis as described herein should result in an increase in the amounts of the major or minor products depending on enzymatic activity. Such overexpression studies, along with the above mentioned structural studies, are in progress in our laboratory.
METHODS
Arabinosyltransferase assay
Enzymatically-active membranes and cell envelope (P60) fractions from M. smegmatis mc2155 were prepared as described previously12. The [14C]-labeled arabinose donor, phosphoribose pyrophosphate (p[14C]Rpp), was generated from uniformly labeled D-[14C] glucose (American Radiochemical Inc.) as described24. AraT assay reaction mixture contained synthetic mannoside acceptors (see Fig. 2a and Supplementary Methods) (0.2 mM), ATP (1 mM), p[14C]Rpp (500,000 dpm), buffer A [50 mM MOPS (pH 8), 5 mM 2-mercaptoethanol, 10 mM MgCl2], membrane (0.5 mg) and P60 (0.3 mg) fractions in a total volume of 200 μL. Negative control reactions lacked acceptor substrates. Reaction mixtures were incubated at 37 °C for 2 h and terminated by the addition of 200 μL of ethanol. Upon centrifugation at 14,000 rpm for 10 min, the resulting supernatant was loaded onto a strong anion exchange column (Hypersep SAX; Thermo scientific) prequilibrated in water. The products were eluted from the column with 2 mL 50 % ethanol solution, and the eluents evaporated to dryness and finally partitioned between 1-butanol and water (1:1). Butanol fractions were recovered and the lower aqueous phase was further extracted twice with 1-butanol. The pooled butanol fractions were backwashed with 1-butanol, dried and resuspended in 200 μL of 1-butanol. Equal volumes of radiolabeled products were applied to aluminum-backed silica gel 60 F254TLC plates and developed in CHCl3/CH3OH/13 M NH4OH/1M NH4OAc/H2O (180:140:9:9:23 by vol.) followed by autoradiography at −80 °C using Biomax MR1 films (Kodak). Non-radioactive AraT assays were performed as above in the presence of DPA (100 μM) synthesized in-house21.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) grants AI064798, and the Alberta Glycomics Centre. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank D. Dick for LC/MS analyses, C. Brockling for MS/MS analyses, and A. Amin for her help with the preparation of pRpp.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet.
References
- 1.World Health Organization. Global Tuberculosis Report 2015. WHO Press; 2015. [Google Scholar]
- 2.Angala SK, Belardinelli JM, Huc-Claustre E, Wheat WH, Jackson M. The cell envelope glycoconjugates of Mycobacterium tuberculosis. Crit Rev Biochem Mol Biol. 2014;49:361–399. doi: 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolly GS, Boldrin F, Sala C, Dhar N, Hartkoorn RC, Ventura M, Serafini A, McKinney JD, Manganelli R, Cole ST. Assessing the essentiality of the decaprenyl-phospho-d-arabinofuranose pathway in Mycobacterium tuberculosis using conditional mutants. Mol Microbiol. 2014;92:194–211. doi: 10.1111/mmi.12546. [DOI] [PubMed] [Google Scholar]
- 4.Birch HL, Alderwick LJ, Appelmelk BJ, Maaskant J, Bhatt A, Singh A, Nigou J, Eggeling L, Geurtsen J, Besra GS. A truncated lipoglycan from mycobacteria with altered immunological properties. Proc Natl Acad Sci USA. 2010;107:2634–2639. doi: 10.1073/pnas.0915082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Škovierová H, Larrouy-Maumus G, Zhang J, Kaur D, Barilone N, Korduláková J, Gilleron M, Guadagnini S, Belanová M, Prevost M-C. AftD, a novel essential arabinofuranosyltransferase from mycobacteria. Glycobiology. 2009;19:1235–1247. doi: 10.1093/glycob/cwp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang N, Torrelles JB, McNeil MR, Escuyer VE, Khoo K-H, Brennan PJ, Chatterjee D. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N-terminal recognition region and a C-terminal synthetic region. Mol Microbiol. 2003;50:69–76. doi: 10.1046/j.1365-2958.2003.03681.x. [DOI] [PubMed] [Google Scholar]
- 7.Berg S, Starbuck J, Torrelles JB, Vissa VD, Crick DC, Chatterjee D, Brennan PJ. Roles of Conserved Proline and Glycosyltransferase Motifs of EmbC in Biosynthesis of Lipoarabinomannan. J Biol Chem. 2005;280:5651–5663. doi: 10.1074/jbc.M411418200. [DOI] [PubMed] [Google Scholar]
- 8.Shi L, Berg S, Lee A, Spencer JS, Zhang J, Vissa V, McNeil MR, Khoo K-H, Chatterjee D. The carboxy terminus of EmbC from Mycobacterium smegmatis mediates chain length extension of the arabinan in lipoarabinomannan. J Biol Chem. 2006;281:19512–19526. doi: 10.1074/jbc.M513846200. [DOI] [PubMed] [Google Scholar]
- 9.Alderwick LJ, Lloyd GS, Ghadbane H, May JW, Bhatt A, Eggeling L, Futterer K, Besra GS. The C-terminal domain of the Arabinosyltransferase Mycobacterium tuberculosis EmbC is a lectin-like carbohydrate binding module. PLoS Pathog. 2011;7:e1001299. doi: 10.1371/journal.ppat.1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korkegian A, Roberts DM, Blair R, Parish T. Mutations in the essential arabinosyltransferase EmbC lead to alterations in Mycobacterium tuberculosis lipoarabinomannan. J Biol Chem. 2014;289:35172–35181. doi: 10.1074/jbc.M114.583112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayers JD, Lowary TL, Morehouse CB, Besra GS. Synthetic arabinofuranosyl oligosaccharides as mycobacterial arabinosyltransferase substrates. Bioorg Med Chem Lett. 1998;8:437–442. doi: 10.1016/s0960-894x(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 12.Khasnobis S, Zhang J, Angala SK, Amin AG, McNeil MR, Crick DC, Chatterjee D. Characterization of a specific arabinosyltransferase activity involved in mycobacterial arabinan biosynthesis. Chem Biol. 2006;13:787–795. doi: 10.1016/j.chembiol.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Brown JR, Field RA, Barker A, Guy M, Grewal R, Khoo KH, Brennan PJ, Besra GS, Chatterjec D. Synthetic mannosides act as acceptors for mycobacterial alpha1-6 mannosyltransferase. Bioorg Med Chem. 2001;9:815–824. doi: 10.1016/s0968-0896(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 14.Tam PH, Besra GS, Lowary TL. Exploring the Substrate Specificity of a Mycobacterial Polyprenol Monophosphomannose-Dependent α-(1→ 6)-Mannosyltransferase. ChemBioChem. 2008;9:267–278. doi: 10.1002/cbic.200700391. [DOI] [PubMed] [Google Scholar]
- 15.Mishra AK, Alderwick LJ, Rittmann D, Tatituri RV, Nigou J, Gilleron M, Eggeling L, Besra GS. Identification of an alpha(1-->6) mannopyranosyltransferase (MptA), involved in Corynebacterium glutamicum lipomanann biosynthesis, and identification of its orthologue in Mycobacterium tuberculosis. Mol Microbiol. 2007;65:1503–1517. doi: 10.1111/j.1365-2958.2007.05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L, Zhou R, Liu Z, Lowary TL, Seeberger PH, Stocker BL, Crick DC, Khoo K-H, Chatterjee D. Transfer of the First Arabinofuranose Residue to Galactan Is Essential for Mycobacterium smegmatis Viability. J Bacteriol. 2008;190:5248–5255. doi: 10.1128/JB.00028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alderwick LJ, Seidel M, Sahm H, Besra GS, Eggeling L. Identification of a Novel Arabinofuranosyltransferase (AftA) Involved in Cell Wall Arabinan Biosynthesis in Mycobacterium tuberculosis. J Biol Chem. 2006;281:15653–15661. doi: 10.1074/jbc.M600045200. [DOI] [PubMed] [Google Scholar]
- 18.Telenti A, Philipp WJ, Sreevatsan S, Bernasconi C, Stockbauer KE, Wieles B, Musser JM, Jacobs WR., Jr The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat Med. 1997;3:567–570. doi: 10.1038/nm0597-567. [DOI] [PubMed] [Google Scholar]
- 19.Belanger AE, Besra GS, Ford ME, Mikusová K, Belisle JT, Brennan PJ, Inamine JM. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci USA. 1996;93:11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goude R, Amin A, Chatterjee D, Parish T. The arabinosyltransferase EmbC is inhibited by ethambutol in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2009;53:4138–4146. doi: 10.1128/AAC.00162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liav A, Huang HR, Ciepichal E, Brennan PJ, McNeil MR. Stereoselective synthesis of decaprenylphosphoryl beta-D-arabinofuranose. Tetrahedron Lett. 2006;47:545–547. [Google Scholar]
- 22.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carb Res. 1984;131:209–217. [Google Scholar]
- 23.Chatterjee D, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology. 1993;3:497–506. doi: 10.1093/glycob/3.5.497. [DOI] [PubMed] [Google Scholar]
- 24.Scherman MS, Kalbe-Bournonville L, Bush D, Xin Y, Deng L, McNeil M. Polyprenylphosphate-pentoses in mycobacteria are synthesized from 5-phosphoribose pyrophosphate. J Biol Chem. 1996;271:29652–29658. doi: 10.1074/jbc.271.47.29652. [DOI] [PubMed] [Google Scholar]
- 25.Guerardel Y, Maes E, Elass E, Leroy Y, Timmerman P, Besra GS, Locht C, Strecker G, Kremer L. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with alpha 1,3-mannopyranose side chains. J Biol Chem. 2002;277:30635–30648. doi: 10.1074/jbc.M204398200. [DOI] [PubMed] [Google Scholar]
- 26.Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J. 5:397–409. [Google Scholar]
- 27.Kaur D, Angala SK, Wu SW, Khoo KH, Chatterjee D, Brennan PJ, Jackson M, McNeil MR. A single arabinan chain is attached to the phosphatidylinositol mannosyl core of the major immunomodulatory mycobacterial cell envelope glycoconjugate, lipoarabinomannan. J Biol Chem. 2014;289:30249–30256. doi: 10.1074/jbc.M114.599415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.