Abstract
Although animal models have consistently demonstrated acute pain-inhibitory effects of nicotine and tobacco, human experimental studies have yielded mixed results. The main goal of this meta-analysis was to quantify the effects of nicotine/tobacco administration on human experimental pain threshold and tolerance ratings. A search of PubMed and PsychINFO online databases identified 13 eligible articles, including k = 21 tests of pain tolerance (N = 393) and k = 15 tests of pain threshold (N = 339). Meta-analytic integration for both threshold and tolerance outcomes revealed that nicotine administered via tobacco smoke and other delivery systems (e.g., patch, nasal spray) produced acute analgesic effects that may be characterized as small to medium in magnitude (Hedges’ g = .35, 95% CI = .21-.50). Publication bias-corrected estimates remained significant and indicated that these effects may be closer to small. Gender composition was observed to be a significant moderator, such that pain threshold effects were more robust among samples that included more men than women. These results help to clarify a mixed literature, and may ultimately help to inform the treatment of both pain and nicotine dependence. Pain and tobacco smoking are both highly prevalent and comorbid conditions, current smoking has been associated with more severe chronic pain and physical impairment, and acute nicotine-induced analgesia could make smoking more rewarding and harder to give up. Future research should employ dynamic measures of experimental pain reactivity and further explore biopsychosocial mechanisms of action.
Keywords: pain, nicotine, tobacco, smoking, analgesia, analgesic, meta-analysis
1. Introduction
Laboratory studies in the area of pain and smoking have primarily focused on how nicotine (delivered directly or via tobacco smoke) may modulate pain responding among animals and humans. Although animal models have consistently demonstrated acute pain-inhibitory effects of nicotine/tobacco administration for over 80 years [19; 44], human experimental studies have generated mixed and somewhat contradictory findings [22; 70]. Indeed, the question of whether nicotine may decrease sensitivity to pain in humans has been a topic of empirical debate since the effects of smoking on human pain reactivity were first examined in 1973 [45; 58].
Although a recent comprehensive review of relations between pain, nicotine, and tobacco smoking noted that just over half of all published human experimental studies demonstrated acute analgesic effects of nicotine/tobacco [22], we are not aware of any previous work that used meta-analytic techniques to synthesize the extant literature and generate estimated effect sizes. Possible explanations for mixed findings include differential timing and dosage of nicotine administration relative to experimental pain onset, variability in the stimuli used to induce experimental pain, gender differences in response to noxious stimulation [32; 67], the recruitment of relatively small samples comprised of both smokers who had been habituated to nicotine and nicotine naïve nonsmokers [70], and the possibility that pain-inhibitory effects among humans may be achieved indirectly via other biopsychosocial mechanisms [22].
In closely examining the relevant empirical literature, we observed substantial variability with regard to how the results of studies that tested the effects of nicotine on human experimental pain reactivity tended to be characterized. For example, whereas many authors simply acknowledged that discrepant results limit or confound interpretability [61], some took a more selective approach by only reviewing a subset of outcomes (e.g., those obtained using cold pressor pain induction [77]), while others drew the more general conclusion that nicotine has been shown to reduce pain among smokers and nonsmokers [8]. Such interpretative variability is understandable given the mixed state of this literature and underscores the need for a meta-analytic approach to estimating effect sizes and testing potential moderators.
Clarifying the extent to which nicotine may produce acute analgesic effects in humans may also inform the treatment of both pain and tobacco addiction. For example, in referencing the broad experimental literature, researchers have suggested that intranasal or transdermal nicotine may have utility as a postoperative analgesic [10], though initial trials have yielded inconsistent results [79]. Researchers have also long proposed that the avoidance and/or relief of pain may serve as a potent reinforcer in the maintenance of tobacco smoking [66], and there are emerging data to support this notion. For example, experimental pain induction has been shown to increase motivation to smoke [21; 23], and treatment-seeking pain patients have reported smoking tobacco to cope with pain [41; 62].
In sum, despite decades of scientific inquiry, the degree to which nicotine may produce acute analgesic effects among humans remains unclear. The goals of this meta-analysis were to quantify the magnitude of effects of nicotine/tobacco administration on human experimental pain threshold and tolerance ratings, and to identify potential moderators of these relations.
2. Methods
2.1. Search procedure
Relevant articles published prior to April 2015 were identified using PubMed and PsycINFO online databases. Searches in PubMed were conducted using the major search term pain in combination with the major search terms smoking, or nicotine, or tobacco. Searches in PsycINFO were conducted with the subject heading pain in combination with the subject heading smoking, or nicotine, or tobacco. Two independent reviewers manually examined the reference lists of all relevant articles. These searches generated 531 unique articles.
2.2. Determination of outcome variables
Responses to painful stimuli have been quantified using measures of pain threshold and/or pain tolerance [43]. Pain threshold has typically been measured by either the duration of time (e.g., seconds) or intensity (e.g., volts) at which participants first report experiencing pain following the stimulus onset. Pain tolerance has typically been measured by either the maximum duration of time or intensity at which participants are no longer willing or able to tolerate the stimulus. Given evidence that pain threshold and tolerance may differentiate sensory-discriminative and affective-motivational pain processes (e.g., Meagher, Arnau, Rhudy, 2001[53]), both pain threshold and tolerance were selected as primary outcomes. Other factors that have been shown to influence laboratory pain responding (e.g., gender and smoking status) were identified as potential moderators.
2.3. Study selection
Studies were included if they met each of the following criteria: (1) sample was comprised of human participants; (2) utilization of an experimental/laboratory method of pain-induction; (3) nicotine administration prior to or during pain induction; (4) designs were either between-subjects (comparing nicotine administration to a non-nicotine control) or within-subjects (assessing laboratory pain reactivity pre-and-post nicotine administration); and (5) incorporated measurement of pain threshold and/or tolerance.
2.4. Validity assessment
The methodological quality of each study was rated on a validity scale that was based on Cochrane Collaboration criteria, PRISMA recommendations, and PEDro guidelines [50; 56; 69]. The 12-item validity scale accounted for relevant aspects of internal, external, and construct validity and yielded a maximum score of 12 (see Table 1).
Table 1.
Study Validity Assessment Items and Ratings
| Validity Items | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Study | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12* | Total |
| Fertig et al. (1986)-a [31] | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| Fertig et al. (1986)-b [31] Jamner | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| et al. (1998)-a, b, c, d [45] Jarvik | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| et al. (1989) [46] | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kanarek & Carrington (2004) [47] | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Lane et al. (1995) [48] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| Nastase et al. (2007)-a, b [57] | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 8 |
| Nesbitt (1973) [58] | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 8 |
| Pauli et al. (1993) [63] | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 6 |
| Perkins et al. (1994)-a, b [64] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 7 |
| Perkins et al. (1994)-c, d [64] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 9 |
| Perkins et al. (1994)-e, f [64] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 9 |
| Pomerleau et al. (1984) [66] | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Shiffman & Jarvik (1984) [71] | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Silverstein (1982) [73] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| Unrod et al. (2004) [78] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 11 |
Notes. Item 1: Were subjects randomly allocated to groups (in a within-subjects design, were subjects randomly allocated an order in which treatments were received)?; Item 2: Was allocation of participants to groups concealed?; Item 3: Was there a description of all participants who did not complete study measures?; Item 4: Were study objectives defined clearly?; Item 5: Were the outcome measures defined clearly?; Item 6: Was there a clear description of the inclusion and exclusion criteria?; Item 7: Was the sample size justified (e.g., power calculation)?; Item 8: Was there a clear description of the interventions (i.e., pain procedure and nicotine administration procedure)?; Item 9: Was there at least one control (comparison) group?; Item 10: Were relevant participant characteristics described?; Item 11: Were complete outcome data reported (i.e., point measures and measures of variability)?; Item 12: Were outcome data reported selectively?; No = “0”; Yes = “1”;
Item 12 was reverse-scored.
2.5. Data extraction
Data were extracted and coded by two independent reviewers. Primary studies were first classified as between- or within-subjects. For between-subjects designs, mean pain ratings (along with standard deviations and standard errors) were recorded for each group. For within-subjects designs, mean pre- and post-nicotine administration pain ratings (along with standard deviations and standard errors of the mean), and mean difference scores (along with standard deviations and standard errors of the difference) were recorded.
Additional data were extracted to test potential moderators. The total number of participants, gender composition, and average age were recorded. Participant smoking status was coded as either smoker or nonsmoker. Data were also extracted to characterize methods of pain induction (e.g., electrical, thermal), nicotine administration (e.g., patch, cigarette), study design (i.e., between vs. within subjects), and active control condition (vs. passive control). Several data extraction decision points were noteworthy. First, in the single study that incorporated more than one method of nicotine administration [31], no differences were observed as a function of experimental condition and a composite effect size was computed to collapse across experimental conditions. Second, when other experimental manipulations were examined in the same study (e.g., distraction, nicotine deprivation), we collapsed across the other manipulations [63; 78]. Third, in the few cases where a single study administered multiple levels of nicotine, the highest dose was used [48; 58; 64; 73]. Fourth, when agents in addition to nicotine were administered (e.g., sucrose or caffeine), means were recorded only from cells that did not include them [47; 57]. Finally, when studies included subsamples of participants that reflected characteristics of interest in our moderation analyses (i.e., gender stratification and smoking status), each subsample was treated as an individual study [31; 45; 57; 64]. In those cases, only the sample size corresponding to each subsample was extracted.
2.6. Quantitative data synthesis
Individual study effect sizes and meta-analytic statistics were calculated using Comprehensive Meta-Analysis [1]. Separate meta-analyses were conducted for pain threshold and tolerance outcomes. Raw data were used when available (threshold: 86%; tolerance: 87%), and t or p statistics were used otherwise. For the two occasions when statistical significance was indicated but no p-value was reported, it was imputed as .05 [66; 73]. When calculating matched group effect sizes, a conservative correlation of .7 was assumed [68]. Hedges’ g was calculated (along with 95% CIs) by use of a random effects approach. Hedges’ g is a summary statistic that corrects for small sample size bias and may be interpreted comparatively with Cohen’s d of .2 representing small, .5 medium, and .8 large effects [14].
2.7. Publication bias analyses
Meta-analytic results may be biased when studies that report significant findings are more likely to be published than those with null findings. To address potential publication bias, we constructed and examined funnel plots for both pain threshold and tolerance outcomes. We then utilized established Trim and Fill methods to estimate the number of missing studies needed to make the plots symmetrical, and present adjusted effect sizes [27]. To further examine potential publication bias, we also conducted two statistical tests of funnel plot asymmetry, including the Begg and Mazumdar (1994) non-parametric test (based on the rank correlation between effect estimates and their sampling variances), and the Egger et al. (1997) regression method, which tests for a linear association between the manipulation effect and its standard error [7; 28].
2.8. Moderator analysis
Six potential moderators (i.e., smoking status, gender composition, study quality, pain induction modality, control condition, and study design) were identified based on previous research and data that were available in published studies [22]. Mixed effects analyses were used to examine smoking status (i.e., smoker vs. nonsmoker), pain induction modality (i.e., electrical vs. thermal vs. cold pressor), control condition (i.e., active vs. passive) and study design (i.e., between-subjects vs. within-subjects), and continuous moderators (i.e., gender composition and study quality) were tested via meta-regressions using method-of-moments for parameter estimation [1; 51]. However, given that residual heterogeneity (i.e., noise not explained by the moderator) is an essential factor in determining when associations between moderator variables and effect sizes can be statistically detected [38], we first conducted two tests of heterogeneity (Q statistic and I2 index) to determine whether moderation analyses should proceed. Briefly, the Q statistic reflects statistical significance (the presence vs. absence) of heterogeneity, and the I2 index reflects the extent of heterogeneity (percentage of the total variability) across effect sizes, with I2 values of 25%, 50%, and 75% corresponding to low, moderate, and high levels of heterogeneity, respectively [39]. If primary analyses revealed evidence of heterogeneity, moderation tests were conducted.
3. Results
3.1. Study selection
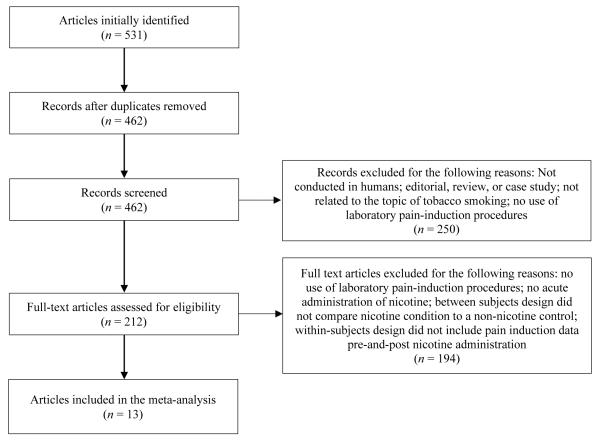
A flowchart of our approach to study selection is presented in Figure 1. Of the 531 articles identified as potentially relevant via initial searches, 69 were found to be duplicates, and the remaining 462 were screened for inclusion. Of these, 212 were deemed potentially eligible and worthy of full-text review by two independent raters. Of these, 194 articles were excluded for not meeting inclusion criteria (e.g., did not induce pain, administer nicotine, or measure pain threshold or tolerance). The remaining 13 articles provided the data necessary to examine both pain threshold (k = 21 comparisons) and tolerance (k = 15 comparisons) outcomes.
Figure 1.
Study selection flowchart.
3.2. Characteristics of the study samples
Individual study characteristics are presented in Table 2. The vast majority of studies utilized a within-subjects design (threshold: 90%; tolerance: 87%), and measured both threshold and tolerance.
Table 2.
Study Sample Characteristics
| Study Sample | N (% Female) | Design | Pain Stimulus | Threshold Comparison | Tolerance Comparison |
|---|---|---|---|---|---|
| Fertig et al. (1986)-a [31] Fertig et al. (1986)-b [31] |
10sm (0) 15e-sm (0) |
Within | Cold Pressor | Smoke + Snuff vs. Zero-Nic Cig + Sham1,2 Snuff vs. Zero-Nic Snuff |
Smoke + Snuff vs. Zero-Nic Cig + Sham1,2 Snuff vs. Zero-Nic Snuff |
|
| |||||
| Jamner et al. (1998)-a [45] Jamner et al. (1998)-b [45] Jamner et al. (1998)-c [45] Jamner et al. (1998)-d [45] |
21sm (0) 17sm (0) 23n-sm (0) 13sm (0) |
Within | Heat | Nic Patch vs. Zero-Nic Patch | Nic. Patch vs. Zero-Nic Patch |
|
| |||||
| Jarvik et al. (1989) [46] | 15sm (27) | Within | Cold Pressor | No Data Reported | Smoke vs. Deprived |
|
| |||||
| Kanarek & Carrington (2004) [47] | 49sm (51) | Within | Cold Pressor | Smoke vs. No Smoke | Smoke vs. No Smoke |
|
| |||||
| Lane et al. (1995) [48] | 18sm (49) | Within | Heat | Smoke vs. Abstinence | Smoke vs. Abstinence |
|
| |||||
| Nastase et al. (2007)-a [57] Nastase et al. (2007)-b [57] |
11sm (100) 12sm (0) |
Within | Cold Pressor | Smoke vs. No Smoke | Smoke vs. No Smoke |
|
| |||||
| Nesbitt (1973) [58] | 30sm (0) | Within | Electrical | Not Tested | Smoke vs. Sham1 |
|
| |||||
| Pauli et al. (1993) [63] | 9sm (0) | Within | Heat | Pre-Post Smoking | Not Tested |
|
| |||||
| Perkins et al. (1994)-a [64] Perkins et al. (1994)-b [64] |
10sm (0) 10n-sm (0) |
Between | Heat | Pre-Post Nic Spray | Not Tested |
| Perkins et al. (1994)-c [64] Perkins et al. (1994)-d [64] |
12sm (50) 12n-sm (50) |
Between | Heat | Nic vs. Zero Nic Spray (pre-post) | Not Tested |
| Perkins et al. (1994)-e [64] Perkins et al. (1994)-f [64] |
18sm (50) 18n-sm (50) |
Between | Heat | Nic vs. Zero Nic Spray (pre-post) | Not Tested |
|
| |||||
| Pomerleau et al. (1984) [66] | 5sm (0) | Within | Cold Pressor | Smoke vs. Zero Nic Cig | Smoke vs. Placebo |
|
| |||||
| Shiffman & Jarvik (1984) [71] | 10sm (80) | Within | Electrical | Smoke vs. Sham1 | Not Tested |
|
| |||||
| Silverstein (1982) [73] | 20sm (0) | Between | Electrical | Smoke vs. No Smoke | Smoke vs. No Smoke |
|
| |||||
| Unrod et al. (2004) [78] | 80sm (50) | Between | Cold Pressor | Smoke vs. No Smoke | Smoke vs. No Smoke |
Notes. Sham = simulated smoking using an unlit cigarette;
data within each condition was collapsed across both manipulations. Between = between-subjects design; Cig = cigarette; e-sm = ex-smoker; Nic. = Nicotine; n-sm = non-smoker; sm = smoker; Within = within-subjects design.
3.2.1. Pain threshold comparisons and sample characteristics
The 21 comparisons included in the pain threshold meta-analysis were comprised of 393 participants (M age = 32 years). Of these, 77% were classified as tobacco smokers (M cigarettes per day = 22). Methods of nicotine administration included tobacco smoking (k = 10), nicotine nasal spray (k = 6), nicotine patch (k = 4), and tobacco snuff (k = 1). Nicotine was administered prior to pain induction in all but two pain threshold comparisons (90%). Most comparisons (k = 12) administered nicotine within 15 minutes of pain induction, and three comparisons administered nicotine during pain induction [58; 71; 73].
3.2.2. Pain tolerance comparisons and sample characteristics
The 15 comparisons included in the pain tolerance meta-analysis were comprised of 339 participants (M age = 33 years). Of these, 84% were classified as tobacco smokers (M cigarettes per day = 22). Methods of nicotine administration included tobacco smoking (k = 10), nicotine patch (k = 4), and tobacco snuff (k = 1). Again, nicotine was administered prior to pain induction in all but two pain tolerance studies (87%) [58; 73].
3.2.3. Quality of included studies
Across studies, quality scores ranged from 6-11 out of 12 (threshold studies M = 8.93, SD = 1.44; tolerance studies M = 9.27, SD = 1.19). Two independent reviewers examined validity criteria for each study (inter-rater r = .73), and 100% agreement was obtained via discussion. Quality scores were not related to meta-analytic findings for threshold (p = .18) or tolerance (p =.30). Across all studies, the validity categories least frequently addressed by the primary studies included justification of sample size and experimenter blinding. Specifically, only two studies provided justification for the sample size (e.g., via power calculations), and only five studies concealed group allocation. We also observed that seven studies failed to provide full inclusion/exclusion criteria.
3.3. Effects of nicotine/tobacco on pain threshold
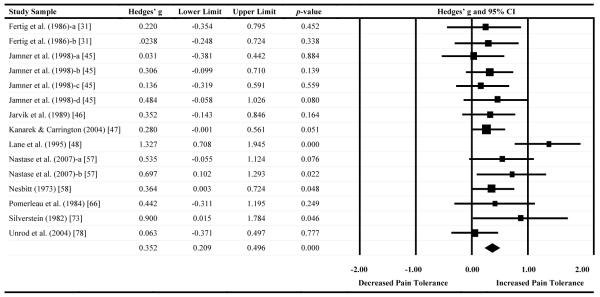
A forest plot of effect sizes for pain threshold as a function of nicotine administration (based on 21 comparisons) is presented in Table 3. Seven comparisons (33%) demonstrated significant analgesic effects of nicotine/tobacco (i.e., that nicotine increased pain threshold). In contrast, no comparisons (0%) demonstrated a significant decrease in pain threshold following nicotine administration, which indicates that nicotine did not increase pain sensitivity. The Hedges’ g effect size was .35 (CI: .21-.50, Z = 4.88, p < .001), indicating that nicotine administered via tobacco smoke and other means had an analgesic effect on pain threshold that may be characterized as small to medium in magnitude [14].
Table 3.
Effect sizes, confidence intervals, and forest plot for pain threshold outcomes
Notes. Effect sizes to the right of zero reflect greater nicotine/tobacco-induced pain inhibition. Confidence intervals that do not include zero reflect significant differences.
Summary statistics were computed via random effects meta-analysis.
There was evidence of publication bias across pain threshold comparisons. Trim and fill methods revealed that seven studies would need to be imputed to create symmetry in the pain threshold funnel plot, and that doing so would reduce the observed effect size from .35 to .20 (CI = .04-.36). Complementary tests, including the Begg-Mazumdar rank correlation test (Kendall’s Tau = .54, p < .001) and Egger’s test (t = 3.89, p < .001), provided further evidence of bias.
3.4. Effects of nicotine/tobacco on pain tolerance
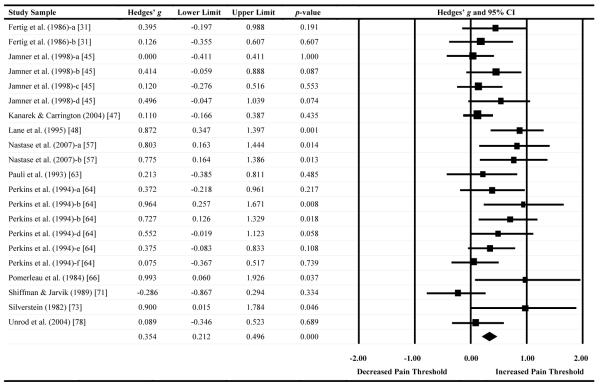
A forest plot of effect sizes for pain tolerance as a function of nicotine administration (based on 15 comparisons) is presented in Table 4. Four comparisons (27%) demonstrated significant analgesic effects (i.e., that nicotine increased pain tolerance). Similar to the results for pain threshold, there was no evidence that nicotine/tobacco administration decreased tolerance to painful stimuli. The Hedges’ g effect size was .35 (CI: .21-.50, Z = 4.81, p < .001), indicating that nicotine administered via tobacco smoke, nicotine patch, or smokeless tobacco resulted in small- to medium-sized analgesic effects on pain tolerance.
Table 4.
Effect sizes, confidence intervals, and forest plot for pain tolerance outcomes
Notes. Effect sizes to the right of zero reflect greater nicotine/tobacco-induced pain tolerance. Confidence intervals that do not include zero reflect significant differences.
Summary statistics were computed via random effects meta-analysis.
Similar to pain threshold, there was evidence of publication bias in the pain tolerance literature. Trim and fill methods estimated that four studies would need to be imputed to create symmetry in the pain tolerance funnel plot, which would reduce Hedges’ g from .35 to .25 (CI: = .08-.42). Again, this finding was corroborated by the results of Begg-Mazumdar’s rank correlation test (Kendall’s Tau = .57, p = .001) and Egger’s test (t = 2.22, p = .02).
3.5. Moderator analyses
We first determined whether there was sufficient heterogeneity to warrant moderation analyses. Significant levels of heterogeneity were observed across comparison effect sizes for pain threshold (Q = 31.21, df = 20, p = .05; I2 = 35.91), but not for pain tolerance (Q = 18.52, df = 14, p =.18; I2 = 24.42). Thus, moderation analyses were limited to pain threshold.
Gender composition was found to be a significant moderator of threshold outcomes (slope = .003, p = .04), such that analgesic effects of nicotine and tobacco were more robust among comparisons that included a larger proportion of men than women. Conversely, neither current smoking status (p = .76), pain induction modality (p = .19), method of nicotine administration (p = .80), control condition (p = .20), nor study design (p = .50) were observed to moderate pain threshold outcomes. Thus, nicotine/tobacco administration was found to produce acute analgesic effects (as measured by pain threshold) regardless of whether participants were current smokers or nonsmokers, and irrespective of whether nicotine was delivered via smoked tobacco or other means. The effects of nicotine/tobacco administration also appeared to remain stable across comparisons that utilized both between- and within-subjects designs, when pain was induced via electrical, thermal, or cold pressor modalities, and when the acute effects of nicotine were compared to either active or passive control conditions.
4. Discussion
4.1. Summary
To determine the extent to which nicotine/tobacco administration may confer acute analgesic effects among humans undergoing experimental pain induction, we extracted data from 13 empirical articles that included 21 comparisons of pain threshold (n = 393 participants) and 15 comparisons of pain tolerance (n = 339 participants). The pooled effect sizes for both pain threshold and tolerance revealed that nicotine administered via tobacco and other means (e.g., nicotine patch and nasal spray) produced acute pain-inhibitory effects that may be characterized as small to medium in magnitude. Publication bias-corrected estimates indicate that these effects may be closer to small than moderate, but still significant. Moderation analyses further revealed that acute analgesic effects may be achieved regardless of nicotine delivery method, current smoking status, pain induction modality, study design, or control condition, and that such effects may be more robust among men than women.
4.2. Clinical implications
Chronic pain and tobacco addiction are both highly prevalent and comorbid disorders that likely interact in a bi-directional manner [22; 84]. Indeed, tobacco smoking has been identified as a unique risk factor in the development of chronic pain [72; 75], pain has been shown to motivate smoking behavior [20; 21], current smoking has been linked to more severe pain and functional impairment among treatment-seeking pain patients [33], and there is mounting evidence that pain may impede smoking cessation [24; 25; 83]. Although it has been suggested that chronic exposure to nicotine and tobacco smoke may increase sensitivity to pain over time [22, 70], these results indicate that nicotine can also produce short-term analgesic effects. One implication of our findings is that, for persons in pain, acute nicotine analgesia could make smoking more rewarding and harder to quit. For example, negative reinforcement models of addiction assert that substance use is largely contingent upon the extent to which it may terminate or mitigate aversive interoceptive states [6; 80]. Based on these data, we would hypothesize that as pain becomes increasingly linked with the self-administration of nicotine to ameliorate pain, the pain experience itself may gain salience as a conditioned interoceptive cue for tobacco smoking. Consistent with theoretical conceptualizations of allostatic load among chronic substance users [29; 30], smokers may become sensitized to the pain experience and evince greater pain reactivity (i.e., hyperalgesia) during the early stages of nicotine withdrawal, which in turn, could motivate relapse to smoking [15; 22; 70; 84].
In addition, our finding that analgesic effects were more robust among studies that included a larger proportion of men suggests that male smokers may be at greater risk for increasing their dependence on nicotine via pain-smoking-analgesia processes than female smokers (though this statement requires direct empirical scrutiny). Finally, that method of nicotine administration was not found to moderate pain threshold outcomes suggests that smokers with comorbid pain may derive a similar degree of analgesic benefit from nicotine replacement therapy (e.g., nicotine patch/gum) as they would from tobacco smoking. Given evidence that smokers with chronic pain are amenable to pharmacotherapy for smoking cessation [82], treatment programs may consider the utility of high-dose and combination nicotine replacement therapy [35; 55] when treating smokers in pain.
4.3. Directions for future research
First, it is imperative that future research move beyond the static measurement of pain threshold/tolerance to more dynamic quantitative sensory testing (QST) that may enhance our understanding of neurobiological mechanisms in acute nicotine analgesia (e.g., Hansson, Backonja, & Bouhassira, 2007 [34]). For example, a QST approach could allow researchers to distinguish the effects of nicotine on ascending pain-facilitatory processes (e.g., via temporal summation) from those on descending pain-inhibitory processes (e.g., via conditioned pain modulation [59]). Second, only three studies used a procedure in which participants were required to smoke during pain induction [57; 58; 71]. It would seem prudent to test the influence of nicotine administered before, relative to during, the experience of pain to determine whether acute analgesic effects vary based on timing of administration. In addition, although we suspect that the act of smoking during pain may function as a behavioral distractor that serves to augment the analgesic properties of nicotine, this hypothesis has not yet been tested. Third, although we did not observe differences in nicotine analgesia as a function of pain induction modality, the vast majority of studies reviewed herein utilized short-acting methods of pain induction that typically last no more than a few seconds. Future research would benefit from testing longer-acting pain stimuli that may better approximate features of clinical pain. The capsaicin experimental pain model, for example, has been shown to evoke pain that steadily increases for approximately 15-20 minutes, resulting in central sensitization and associated symptoms (e.g., hyperalgesia and allodynia) that have been described as essential characteristics of clinical pain [60]. Fourth, additional work is needed to examine the uniquely-human role of cognitive expectations for pain reduction and/or pain-coping via tobacco smoking [26], as chronic pain patients have reported that tobacco smoking influences both their experience of pain and their use of prescription opioid medications [42; 83]. Given that smokers readily endorse use of smoking for pain-coping [26; 42; 62], it is also possible that the small to medium effect sizes observed in the current meta-analysis may underestimate the acute analgesic effects of nicotine on clinical pain, and additional studies should also measure clinical pain reporting in the context of acute nicotine administration. Finally, future research should examine whether nicotine-induced pain reduction covaries with reinforcing effects/value derived from tobacco smoking. For example, smokers who experience greater nicotine analgesia may also demonstrate greater satisfaction from smoking, increased pain-induced urge to smoke, and greater cigarette consumption during painful episodes.
4.4. Potential mechanisms of nicotine/tobacco-induced analgesia
Although not a focus of the current meta-analysis, future research would also benefit from exploring potential mechanisms in acute nicotine analgesia. For example, there is an emerging consensus that acute analgesic effects of nicotine/tobacco likely involve the activation of nicotinic acetylcholine receptors, particularly the α4β2 subtype [17], which are widely distributed throughout the central and peripheral nervous systems [9; 16]. There is also evidence that nicotine-analgesic effects may be mediated via activation of endogenous opioid systems [52] and the release of beta-endorphins [65]. Indeed, endogenous opioid peptides and their receptor subtypes have demonstrated effects of nicotine in spinally-mediated antinociception [13], and animal models have further demonstrated reduced nicotine analgesia in mu-opioid receptor gene knockout mice [11]. Nicotine may also modulate pain via pressor actions on the cardiovascular system [22; 45]. Nicotine has been shown to increase cardiovascular activity [5; 74; 76], which in turn has been associated with reduced pain sensitivity [2; 3; 12; 49]. Other promising mechanisms of interest include the pain-modulating role of serotonin [18; 70], nicotine effects on executive functioning and self-control processes [36; 37], smoking-related blunting of the stress response [4; 17; 32], and nicotine-induced anti-inflammatory actions [54; 81].
4.5. Strengths and limitations
To our knowledge, this is the first study to employ meta-analytic techniques to quantify the magnitude with which nicotine and tobacco may produce acute analgesia among humans. Our findings that nicotine can reduce pain sensitivity and increase pain tolerance among humans helps to clarify a mixed literature that has long been susceptible to misinterpretation. Furthermore, these data have the potential to stimulate future research that may inform the treatment of both pain and tobacco smoking. Several limitations also warrant noting. First, these findings are inherently dependent on factors such as quality of included studies and statistical assumptions about true values [40]. For example, we observed substantial variability across studies in terms of nicotine delivery method, nicotine dose, and the stimuli used to induce experimental pain. In general, the individual study samples were relatively small, females were underrepresented, and heavy alcohol use was not an exclusion criterion for all samples. Second, our analyses were limited to threshold and tolerance outcomes of experimental studies that tend to maximize internal validity at the expense of external validity. Thus, the extent to which these findings may generalize to real-world pain-nicotine/tobacco-analgesia processes remains unclear. Third, included studies generally failed to report other data that would have been of interest in these analyses (e.g., number of years smoking, number of cigarettes smoked per day, presence of chronic pain, and ratings of pain intensity). Finally, we were unable to account for the degree to which pain-inhibitory effects of nicotine/tobacco may be achieved indirectly via other mediating psychological or physiological factors (e.g., expectancies for pain coping/reduction via smoking [23]).
Acknowledgements
This research was supported by NIH Grant Nos. R21DA034285 and R21DA038204 awarded to Joseph W. Ditre, NIH Grant Nos. F31DA033058 and T32DA007288 awarded to Bryan W. Heckman, NIH Grant No. F31DA039628 awarded to Emily L. Zale, and NIH Grant No. 2K05 AA16928 awarded to Stephen A. Maisto.
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
References
- [1].Comprehensive Meta-Analysis. Biostat; Englewood Cliffs, NJ: 2010. [Google Scholar]
- [2].al'Absi M, Buchanan T, Lovallo WR. Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology. 1996;33(6):655–661. doi: 10.1111/j.1469-8986.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- [3].al'Absi M, Petersen KL, Wittmers LE. Blood pressure but not parental history for hypertension predicts pain perception in women. Pain. 2000;88(1):61–68. doi: 10.1016/S0304-3959(00)00306-7. [DOI] [PubMed] [Google Scholar]
- [4].al'Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted Opiate Modulation of Hypothalamic-Pituitary-Adrenocortical Activity in Men and Women Who Smoke. Psychosom Med. 2008;70(8):928–935. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Argacha JF, Adamopoulos D, Gujic M, Fontaine D, Amyai N, Berkenboom G, van de Borne P. Acute effects of passive smoking on peripheral vascular function. Hypertension. 2008;51(6):1506–1511. doi: 10.1161/HYPERTENSIONAHA.107.104059. [DOI] [PubMed] [Google Scholar]
- [6].Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- [7].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- [8].Belfer I. Nature and nurture of human pain. Scientifica (Cairo) 2013;2013:415279. doi: 10.1155/2013/415279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121(4 Suppl 1):S3–10. doi: 10.1016/j.amjmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- [10].Benowitz NL. Nicotine and postoperative management of pain. Anesth Analg. 2008;107(3):739–741. doi: 10.1213/ane.0b013e3181813508. [DOI] [PubMed] [Google Scholar]
- [11].Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci. 2002;22(24):10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bruehl S, Chung OY, Ward P, Johnson B, McCubbin JA. The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: the effects of opioid blockade. Pain. 2002;100(1-2):191–201. doi: 10.1016/s0304-3959(02)00295-6. [DOI] [PubMed] [Google Scholar]
- [13].Campbell VC, Taylor RE, Tizabi Y. Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception. Alcohol Clin Exp Res. 2007;31(8):1435–1440. doi: 10.1111/j.1530-0277.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- [14].Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Inc; Hillsdale: 1988. [Google Scholar]
- [15].Cosgrove KP, Esterlis I, McKee S, Bois F, Alagille D, Tamagnan GD, Seibyl JP, Krishnan-Sarin S, Staley JK. Beta2* nicotinic acetylcholine receptors modulate pain sensitivity in acutely abstinent tobacco smokers. Nicotine Tob Res. 2010;12(5):535–539. doi: 10.1093/ntr/ntq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Daly JW, Garraffo HM, Spande TF, Decker MW, Sullivan JP, Williams M. Alkaloids from frog skin: the discovery of epibatidine and the potential for developing novel non-opioid analgesics. Nat Prod Rep. 2000;17(2):131–135. doi: 10.1039/a900728h. [DOI] [PubMed] [Google Scholar]
- [17].Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, Collins AC, Martin BR. Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. J Pharmacol Exp Ther. 2007;321(3):1161–1169. doi: 10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- [18].Damaj MI, Glennon RA, Martin BR. Involvement of the serotonergic system in the hypoactive and antinociceptive effects of nicotine in mice. Brain Res Bull. 1994;33(2):199–203. doi: 10.1016/0361-9230(94)90252-6. [DOI] [PubMed] [Google Scholar]
- [19].Davis L, Pollock L, Stone T. Visceral pain. Surgery Gynecology and Obstetrics. 1932;55:418–427. [Google Scholar]
- [20].Dhingra LK, Homel P, Grossman B, Chen J, Scharaga E, Calamita S, Shin J, Portenoy R. Ecological Momentary Assessment of Smoking Behavior in Persistent Pain Patients. Clin J Pain. 2013;30(3):205–213. doi: 10.1097/AJP.0b013e31829821c7. [DOI] [PubMed] [Google Scholar]
- [21].Ditre JW, Brandon TH. Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117(2):467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychol Bull. 2011;137(6):1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. J Abnorm Psychol. 2010;119(3):524–533. doi: 10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ditre JW, Kosiba JD, Zale EL, Zvolensky MJ, Maisto SA. Chronic Pain Status, Nicotine Withdrawal, and Expectancies for Smoking Cessation among Lighter Smokers. Ann Behav Med. 2016 doi: 10.1007/s12160-016-9769-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ditre JW, Langdon KJ, Kosiba JD, Zale EL, Zvolensky MJ. Relations between pain-related anxiety, tobacco dependence, and barriers to quitting among a community-based sample of daily smokers. Addict Behav. 2014;42C:130–135. doi: 10.1016/j.addbeh.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ditre JW, Zale EL, Heckman BW, Hendricks PS. A Measure of Pain and Smoking Expectancies: Development and Initial Validation of the Pain and Smoking Inventory. under review.
- [27].Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- [28].Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36(10):2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elman I, Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 2016;89(1):11–36. doi: 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- [31].Fertig JB, Pomerleau OF, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addict Behav. 1986;11(3):239–248. doi: 10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- [32].Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114(3):372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- [33].Goesling J, Brummett CM, Meraj TS, Moser SE, Hassett AL, Ditre JW. Associations between Pain, Current Tobacco Smoking, Depression, and Fibromyalgia Status Among Treatment-Seeking Chronic Pain Patients. Pain Med. 2015;16(7):1433–42. doi: 10.1111/pme.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. 2007;129(3):256–259. doi: 10.1016/j.pain.2007.03.030. [DOI] [PubMed] [Google Scholar]
- [35].Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacol Biochem Behav. 2007;86(1):132–139. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Heckman BW, Ditre JW, Brandon TH. The restorative effects of smoking upon self-control resources: A negative reinforcement pathway. J Abnorm Psychol. 2011;121(1):244–9. doi: 10.1037/a0023032. [DOI] [PubMed] [Google Scholar]
- [37].Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hempel S, Miles JN, Booth MJ, Wang Z, Morton SC, Shekelle PG. Risk of bias: a simulation study of power to detect study-level moderator effects in meta-analysis. Syst Rev. 2013;2:107. doi: 10.1186/2046-4053-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hofmann SG, Smits JA. Pitfalls of meta-analyses. J Nerv Ment Dis. 2008;196(9):716–717. doi: 10.1097/NMD.0b013e318183fd90. author reply 717-718. [DOI] [PubMed] [Google Scholar]
- [41].Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152(1):223–229. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hooten WM, Vickers KS, Shi Y, Ebnet KL, Townsend CO, Patten CA, Warner DO. Smoking cessation and chronic pain: patient and pain medicine physician attitudes. Pain Practice. 2011;11(6):552–563. doi: 10.1111/j.1533-2500.2011.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].IASP . Part III: Pain Terms, A Current List with Definitions and Notes on Usage. In: Merskey HB, N., editors. Classification of Chronic Pain. Second Edition IASP Press; Seattle: 1994. [Google Scholar]
- [44].Jackson KJ, Damaj MI. Calcium/calmodulin-dependent protein kinase IV mediates acute nicotine-induced antinociception in acute thermal pain tests. Behav Pharmacol. 2013;24(8):689–692. doi: 10.1097/FBP.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jamner LD, Girdler SS, Shapiro D, Jarvik ME. Pain inhibition, nicotine, and gender. Exp Clin Psychopharmacol. 1998;6(1):96–106. doi: 10.1037//1064-1297.6.1.96. [DOI] [PubMed] [Google Scholar]
- [46].Jarvik ME, Caskey NH, Rose JE, Herskovic JE, Sadeghpour M. Anxiolytic effects of smoking associated with four stressors. Addict Behav. 1989;14(4):379–386. doi: 10.1016/0306-4603(89)90025-7. [DOI] [PubMed] [Google Scholar]
- [47].Kanarek RB, Carrington C. Sucrose consumption enhances the analgesic effects of cigarette smoking in male and female smokers. Psychopharmacology (Berl) 2004;173(1-2):57–63. doi: 10.1007/s00213-003-1699-0. [DOI] [PubMed] [Google Scholar]
- [48].Lane JD, Lefebvre JC, Rose JE, Keefe FJ. Effects of cigarette smoking on perception of thermal pain. Exp Clin Psychopharmacol. 1995;3(2):140–147. [Google Scholar]
- [49].Lewkowski MD, Young SN, Ghosh S, Ditto B. Effects of opioid blockade on the modulation of pain and mood by sweet taste and blood pressure in young adults. Pain. 2008;135(1-2):75–81. doi: 10.1016/j.pain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [50].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lipsey MW, Wilson D. Practical meta-analysis. Sage; Thousand Oaks: 2001. [Google Scholar]
- [52].Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, Changeux JP. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398(6730):805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- [53].Meagher MW, Arnau RC, Rhudy JL. Pain and emotion: effects of affective picture modulation. Psychosom Med. 2001;63(1):79–90. doi: 10.1097/00006842-200101000-00010. [DOI] [PubMed] [Google Scholar]
- [54].Miao FJ, Green PG, Benowitz N, Levine JD. Central terminals of nociceptors are targets for nicotine suppression of inflammation. Neuroscience. 2004;123(3):777–784. doi: 10.1016/j.neuroscience.2003.10.027. [DOI] [PubMed] [Google Scholar]
- [55].Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. 2012;44(6):588–597. doi: 10.3109/07853890.2012.705016. [DOI] [PubMed] [Google Scholar]
- [56].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- [57].Nastase A, Ioan S, Braga RI, Zagrean L, Moldovan M. Coffee drinking enhances the analgesic effect of cigarette smoking. Neuroreport. 2007;18(9):921–924. doi: 10.1097/WNR.0b013e32811d6d0d. [DOI] [PubMed] [Google Scholar]
- [58].Nesbitt PD. Smoking, physiological arousal, and emotional response. J Pers Soc Psychol. 1973;25(1):137–144. doi: 10.1037/h0034256. [DOI] [PubMed] [Google Scholar]
- [59].Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9(2):131–137. doi: 10.1097/SPC.0000000000000126. [DOI] [PubMed] [Google Scholar]
- [60].O'Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev. 2012;64(4):939–971. doi: 10.1124/pr.112.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Palmer KT, Syddall H, Cooper C, Coggon D. Smoking and musculoskeletal disorders: findings from a British national survey. Ann Rheum Dis. 2003;62(1):33–36. doi: 10.1136/ard.62.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, Morasco BJ. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. J Pain. 2012;13(3):285–292. doi: 10.1016/j.jpain.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pauli P, Rau H, Zhuang P, Brody S, Birbaumer N. Effects of smoking on thermal pain threshold in deprived and minimally-deprived habitual smokers. Psychopharmacology (Berl) 1993;111(4):472–476. doi: 10.1007/BF02253538. [DOI] [PubMed] [Google Scholar]
- [64].Perkins KA, Grobe JE, Stiller RL, Scierka A. Effects of nicotine on thermal pain detection in humans. Exp Clin Psychopharmacol. 1994;2(1):95–106. [Google Scholar]
- [65].Pomerleau OF. Nicotine and the central nervous system: biobehavioral effects of cigarette smoking. Am J Med. 1992;93(1A):2S–7S. doi: 10.1016/0002-9343(92)90619-m. [DOI] [PubMed] [Google Scholar]
- [66].Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addict Behav. 1984;9(3):265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- [67].Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2-3):181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- [68].Rosenthal R. Effect sizes: Pearson's correlation, its display via the BESD, and alternative indices. Am Psychol. 1991;46(10):1086–1087. [Google Scholar]
- [69].Sherrington C, Herbert RD, Maher CG, Moseley AM. PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther. 2000;5(4):223–226. doi: 10.1054/math.2000.0372. [DOI] [PubMed] [Google Scholar]
- [70].Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. Smoking and pain: Pathophysiology and clinical implications. Anesthesiology. 2010;113(4):977–992. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- [71].Shiffman S, Jarvik ME. Cigarette smoking, physiological arousal, and emotional response: Nesbitt's paradox re-examined. Addict Behav. 1984;9(1):95–98. doi: 10.1016/0306-4603(84)90012-1. [DOI] [PubMed] [Google Scholar]
- [72].Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. Am J Med. 2010;123(1):87, e87–35. doi: 10.1016/j.amjmed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- [73].Silverstein B. Cigarette smoking, nicotine addiction, and relaxation. J Pers Soc Psychol. 1982;42(5):946–950. doi: 10.1037//0022-3514.42.5.946. [DOI] [PubMed] [Google Scholar]
- [74].Srivastava ED, Russell MA, Feyerabend C, Masterson JG, Rhodes J. Sensitivity and tolerance to nicotine in smokers and nonsmokers. Psychopharmacology (Berl) 1991;105(1):63–68. doi: 10.1007/BF02316865. [DOI] [PubMed] [Google Scholar]
- [75].Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, Kumagai S. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- [76].Tanus-Santos JE, Toledo JC, Cittadino M, Sabha M, Rocha JC, Moreno H., Jr. Cardiovascular effects of transdermal nicotine in mildly hypertensive smokers. Am J Hypertens. 2001;14(7 Pt 1):610–614. doi: 10.1016/s0895-7061(01)01301-2. [DOI] [PubMed] [Google Scholar]
- [77].Umana IC, Daniele CA, McGehee DS. Neuronal nicotinic receptors as analgesic targets: it's a winding road. Biochem Pharmacol. 2013;86(8):1208–1214. doi: 10.1016/j.bcp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Unrod M, Kassel JD, Robinson M. Effects of smoking, distraction, and gender on pain perception. Behav Med. 2004;30(3):133–139. doi: 10.3200/BMED.30.3.133-140. [DOI] [PubMed] [Google Scholar]
- [79].Weingarten TN, McGlinch BP, Liedl L, Kendrick ML, Kellogg TA, Schroeder DR, Sprung J. Intranasal nicotine increases postoperative nausea and is ineffective in reducing pain following laparoscopic bariatric surgery in tobacco-Naive females: a randomized, double blind trial. Obes Surg. 2015;25(3):506–513. doi: 10.1007/s11695-014-1431-7. [DOI] [PubMed] [Google Scholar]
- [80].Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. Am J Psychiatry. 1948;105(5):329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- [81].Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146(1):116–123. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zale EL, Ditre JW. Associations Between Chronic Pain Status, Attempts to Quit Smoking, and Use of Pharmacotherapy for Smoking Cessation. Psychol Addict Behav. 2013;28(1):294–9. doi: 10.1037/a0032515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zale EL, Ditre JW, Dorfman ML, Heckman BW, Brandon TH. Smokers in Pain Report Lower Confidence and Greater Difficulty Quitting. Nicotine Tob Res. 2014;16(9):1272–1276. doi: 10.1093/ntr/ntu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zale EL, Maisto SA, Ditre JW. Anxiety and Depression in Bidirectional Relations Between Pain and Smoking: Implications for Smoking Cessation. Behav Modif. 2015 doi: 10.1177/0145445515610744. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]