Abstract
Cyclooxygenase (COX), commonly overexpressed in cancer cells, is a major lipid peroxidizing enzyme that metabolizes polyunsaturated fatty acids (ω-3s and ω-6s). The COX-catalyzed free radical peroxidation of arachidonic acid (ω-6) can produce deleterious metabolites (e.g. 2-series prostaglandins) that are implicated in cancer development. Thus, COX inhibition has been intensively investigated as a complementary therapeutic strategy for cancer. However, our previous study has demonstrated that a free radical-derived by product (8-hydroxyoctanoic acid) formed from COX-catalyzed peroxidation of dihomo-γ-linolenic acid (DGLA, the precursor of arachidonic acid) can inhibit colon cancer cell growth. We thus hypothesize that the commonly overexpressed COX in cancer (~90% of colon cancer patients) can be taken advantage to suppress cell growth by knocking down delta-5-desaturase (D5D, a key enzyme that converts DGLA to arachidonic acid). In addition, D5D knockdown along with DGLA supplement may enhance the efficacy of chemotherapeutic drugs. After knocking down D5D in HCA-7 colony 29 cells and HT-29 cells (human colon cancer cell lines with high and low COX levels, respectively), the antitumor activity of DGLA was significantly enhanced along with the formation of a threshold range (~0.5–1.0 µM) of 8-hydroxyoctanoic acid. In contrast, DGLA treatment did not inhibit cell growth when D5D was not knocked down and only limited amount of 8-hydroxyoctanoic acid was formed. D5D knockdown along with DGLA treatment also enhanced the cytotoxicities of various chemotherapeutic drugs, including 5-fluorouracil, regorafenib, and irinotecan, potentially through the activation of pro-apoptotic proteins, e.g. p53 and caspase 9. For the first time, we have demonstrated that the overexpressed COX in cancer cells can be utilized in suppressing cancer cell growth. This finding may provide a new option besides COX inhibition to optimize cancer therapy. The outcome of this translational research will guide us to develop a novel ω-6-based diet-care strategy in combination with current chemotherapy for colon cancer prevention and treatment.
Keywords: Cancer chemotherapy, Cell cycle and apoptosis, Colon cancer cell lines (HCA-7 colony 29 and HT-29), Cyclooxygenase and delta-5-desaturase, Dihomo-γ-linolenic acid and ω-6, peroxidation
1. Introduction
Colon cancer is the third most prevalent cancer and the second leading cause of cancer deaths in the United States. A variety of chemotherapy drugs as well as nutritional supplements, including polyunsaturated fatty acids (PUFAs), have been studied and applied to colon cancer treatment [1–9]. There are two different types of PUFAs (ω-3s and ω-6s) that are both essential for human health and must be provided from dietary sources. ω-3s, mainly found in marine products, have been reported to have many beneficial effects for human health and are commonly used in complementary therapeutic strategies for cancer treatment [7–13]. However, as the more abundant dietary source (available from meat, cereals and plant oils), the consumption of ω-6s has been shown to correlate with cancer development [14–19]. Increasing evidence suggests that the pro-cancer effect of ω-6s may mainly be attributed to the formation deleterious metabolites (e.g., prostaglandin E2, PGE2) from COX-catalyzed peroxidation of arachidonic acid (AA) [20–26]. On the other hand, the precursors of AA, e.g., dihomo-γ-linolenic acid (DGLA) as well as γ-linolenic acid (GLA), were reported to possess certain anti-proliferative activities towards cancer cells [27–33]. DGLA might therefore represent a promising dietary source for cancer prevention and therapy, but the molecular mechanisms of its anti-cancer activities remain unclear.
COX is a membrane-bound bi-functional enzyme that peroxidizes ω-6s (e.g. DGLA and AA) as well as ω-3s (e.g. docosahexaenoic acid and eicosapentaenoic acid) into various bioactive products. Two isoforms of COX have been identified, e.g. COX-1, the constitute form, and COX-2, the inducible form. COX-2 can be readily induced by various pro-inflammatory stimuli, such as lipopolysaccharide and cytokines [34–37], and is commonly overexpressed in human adenocarcinomas (80–90% [38–40]). High expression levels of COX-2 are implicated in inflammatory disorders and cancer, mainly due to formation of 2-series prostaglandins from COX-catalyzed AA peroxidation [38–45]. Therefore, COX inhibition strategies aiming at limiting COX-catalyzed AA peroxidation has been extensively studied and applied as a complementary therapeutic approach for cancer as well as inflammatory conditions [1–3,41–45].
Using a novel HPLC/ESR/MS combined technique coupled with spin trapping method, our lab has demonstrated that there are different free radical mechanisms to generate distinct free radical metabolites from COX-catalyzed peroxidation of DGLA vs. AA, and that 8-hydroxyoctanoic acid (8-HOA) is an exclusive free radical-derived by product from DGLA peroxidation [46–52]. We also observed that 8-HOA inhibits cell growth, causes cell cycle arrest, and promotes apoptosis in colon cancer cells, while similar concentration of exogenous or endogenous prostaglandin E1 and E2 (commonly viewed as bioactive products of DGLA vs. AA) has no effect on cancer cell growth [48–49]. Thus, 8-HOA might be the beneficial bio-product that is responsible for DGLA's anti-cancer activity. Here we tested the hypothesis that the overexpression of COX-2 in cancer cells can be targeted to suppress cancer cell growth through the formation of 8-HOA, and that knocking-down of delta-5-desaturase (D5D, a key enzyme converting DGLA to AA) to limit the conversion of DGLA to AA and to maintain 8-HOA at a threshold range can be used to elicit DGLA's anti-cancer effect.
A large body of work including ours has showed that supplementation of ω-3s/ω-6s and 8-HOA could enhance the cytotoxicity of chemo-drugs in cancer cells [12,33,49,53]. In this study, we have further demonstrated that DGLA supplement along with D5D knockdown could be used to sensitize colon cancer cells to various chemo-drugs, including 5-fluorouracil (5-FU), regorafenib, and irinotecan, potentially through promoting chemotherapy-induced cell cycle arrest and apoptosis (e.g., activation of p53, caspase 9, and PARP). The results from this work could provide a biochemical rationale for the development of novel ω-6-based diet care strategies to combine with chemo-drugs for colon cancer treatment, by taking advantage of the wide availability of dietary ω-6s as well as the overexpression of COX-2 in colon cancer cells.
2. Materials and methods
2.1. Cell lines and materials
The human colon cancer cell lines HCA-7 colony 29 (high COX-2 expression, European Collection of Cell Cultures, Salisbury, UK) and HT-29 (low COX-2 expression, ATCC) were grown in Dulbecco's Modified Eagle's Medium and McCoy’s 5A Medium (with 10% fetal bovine serum, Thermo Fisher Scientific, UT, USA), respectively. Cells were cultured in an incubator containing a 95% humidified atmosphere with 5% CO2 at 37 °C.
DGLA was purchased from Nu-Chek-Prep (MN, USA); 8-HOA and 5-FU were purchased from Sigma-Aldrich (MO, USA); regorafenib was obtained from Adooq Biosciences (CA, USA); irinotecan, PGE1, DGLA-d6 and PGE1-d4 were purchased from Cayman Chemicals (MI, USA).
2.2. SiRNA transfection
Negative control siRNA (NC-si), D5D-targeting siRNA (catalog # 4390825) and Lipofectamine™ RNAiMAX transfection reagent were purchased from Life Technologies (Grand Island, NY, USA). GlutaMAX™ Opti-MEM reduced serum medium was purchased from Thermo Fisher Scientific (MA, USA). Briefly, colon cancer cell lines (HCA-7 and HT-29) were seeded at 3.0 × 105 cells per well in a 6-well plate or 8000 cells per well in a 96-well plate for different experiments. After overnight incubation and removing culture medium, cells were washed by phosphate buffered saline (PBS) and treated with siRNA transfection mixture containing D5D siRNA (final concentration at 150 nM) and Lipofectamine™ RNAiMAX transfection reagent (both diluted in GlutaMAX™ Opti-MEM reduced serum medium). After 6 h transfection, the reduced serum medium was replaced by Dulbecco's Modified Eagle's Medium (for HCA-7) or McCoy’s 5A Medium (for HT-29) with 10% fetal bovine serum. After 48 h, the transfected cells were ready for further treatments (e.g. 8-HOA, ω-6s, chemo-drugs) and other experiments, e.g. western blot, MTS assay, colony formation assay, LC/MS analysis, GC/MS analysis, cell cycle distribution and apoptosis analysis. Cells transfected with a non-target control siRNA were used as controls.
2.3. MTS assay
Cell proliferation of D5D-KD colon cancer cell lines and negative control cells upon treatments (e.g. 8-HOA, ω-6s and/or chemo-drugs) was assessed using CellTiter® 96 Aqueous One Solution Reagent (Promega, Madison, WI, USA). Briefly, cells were seeded at 8000 cells (in 100 µL medium) per well into 96-well plates, incubated overnight and transfected with D5D siRNA or negative control siRNA for 48 h. Upon 48 h treatments of ω-6s and/or chemo-drugs, 20 µL per well of CellTiter® 96 Aqueous One Solution Reagent was added. After up to 4 h incubation, the quantity of formazan product was assessed by recording the absorbance at 490 nm with a 96-well plate reader (SpectraMax M5; Molecular Devices). Cell viability was calculated as a percentage of the control group (treated with vehicle).
2.4. Clonogenic cell survival assay (colony formation assay)
Colony formation of D5D-KD HCA-7 colony 29 cells and negative control cells upon treatments (e.g. ω-6s and/or chemo-drugs) was assessed for cell survival study. Briefly, cells were seeded at 3.0 × 105 cells per well into a 6-well plate, incubated overnight, and transfected with D5D siRNA or negative control siRNA. After 24 h transfection, the cells were trypsinized, collected, seeded at 2000 cells per well into a 6-well plate, and then exposed to 48 h treatments of ω-6s, chemo-drugs, or their combination. The cells were then washed with PBS and incubated with fresh medium for 10 days. After incubation, the cells were washed with PBS, fixed with 10% neutral buffered formalin, and stained with 0.05% crystal violet solution. Cell colonies (more than 30 cells) formed in each well were counted and plate efficiency was calculated as number of colonies divided by number of cells seeded; surviving fraction was calculated as the plate efficiency of treatment group vs. the plate efficiency of control groups (e.g., vehicle treatment). Untreated 2000 cells were also plated in 6-well plates, and the average plate efficiencies were range from 0.127 to 0.144.
2.5. Detection of ω-6s and PGs from cells treated with DGLA
The free ω-6s and PGs from D5D-KD HCA-7 colony 29 cells and negative control cells treated with/without DGLA were quantified via LC/MS analysis as described elsewhere [48–49]. Briefly, 3.0 × 105 cells per well in a 6-well plate were seeded overnight and transfected with D5D siRNA or negative control siRNA for 48 h (during which the cell number grew to ~1.0 × 106). Then the cells were treated with 100 µM of DGLA (supplemented as 1.0 µL of ethanol solution into 1.0 mL of complete cell culture medium). At different time points, the cells (scratched off from well) with 1.0 mL of culture medium were collected and mixed with 0.45 mL of methanol and 1.55 mL of water. After adding internal standards (DGLA-d6, PGE1-d4), the mixture was vortexed for 1 min and set on ice for 30 min. After centrifuged for 15 min at 3000 rpm, the supernatant of mixture was collected and adjusted to pH 3.0, then subjected to solid phase extraction (SPE) using a reverse phase SPE cartridge (SampliQ Silica C18 ODS, Agilent, CA, USA). After eluted with 2.0 mL ethyl acetate from cartridge, the elution containing analytes was vacuumed to dryness and reconstituted with 100 µL ethanol for LC/MS analysis.
The LC/MS system, Agilent 1200 series HPLC system and Agilent 6300 LC/MSD SL ion trap mass, was used to quantify the free ω-6s and PGs in reconstituted sample solution. LC separations were performed on a C18 column (Zorbax Eclipse-XDB, 4.6 × 75 mm, 3.5 µm) with 5.0 µL sample injection at a flow rate of 0.8 mL/min of gradient mobile phases (A: H2O-0.1% Acetic acid and B: ACN-0.1% Acetic acid): 0–12 min (isocratic), 32% B; 12–14 min, 32–56% B; 14–28 min (isocratic), 56% B; 28–30 min, 56–86% B; 30–38 min, 86–95% B; and 38–44 min (isocratic), 95% B. MS settings are as follows: electrospray ionization in negative mode; total ion current chromatograms in full mass scan mode (m/z 50 to m/z 600) were performed; nebulizer press, 15 psi; dry gas flow rate, 5.0 L/min; dry temperature, 325 °C; compound stability, 20%; number of scans, 50. DGLA and PGE1 were monitored by extracted ion current chromatogram (m/z 305 and 353 respectively) projected from total ion current chromatograms. For quantification, standard curves for DGLA and PGE1 were established respectively with a series concentrations of DGLA or PGE1 and constant concentration of deuterium internal standards. The levels of DGLA or PGE1 in cell culture samples were determined by comparing the ratios of DGLA or PGE1 to corresponding internal standards with those in standard curves.
2.6. Detection of 8-HOA (in pentafluorobenzyl derivative form) from cells
8-HOA produced from D5D-KD HCA-7 colony 29 cells and negative control cells treated with/without DGLA were quantified via GC/MS analysis in its derivative of pentafluorobenzyl (PFB) bromide [54]. Briefly, 3.0 × 105 cells were seeded overnight in each well of 6-well plate, transfected with D5D siRNA or negative control siRNA for 48 h (during which the cell number grew to ~1.0 × 106), and treated with 0–100 µM of DGLA (in ethanol, final volume < 0.1%) for up to 48 h. At experimental time points, the cells (scratched off from plate) and ~1.0 mL medium were collected, and mixed with 500 µL of methanol containing internal standard (hexanoic acid), 50 µL of 1.0 N HCl, as well as 3.0 mL of dichloromethane. The mixture was then vortexed for 30 s, centrifuged at 3000 rpm for 4 min and the organic layer was collected. The same extraction was repeated once, and the dichloromethane layers were combined and evaporated to dryness using a vacuum evaporator, and reconstituted in 50 µL of 1.0% diisopropylethylamine in acetonitrile and derivatized with 50 µL of 1% PFB-bromide in acetonitrile at 37 °C for 30 min. After the acetonitrile was removed using a vacuum evaporator, the residue was reconstituted in 100 µL dichloromethane ready for GC/MS analysis.
Reconstituted sample solution (2.0 µL) was injected into an Agilent 7890A gas chromatograph. The GC oven temperature is programmed from 60 to 300 °C at 25°C/min. The injector and transfer line are kept at 280 °C. Quantitative analysis was performed using an Mass selective detector with a source temperature of 230 °C and nebulizer pressure of 15 psi. The formation of 8-HOA (in PFB derivative form) was measured in selected ion monitoring mode with m/z 181, the base peak of 8-HOA-PFB, by comparing with the similar base peak of internal standard (hexanoic acid-PFB derivative).
2.7. Cell cycle analysis
Cell cycle distribution of D5D-KD HCA-7 colony 29 cells and negative control cells upon treatments (e.g. ω-6s and/or chemodrugs) was analyzed using propidium iodide (PI) staining method. Briefly, 3.0 × 105 cells were seeded overnight in each well of 6-well plate, transfected with D5D siRNA or negative control siRNA, and treated with ω-6s and/or chemo-drugs. Then the cells were harvested by trypsinization, washed with PBS and fixed with 70% ethanol at 1.0 × 106 cells/mL at 4 °C for 30 min. After centrifuged for 5 min at 1000 rpm, the supernatant of mixture was discarded, and the cells were washed with PBS, centrifuged again for 5 min at 1000 rpm and treated with 10 µL ribonuclase (10 mg/mL) at room temperature for 5 min. Then 400 µL of PI (50 µg/mL) was added into each sample. The cell cycle distribution was measured after 30 min incubation using an Accuri C6 flow cytometer (Becton–Dickinson, NJ, USA), 10,000 cells were counted for each sample. Data was analyzed by FlowJo (TreeStar, Ashland, OR, USA).
2.8. Cell apoptosis assay
Cell apoptosis of D5D-KD HCA-7 colony 29 cells and negative control cells upon treatments (e.g. ω-6s and/or chemo-drugs) was analyzed using Annexin V Apoptosis Detection Kit I (BD Pharmingen ™, NJ, USA) according to the manufacturer's instruction. Briefly, 3.0 × 105 cells were seeded overnight in each well of 6-well plate, transfected with D5D siRNA or negative control siRNA, and treated with ω-6s and/or chemo-drugs. Then the cells were harvested by trypsinization, washed with PBS and re-suspended in 1 × binding buffer at a concentration of 1.0 × 106 cells/ml. 100 µl of such cell suspension was treated with 5.0 µL each of FITC Annexin V and PI solution, and gently vortex and incubated for 15 min at 25 °C in the dark, then mixed with 400 µL of 1 × binding buffer. The effect of different treatment on cell apoptosis was determined by Accuri C6 flow cytometer within 1 h, 10,000 cells were counted for each sample. Unstained cells, cells stained with FITC Annexin V only and PI only was used to set up compensation and quadrants. Data was analyzed by FlowJo (TreeStar, Ashland, OR, USA).
2.9. Western blotting
Expression of D5D, COX-2 and cell signaling proteins in D5D-KD HCA-7 colony 29 cells and negative control cells upon treatments (e.g. siRNA transfection, ω-6s and/or chemo-drugs) was assessed by western blot. D5D primary antibody (from rabbit) and β-actin primary antibody (from mouse) were purchased from Sigma-Aldrich (MO, USA). COX-2 primary antibody produced in rabbit was purchased from Abcam (MA, USA). γH2AX primary antibody was purchase from Bethyl Laboratories (TX, USA). All other primary and secondary antibodies were purchased from cell signaling (MA, USA). The cells were seeded in a 6-well plate, transfected with D5D siRNA or negative control siRNA, and treated with ω-6s and/or chemo-drugs 48 h. Then the proteins were extracted and loaded into 10% SDS-PAGE gels. The gel was run at a constant current of 30 mA for 1 h, proteins were then transferred to nitrocellulose membranes at a constant voltage of 80 V for 2 h on ice. Then the membranes were incubated with primary antibodies (used as 1:600 dilution) overnight at 4 °C with and horseradish peroxidase (HRP)-conjugated secondary antibody (used as 1:2000 dilution) for 1 h at room temperature with continuous rocking. Then the membranes were incubated in ECL western blot substrates (Pierce, Thermo Fisher Scientific, UT, USA) for 1 min, and exposed to X-ray film (Phoenix Research Products, NC, USA). Luminescent signals were captured on a Mini-Medical Automatic Film Processor (Imageworks). Image was acquired by HP Scanjet G4000 photo scanner and analyzed by ImageJ software.
2.10. Statistic analysis
All data were assessed using an unpaired student-test. A significant difference was considered with a p value ≤ 0.05.
3. Results
3.1. Delta-5-desaturase knockdown (D5D-KD) promoted DGLA's anti-proliferation effect on colon cancer cells expressing COX-2
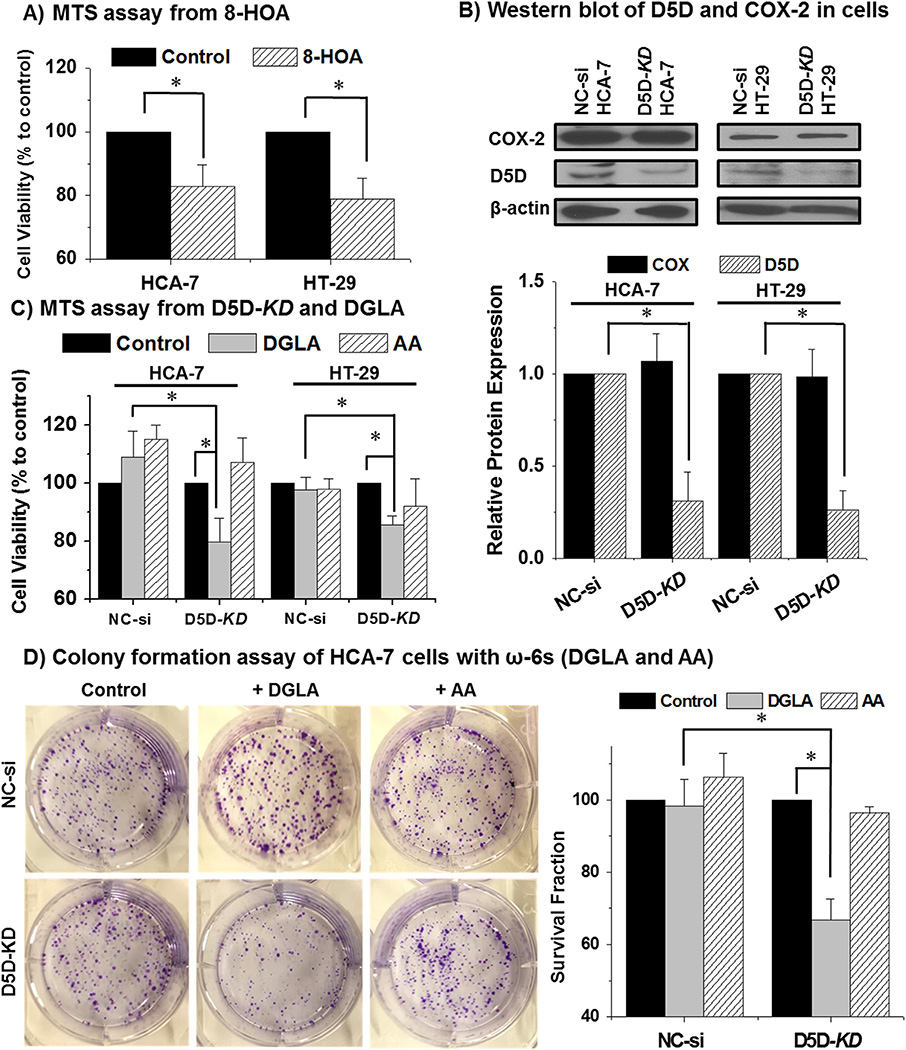
Consistent with our previous observation that 8-HOA inhibited cell proliferation of HCA-7 colony 29 cell line (with high COX-2 expression) [49], we found that 8-HOA also suppressed the growth of HT-29 cells (with low COX-2 expression) (Fig. 1A). When D5D was knocked down (KD) using siRNA to inhibit the conversion of DGLA to AA, DGLA supplementation significantly reduced the cell viability at 48 h in HCA-7 and HT-29 cell lines (Fig. 1C), while no growth inhibition was observed in the control siRNA transfected cells (Fig. 1C, MTS assay). D5D-KD along with DGLA treatment also significantly inhibited the colony formation in HCA-7 colony 29 cells (surviving fraction was reduced to ~66.8 ± 5.7%), but had no effect on the control siRNA transfected cells (surviving fraction ~98.4 ± 7.3% in clonogenic cell survival assays, Fig. 1D). We also observed that there was no inhibitory effect on cell growth of AA (100 µM) treatment in both control siRNA transfected and D5D-KD cells (Fig. 1C–D). These observations suggested that D5D-KD could be an effective strategy to elicit DGLA's anti-cancer activities in the cancer cells that express COX-2.
Fig. 1.
D5D-KD promoted DGLA's anti cancer effect in colon cancer cells. (A) MTS assay of HCA-7 colony 29 and HT-29 cells treated by 1.0 µM 8-HOA; (B) Western blot and relative protein expression of D5D and COX-2 expression in HCA-7 colony 29 and HT-29 cell lines after D5D siRNA transfection (loading control: β-actin). The relative ratios of D5D or COX to β-actin for each cell line were normalized to 1 respectively; (C) MTS assay of HCA-7 colony 29 and HT-29 cell lines (control siRNA transfected vs. D5D-KD) after 48 h of DGLA or AA treatment (100 µM). The control siRNA transfected and D5D-KD cells without fatty acid treatment were used as controls; (D) Colony formation of HCA-7 cells (control siRNA transfected vs. D5D-KD) at 10 days after DGLA or AA treatment (100 µM for 48 h). The control siRNA transfected and D5D-KD cells without fatty acid treatment were used as controls (*: significant difference with p < 0.05 from n ≥ 3).
3.2. Delta-5-desaturase knockdown led to accumulation of DGLA, PGE1 and 8-HOA
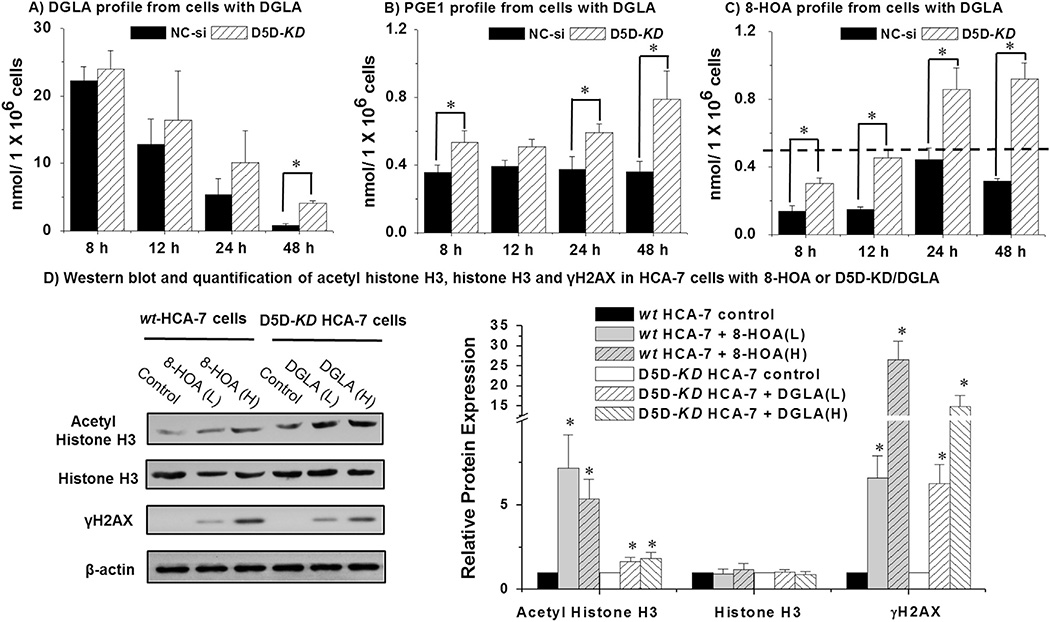
Results showed that supplement of 100 µM DGLA in HCA-7 cells led to a dramatic increase of the levels of free fatty acids and their metabolites (Fig. 2A–C) compared with the basal level in cells without DGLA treatment (Supplement Table 1). After DGLA supplement, limited conversion of DGLA to AA in D5D-KD cells was evident by the increase of remaining free DGLA (Fig. 2A) and increased formation of PGE1 and 8-HOA (two potential bioactive metabolites from DGLA peroxidation, Fig. 2B–C), along with decreased level of free AA and formation of PGE2 (metabolite from AA peroxidation, Supplement Fig. 1A–B), comparing to that in the cells transfected with the negative siRNA controls. In control siRNA transfected HCA-7 cells with 48 h treatment of 100 µM DGLA, PGE1 retained a stable concentration range (0.35–0.39 µM), while PGE1 in D5D-KD HCA-7 cells treated with DGLA was able to be accumulated from ~0.5 µM (8–12 h) to 0.8 µM in 48h (Fig. 2C). Consistent with our previous finding in which PGE1 (dose from 0.1 µM to 10µM) did not inhibit cancer cell growth [49], direct treatment of PGE1 (< 10 µM) was unable to suppress growth of cancer cell lines (data not shown). Since 8-HOA has been most recently reported to exert an inhibitory effect on cancer cell growth [49], it seems logical to conclude 8-HOA is the bioactive metabolite responsible for the growth inhibitory effect of DGLA. In fact, the most significant differences on 8-HOA production at all experimental time points were observed between D5D-KD and control siRNA transfected cells.
Fig. 2.
D5D-KD increased the levels of free DGLA, PGE1 and 8-HOA in HCA-7 colony 29 cells. (A) LC/MS quantification of DGLA from cell medium containing 1.0 × 106 of control siRNA transfected or D5D-KD HCA-7 cells after DGLA treatment (100 µM); (B) LC/MS quantification of PGE1 from cell medium containing 1.0 × 106 of control siRNA transfected or D5D-KD HCA-7 cells after DGLA treatment (100 µM); (C) GC/MS quantification of 8-HOA from cell medium containing 1.0 × 106 of control siRNA transfected or D5D-KD HCA-7 cells after DGLA treatment (100 µM). Note: in the cell experiments without DGLA treatment, only trace amount of DGLA were detected within 48 h (≤ 0.03 respectively), while the formation of PGE1 and 8-HOA was under detect limit; (D) Western blots of acetyl-histone H3, histone H3 and γH2AX in HCA-7 cells treated with 8-HOA (10 and 25 µM) and D5D-KD HCA-7 cells treated by DGLA (50 and 100 µM). The relative ratios of different proteins to β-actin were normalized to 1 respectively; (*: significant difference vs. the corresponding controls with p < 0.05 from n ≥ 3).
We proposed that there should be a threshold range of endogenous 8-HOA produced in our cell experiments that is required to elicit the anti-cancer activity of DGLA. We observed that 8-HOA accumulated ~0.5 µM at 12 h and reached a plateau at 24 h to 48 h (0.92 ± 0.09 µM at 48 h detected as PFB-derivative form in GC/MS analysis, Fig. 2C) in D5D-KD HCA-7 cells treated with DGLA. However, in control siRNA transfected HCA-7 cells treated with DGLA, 8-HOA only reached ~0.5 µM at 24 h time point (Fig. 2C). To maintain a threshold range of endogenous 8-HOA (~0.5 µM to 1.0 µM), instead of forming PGE1 (0.5–0.8 µM), would be essential for eliciting DGLA's anti-cancer activity. Furthermore, we found that 8-HOA could act as a DNA damaging reagent possibly via inhibiting the histone deacetylase (HDAC) as many other short chain fatty acids have been reported to inhibit the HDACs [55]. Treatment of 8-HOA significantly increased acetyl histone H3 expression and also the DNA damage marker γH2AX (Fig. 2D) [56]. Consistently, we also observed a significant increase of acetyl histone H3 and γH2AX from DGLA treatment in D5D-KD HCA-7 cells, indicating the anti-cancer effect of DGLA is derived from formation of 8-HOA.
3.3. D5D-KD along with DGLA supplement enhanced 5-FU's cytotoxicity
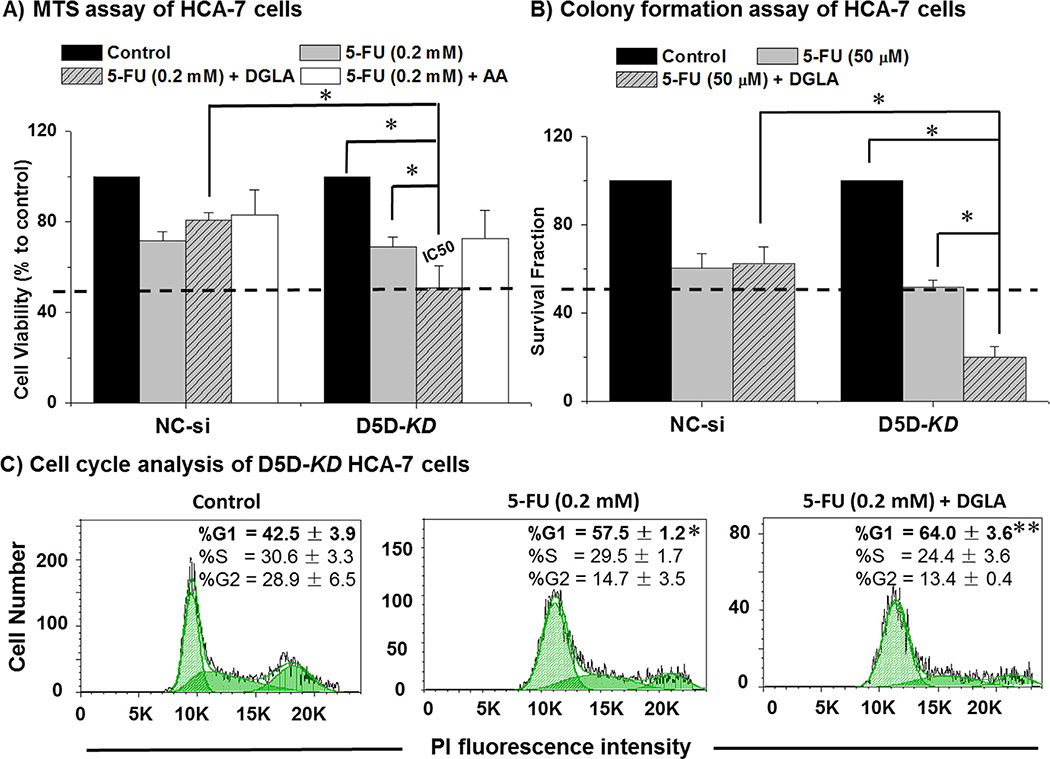
We have previously shown that supplementation of 8-HOA (DGLA's byproduct) sensitizes HCA-7 cells to 5-FU [49], one of the main chemo-drugs used as a first line of standard chemotherapy for patients with advanced colorectal cancer. Here we tested whether DGLA supplementation along with D5D-KD could enhance the efficacy of 5-FU in HCA-7 cells. Co-treatment of DGLA (100 µM) and 5-FU (0.2 mM) in D5D-KD HCA-7 cells enhanced cell growth inhibition (cell viabillity at 48 h ~50.9 ± 9.7%) comparing to 5-FU treatment alone (cell viabillity ~69.0 ± 4.2%, Fig. 3A). In contrast, DGLA attenuated 5-FU's cytotoxicity on control siRNA transfected HCA-7 cells (~80.9 ~ 3.2%) comparing to the cell viability (71.7 ± 3.8%) at 48 h with 5-FU treatment alone. As expected, co-treatment with AA did not improve the efficacy of 5-FU and appeared to promote cell proliferation in both control siRNA transfected and D5D-KD HCA-7 cells (Fig. 3A). Co-treatment with DGLA significantly decreased the IC50 of 5-FU in D5D-KD HCA-7 cells (~0.2 mM in Fig. 3A, vs. the reported IC50 in wt-HCA-7 cells ~1.0 mM [2,49]). There was no colony formation from the D5D-KD cells treated by such high doses of 5-FU. Significant inhibition of colony formation was observed when 50 µM of 5-FU and DGLA (100 µM) were combined to treat D5D-KD HCA-7 cells (surviving fraction ~20.1 ± 4.8%), while 5-FU treatment alone resulted in a surviving fraction of 51.9 ± 2.9% Fig. 3B).
Fig. 3.
Enhanced 5-FU's efficacy by DGLA in D5D-KD HCA-7 colony 29 cells. (A) MTS assay for proliferation of HCA-7 cells (control siRNA transfected vs. D5D-KD) treated with 5-FU (0.2 mM), 5-FU + DGLA (100 µM) or 5-FU + AA (100 µM) for 48 h. The control siRNA transfected and D5D-KD cells without fatty acid and drug treatment were used as controls. The IC50 of 5-FU was improved to ~0.2 mM from 1.0 mM [2,49]. (B) Colony formation assay of HCA-7 cells (control siRNA transfected vs. D5D-KD) at 10 days with treatment of DGLA (100 µM), 5-FU (50 µM) or 5-FU + DGLA (100 µM) for 48 h. The control siRNA transfected and D5D-KD cells without fatty acid and drug treatment were used as controls. (*: significant difference with p < 0.05 from n ≥ 3). (C) Cell Cycle distribution was examed via flow cytometer afterD5D-KD HCA-7 cells were treated with 5-FU (0.2 mM), or 5-FU + DGLA (100 µM) for 48 h, followed by PI staining. At least 10,000 cells were counted for each sample. Cells treated with vehicle served as controls. (*: significant difference vs. control with p < 0.05; **: significant difference vs. 5-FU group with p < 0.05, n ≥ 3).
5-FU has been known to block DNA synthesis by inhibition of thymidylate synthase activity and target cycling cells at G1/S point (G1 arrest) [57]. We observed that 5-FU induced G1 arrest in D5D-KD HCA-7 cells (57.5 ± 1.2% vs. 42.5 ± 3.9% in control, Fig. 3C), while DGLA further promoted 5-FU-induced G1 arrest in D5D-KD cells to 64.0 ± 3.6%. These data indicated that DGLA might further improve the efficacy of 5-FU in D5D-KD HCA-7 cells exposed to a threshold level of 8-HOA which has also been reported to cause G1 arrest [49].
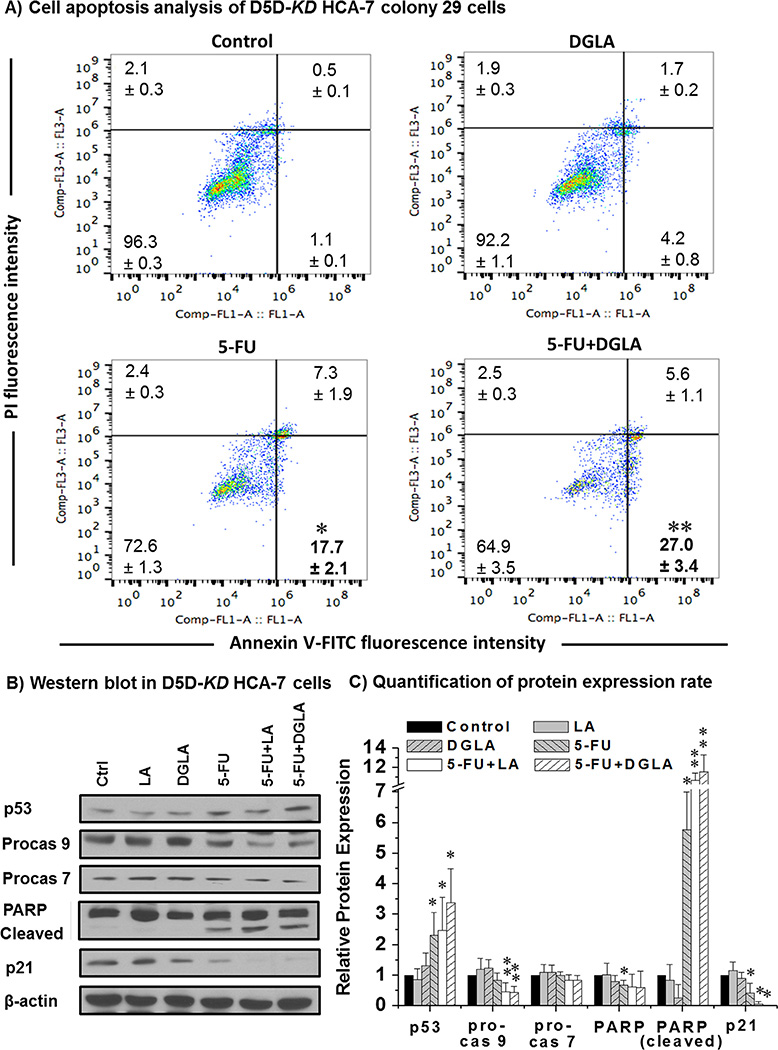
5-FU is also known to inhibit cancer cell growth by inducing p53-dependent cell apoptosis in which caspases and PARP activation are involved [58–59]. We also found that 5-FU could induce apoptosis in D5D-KD HCA-7 cells as demonstrated by the annexin V-positive/PI-negative staining (population of early apoptotic cells ~17.7 ± 2.1% vs. 1.06 ± 0.1 in the control, Fig. 4A). DGLA treatment (100 µM) further promoted 5-FU-induced apoptosis of D5D-KD cells (27.0 ± 3.4%). We also observed that 5-FU treatment alone could up-regulate the tumor suppressor p53, activate procaspase 9, cleave PARP and decrease p21 in D5D-KD HCA-7 cells (Fig. 4B–C). When the cells were co-treated with DGLA (100 µM) and 5-FU, an ~1.5 fold p53 upregulation, ~2 fold decreased procaspase 9, and ~2 fold increase of cleaved PARP were observed compared to 5-FU treatment alone (Fig. 4B–C). Our results suggested that DGLA and D5D-KD can improve the efficacy of 5-FU potentially through the activation of pro-apoptotic proteins, e.g., p53, caspase 9. In addition, linoleic acid (LA, anther upstream ω-6) could also further activate the pro-apoptotic proteins in D5D-KD cells treated with 5-FU (Fig. 4), and promoted cell apoptosis and inhibited cell growth similar to DGLA (data not shown).
Fig. 4.
DGLA promoted 5-FU-induced cell apoptosis in D5D-KD HCA-7 colony 29 cells. (A) Cell apoptosis was examed via flow cytometer after D5D-KD HCA-7 cells were treated with DGLA (100 µM), 5-FU (0.2 mM), or 5-FU + DGLA (100 µM) for 48 h, followed by Annexin V-FITC/PI double staining. At least 10,000 cells were counted for each sample. Cells treated with vehicle served as controls; (B) Western blot of p53, procaspase 9, procaspase 7, PARP, cleaved PARP and p21 from D5D-KD HCA-7 cells treated with 5-FU (0.2 mM), DGLA (100 µM), LA (100 µM) 5-FU + DGLA, and 5-FU + LA 48 h; (C) Quantification of western blot. Protein expression rate was normalized using β-actin as a loading control. Cells treated with vehicle served as control (*: significant difference vs. control with p < 0.05; and **: significant difference vs. 5-FU group, p < 0.05, n ≥ 3).
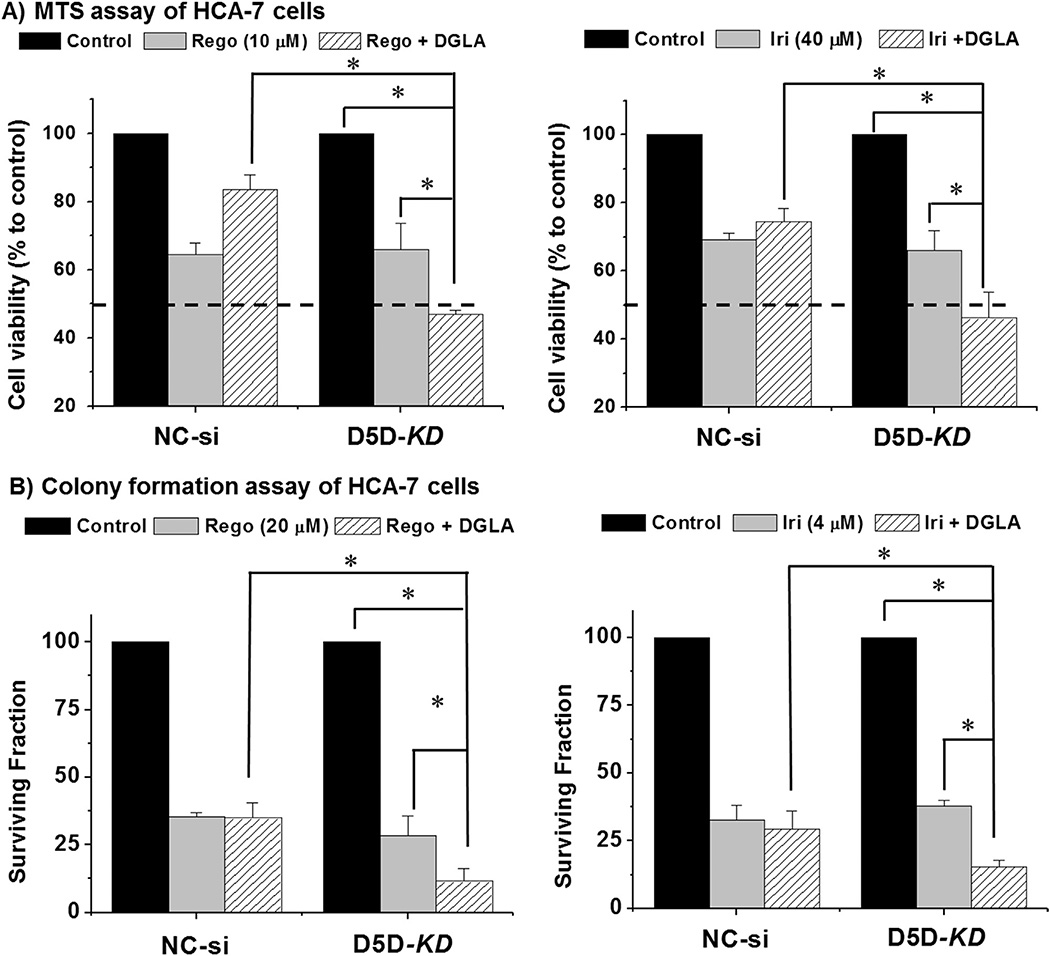
3.4. D5D-KD along with DGLA enhanced the cytotoxicity of several chemotherapies
We also tested whether DGLA supplementation in combination with D5D-KD could enhance the cytotoxicity of other common chemotherapeutic drugs, such as irinotecan and a recently FDA-approved targeted therapy drug, regorafenib. Treatment with regorafenib (10 µM) or irinotecan (40 µM) alone for 48 h decreased D5D-KD cells’ viability to ~65.9 ± 7.77% and 66.2 ± 5.7%, respectively (Fig. 5A). DGLA supplementation (100 µM, 48 h) enhanced the growth inhibitory effects of both drugs in D5D-KD cells (cell viability ~46.9 ± 1.3% for regorafenib and ~46.2 ± 7.5% for irinotecan), relative to control siRNA transfected HCA-7 cells (Fig. 5A). DGLA supplementation further inhibited colony formation in D5D-KD HCA-7 cells co-treated with 20 µM regorafenib and 4.0 µM irinotecan (surviving fraction ~11.6 ~ 4.6% and 15.5 ± 2.6%, respectively), while regorafenib and irinotecan alone resulted in the surviving fractions at 28.4 ± 7.3%, and 37.9 ± 2.0%, respectively (Fig. 5B).
Fig. 5.
Enhancement of regorafenib's and irinotecan's cytotoxicities by DGLA in D5D-KD HCA-7 colony 29 cells. (A) MTS assay for cell proliferation of control siRNA transfected or D5D-KD HCA-7 cells treated with 10 µM regorafenib, regorafenib and 100 µM DGLA, 40 µM irinotecan, and irinotecan with 100 µM DGLA for 48 h. The control siRNA transfected and D5D-KD cells without fatty acid and drug treatment were used as controls. (*: significant difference with p < 0.05 from n ≥ 3); (B) colony formation assay of control siRNA transfected or D5D-KD HCA-7 cells treated with regorafenib (20 µM), regorafenib + DGLA (100 µM), irinotecan (4 µM), irinotecan + DGLA (100 µM). The control siRNA transfected and D5D-KD cells without fatty acid and drug treatment were used as controls (*: significant difference with p < 0.05 from n ≥ 3).
4. Discussion
Our recent work has shown that distinct free radical-derived byproducts 8-HOA generated from COX-catalyzed peroxidation of ω-6 DGLA could inhibit colon cancer cell growth [49]. In this study, we demonstrate that the overexpression of COX-2 can be targeted in colon cancer cells by knocking down D5D to enhance DGLA peroxidation for generating more growth inhibitory 8-HOA. Furthermore, D5D knockdown along with DGLA supplement could enhance the efficacies of chemotherapeutic drugs.
Treatment of 8-HOA and supplementation of DGLA could significantly inhibit the growth of D5D-KD HCA-7 cells and D5D-KD HT-29 cells (Fig. 1A–D). Treatment with another upstream ω-6 (e.g., LA) also significantly suppressed the growth of these D5D-KD cell lines and sensitized to chemotherapeutic drugs (Supplement 2C). Although the effect from LA was moderate from current 48 h treatment regimen, we expect more significant anti-cancer effect in our future animal or clinical study in which we will modulate fatty acids consumption on a daily basis. These results may provide us a practical ω-6-based diet care strategy in cancer treatment since linoleic acid is prevalent in the human diet. In order to maintain potential benefit of ω-6s, down-regulating D5D should be an effective as well as an essential approach to elicit DGLA's anti-cancer activity.
Our previous work demonstrated that, supplement of 8-HOA (1.0–10 µM), but not the same amount of PGEs, could significantly inhibit colon cancer cell growth. Here we have made the first effort to quantify endogenous 8-HOA (by GC/MS for their PFB derivative) from COX-catalyzed DGLA peroxidation in cancer cells in order to test whether D5D-KD could lead to the accumulation of 8-HOA to the threshold levels that are required for delivering DGLA's anticancer effect. The threshold level of endogenous 8-HOA (0.5–1.0 µM, Fig. 2C) was relatively lower than the 8-HOA (1.0–10 µM, single dose) used to inhibit cell growth in previous study [49]. However, 8-HOA was able to be retained at the threshold level most of time due to continuous COX-catalyzed DGLA peroxidation. Lower doses of DGLA (10–75 µM) were also tested to define whether the threshold level of 8-HOA could also be retained to suppress cancer cell growth. Treatment of DGLA (≥ 50 µM) to D5D-KD HCA-7 cells was also able to maintain the threshold level of 8-HOA (> 0.5 µM), therefore ensuring the anti-cancer effect of DGLA from its COX peroxidation. Our results also showed that direct 8-HOA treatment, or formation of threshold level of 8-HOA from DGLA supplement along with D5D knockdown, can both inhibit histone deacetylase and lead to DNA damage (Fig. 2D). Thus the anti-cancer effect of DGLA should be derived from 8-HOA as treatment with similar amount of PGE1, e.g., another bioactive metabolites of DGLA, did not inhibit histone deacetylase (data not shown).
Chemo-drug 5-FU, a pyrimidine analog which induces cell apoptosis by interfering DNA replication, has been used as a first line of standard chemotherapy for patients with advanced colorectal cancer. To overcome drug resistance to 5-FU, various drug combination strategies have been studied in which 5-FU was concurrently administrated with other therapeutic agents [1–2,6]. For the first time, we have demonstrated that 8-HOA enhanced 5-FU's efficacy in colon cancer cells [49]. In this study, we observed that DGLA supplementation along with D5D-KD increased 8-HOA formation and significantly enhanced the cytotoxicity of 5-FU (Fig. 3). In addition, DGLA was also found to enhance the efficacies of other commonly used chemo-drugs in D5D-KD cells, e.g. regorafenib and irinotecan (Fig. 5), which have been reported to induce cell apoptosis in colon cancer cells [4,60]. D5D-KD along with DGLA may represent a novel and effective way to improve chemo-therapy for cancer.
We have shown previously that 8-HOA induces G1 arrest and apoptosis in HCA-7 colony 29 cells, and promotes 5-FU-induced apoptosis [49]. Consistent with these observations, DGLA treatment significantly promoted 5-FU-induced G1 arrest and apoptosis in D5D-KD HCA-7 colony 29 cells (Fig 3–4). The enhanced apoptosis was likely associated with further activation of apoptotic signaling proteins, e.g. p53, procaspase 9, procaspase 7 and PARP. A down-regulation of p21, an anti-apoptotic protein [61–63], was also observed upon combination treatment. 5-FU has also been reported to induce cell death via both p53-dependent and p53-independent pathways [58–59,64–65]. Cell growth of HCA-7 cells (wt-/mutant p53 heterozygotes) and HT-29 cells (mutate p53) were both significantly inhibited by DGLA along with D5D-KD (Fig. 1), indicating that 8-HOA could cause cancer cell death via p53-dependent and p53-independent mechanisms. Considering the fact that many cancer cells bear mutant p53 gene or wt-/mutant combination (e.g., HCA-7), to identify p53-dependent as well as p53-independent cell death pathways may guide us to develop a ω-6-based diet-care strategy for colon cancer chemotherapy.
To confirm whether different COX-2 levels could lead to different cell growth responses, and to confirm the promoted formation of 8-HOA, instead of decreased formation of AA, would be responsible for anti-cancer effect of our D5D-KD strategy, we also conducted DGLA treatment to D5D-KD HCT-116 cell line (COX-2 deficient) and HCA-7 cell line whose COX-2 and D5D were simultaneously knocked down (Supplement Fig 2–3). We found that while DGLA could inhibit the growth of D5D-KD HT-29 cells (low COX-2) and D5D-KD HCA-7 (high COX-2, Fig. 1), DGLA had no inhibitory effect on the D5D-KD HCT-116 cells and the double knockdown (D5D/COX-2 KD) HCA-7 cells (Supplement Figs. 3C and 2C). These results suggested that COX-2 is essential for DGLA's anti-cancer activity. However, regardless of the D5D and COX-2 expression levels, the growth of colon cancer cell lines (including their genetic variants) could all be significantly suppressed by the treatment of 8-HOA (Supplement Figs. 2B and 3B). Thus, our study suggested that the overexpression of COX-2 in cancer cells (~90% of colon cancer patients) can be exploited to promote the formation of 8-HOA (local action) from DGLA peroxidation to kill colon cancer cells. Growth of cancer cells with deficient COX-2 level can also be suppressed by 8-HOA in a paracrine manner (or a bystander effect) as DGLA peroxidation continually takes place in the nearby cells that (over)express COX-2.
In conclusion, our work demonstrates that the COX enzymes in cancer cells along with D5D-KD can be exploited to enhance cancer cell killing as well as enhancing the cytotoxicities of chemo-drugs. D5D has not received much research attention as a cancer therapeutic target. Our study indicated that D5D regulation may be an effective way to control ω-6 metabolism to promote DGLA's anti-cancer activity. The outcome of this research will allow for the development of a novel ω-6-based diet-care strategy in combination with current chemotherapy for cancer prevention and treatment.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants 1R15CA140833 (S Qian) and 1R01CA186100-01A1 (B Guo).
Abbreviations
- AA
arachidonic acid
- COX
Cyclooxygenase
- DGLA
dihomo-γ-linolenic acid
- D5D
Delta-5-desaturase
- ESR
electron spin resonance
- GLA
γ-linolenic acid
- GC
gas chromatography
- HPLC
high performance liquid chromatography
- LA
linoleic acid
- MS
mass spectrometry
- NC-si
negative control siRNA transfection
- PBS
phosphate buffered saline
- PFB
pentafluorobenzyl
- PI
propidium iodide
- PGE1
prostaglandin E1
- PGE2
prostaglandin E2
- PUFAs
polyunsaturated fatty acids
- wt-
wild type
- 5-FU
5-Fluorouracil
- 8-HOA
8-hydroxyoctanoic acid
Footnotes
Conflict of interest
The authors claim no Conflict of interest.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.freeradbiomed.2016.04.016.
References
- 1.Gasparini G, Gattuso D, Morabito A, Longo R, Torino F, Sarmiento R, Vitale S, Gamucci T, Mariani L. Combined therapy with weekly irinotecan, infusional 5-fluorouracil and the selective COX-2 inhibitor rofecoxib is a safe and effective second-line treatment in metastatic colorectal cancer. Oncologist. 2005;10:710–717. doi: 10.1634/theoncologist.10-9-710. [DOI] [PubMed] [Google Scholar]
- 2.Réti A, Barna G, Pap E, Adleff V, Komlósi L, Jeney V, Kralovánszky A, Budai J. B. Enhancement of 5-fluorouracil efficacy on high COX-2 expressing HCA-7 cells by low dose indomethacin and NS-398 but not on low COX-2 expressing HT-29 cells. Pathol. Oncol. Res. 2009;15:335–344. doi: 10.1007/s12253-008-9126-9. [DOI] [PubMed] [Google Scholar]
- 3.Lin J, Hsiao PW, Chiu TH, Chao JI. Combination of cyclooxygenase-2 inhibitors and oxaliplatin increases the growth inhibition and death in human colon cancer cells. Biochem. Pharmacol. 2005;70:658–667. doi: 10.1016/j.bcp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Wei L, Yu J, Zhang L. Regorafenib inhibits colorectal tumor growth through PUMA-mediated apoptosis. Clin. Cancer Res. 2014;20:3472–3484. doi: 10.1158/1078-0432.CCR-13-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, Lederle W. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol. Cancer Ther. 2013;12:1322–1331. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 6.Schultheis B, Folprecht G, Kuhlmann J, Ehrenberg R, Hacker UT, Köhne CH, Kornacker M, Boix O, Lettieri J, Krauss J, Fischer R, Hamann S, Strumberg D, Mross KB. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study. Ann. Oncol. 2013;24:1560–1567. doi: 10.1093/annonc/mdt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 8.Wen B, Deutsch E, Opolon P, Auperin A, Frascognal V, Connault E, Bourhis J. n-3 polyunsaturated fatty acids decrease mucosal/epidermal reactions and enhance antitumour effect of ionising radiation with inhibition of tumour angiogenesis. Br. J. Cancer. 2003;89:1102–1107. doi: 10.1038/sj.bjc.6601136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, Renkema JM, Bakker EJ, van’t Veer P, Kampman E. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am. J. Epidemiol. 2007;166:1116–1125. doi: 10.1093/aje/kwm197. [DOI] [PubMed] [Google Scholar]
- 10.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, Berry D, Steward W, Dennison A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur. J. Cancer. 2009;45:2077–2086. doi: 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 11.D’Eliseo D, Manzi L, Merendino N, Velotti F. Docosahexaenoic acid inhibits invasion of human RT112 urinary bladder and PT45 pancreatic carcinoma cells via down-modulation of granzyme B expression. J. Nutr. Biochem. 2012;23:452–457. doi: 10.1016/j.jnutbio.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui RA, Harvey KA, Xu Z, Bammerlin EM, Walker C, Altenburg JD. Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors. 2011;37:399–412. doi: 10.1002/biof.181. [DOI] [PubMed] [Google Scholar]
- 13.Kokura S, Nakagawa S, Hara T, Boku Y, Naito Y, Yoshida N, Yoshikawa T. Enhancement of lipid peroxidation and of the antitumor effect of hyperthermia upon combination with oral eicosapentaenoic acid. Cancer Lett. 2002;185:139–144. doi: 10.1016/s0304-3835(02)00262-8. [DOI] [PubMed] [Google Scholar]
- 14.Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011;31:1–8. doi: 10.1016/j.nutres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on meta- static spread from prostate cancer. Br. J. Cancer. 2010;102:403–413. doi: 10.1038/sj.bjc.6605481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Bénichou J, Clavel-Chapelon F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int. J. Cancer. 2009;124:924–931. doi: 10.1002/ijc.23980. [DOI] [PubMed] [Google Scholar]
- 17.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case–control study. Int. J. Cancer. 2008;123:1974–1977. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 18.Sauer LA, Blask DE, Dauchy RT. Dietary factors and growth and metabolism in experimental tumors. J. Nutr. Biochem. 2007;18:637–649. doi: 10.1016/j.jnutbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Kono S, Toyomura K, Nagano J, Mizoue T, Moore MA, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, Yasunami Y, Maekawa T, Takenaka K, Ichimiya H, Imaizumi N. Meat, fish and fat intake in relation to subsite-specific risk of colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci. 2007;98:590–597. doi: 10.1111/j.1349-7006.2007.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Zhao H, Wang Y, Zheng H, Yu W, Chai H, Zhang J, Falck JR, Guo AM, Yue J, Peng R, Yang J. Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of PI3K/Akt in human breast cancer. Toxicol. Appl. Pharmacol. 2013;272:37–48. doi: 10.1016/j.taap.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Zhao X, Wu Z, Li Y, Zhu L, Cui B, Dong X, Tian S, Hu F, Zhao Y. Polymorphisms in arachidonic acid metabolism-related genes and the risk and prognosis of colorectal cancer. Fam. Cancer. 2013;12:755–765. doi: 10.1007/s10689-013-9659-2. [DOI] [PubMed] [Google Scholar]
- 22.Pender-Cudlip MC, Krag KJ, Martini D, Yu J, Guidi A, Skinner SS, Zhang Y, Qu X, He C, Xu Y, Qian SY, Kang JX. Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue. Cancer Sci. 2013;104:760–764. doi: 10.1111/cas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakai M, Kakutani S, Horikawa C, Tokuda H, Kawashima H, Shibata H, Okubo H, Sasaki S. Arachidonic acid and cancer risk: a systematic review of observational studies. BMC Cancer. 2012;12:606. doi: 10.1186/1471-2407-12-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang P, Cartwright CA, Li J, Wen S, Prokhorova IN, Shureiqi I, Troncoso P, Navone NM, Newman RA, Kim J. Arachidonic acid metabolism in human prostate cancer. Int. J. Oncol. 2012;41:1495–1503. doi: 10.3892/ijo.2012.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amirian ES, Ittmann MM, Scheurer ME. Associations between arachidonic acid metabolism gene polymorphisms and prostate cancer risk. Prostate. 2011;71:1382–1389. doi: 10.1002/pros.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang CM, Hsieh HL, Lee CW. Intracellular signaling mechanisms underlying the expression of pro-inflammatory mediators in airway diseases. Chang Gung Med. J. 2005;28:813–823. [PubMed] [Google Scholar]
- 27.Fan YY, Ramos KS, Chapkin RS. Cell cycle related inhibition of mouse vascular smooth muscle cell proliferation by prostaglandin E1: Relationship between prostaglandin E1 and intracellular cAMP levels. Prostaglandins Leukot. Essent. Fatty Acids. 1996;54:101–107. doi: 10.1016/s0952-3278(96)90066-6. [DOI] [PubMed] [Google Scholar]
- 28.Gianetti J, de Caterina M, de Cristofaro T, Ungaro B, del Guercio R, de Caterina R. Intravenous prostaglandin E1 reduces soluble vascular cell adhesion molecule-1 in peripheral arterial obstructive disease. Am. Heart J. 2001;142:733–739. doi: 10.1067/mhj.2001.118109. [DOI] [PubMed] [Google Scholar]
- 29.Takai S, Jin D, Kawashima H, Kimura M, Shiraishi-Tateishi A, Tanaka T, Kakutani S, Tanaka K, Kiso Y, Miyazaki M. Anti-atherosclerotic effects of dihomo-γ-linolenic acid in ApoE-deficient mice. J. Atheroscler. Thromb. 2009;16:480–489. doi: 10.5551/jat.no430. [DOI] [PubMed] [Google Scholar]
- 30.Fang W, Li H, Zhou L, Su L, Liang Y, Mu Y. Effect of prostaglandin E1 on TNF-induced vascular inflammation in human umbilical vein endothelial cells. Can. J. Physiol. Pharmacol. 2010;88:576–583. doi: 10.1139/y10-028. [DOI] [PubMed] [Google Scholar]
- 31.Tabolacci C, Lentini A, Provenzano B, Gismondi A, Rossi S, Beninati S. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16-F10 murine melanoma cells. Melanoma Res. 2010;20:273–279. doi: 10.1097/CMR.0b013e328339d8ac. [DOI] [PubMed] [Google Scholar]
- 32.Sagar PS, Das UN. Cytotoxicaction of cis-unsaturated fatty acids on human cervical carcinoma (HeLa) cells in vitro. Prostaglandins Leukot. Essent. Fatty Acids. 1995;53:287–299. doi: 10.1016/0952-3278(95)90129-9. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Qian S. Anti-cancer activity of ω-6 polyunsaturated fatty acids. Biomed. J. 2014;37:112–119. doi: 10.4103/2319-4170.131378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JB, Han AR, Park EY, Kim JY, Cho W, Lee J, Seo EK, Lee KT. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull. 2007;30:2345–2351. doi: 10.1248/bpb.30.2345. [DOI] [PubMed] [Google Scholar]
- 35.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell JA, Belvisi MG, Akarasereenont P, Robbins RA, Kwon OJ, Croxtall J, Barnes PJ, Vane JR. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br. J. Pharmacol. 1994;113:1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akarasereenont P, Bakhle YS, Thiemermann C, Vane JR. Cytokine-mediated induction of cyclo-oxygenase_2 by activation of tyrosine kinase in bovine endothelial cells stimulated by bacterial lipopolysaccharide. Br. J. Pharmacol. 1995;115:401–408. doi: 10.1111/j.1476-5381.1995.tb16347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and-2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 39.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 40.Tomozawa S, Tsuno NH, Sunami E, Hatano K, Kitayama J, Osada T, Saito S, Tsuruo T, Shibata Y, Nagawa H. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br. J. Cancer. 2000;83:324–328. doi: 10.1054/bjoc.2000.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fournier DB, Gordon GB. COX-2 and colon cancer: potential targets for chemoprevention. J. Cell Biochem. Suppl. 2000;34:97–102. doi: 10.1002/(sici)1097-4644(2000)77:34+<97::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 42.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin. Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 43.Ferrández A, Prescott S, Burt RW. COX-2 and colorectal cancer. Curr. Pharm. Des. 2003;9:2229–2251. doi: 10.2174/1381612033454036. [DOI] [PubMed] [Google Scholar]
- 44.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. [PubMed] [Google Scholar]
- 45.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J. Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Q, Purwaha P, Ni K, Sun C, Mallik S, Qian SY. Characterization of novel radicals from COX-catalyzed arachidonic acid peroxidation. Free Radic. Biol. Med. 2009;47:568–576. doi: 10.1016/j.freeradbiomed.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao Y, Gu Y, Purwaha P, Ni K, Law B, Mallik S, Qian SY. Characterization of free radicals formed from COX-catalyzed DGLA peroxidation. Free Radic. Biol. Med. 2011;50:1163–1170. doi: 10.1016/j.freeradbiomed.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Y, Xu Y, Law B, Qian SY. The first characterization of free radicals formed from cellular COX-catalyzed peroxidation. Free Radic. Biol. Med. 2013;57:49–60. doi: 10.1016/j.freeradbiomed.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y, Qi J, Yang XY, Wu E, Qian SY. Free radical derivatives formed from COX-catalyzed DGLA peroxidation can attenuate colon cancer cell growth and enhance 5-FU's cytotoxicity. Redox Biol. 2014;2:610–618. doi: 10.1016/j.redox.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Qian SY. Anti-cancer activities of ω-6 polyunsaturated fatty acids. Biomed. J. 2014;30:112–119. doi: 10.4103/2319-4170.131378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y, Gu Y, Qian SY. An advanced electron spin resonance (ESR) spin-trapping and LC/(ESR)/MS technique for the study of lipid peroxidation. Int. J. Mol. Sci. 2012;13:14648–14666. doi: 10.3390/ijms131114648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian SY, Xu Y, Gu Y. Combination of spin-trapping, LC/ESR and LC/MS technique in characterization of PUFA-derived free radicals in lipid peroxidation. Acta Biophys. Sin. 2012;28:355–372. [Google Scholar]
- 53.Shaikh IA, Brown I, Wahle KW, Heys SD. Enhancing cytotoxic therapies for breast and prostate cancers with polyunsaturated fatty acids. Nutr. Cancer. 2010;62:284–296. doi: 10.1080/01635580903407189. [DOI] [PubMed] [Google Scholar]
- 54.Quehenberger O, Armando A, Dumlao D, Stephens DL, Dennis EA. Lipidomics analysis of essential fatty acids in macrophages, Prostaglandins Leukot. Essent. Fatty Acids. 2008;79:123–129. doi: 10.1016/j.plefa.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen JS, Faller DV, Spanjaard RA. Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics? Curr. Cancer Drug Targets. 2003;3:219–236. doi: 10.2174/1568009033481994. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Li X, Guo B. Chemopreventive agent 3,3’-diindolylmethane selectively induces proteasomal degradation of class I histone deacetylases. Cancer Res. 2010;70:646–654. doi: 10.1158/0008-5472.CAN-09-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo X, Goessl E, Jin G, Collie-Duguid ES, Cassidy J, Wang W, O’Brien V. Cell cycle perturbation and acquired 5-fluorouracil chemoresistance. Anticancer Res. 2008;28:9–14. [PubMed] [Google Scholar]
- 58.Sun XX, Dai MS, Lu H. 5-fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J. Biol. Chem. 2007;282:8052–8059. doi: 10.1074/jbc.M610621200. [DOI] [PubMed] [Google Scholar]
- 59.Thant AA, Wu Y, Lee J, Mishra DK, Garcia H, Koeffler HP, Vadgama JV. Role of caspases in 5-FU and selenium induced growth inhibition of colorectal cancer cells. Anticancer Res. 2008;28:3579–3592. [PMC free article] [PubMed] [Google Scholar]
- 60.Rudolf E, John S, Cervinka M. Irinotecan induces senescence and apoptosis in colonic cells in vitro. Toxicol. Lett. 2012;214:1–8. doi: 10.1016/j.toxlet.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Jänicke RU, Sohn D, Essmann F, Schulze-Osthoff K. The multiple battles fought by anti-apoptotic p21. Cell Cycle. 2007;6:407–413. doi: 10.4161/cc.6.4.3855. [DOI] [PubMed] [Google Scholar]
- 62.Javelaud D, Besancon F. Inactivation of p21WAF1 sensitizes cells to apoptosis via an increase of both p14ARF and p53 levels and an alteration of the Bax/Bcl-2 ratio. J. Biol. Chem. 2002;277:37949–37954. doi: 10.1074/jbc.M204497200. [DOI] [PubMed] [Google Scholar]
- 63.Fan X, Liu Y, Chen JJ. Down-regulation of p21 contributes to apoptosis induced by HPV E6 in human mammary epithelial cells. Apoptosis. 2005;10:63–73. doi: 10.1007/s10495-005-6062-y. [DOI] [PubMed] [Google Scholar]
- 64.Nita ME, Nagawa H, Tominaga O, Tsuno N, Fujii S, Sasaki S, Fu CG, Takenoue T, Tsuruo T, Muto T. 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br. J. Cancer. 1998;78:986–992. doi: 10.1038/bjc.1998.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu HC, Chen GG, Vlantis AC, Leung BC, Tong MC, van Hasselt CA. 5-fluorouracil mediates apoptosis and G1/S arrest in laryngeal squamous cell carcinoma via a p53-independent pathway. Cancer J. 2006;12:482–493. doi: 10.1097/00130404-200611000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.