Abstract
Humans and many animals exhibit freezing behavior in response to threatening stimuli. In humans, inappropriate threat responses are fundamental characteristics of several mental illnesses. To identify small molecules that modulate threat responses, we developed a high-throughput behavioral assay in zebrafish (Danio rerio) and characterized the effects of 10,000 compounds on freezing behavior. We found three classes of compounds that switch the threat response from freezing to escape-like behavior. We then screened these for binding activity across 45 candidate targets. Using target profile clustering we implicated the sigma-1 receptor in the mechanism of behavioral switching and confirmed that known sigma-1 ligands also disrupt freezing behavior. Furthermore, mutation of the sigma-1 gene prevented the behavioral effect of escape-inducing compounds. The compound ‘finazine’ potently bound mammalian sigma-1 and altered rodent threat response behavior. Thus, pharmacological and genetic interrogation of the freezing response revealed sigma-1 as a mediator of vertebrate threat responses.
SUBJECT TERMS: Chemical Biology, Neuroscience, Behavior, Small Molecules, Threat Responses, Innate, Unconditioned, Fear, Freezing, Escape, Sigma-1, sigmar1, Sigma-2, pgrmc1
INTRODUCTION
Most animals, including humans and fish, freeze instinctively in response to aversive stimuli. The ability to choose an appropriate defense response during a threatening situation can be essential for survival, but can also have consequences for psychiatric health.1 Altered responses to threatening stimuli and breakdown in limbic control are fundamental characteristics of several psychiatric disorders including schizophrenia2–4 and post-traumatic stress disorder.5 Therefore, studying the biological responses to threatening stimuli could lead to greater understanding of these fundamental behaviors while also providing new avenues for treatment of mental illnesses.
Polygenic mental health disorders will likely require systems-modulating therapeutics given the observations that hundreds to thousands of susceptibility genes exist. Target- and cell-based CNS discovery platforms facilitate rapid drug screening but are generally too simple to replicate the integrated networks required for complex brain processes.6 Conversely, rodent models offer sufficient biological complexity to study threat responses and other behaviors, but they are too large and unwieldy for high-throughput discovery. Zebrafish behavioral screens are an effective means for discovering neuroactive compounds because they enable a throughput comparable to many in vitro screening platforms, but allow screening to occur in the context of an integrated, vertebrate nervous system.7
We found that zebrafish larvae exhibit a robust freezing response to strobe light. Using this innate behavior, we conducted the first ever high-throughput freezing assay in animals, cataloging the behavioral effects of more than 10,000 synthetic compounds with unknown pharmacology. Among the behavioral modifiers we discovered were three structurally distinct classes of small molecules that completely switch the innate threat response from freezing to escape behavior, suggesting the existence of a druggable pathway regulating a decision between passive and active threat responses. We tested one of these compound classes in a rodent fear assay and found that its behavioral activity is indeed conserved in mammals. We then combined the in vivo zebrafish screen with a secondary in vitro target screen and identified the sigma-1 receptor as a major target for small molecules that induce the dramatic switch from freezing to escape behavior.
RESULTS
Zebrafish larvae freeze in response to strobe light
To discover behavior-modifying small molecules with complex mechanisms of action, we developed a high-throughput behavioral assay using live, freely behaving zebrafish. We chose zebrafish larvae at an age when the fish are developed enough to swim freely and exhibit complex behaviors, yet small enough to fit in 96-well plates. Adult zebrafish exhibit defensive behaviors, including freezing and escape, in response to predators and olfactory cues.8–10 Larvae also exhibit avoidance and escape behaviors by 7 days post fertilization (dpf) and reduced locomotor activity in response to steady light.11,12 Nevertheless, no consistent freezing response has been reported for larval zebrafish. We tested several potential freeze-inducing stimuli and found that 10 Hz strobe light elicits rapid (within seconds) and robust hypolocomotion in 7 dpf larval zebrafish (Fig 1a). This larval behavior was found to be dependent on the strobe frequency (Supplementary Results, Supplementary Fig 1) and distinct from that of adults, which show hyperactive escape behavior in response to strobe light13 (Supplementary Fig 2). These findings suggested that the zebrafish strobe light response would be a useful tool for high-throughput identification of neuroactive compounds.
Figure 1. High-throughput chemical screen for compounds that disrupt zebrafish freezing behavior.
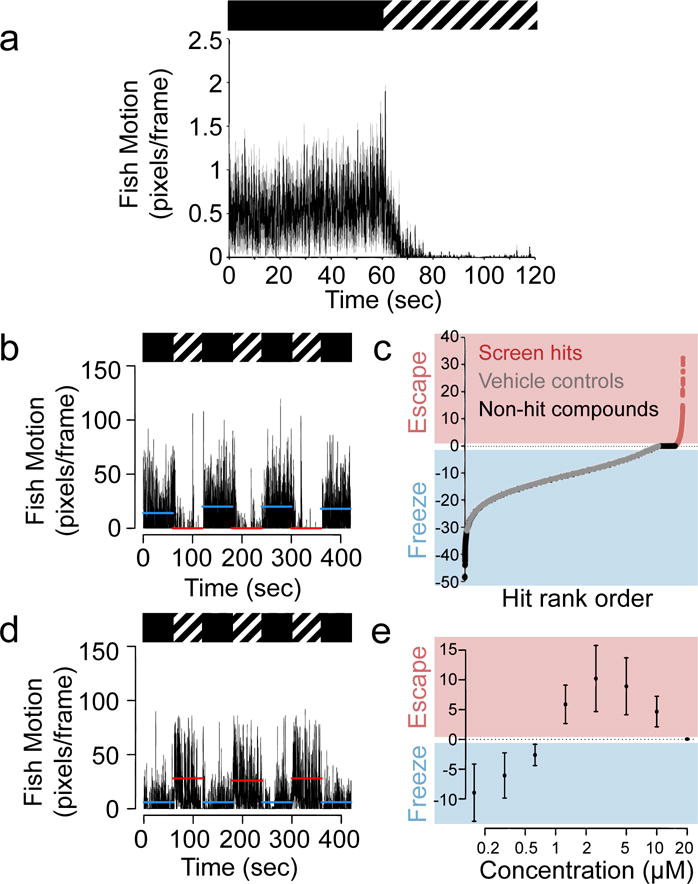
(a) Aggregate motor activity (in pixels per frame) over time from one 7 dpf larva per well of a 96-well plate during a two-minute experiment, n = 48 larvae. Boxes above mark 1 min intervals when the fish are in darkness (black box) or strobe light (hashed box). Data are shown as mean (black line) ± s.d. (gray regions) and are representative of three independent experiments. (b) Motor activity from all larvae in a single control, vehicle (DMSO) treated well during the SLR chemical screen. Horizontal lines represent 1 min averages for motion in darkness (blue) or in strobe light (red), n = 10 larvae. Data are representative of 2,000 wells in 125 independent experiments. (c) High-throughput screen results from >10,000 compounds. The y-axis represents the freezing index (a measure of the difference in motion between strobe light and dark periods, see Online Methods) for each well tested. The x-axis represents well position (for 12,000 wells, n = 10 larvae per well) ranked by freezing index value. Wells with test compounds are labeled either pink (hit compounds, freezing index > 0) and black (non-hit compounds, freezing index ≤ 0). Negative control wells (DMSO vehicle alone) are labeled in gray. (d) Fish motion during the strobe assay in wells treated with compound 1, n = 10 larvae. Data are representative of 36 wells from 3 independent experiments. (e) Dose curves showing the degree of behavioral switching. Data are presented as mean of the freezing index ± s.d. (n = 12 wells per dose) and are representative of 3 independent experiments.
Because hypolocomotion could be the result of freezing or sedation, we tested the fish for the hallmarks of freezing, namely rapid onset hypolocomotion in response to an aversive stimulus. To determine if the strobe light was aversive to 7 dpf larvae or having a calming effect, we measured cortisol levels in larvae exposed to strobe light. As controls, we exposed larvae to light (negative control) or to darkness or high salt, which are known to increase cortisol in larvae.14 We found that strobe light exposure leads to a robust increase in cortisol levels in 7 dpf larvae, indicating that strobe light is aversive rather than calming at this age, consistent with a freezing response (Supplementary Fig 3). Because strobe-induced hypolocomotion in zebrafish exhibits several hallmarks of freezing, we refer to it as such throughout this manuscript, although the degree of conservation with mammalian freezing has not been determined at molecular or circuitry levels.
Small molecule disruptors of larval freezing behavior
We designed a robust high-throughput screening assay to measure the zebrafish strobe light response (SLR) where we loaded 7 dpf zebrafish into 96-well plates, 10 animals per well (Supplementary Fig 4), and subjected them to dark and 10 Hz strobe light in alternating one minute time intervals for a total of 7 minutes. (For assay optimization results, see Supplementary Fig 5) During periods of darkness, larvae swam actively, consistent with previous observations.15 However, when subjected to strobe light, larvae rapidly and consistently exhibited a freezing behavior (Fig 1b and Supplementary Video 1). When returned to dark, larvae immediately resumed their normal, active swimming. To quantify the degree of freezing behavior, we developed a ‘freezing index’ by subtracting the average fish motion during the dark periods from the average motion during the periods of strobe light (see Online Methods).
Using this SLR assay and about 120,000 zebrafish larvae, we rapidly screened more than 10,000 synthetic compounds of unannotated pharmacology from the DIVERSetE (ChemBridge) compound library and characterized their effects on freezing behavior (Supplementary Table 1). The DIVERSetE library contains relatively small, structurally diverse synthetic molecules with molecular weights in the 100–500 g/mol range. Whereas the 2,000 vehicle-treated control wells always gave a freezing index value less than or equal to zero (mean value –9.22 with a standard deviation of ± 4.86, Fig 1c), we identified several small molecules that completely switched the response to strobe light from freezing to ‘escape’-like behavior characterized by rapid motion in the presence of strobe light (Fig 1d, Supplementary Fig 6, Supplementary Table 2). We reordered some of the active compounds, retested them over a range of doses, and confirmed that they each disrupted larval freezing and switched the response to a hyperlocomotive, ‘escape’-like behavior in a dose-dependent manner (Fig 1e, Supplementary Video 2). The hyperlocomotion observed was restricted to the strobe period and not seen during background swimming. While general hyperlocomotion is a non-specific phenotype that psychostimulants can induce, we found that psychostimulants (including amphetamine, cocaine, methylphenidate and nicotine) did not cause strobe-induced ‘escape’ in our SLR assay (Supplementary Fig 7).
To characterize the observed hyperactive behavior further, we tested whether hit compounds could cause 7 dpf larvae to escape from an uncovered half of a 10 cm plate (exposed to strobe light) to a covered (unexposed) half. We found that strobe light caused a statistically significant movement of larvae from the exposed half of the plate to the unexposed half when treated with 5 μM compound 1 (Supplementary Fig 8), further confirming that the strobe light stimulus is aversive and suggesting that the observed hyperlocomotion in the presence of compound 1 can enable the larvae to escape the aversive stimulus. These data indicate the zebrafish SLR assay can be used to quickly and inexpensively identify potent small molecules able to modulate a switch between passive and active threat responses in zebrafish.
We then used the ChemMine chemical structure comparison tool16 to group hit compounds based on molecular similarity and found that several of our hits clustered together in structurally related classes. Three classes of compounds were of particular interest given their potencies, wide dose ranges of neuroactivity and favorable tolerability. The largest group of compounds we identified in the screen consisted of 11 hit compounds, 1–11. These had as their core structure a nitrogen-containing ring, usually a piperazine, linked to a substituted N-phenylpropanamide (Fig 2a, Supplementary Fig 9a). Thus we named these compounds “finazines.” Each of these 11 small molecules produced a dose-dependent switch from freezing to escape behavior in the zebrafish SLR assay, though some were more potent and more effective at producing escape behavior than others (Supplementary Fig 10). The second largest group of hits comprised six compounds, 12 a substituted 2-methoxy-N-(2-phenoxyethyl)ethanamine and 13–17 which shared a similar N,N-dimethyl-2-(2-phenoxyethoxy)ethanamine structural core (Fig 2b, Supplementary Fig 9b). We named this class ‘finoxetines’ and confirmed each member of this class also produces a dose-dependent effect in zebrafish at a concentration range that included the original screening dose (~15 μM) (Supplementary Fig 11). The third group of compounds identified consisted of four members, 18–21. Due to the structural similarity between these compounds and the known neuroactive compound naftopidil (22) (Fig 2c), we named these compounds ‘finopidils.’ We confirmed the dose-dependent activity of each of these four compounds and also tested naftopidil. Naftopidil just barely switched the zebrafish response from freezing to escape at 5 μM, before it completely immobilized the larvae at 10 μM, presumably due to toxicity (Supplementary Fig 12). These data indicate that the assay can quickly discover multiple structurally diverse classes of compounds that produce the same behavioral effect.
Figure 2. Chemical structures.
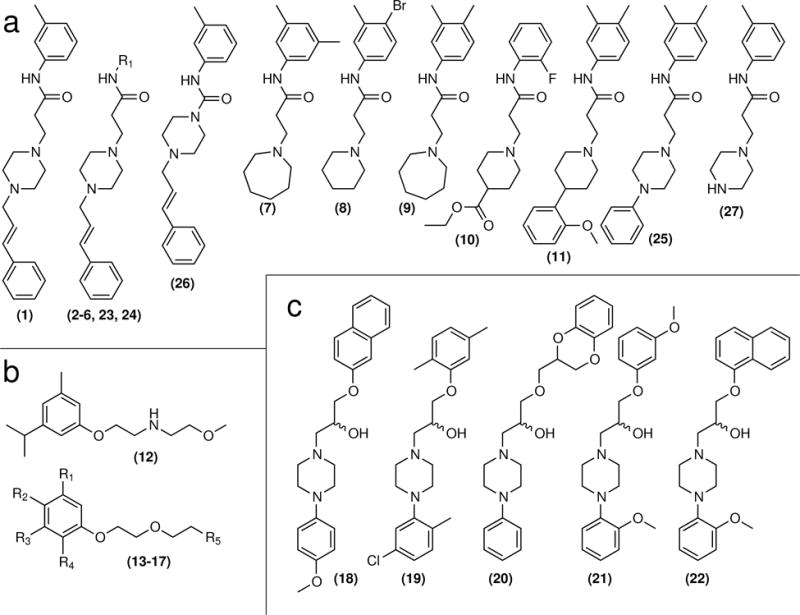
(a) Finazine compound class. (b) Finoxetine compound class. (c) Finopidil compound class. 1–21 are confirmed hits from the screen. 22–27 were not initial hits but later tested due to their structural similarity.
Testing of finazine analogues
To identify additional analogues of the hit compounds, we purchased four more commercially available finazine-like small molecules, 23–26, and tested them in the zebrafish SLR assay. We found that each of these compounds induced escape-like behavior in a dose-dependent manner, although 24 and 25 were significantly less potent (Supplementary Fig 10). We determined the concentration at which each compound switched the strobe light response from freezing to escape by calculating the initial zero intercept from each dose curve plot. By calculating this value, which we call the in vivo ‘effective concentration’ (or ‘Fish EC’), we were able to quantitatively compare the potencies of each compound tested (Supplementary Table 3). Because all of the purchased analogues were active to some degree, we synthesized compound 27 as a negative control (Supplementary Note 1). We intentionally designed this analogue to have a more dramatic change in structure to disrupt its function. Indeed, when we tested it in our zebrafish strobe light assay it had no effect (Supplementary Fig 10).
Identifying neuronal targets
To begin to determine the mechanism(s) of action of the newly discovered small molecules, we screened each of them against a panel of 45 candidate neuronal targets in vitro. In each case we tested their ability to disrupt the interaction between a candidate neuronal protein and a canonical high-affinity radiolabeled ligand. We found that the compounds bound to a wide range of targets, indicating complex mechanisms of action, as expected (Supplementary Table 3).
To determine which of the 45 targets is most likely to be functionally important in the switch from passive to active fear responses, we used a simple clustering algorithm to compare in vitro Ki values from each of the small molecules with their in vivo activities. To our surprise, the hit compounds did not cluster discretely into their three structural families (Fig 3a). Instead, compounds from all three classes bound to multiple targets (ranging from 5–30). Interestingly, of all the targets tested, binding to sigma-1 and -2 correlated most closely with in vivo activity. Of the 1170 binding assays performed, the six best Ki values were all obtained for sigma-1 (0.5, 0.8, 0.8, 1.0, 1.4 and 1.7 nM for compounds 15, 8, 16, 17, 7, and 9, respectively). The prototypical finazine compound 1, for example, had low nM Ki values for sigma-1 and sigma-2 receptors similar to the known non-specific sigma compound haloperidol (Supplementary Fig 13). The finding that every one of the compounds with in vivo activity exhibited binding activity at sigma-1 and/or -2 implicated sigma receptors in the behavioral switch between passive and active fear responses.
Figure 3. Target profiling.
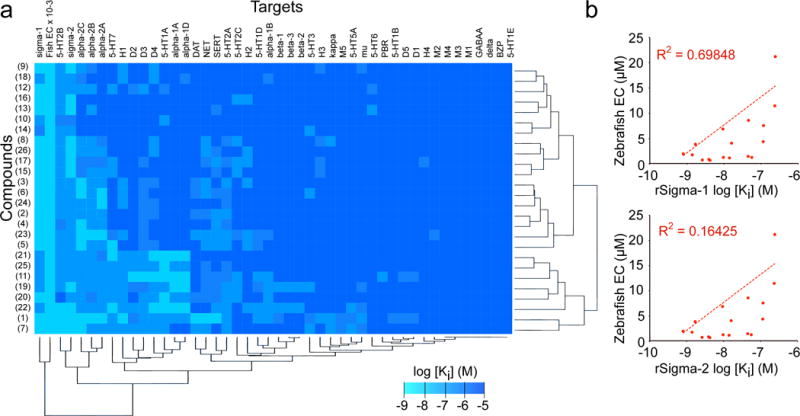
(a) Heat map depiction of in vitro mammalian target binding assay results across 45 candidate targets. Small molecules were computationally clustered by target profile similarity (vertical brackets) and targets were clustered by chemical binding profiles (horizontal brackets). Legend shows Ki values in log scale (range ≥ 10 μM to 1 nM, with the exception of the ‘fish activity’ column where the EC values were scaled for comparison, range 100 to 0.1 μM). (b) Correlation plots of compound potency in the zebrafish strobe assay versus in vitro potency in simga-1 or -2 binding assays, as indicated. Each point represents a different compound in the finazine class. Regression line and R2 value was calculated using Microsoft Excel. For all Ki determinations, n = 3 replicates per dose over a 12 dose range. For zebrafish assays n = 12 wells per dose over a 12 dose range.
To determine whether or not there was a correlation between sigma receptor binding and behavioral function we plotted the effective concentrations of each finazine analogue in vivo versus their Ki values in vitro, then also plotted a best-fit linear regression line and determined its R2 value. We found that activity at sigma-1 correlated fairly well with behavioral activity; almost 70% of the behavioral variability between finazine analogues could be explained by sigma-1 binding (Fig 3b). On the other hand, activity at sigma-2 was not well correlated; neither was activity at any of the other candidate targets we examined, including serotonin (5-HT), dopamine D2 family, or adrenergic alpha-2 receptors (Supplementary Fig 14). We also profiled our inactive finazine analogue, compound 27, and found that not only was it ineffective at modulating behavior (and thus excluded from our correlation plots), it also lost its binding potency at sigma-1 (Supplementary Table 3). Together, these data implicated sigma-1 in the mechanism of action of the finazine family of compounds.
Known sigma-1 ligands affect freezing and escape behavior
Because we identified sigma-1 as a major target of three structurally distinct classes of threat response modulators, we hypothesized that known sigma-1 ligands should also switch the zebrafish strobe light response from freezing to escape behavior. We tested a panel of known sigma-1 ligands from distinct structural classes in zebrafish and found that sigma-1 agonists robustly induced a dose-dependent switch from freezing to escape behavior. These include the sigma-1 selective agonists (+)-SKF-10,04717 (28) and cutamesine18 (29) as well as non-selective agonists – e.g. phencyclidine (30), noscapine (31), eliprodil (32) and donepezil (33) (Fig 4a). The doses required for behavioral switching were relatively low, ranging from about 1 to 20 μM, and the peak freezing index values ranged from +5 to +10 (Fig 4b). These values are very similar to the sigma-1 ligands identified by our screen. Of course, additional mechanisms of action for all six of these compounds may exist which could also contribute to the escape phenotype. To our knowledge no other target has been described for cutamesine (also known as SA 4503); however (+)-SKF-10,047, phencyclidine and eliprodil are all known to have some activity as N-Methyl-D-aspartate (NMDA) receptor antagonists, a common feature of many sigma-1 ligands.19 To rule out the involvement of the NMDA receptor in the finazines’ mechanism(s) of action, we tested our lead finazine (1, from here on referred to as ‘finazine’) for its ability to bind to the NMDA receptor and found that unlike control compound MK-801, finazine was unable to bind (Supplementary Fig 15).
Figure 4. Sigma-1 is a functional target of finazine.
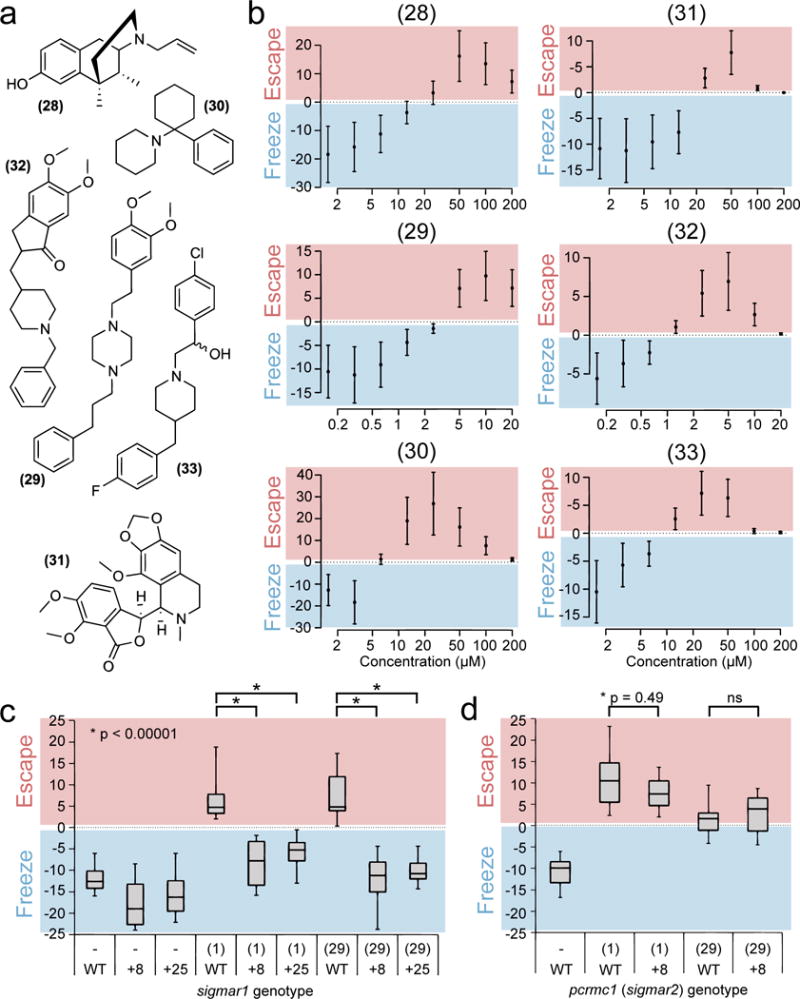
(a) Chemical structure of known sigma-1 ligands: (+)-SKF-10,047 (28), cutamesine (29), phencyclidine (30), noscapine (31), eliprodil (32) and donepezil (33). (b) Dose curves showing the degree of behavioral switching, measured by the freezing index. Data represent mean ± s.d. for n = 12 wells per dose and are representative of three independent experiments. (c) Full-range boxplots showing all data from n = 12 wells of wild-type sibling (WT) or homozygous mutant sigmar1 fish (8 or +25) treated with 10 μM scopolamine and either DMSO (−), 5 μM finazine or 10 μM cutamesine. Boxes represent the interquartile range, whiskers represent maximum and minimum values. Data are representative of two independent experiments. (d) Same as (c), but with wild-type sibling (WT) or homozygous mutant pgrmc1 fish (+8). Statistical significance was calculated using a student’s t-test (2-tailed, unpaired, unequal variance).
To test the potential involvement of sigma-2, a frequent secondary target of sigma-1 ligands, in the switch from freezing to escape behaviors, we tested several sigma-2 specific ligands, but none of these had the same robust behavioral effect in zebrafish exhibited by sigma-1 ligands (Supplementary Fig 16). Because some of our hits exhibited some in vitro affinity for 5-HT2B and adrenergic alpha-2 receptors (Fig 3a and Supplementary Table 3), we also tested several known ligands for these targets in the SLR assay, but obtained mostly negative results. The compounds tested were 5-HT2B agonists fenfluramine, dyhidroergotamine and BW 723C86; 5-HT2B antagonists clomipramine and chlorprothixene (Supplementary Fig 17); alpha-2 agonists norepinephrine, clonidine and dexmedetomidine; and alpha-2 antagonists yohimbine, BRLR 44408 and mirtazapine (Supplementary Fig 18). We found that clonidine did cause escape behavior at the highest dose tested, though this is may be an off-target effect given its poor potency in the SLR assay.
Because the antipsychotic haloperidol, like the finazines, is a high-affinity sigma-1 ligand (Supplementary Fig 13), and because we also identified five finazines using a computational phenotype-based (phenoBLAST) approach for identifying antipsychotic-like compounds (see our companion paper in this issue20), we tested haloperidol in our SLR assay. We found that haloperidol also induced escape behavior in 7 dpf zebrafish in the SLR assay, albeit to a much lesser extent than the finazines (Supplementary Fig 19). These data are consistent with the findings reported in Bruni et al.20 and further demonstrate that the zebrafish SLR assay is useful for the identification of sigma-1 ligands.
Genetic knockout of sigma-1 rescues freezing behavior
In addition to providing chemical evidence for the involvement of sigma-1 in the mechanism of finazine-induced escape behavior, we sought to provide genetic evidence. Using CRISPR-cas9 mediated mutagenesis we generated two different lines of zebrafish harboring frameshift insertions (+8 and 25, Supplementary Fig 20a) within exon 2 of the sigmar1 gene. Each mutation was created just upstream of the gene sequence that encodes the most highly conserved part of the sigma-1 protein, from yeast to humans (Supplementary Fig 20b). The result of each mutation was loss of sigma-1 protein (Supplementary Fig 20c); however fish homozygous for either mutant allele showed no obvious abnormalities (Supplementary Fig 20d) and grew up to be viable, fertile adults.
When tested in the SLR assay, larvae from wild-type sibling crosses showed escape responses when treated with 5 μM finazine or 10 μM cutamesine; however mutant fish homozygous for either the +8 or +25 allele showed significantly impaired responses to either drug (Fig 4c). CRISPR-cas9 mediated frameshift mutagenesis of the putative sigma-2 gene (pgrmc1, Supplementary Fig 21) had no effect in the context of either drug (Fig 4d). These data further support the hypothesis that sigma-1 is a functional target of the finazine family of compounds and that the sigma-1 protein is involved in the switch between active and passive threat responses.
Finazine has behavioral effects in rodents
To determine whether or not compounds discovered in the zebrafish assay retain activity in mammals, we turned to a well-established assay of behavioral freezing in mice, contextual fear conditioning. The basis for this assay is the tendency of mice to freeze when re-exposed to a context where they received an aversive stimulus (e.g., a footshock). Mice were treated with either 10% DMSO vehicle or finazine, and evaluated for their freezing response to the conditioning chamber twenty-four hours after receiving a series of mild (0.5 mA) footshocks. Vehicle-treated mice responded to the conditioning context by exhibiting a high level of freezing behavior, freezing about 60% of the time, as expected (Fig 5). Finazine-treated mice, however, spent less than 40% of their time immobile, suggesting impaired freezing in this contextual fear conditioning assay. There was no evidence of elevated freezing behavior when mice from either group were exposed to an altered (neutral) context (Fig 5). These data suggest that finazine is able to penetrate the blood-brain barrier in mice as well as zebrafish and modulate the behavior of both species.
Figure 5. Finazine has neuroactive effects in a mouse freezing assay.
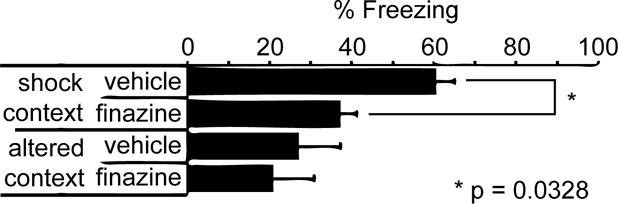
Contextual Fear Conditioning Test using male C57BL/6J mice (n = 12 per condition) treated with 10% DMSO vehicle or finazine, as indicated. Locomotion was measured during the exposure to the conditioned context 24 h post shock treatment. Data represent mean ± s.e.m. Statistical analysis was performed using one-way analysis of variance (ANOVA) and the Fisher’s PLSD post hoc test.
DISCUSSION
This study represents the first high-throughput approach to discovery of compounds that modulate threat response behaviors. Zebrafish can respond to threats in two mutually exclusive ways—hypolocomotion or hyperlocomotion. These responses share hallmarks of the stereotypical freezing and escape responses described for numerous other animals. Hypolocomotion is a non-specific behavior that can reflect sedation, fatigue, sickness, or paralysis, making it important to determine if strobe-induced hypolocomotion in zebrafish is freezing or some other immobile state. We observed that in our model, hypolocomotion is triggered by aversive stimuli, is rapidly reversible, and is capable of being switched to escape-like behavior, all of which are more consistent with freezing than sedation, fatigue, sickness, or paralysis. Nevertheless, more work will be needed to characterize fully this strobe-induced response in zebrafish and determine the extent to which it parallels the freezing responses of mammals.
Using a whole-organism behavioral screen of more than 10,000 small molecules, we discovered that the default freezing behavior of zebrafish larvae could be completely switched to escape behavior by three classes of small molecules, the finazines, finoxetines, and finopidils. These compounds cause zebrafish to swim vigorously in the presence of strobe light, a stimulus that normally elicits a freezing response. We also note that most of the compounds also produce mild to moderate sedation in the absence of the strobe stimulus, indicating that the compounds are not general stimulants. It is currently unclear whether the compounds’ mild sedation effects under dark conditions are independent of their ability to switch the threat response from freezing to escape. Regardless, the existence of such behavior-switching compounds motivated us to try to discover their functional targets, with the hope that we might uncover a key pathway regulating the response to threatening stimuli.
Determining the mechanism by which neuroactive drugs exert their effects remains one of the great challenges in neuropharmacology. Most neuroactive compounds exhibit activity at multiple targets, making it difficult to ascertain which target(s) are responsible for the compounds’ in vivo effects. The same was true for the finazines, finoxetines, and finopidils. Each compound we profiled exhibited binding activity at 5–30 of the major known CNS drug targets. Therefore, for any individual compound, elucidation of the relevant target would have been difficult. However, because we discovered multiple behavior-modifying compounds from distinct structural classes and profiled their binding activity systematically, we were able to use computational clustering to determine that most of the active compounds exhibited potent binding to the sigma receptors, suggesting that sigma-1 and/or -2 were responsible for the behavioral effects of all three compound classes. Further testing with structurally distinct sigma receptor ligands and sigma-1 knockout animals added further support to the idea that the sigma-1 receptor is a central regulator of the switch between active and passive fear responses. Therefore, the combination of large-scale behavioral screening and systematic receptor profiling was proven to be a powerful means of identifying behavior-modifying neuronal targets. Of course, one limitation of this approach is that it can only identify targets that are contained within the set of potential targets tested. In this case, the correlation between sigma-1 receptor binding and in vivo activity was excellent, but that finding does not exclude the possibility that additional, untested proteins may also be targeted by the finazines, finoxetines, and finopidils. In this regard, our ability to generate targeted mutations in sigma-1 was critical for confirming the relevance of sigma-1 as a finazine target. The fact that sigma-1 mutation prevented the escape response in finazine-treated animals confirms its importance in mediating finazine’s effect.
In mammals the limbic system plays a central role in the modulation of freezing and escape behaviors. Sigma-1 receptors are expressed in limbic structures including the dentate gyrus and pyramidal cell layer of the hippocampus, superficial layers of the cortex, amygdala, basal forebrain nuclei and olfactory bulb. These regions are involved in motivation and emotional behaviors, including unconditioned fear responses.21–23 To our knowledge, only one previous report has examined the role of sigma-1 in zebrafish, describing a role for sigma-1 in turning off microglial trafficking in the brain after response to larval brain injury.24 Our findings that sigma-1 ligands are able to control a switch between active and passive threat (fear) responses suggest an important role for sigma-1 in motivation and emotional processing. Sigma-1 receptor ligands show behavioral effects in a number of rodent tests for anxiety and depression. For example, (+)-SKF-10,047 and dextromethorphan reduce animal freezing in response to footshock stress.25 Our mouse data show that finazine also affects freezing in response to footshock stress. Chemically unrelated sigma-1 agonists, such as (+)-pentazocine, 1,3-di-o-tolyguanidine (DTG), igmesine and various neurosteroids ligands dose-dependently reduce immobility in the forced swim test (FST),26–28 an animal model of behavioral despair, and the antidepressant-like action induced by these ligands is reversed by sigma-1 antagonists.29 The importance of sigma-1 activity in normal forced swim behavior was further demonstrated by experiments using two different lines of sigma-1 knockout mice, which each showed increased immobility in the FST.30,31 Many of these rodent data suggest an antidepressant role for sigma-1 activation.32
Beyond improving our understanding of a fundamental behavior, discovery of small molecules that alter the zebrafish freezing response could have important therapeutic implications. Disruption of the limbic system and inappropriate responses to perceived threats are thought to be central features of mental health disorders including schizophrenia, post-traumatic stress disorder (PTSD) and depression. These disorders affect millions and remain poorly treated, despite large investment in discovery of therapies using targeted approaches. Our data demonstrate that zebrafish can be used as a tool to rapidly discover complex, system-modulating compounds. By using live organisms with intact brains during the very first phase of the discovery process, this whole-organism screening approach allows assessment not only of the behavioral efficacy of test compounds, but also their bioavailability and toxicity.
Although sigma-1 has not previously been associated with regulation of threat responses, sigma-1 ligands may have therapeutic utility for conditions associated with inappropriate threat responses. Accumulating evidence suggests that sigma-1 ligands can be used to treat the cognitive deficits associated with schizophrenia and patients suffering from PTSD.33,34 Moreover, several clinically important CNS drugs are potent sigma-1 ligands, including the antidepressant medications citalopram, fluoxetine/fluvoxamine, imipramine, and sertraline; and butyrophenone antipsychotics such as haloperidol. These sigma-1-modifying drugs all have complex mechanisms of action involving additional neuronal targets; however positron emission tomography (PET) studies have shown that these drugs can bind sigma-1 sites within the human brain at commonly used, orally administered doses. Therefore, the beneficial clinical effects of these antipsychotics and antidepressants may be due in part to their activity at the sigma-1 receptor.
The in vivo function of the sigma-1 receptor has been a matter of much investigation and debate. Discovery of a role for sigma-1 in switching between passive and active threat responses opens up new avenues for investigation of this enigmatic receptor. Additional studies will be required to elucidate the precise mechanisms by which sigma-1 regulates threat responses, including the neuronal circuits involved, the cellular signaling events triggered and the existence of any endogenous signals that might influence sigma-1 activity. Nevertheless, we hope the robust zebrafish freezing assay described here will be a valuable tool for future investigation of threat responses, as will the finazines, finoxetines, and finopidils.
ONLINE METHODS
Aquaculture
We collected a large number of fertilized eggs (up to 10,000 embryos per day) from group matings of EkkWill strain zebrafish (Danio rerio) (EkkWill Waterlife Resources). Embryos were raised in HEPES (10 mM) buffered E3 medium at 28 °C on a normal 14/10 hr on/off light cycle because larval fish show dramatic changes in visual responsiveness when maintained in continuous darkness.35 At 3 days post fertilization (dpf), chorion debris was removed and larvae were transferred into fresh medium until 7 dpf. Larvae (7 dpf, unless otherwise noted) were counted and manually pipetted (10 larvae per well, unless otherwise noted) into clear 96-well, U-bottom plates (250 μl/well) in the same medium. All larvae were used regardless of sex; zebrafish sex is indeterminable at this early stage in life.
Automated freezing assay and measurement
We use 10 larvae/well of a 96-well plate when conducting the behavioral assays. This number was determined empirically; fewer or more than 8–10 fish/well yield smaller signal to noise ratios. Larvae from group matings of ≥ 180 adult fish were pooled and plated randomly. 96-well plates (Fisher Scientific, 12-565-500) containing ten 7 dpf larvae per well were loaded into a ZebraBox (ViewPoint) containing a computer-controlled light box and a video camera with an infrared filter. Infrared light was used to illuminate the chamber and the temperature was maintained at 28 °C for the duration of the 7 mim experiment. White strobe light was automated using the ZebraLab software (ViewPoint) as follows: dark for minutes 1, 3, 5 and 7; strobing for minutes 2, 4 and 6. During the dark periods white light was set at 0% (no white light), and during the strobing periods white light alternated between 100 ms at the 100% setting (white light on) and 100 ms at 0% to set strobe at 10 Hz. Strobe light generated using the ‘Strobe Light’ tachometer application for iPhone (Peter Thew, Grappetite) yielded similar results. Light intensity at 100% was measured using a PM100D power meter attached to a S130VC photodiode power sensor (Thorlabs) and found to be 7.87 μW/mm2. Fish movement was recorded by an infrared camera using the ZebraLab Videotrack ‘quantization mode’ at 30 frames s−1 with parameters set as follows: sensitivity threshold, 15; burst (threshold for very large movement), 100; freeze (threshold for no movement), 50; bin size, 1 s. 96 evenly-spaced regions of equal size were drawn around each well of the assay plate using the Viewpoint software. The software then tracks the change in pixel intensity for each region over time producing our ‘Fish Motion’ (pixels/frame) index, which correlates with the overall amount of motion in the well. Each video was saved for review and the data were further analyzed using custom R scripts. A freezing index was calculated by subtracting the third quartile Fish Motion values from the dark periods from that of the strobe periods. The bigger the difference between motion in dark and motion in strobe light the more negative the freezing index. Because the analysis was automated, blinding was unnecessary. We used a student’s t-test (2-tailed, unpaired, unequal variance) to analyze freezing index values. An effect was considered significant if p < 0.0005. Data from the entire plate was excluded if any of the DMSO-only (negative control) wells gave a freeing index score ≥ 0.
Chemical treatment
Chemicals libraries were screened at a 1:100 final dilution in HEPES (10 mM) buffered E3 medium for a final concentration of 3.33 μg/ml (~15 μM on average). In the genetic tests, 10 μM scopolamine was added to the assay buffer to reduce non-sigma-1 related escape activity. DMSO was the solvent for more than 90% of library compounds; all others were dissolved in water. Stock solutions were added directly to zebrafish in the wells of a 96 well plate, mixed, and allowed to incubate for 1 hr at 28 °C in ambient light prior to behavioral evaluation. Dose curve validations were also performed using 1:100 final dilutions. All compounds were dissolved in DMSO (Sigma). The final DMSO concentration was always 1%.
Chemical stocks
The DiverSetE library (10,000 compounds, ChemBridge, ≥95% purity) was used for the initial high-throughput screen. The following compounds were re-ordered and used in all confirmatory and further assays: 1 finazine (ChemBridge, 6557321, ≥95%), 2 (ChemBridge, 6531872, ≥95%), 3 (ChemBridge, 6558983, ≥95%), 4 (ChemBridge, 6548654, ≥95%), 5 (ChemBridge, 6544610, ≥95%), 6 (ChemBridge, 6518299, ≥95%), 7 (ChemBridge, 6524759, ≥95%), 8 (ChemBridge, 6498979, ≥95%), 9 (ChemBridge, 6553891, ≥95%), 10 (ChemBridge, 6527322, ≥95%), 11 (ChemBridge, 6541071, ≥95%), 12 (ChemBridge, 7021430, ≥95%), 13 (ChemBridge, 7318202, ≥95%), 14 (ChemBridge, 7004866, ≥95%), 15 (ChemBridge, 7317879, ≥95%), 16 (ChemBridge, 7317926, ≥95%), 17 (ChemBridge, 7323150, ≥95%), 18 (ChemBridge, 6944181, ≥95%), 19 (ChemBridge, 6942573, ≥95%), 20 (ChemBridge, 5767877, ≥95%), 21 (ChemBridge, 6238692, ≥95%), 23 (ChemBridge, 6520799, ≥95%), 24 (ChemBridge, 6500628, ≥95%), 25 (ChemBridge, 6546312, ≥95%), 26 (ChemBridge, 9045050, ≥95%), naftopidil (Tocris, 0597, ≥98%), (+)-SKF-10,047 (Tocris, 1097, ≥98%), cutamesine (SA 4503, Tocris, 4951, ≥98%), phencyclidine (Sigma, P3029, ≥98%), noscapine (Tocris, 1697, ≥99%), eliprodil (Tocris, 2195, ≥98%), donepezil (Tocris, 4385, ≥99%) and scopolamine (Tocris, 1414, ≥99%). 3-(piperazin-1-yl)-N-(m-tolyl)propanamide 27 was synthesized in house (for synthetic methods, see Supplementary Note 1): 1H NMR (500 MHz, CDCl3): δ 10.60 (br, 1H), 7.39 (s, 1H), 7.30–7.28 (m, 1H), 7.20 (t, 1H, J = 7.5 Hz), 6.90 (d, 1H, J = 7.5 Hz), 3.70 (br s, 1H), 3.07–3.02 (m, 4H), 2.74 (t, 2H, J = 5.5 Hz), 2.64–2.58 (m, 4H), 2.53 (t, 2H, J = 6.0 Hz), 2.33 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 170.5, 138.9, 138.6, 128.8, 124.6, 120.2, 116.6, 54.2, 53.1, 46.0, 32.4, 21.6; ESI-MS: m/z 248 (M +H) +; HPLC Purity 95.0% (tR 8.52 min). HPLC condition, instrument, SHIMADZU; column, Pinnacle C18, 3 μm; UV/Vis, = 254 nm; flow rate, 0.2 mL/min, HPLC gradient went from 5% acetonitrile/95% water to 95% acetonitrile/5% water (both solvents contain 0.1% folic acid) over 30 min. The following compounds were also used in supplementary experiments: AG-205 (Sigma, A1487, ≥98%), amphetamine (Tocris 2813, ≥99%), BRL 44408 (Tocris, 1133, >98%), BW 723C86 (Tocris, 1059, >99%), chlorprothixene (Sigma, C1671, ≥98%), clomipramine (Tocris, 0457, >98%), clonidine (Tocris, 0690, >99%), cocaine (Tocris, 2833, >99%), dexmedetomidine (Tocris, 2749, >99%), dihydroergotamine (Tocris, 0475, >98%), fenfluramine (Sigma, F112, ≥98%), haloperidol (Sigma, H1512, ≥98%), methylphenidate (Sigma, M2892, ≥98%), mirtazapine (Tocris, 2018, >99%), (−)-nicotine (Sigma, N5260, ≥98%), (−)-norepinephrine (Sigma, A7257, ≥98%), PB 28 (Tocris, 2562, >99%), rimcazole (Tocris, 1497, ≥98%), siramesine (Sigma, SML0976, ≥98%), SM-21 (Tocris, 0751, >99%), tamoxifen (Cayman,11629, ≥98%), and yohimbine (Sigma, Y3125, ≥98%).
Cortisol luminescence immunoassay
Zebrafish embryos were raised 30/well in a 6-well plate with 5 ml E3 medium, which was replaced once after hatching at 2 dpf. At 7 dpf, larvae were subjected to 10 min treatments of 250 mM NaCl (positive control), darkness, strobe light or ambient light (negative control). After treatments, each well was filled completely with ice-water, half of the volume in each well was removed (without sucking up fish) and the well again filled with ice-water. Larvae were transferred to pre-chilled 1.5 ml tube, 30 per tube, spun down for 5 sec and transferred back to ice. Water was removed down to 20 μl and fish were frozen using an ethanol/dry-ice bath. Samples were stored at −20 °C. For cortisol measurements, samples were thawed, combined with 150 μl H2O and homogenized for 20 sec with a pellet mixer (VWR International). 1 ml ethyl acetate (Sigma) was added per sample and tubes were vortexed at maximum speed for 30 sec. Solvent and aqueous phases were separated by centrifuging the tubes for 5 m at 3000 × g and 4 °C. Solvent (top) layer was transferred into a new 1.5 ml tube and evaporated at 25 °C for 18 h. Remaining cortisol was dissolved in 60 μl 0.2 % BSA in PBS and used directly in the Cortisol Saliva Luminescence Immunoassay Kit (IBL International) per the manufacturer’s instructions.
Place preference assay
7 dpf fish were plated in to 10 cm plates at 100 fish/plate. Half of each plate was covered with opaque black tape. Fish in each plate were placed in darkness and permitted to acclimate for 10 min. After 10 min, 2 min recordings were started. For the first min the fish remained in darkness. For the second min fish in the uncovered half of the plate were exposed strobe light (10 Hz). Numbers of fish in the strobe-exposed (open) area of the plate were counted from movie frames at 8 sec intervals.
Zebrafish mutagenesis
The complete protocol used to generate the mutant lines used in the study has been previously described.36 Briefly, frameshift mutational insertions were reverse engineered into the sigmar1 and pgrmc1 genes of TuAB strain zebrafish at exon 2 and 1 respectively, using CRISPR-cas9 mediated DNA disruption followed by endogenous error-prone repair. The CRIPSR gRNA variable spacer sequences used were GGCCTTCTCTAAGGTGGTTGTGG (sigmar1) and GAAGCAGTCGAGCAAACTTCTGG (pgrmc1) from 5′ to 3′ (PAM sequence underlined). The primers used for genotyping (also 5′ to 3′) were gtgctgtgcactatagaagctg (sigmar1Fwd), gacctgaatgtccaccggtg (sigmar1Rev), acacaccccagaacatccac (pgrmc1Fwd), and CTCAACCGGGCCATAGTCTG (pgrmc1Rev). Sanger sequencing was performed by the MGH DNA Core Facility. A BigDye v3.1 Cycle Sequencing Kit (Applied Biosystems) was used to generate extension products from purified PCR reactions. These were purified using SPRI technology. Subsequently, fragment separation and sequence detection was carried out by capillary electrophoresis on the 96-well capillary matrix of an ABI3730XL DNA Analyzer (Applied Biosystems).
Immunoblotting
7 dpf fish were aliquot 10 per tube into 1.7 ml tubes, spun down at 14 K rpms for 5 min. E3 medium was aspirated and 50 μl of 2 × SDS Sample Buffer (6 mM Tris, pH 6.8, 20% Glycerol, 7% SDS, protease inhibitors) was added. Next 50 μl of 7 M Urea (3.5 M final concentration) was added and samples were homogenized using a motorized pestle, incubated at 25 °C for 30 min and spun down again. Supernatants were saved. A BCA Assay (Pierce) with a BSA standard was used to determine total protein concentration. Samples were loaded 20 μg/lane in Laemmli Buffer with 2-mercaptoethanol onto a NuPage 4–15% Tris-Bis Gel in MES Running Buffer and run at 110 V for 2 hrs. Proteins were transferred to a MeOH-soaked PVDF membrane using a Tris-Glycine Transfer Buffer with 20% MeOH (110 V for 1 hour). Blot was blocked in TBS-T with 5% BSA and probed with primary rabbit polyclonal antibody SIGMAR1 [N1C3] (Genetex, GTX115389, used at 1:5000) followed by secondary mouse anti-rabbit horseradish peroxidase (HRP) conjugated antibody (Cell Signaling, 7074, used at 1:5000).
Receptor binding assays
In vitro binding assay and Ki data were performed by the National Institute of Mental Health’s Psychoactive Drug Screening Program, contract no. HHSN-271-2008-00025-C (NIMH PDSP) using previously published protocols.37 Detailed descriptions of known ligands used can be found on the PDSP website: https://pdspdb.unc.edu/pdspWeb/content/PDSP%20Protocols%20II%202013-03-28.pdf. To generate heat map and cluster data a simple script was written in R programming language using the ‘heatmap’ function.
Mouse husbandry
Male C57BL/6J mice (Mus musculus) were obtained from Jackson Laboratories. Mice were group-housed 4 per cage in Tecniplast ventilated cages and were maintained on a 12/12 hr light/dark cycle (lights on 0700 EST). The room temperature was maintained at 20–23 °C with relative humidity at approximately 50%. Food and water were available ad libitum for the duration of the study, except during testing and all testing was conducted during the light phase of the light dark cycle.
Mouse behavior
C57BL/6J male mice (Jackson Laboratory), at 9–10 weeks of age, were exposed to a conditioning chamber (31 cm-L 25 cm-W × 25 cm-H, Med Associates, St. Albans, VT) with Plexiglas sidewalls, stainless steel end-walls, and a floor consisting of steel bars. Mice were randomly given a 30 min pretreatment with 10% DMSO vehicle or 25 mg/kg of finazine in 10% DMSO (ip, 10 ml/kg injection volume) and allowed to explore the chamber for 2 min (baseline). Mice were then given 4 presentations of a 2 sec foot shock (0.5mA) separated by 2 min intertrial intervals. Mice were removed from the chamber 2 min after the last foot shock (postshock 1–4). Approximately twenty-four hours after the training session, mice were placed back into the conditioning chamber for 5 min (no electric shock was delivered during this session). After all mice were tested in the conditioning context, the test chamber was altered (smooth white plastic surfaces were inserted to cover the grid floor and side walls) and mice were tested in the altered context for 5 min. Mice were videotaped and the percent time freezing was scored by Topscan software (CleverSys). Because the analysis was automated, blinding was unnecessary. The level of freezing behavior in response to the conditioning context was compared to the amount of freezing observed in the altered (neutral) context. Power calculations were used to determine the numbers of animals required, based on standard deviations from previous studies in wild-type and transgenic mice. The probability of a type I error was set at 0.05 and type II error was set to give a power of 0.80. On the basis of the most variable data, to identify a 30% change as significant would require at least 11 animals per group (n = 2 × [(1.96+0.841)*6307/7557]2 = 10.93 animals). Therefore we used n = 12 mice per condition. No animals were excluded from the final analysis.
Animal welfare
All animal research was conducted humanely according to established protocols approved by the MGH Institutional Animal Care and Use Committee (for zebrafish) and the Harvard Medical Area (HMA) Standing Committee on Animals IACUC (for mice) in AALAC-accredited facilities, and in accordance with the Guide to Care and Use of Laboratory Animals (National Institutes of Health 1996).
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) and the Fisher’s PLSD post hoc test to test for significant differences between groups, generate 95% confidence intervals and identify groups with significantly different means. For groups with significant differences, we used the two-tailed t-test to test the null hypothesis and calculate the P value. All error bars represent s.d. unless otherwise noted.
Supplementary Material
Acknowledgments
This work was supported by NIH grant T32 HL 007208 (AJR), by NIH grants R01 MH 086867 (RTP), R01 GM 088040 (RTP), and U01 MH 105027 (DK and JRJY), by the Charles and Ann Sanders MGH Research Scholar Award (RTP), and by the Harvard NeuroDiscovery Center. Receptor binding profiles and Ki data were generously provided by the NIH Psychoactive Drug Screening Program, contract HHSN-271-2008-025C. We thank Colleen Brady, Jessica J. Colund, I. Taneli Helenius, Youngnam N. Jin and Anjali Nath for helpful comments. This paper is dedicated to Stuart L. Schreiber on the occasion of his 60th birthday.
Footnotes
AUTHOR CONTRIBUTIONS
AJR designed the experiments and performed the zebrafish behavioral and assays (including the screen), created the zebrafish mutants, analyzed the data and wrote the manuscript with RTP. SP and LC assisted AJR with zebrafish assays. APWG assisted with zebrafish mutagenesis. JRJY designed the zebrafish mutagenesis strategies. XPH performed the in vitro binding assays, which were designed by BLR. YW performed the chemical synthesis and purity analyses. PJL performed the mouse experiments and analyzed the data with BJC. BJC, DK and BLR contributed reagents and to research design. All authors contributed to data interpretation and commented on the manuscript.
Competing financial interests
AJR, DK, YW and RTP declare competing financial interests in the form of a pending patent application, No. PCT/US2015/037755, covering the sigma-1 ligands described in this manuscript.
References
- 1.Hartley CA, Phelps EA. Anxiety and decision-making. Biol Psychiatry. 2012;72:113–8. doi: 10.1016/j.biopsych.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laviolette SR. Dopamine modulation of emotional processing in cortical and subcortical neural circuits: evidence for a final common pathway in schizophrenia? Schizophr Bull. 2007;33:971–81. doi: 10.1093/schbul/sbm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams LM, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161:480–9. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 4.Paradiso S, et al. Emotions in unmedicated patients with schizophrenia during evaluation with positron emission tomography. Am J Psychiatry. 2003;160:1775–83. doi: 10.1176/appi.ajp.160.10.1775. [DOI] [PubMed] [Google Scholar]
- 5.Brewin CR. What is it that a neurobiological model of PTSD must explain? Prog Brain Res. 2008;167:217–28. doi: 10.1016/S0079-6123(07)67015-0. [DOI] [PubMed] [Google Scholar]
- 6.Hyman SE. Revolution stalled. Sci Transl Med. 2012;4:155cm11. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- 7.Rennekamp AJ, Peterson RT. 15 years of zebrafish chemical screening. Curr Opin Chem Biol. 2015;24C:58–70. doi: 10.1016/j.cbpa.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–17. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: towards the development of an automated aversive task. Behav Brain Res. 2009;201:318–24. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–77. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Rev Neurosci. 2011;22:63–73. doi: 10.1515/RNS.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emran F, Rihel J, Dowling JEA. behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J Vis Exp. 2008:e923. doi: 10.3791/923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullen CR, Carlson TJ. Non-physical fish barrier systems: their development and potential applications to marine ranching. Reviews in Fish Biology and Fisheries. 2003;13:201–212. [Google Scholar]
- 14.Yeh CM, Glock M, Ryu S. An optimized whole-body cortisol quantification method for assessing stress levels in larval zebrafish. PLoS One. 2013;8:e79406. doi: 10.1371/journal.pone.0079406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol. 2007;210:2526–39. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- 16.Backman TW, Cao Y, Girke T. ChemMine tools: an online service for analyzing and clustering small molecules. Nucleic Acids Res. 2011;39:W486–91. doi: 10.1093/nar/gkr320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Costa BR, et al. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989;251:53–8. doi: 10.1016/0014-5793(89)81427-9. [DOI] [PubMed] [Google Scholar]
- 18.Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse. 2006;59:350–8. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- 19.Walker JM, et al. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- 20.Bruni G, et al. Systematic behavioral profiling identifies antipsychotic-like compounds with multi-target polypharmacology. Nat Chem Biol. 2016 doi: 10.1038/nchembio.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniadis EA, McDonald RJ. Amygdala, hippocampus, and unconditioned fear. Exp Brain Res. 2001;138:200–9. doi: 10.1007/s002210000645. [DOI] [PubMed] [Google Scholar]
- 22.Adolphs R. The biology of fear. Curr Biol. 2013;23:R79–93. doi: 10.1016/j.cub.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- 24.Moritz C, Berardi F, Abate C, Peri F. Live imaging reveals a new role for the sigma-1 (sigma1) receptor in allowing microglia to leave brain injuries. Neurosci Lett. 2015;591:13–8. doi: 10.1016/j.neulet.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Kamei H, Kameyama T, Nabeshima T. (+)-SKF-10,047 and dextromethorphan ameliorate conditioned fear stress through the activation of phenytoin-regulated sigma 1 sites. Eur J Pharmacol. 1996;299:21–8. doi: 10.1016/0014-2999(95)00830-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Mack AL, Coop A, Matsumoto RR. Novel sigma (sigma) receptor agonists produce antidepressant-like effects in mice. Eur Neuropsychopharmacol. 2007;17:708–16. doi: 10.1016/j.euroneuro.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy DS, Kaur G, Kulkarni SK. Sigma (sigma1) receptor mediated anti-depressant-like effects of neurosteroids in the Porsolt forced swim test. Neuroreport. 1998;9:3069–73. doi: 10.1097/00001756-199809140-00028. [DOI] [PubMed] [Google Scholar]
- 28.Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharmacol Exp Ther. 2001;298:1269–79. [PubMed] [Google Scholar]
- 29.Matsuno K, Kobayashi T, Tanaka MK, Mita S. Sigma 1 receptor subtype is involved in the relief of behavioral despair in the mouse forced swimming test. Eur J Pharmacol. 1996;312:267–71. doi: 10.1016/0014-2999(96)00497-9. [DOI] [PubMed] [Google Scholar]
- 30.Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–6. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chevallier N, Keller E, Maurice T. Behavioural phenotyping of knockout mice for the sigma-1 (sigma(1)) chaperone protein revealed gender-related anxiety, depressive-like and memory alterations. J Psychopharmacol. 2011;25:960–75. doi: 10.1177/0269881111400648. [DOI] [PubMed] [Google Scholar]
- 32.Fishback JA, Robson MJ, Xu YT, Matsumoto RR. Sigma receptors: potential targets for a new class of antidepressant drug. Pharmacol Ther. 2010;127:271–82. doi: 10.1016/j.pharmthera.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishimoto A, Kaneko M, Gotoh Y, Hashimoto K. Ifenprodil for the treatment of flashbacks in female posttraumatic stress disorder patients with a history of childhood sexual abuse. Biol Psychiatry. 2012;71:e7–8. doi: 10.1016/j.biopsych.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki T, et al. Ifenprodil for the treatment of flashbacks in adolescent female posttraumatic stress disorder patients with a history of abuse. Psychother Psychosom. 2013;82:344–5. doi: 10.1159/000348585. [DOI] [PubMed] [Google Scholar]
- 35.Emran F, Rihel J, Adolph AR, Dowling JE. Zebrafish larvae lose vision at night. Proc Natl Acad Sci U S A. 2010;107:6034–9. doi: 10.1073/pnas.0914718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang WY, et al. Targeted Mutagenesis in Zebrafish Using CRISPR RNA-Guided Nucleases. Methods Mol Biol. 2015;1311:317–34. doi: 10.1007/978-1-4939-2687-9_21. [DOI] [PubMed] [Google Scholar]
- 37.Besnard J, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–20. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


