Abstract
Phosphorylation is an important post-translational event that has a wide array of functional consequences. With advances in the ability of various technologies in revealing and mapping new phosphosites in proteins, it is equally important to develop affinity reagents that can monitor such post-translational modifications in eukaryotic cells. While monoclonal and polyclonal antibodies have been shown to be useful in assessing the phosphoproteome, we have expanded our efforts to exploit the Forkhead Associated 1(FHA1) domain as scaffold for generating recombinant affinity reagents that recognize phosphothreonine-containing peptides. A phage display library of FHA1 variants was screened by affinity selection with 14 phosphothreonine-containing peptides corresponding to various human transcription factors and kinases, including human Myc, calmodulin-dependent protein kinase II (CaMKII), and extracellular-signal regulated kinases 1 and 2 (ERK1/2). The library yielded binding variants against 9 targets (64% success rate); success was largely determined by what residue occurred at the +3 position (C-terminal) to the pThr moiety (i.e., pT+3). The FHA domains binding Myc, CaMKII, and ERK1/2 were characterized and compared against commercially available antibodies. All FHA domains were shown to be phosphorylation-dependent and phosphothreonine-specific in their binding, unlike several commercial monoclonal and polyclonal antibodies. Both the pThr and the residue at the pT+3 position were major factors in defining the specificity of the FHA domains.
Keywords: Forkhead-associated domain, Phage display, Antibody, Phosphorylation, Phosphothreonine
Introduction
Protein phosphorylation is an important post-translational modification that principally occurs on serine (89%), threonine (10%), and tyrosine (<1%) residues (1-4). With over 100,000 phosphosites reported to date (5), there is a tremendous need for highly sensitive and specific probes to monitor the phosphorylation of particular residues in proteins during cell growth, differentiation, and disease (6). One such class of reagents are antibodies, which can be generated by immunizing animals with phosphopeptides; such antibodies have allowed the identification of physiologically important phosphosites, changes in phosphorylation states, and subcellular translocation of particular proteins upon phosphorylation (7-10).
While monoclonal and polyclonal antibodies have been historically invaluable to the field of eukaryotic cell signaling, drawbacks include production cost, renewability (11), and limited control over specificity, which can result in cross-reactive reagents (12-16). One strategy to overcome these limitations is to use recombinant affinity reagents, as they eliminate the need for animals, there is more control in epitope recognition, they are sequenced and renewable reagents and they are amenable to protein engineering (17, 18). To this extent, several engineered phosphate-binding domains, such as the Src Homology 2 (SH2) domain (19), a recombinant phosphospecific antibody fragment (20), the 10th fibronectin type III domain (10FnIII) (21), and the Forkhead-associated 1 (FHA) domain (22), have all been used successfully for generating recombinant affinity reagents to phosphopeptides.
A major advantage of the FHA domain, compared to other engineered scaffolds, is its natural ability to recognize a phosphothreonine (pThr, pT) residue in a post-translationally modified protein (23). Within the FHA domain, there is a pocket that interacts with the γ-methyl group and phosphate of pThr, which allows the domain to discriminate between phosphoserine (pSer) and pThr (24). Utilizing the domain's natural ability to discriminate between pSer and pThr, the specificity of one particular FHA domain, the FHA1 domain of yeast Rad53 protein, was reengineered through phage display (22). In this report, we demonstrate that the engineered FHA domains are exquisitely selective in binding pThr-, and not pSer- or phosphotyrosine (pTyr)-containing peptides, unlike several polyclonal and monoclonal antibodies tested. Furthermore, we also show that our library is capable of producing a variant that recognizes a doubly-phosphorylated peptide. In this regard, the FHA domain offers great promise in generating highly specific pThr-binding reagents, a feat not readily achievable through traditional immunological means.
Materials & Methods
Reagents
Peptides were synthesized at University of Illinois at Chicago's Research Resource Center, with > 90% purity. All peptides were biotinylated at their N-terminus and amidated at their C-terminus, and included lysine and tyrosine residues to increase peptide solubility and for measuring absorbance, respectively. The cognate targets for the Myc, ERK1/2, and CaMKII FHA domain affinity reagents are FELLPpTPPLSPS (Myc-pT58), HTGFLpTEpYVATRW (ERK1-pT202/pY204+ERK2-pT185/pY187), and LKGAILpTTMLATRN (CaMKII-pT305), respectively. The following peptides were used in a pThr substitution study: FELLPpTPPLSPS (pT58), FELLPpSPPLSPS (pT58pS), FELLPpYPPLSPS (pT58pY), FELLPTPPLSPS (T58), HTGFLpTEpYVATRW (pT202), HTGFLpSEpYVATRW (pT202pS), HTGFLpYEpYVATRW (pT202pY), HTGFLTEYVATRW (T202), LKGAILpTTMLATRN (pT305), LKGAILpSTMLATRN (pT305pS), LKGAILpYTMLATRN (pT305pY), LKGAILTTMLATRN (T305).
Three commercial anti-phosphopeptide antibodies were compared to the recombinant FHA domains generated in this report. Two were polyclonal antibodies (pAb), pAbαMyc (Abnova, catalog# PAB0541) and pAbαCaMKII (Thermo Scientific, catalog# PA5-35521), and one was a monoclonal antibody (mAb) mAbαERK1-pT202/pY204+ERK2-pT185/pY187 (mAbαERK1/2) (Abcam, catalog# ab136926). As all three are rabbit antibodies, a goat anti-rabbit immunoglobulin G (IgG), conjugated to Horseradish peroxidase (HRP; Abcam, catalog# ab97051), served as the common secondary reagent. Another secondary reagent was the anti-Flag epitope mAb, M2, which was conjugated to HRP (Sigma-Aldrich, catalog# A8592).
DNA constructs
The coding sequences for individual FHA domains were amplified from virions by the polymerase chain reaction (PCR). The double-stranded DNA product was digested with Nco I and Not I restriction endonucleases and subcloned into the pET29b expression vector. These constructs included a 3XFlag®-tag sequence (DYKDHDGDYKDHDIDYKDDDDK), followed by a His6-tag, at the C-terminus of the fusion proteins. All constructs were verified by DNA sequencing.
Protein purification
Overexpression of the constructs and their purification was carried out using standard methods (25). Briefly, BL21DE3 cells containing the expression vector was grown at 30°C for 24 hours (h) using the Overnight Express™ Autoinduction System 1 (Novagen). Bacterial cells were lysed using a Sonic Dismembrator (Branson Model 500). The lysate was mixed with Clontech His-60 Ni Superflow resin (Clontech Laboratories), and the His6-tagged proteins eluted with 50 mM sodium phosphate, 300 mM sodium chloride, 250 mM imidazole (pH 8.0).
Enzyme-linked immunosorbent assays (ELISA)
ELISAs were performed using an established protocol (25), except that non-specific binding in microtiter plate wells was blocked with 1% casein in phosphate buffered saline (PBS; 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4).The absorbance was read at 405 nm wavelength in 10 minute (min) intervals, for a total of 40 min. All experiments were performed in triplicate, and repeated at least three times to confirm reproducibility of the data.
Results & Discussion
Production of FHA domains by recombinant phage display
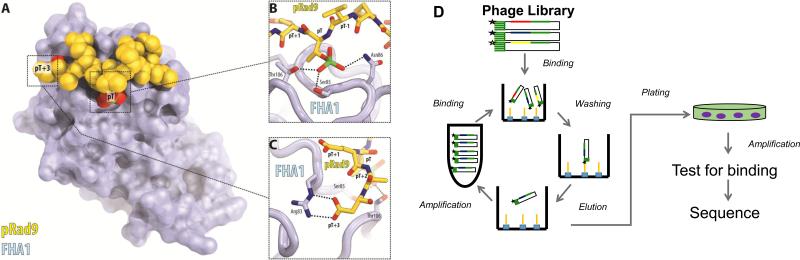
Phage display is a powerful technique that allows for the rapid and efficient production of affinity reagents, such as antibodies (26), without the need to immunize animals (27). To generate recombinant affinity reagents that are phosphothreonine-specific, a phage display library was constructed by randomizing residues in the β4-β5 and β10-β11 loop regions of a thermostable variant (FHA1G2) of the FHA1 domain of the yeast Rad53 protein (22, 28) (Fig. 1A). The library was incubated separately with a variety of phosphothreonine-containing peptides, which were chosen based on the physiological importance of the pThr residue in a eukaryotic signaling pathway, and included protein kinases and transcription factors. After three rounds of affinity selection, individual clones were tested by an enzyme-linked immunosorbent assay (ELISA), and unique clones were identified by DNA sequencing (Fig. 1D). With biotinylated, phosphorylated forms of the peptides as targets, we were able to produce recombinant affinity reagents in less than two weeks for 9 out of 14 peptide attempted, reflecting a 64% success rate (Table 1).
Figure 1. Generation of FHA affinity reagents via phage display.
A. The FHA1 domain (PDB: 1G6G) interacting with its native peptide (SLEVpTEAD) from pRad9. The FHA1 domain and peptide are represented in surface view and as spheres, respectively, with the PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC. B. A magnification of Ser85, Asn86, and Thr106 on FHA1 domain interacting with the phosphate on the pThr residue. C. A magnification of Arg83 on FHA1 domain interacting with Asp on pRad9 in the pT+3 position. D. Schematic of the process for isolating binders to phosphopeptides from a phage library displaying FHA1G2 variants. The biotinylated pThr-containing peptide is immobilized by Streptavidin. The library is incubated with the target and undergoes a series of washes. The phage is eluted and amplified to undergo two more rounds of selection. After the third round, Escherichia coli is infected with eluted phage and plated for amplification. Binding of individual clones is tested by phage ELISA. Clones are sequenced to check for any unique sequences.
Table 1.
A list of FHA variants isolated against phosphothreonine peptides corresponding to various human cell signaling proteins.
| Protein | Phosphosite | Peptide Sequence | FHA Reagent |
|---|---|---|---|
| Ca2+/calmodulin-dependent protein kinase II | CaMKII-pT305 | LKGAILpTTMLATRN | FHAαCaMKII |
| Family with Sequence Similarity 38, Member A | FAM38A-pT1811 | NTRPQSDpTPE/RKYK | FHAαFAM38A |
| Mitogen-activated protein kinase kinase kinase kinase 4 | MAP4K4-pT915 | KRELYNGpTAD/TLRF | FHAαMAP4K4 |
| Mitogen-activated protein kinase 3 | MAPK3-pT197 | ADPEHDHpTGFLTE | FHAαMAPK3* |
| Mitogen-activated protein kinase 1 | MAPK1-pT185 | HDHTGFLpTEYVAT | FHAαMAPK1* |
| Src homology 2 domain containing transforming protein 1 | Shc-1-pT35 | GSFVNKPpTRGWLH | FHAαShc-1 |
| Transcription factor jun-B | JunB-pT255 | EARSRDApTPPVSP | FHAαJunB* |
| Transcription factor jun-D | JunD-pT245 | ALKDEPQpTVPDVP | FHAαJunD* |
| Transcription factor Myc | Myc-pT58 | FELLPpTPPLSPS | FHAαMyc |
| RAF proto-oncogene serine/threonine protein kinase | Raf1-pT491 | IGDFGLApTVKSRWSG | FHAαRaf1 |
The “p” proceeding the “T” indicates the phosphate attached to the T residue (bold). Italicized residues are in the +3 position, where the pT is assigned as the “0” position, and residues N-terminal and C-terminal to the pT are denoted as “-” and “+,” respectively.
Previously reported in (22).
Biochemical and structural studies (29) have revealed that a major determinant of specificity for FHA domains is the +3 position (C-terminal) to the pThr moiety. To date, FHA domains can be categorized into three groups based on their recognition of the pT+3 position - pTxxD, pTxx(I/L), and pTxx(A/S) - with the yeast Rad53 protein FHA1 domain falling into the first category. We also confirmed (see below) this position to be important for binding to our FHA domains. As seen in Table I, we isolated FHA domain variants to peptides with D, L, V, P, S, and W, in the +3 position. We have yet to test phosphothreonine-containing peptides with A, C, Q, E, H, M, F, N, T, and Y at the +3 position.
The five peptides that failed to yield binders included pThr-containing phosphopeptides corresponding to nucleolin (NCL), histone H1, polo-like kinase 1 (PLK1), mitogen-activated protein kinase kinase 2 (MAP2K2), and isoform 1 of epidermal growth factor receptor precursor (EGFR). The inability to isolate FHA1 domains that bound to these particular phosphopeptides was reproducible; their sequences either contained K, R, and G at the +3 position. To our knowledge, an FHA domain that binds to any of these three amino acids at this position has not been observed before in nature. In the future, it will be interesting to see if an FHA domain scaffold can be devised, through directed evolution or computational design that recognizes such residues in the +3 position.
FHA domain variants are phosphorylation-dependent in binding
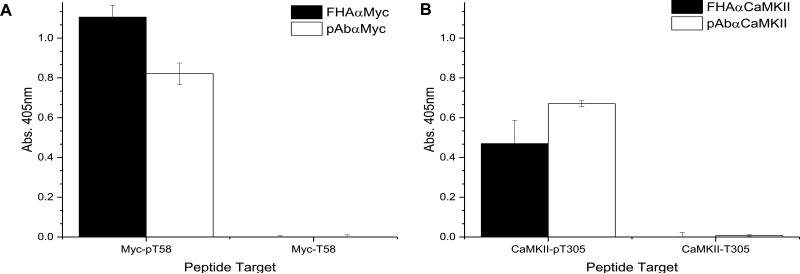
In order to evaluate the specificity of the isolated FHA variants, their open reading frames (ORFs) were subcloned into an expression vector containing 3XFlag®- and His6-tags. The recombinant proteins were purified using immobilized-metal affinity chromatography (IMAC). Each of the variants produced high yields (>150 mg/L) and was shown to be > 95% pure and properly folded by a fluorescence thermal shift assay. Binding of two variants, FHAαMyc (Fig. 2A) and FHAαCaMKII (Fig. 2B), to their cognate phosphorylated targets were assessed by ELISA and compared against commercially available antibodies. The ELISA is an ideal assay to test for peptide binding as it is a sensitive assay format, as compared to western blotting where the peptides are too small to resolve properly by SDS-PAGE. All reagents showed a >1000 fold difference in signal between the phosphorylated and non-phosphorylated peptide targets. These data indicate that binding of all reagents is phosphorylation-dependent.
Figure 2. FHA variants are phosphorylation-dependent.
A phosphorylated or unphosphorylated peptide was used as a target in an ELISA. Phosphospecific reagents were used as probe targets to test for phosphorylation dependence. The M2-HRP and goat α-rabbit-HRP antibodies were used to detect binding of the FHA variant or antibody, respectively. A. Binding of the FHAαMyc and pAbαMyc to the target peptide. B. Binding of the FHAαCaMKII and pAbαCaMKII to the target peptide.
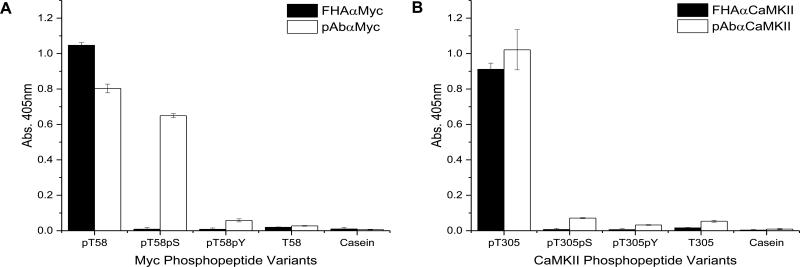
A major challenge in generating pThr-specific affinity reagents is preventing cross-reactivity between peptides that contain pSer or pThr residues, which differ by the γ-methyl group. While the FHA1 naturally recognizes a pThr residue on pRad9 (29), it was uncertain whether the engineered variants would cross-react with pSer-containing versions of the phosphopeptides. To test for specificity, variants of the peptide sequences were synthesized with pSer or pTyr in place of pThr. The cognate target, pSer, pTyr, and unphosphorylated variant peptides were then used as targets in an ELISA (Fig. 3). Both FHAαMyc and FHAαCaMKII bound to their cognate peptide 100 fold better than phosphopeptides that carried pSer or pTyr in place of the pThr residue. These data demonstrate that the FHA domain variants are truly pThr-specific.
Figure 3. FHA variants are phosphothreonine-specific.
The pThr for each of the cognate peptides was substituted with pSer or pTyr. These phosphopeptide variants, the cognate target, unphosphorylated target, and casein (negative control) served as targets in the ELISA. Phosphospecific reagents were used to probe targets to test for pThr-specificity. The M2-HRP and goatαrabbit-HRP were used to detect binding of the FHA1 variant or antibody, respectively. A. Binding of the FHAαMyc and pAbαMyc to the target peptides. B. Binding of the FHAαCaMKII and pAbαCaMKII to the target peptides.
Soluble forms of the FHA domains were then compared against commercially available monoclonal and polyclonal antibodies to the same targets. Like the FHA variants, all antibodies were shown to be phosphorylation-dependent in binding (Fig. 2). However, in evaluating the commercial antibodies for discrimination between peptides containing pSer, pThr, and pTyr, we observed that the pAbαMyc reagent binds equally well to the phosphopeptide variant containing pSer and pThr, but not pTyr (Fig. 3A). In contrast, for the polyclonal antibody against the pThr-containing phosphopeptide of CaMKII, we observed that the pAbαCaMKII reagent did not cross-react with the other phosphoresidues (Fig. 3B). Without the details of how these two polyclonal antibodies were prepared, it is difficult to speculate why one antibody is more selective than the other. Nevertheless, these data demonstrate that the FHA1 domains are more consistent in discriminating between pThr, pSer, and pTyr than commercial antibodies.
In the FHA1 domain, the β4-β5 and β6-β7 loops create a structural pocket for the γ-methyl of the pThr to fill. More specifically, the histidine at position 88 (His88) of the β4-β5 loop interacts with Ser85 (β4-β5), Thr106 (β6-β7), Ile104 (β6-β7), and Gly108 (β6-β7) to create a pocket for the γ-methyl group as well as interacting with the phosphate (Fig. 1B) (24). Given the structure of the FHA1 domain, and because we have been unable to isolate any variants against pSer- or pTyr-containing peptides, we are confident that FHA domain variants from the library share the same selectivity for pThr. Thus, one major advantage of the FHA1 domain as a scaffold for recombinant affinity reagent generation is its ability to discriminate between pThr and pSer residues.
Identifying positions important for FHA-peptide interaction
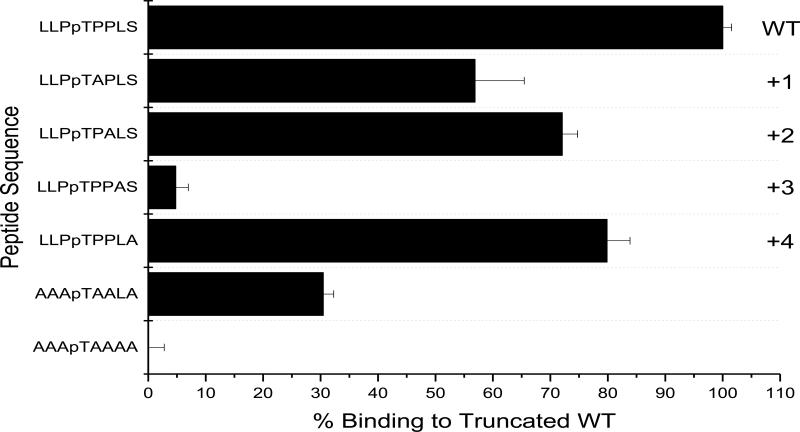
It has previously been reported that a major recognition determinant for naturally occurring FHA domains is the residue at the pT+3 position in the peptide ligand. Specifically, in the Rad53-FHA1, Arg83 interacts with the Asp in the pT+3 of the pRad9 peptide ligand (Fig. 1C). To confirm this for the recombinant FHA domain variants, alanine scanning was performed on the peptide ligand for the FHAαMyc domain; each residue from the pT+1 to the pT+4 was substituted to Ala. Two control peptides were used to confirm residue contribution: the first control peptide contains Ala at every position from pT+1 through pT+4 (AAApTAAAA), and the second control peptide contains Ala at positions pT+1, pT+2, and pT+4 with Leu at the +3 position (AAApTAALA). The signals for each phosphopeptide variant was normalized against the truncated cognate sequence (LLPpTPPLS). There was a 45%, 28%, and 20% reduction in signal using the phosphopeptide variants containing the Ala substituted at positions pT+1, pT+2, and pT+4, respectively. A 96% reduction in signal was observed substituting the Leu (pT+3) for Ala (Fig. 4). Our findings confirm that the pT+3 position is critical for binding for this FHA domain variant. This is consistent with the previous finding of Pershad et al. (22), which demonstrated the importance of the pT+3 in the peptide ligand for the FHA domain that binds MAPK3. However, it is likely that other positions in the peptide likely contribute somewhat to binding, as the peptide AAApTAALA does not bind to the same level as the target sequence, LLPpTPPLS.
Figure 4. Identification of important residues for the FHA-peptide.
An alanine scanning of the cognate peptide for FHAαMyc. Ala was substituted at positions +1, +2, +3, +4 in the cognate peptide ligand. Binding of the FHAαMyc to its cognate truncated target was set to 100% and the Myc phosphopeptide variants were compared against it.
Identifying the important phosphoresidues for binding in dual-phosphorylated targets
As many proteins are doubly-phosphorylated during signal transduction in eukaryotes, we surveyed the phage-display library for members capable of binding a doubly-phosphorylated peptide target. We selected three proteins, activating transcription factor 2 (ATF2), extracellular signal-regulated kinase1/2 (ERK1/2), and myc, as important biological proteins that are dually phosphorylated, as targets for affinity selection. We were able to isolate FHA domain variants that bind to each of the three peptides. This prompted us to examine how doubly-phosphorylated peptides are recognized by FHA domain variants.
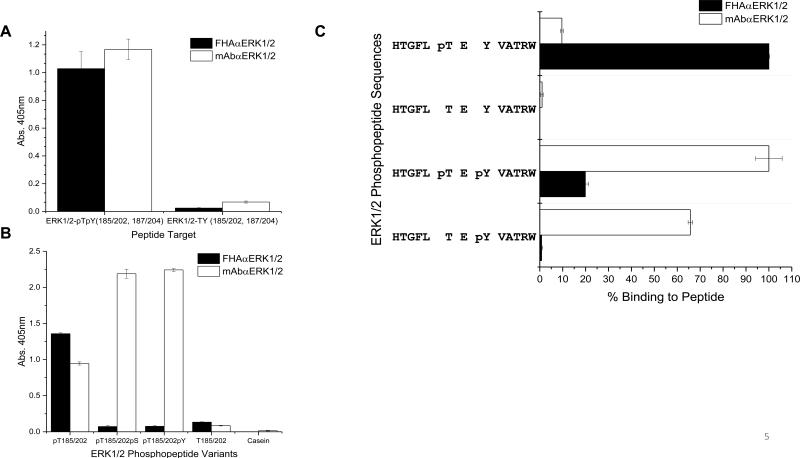
The cognate target for the FHAαERK1/2 variant contains a pThr residue as well as a pTyr residue at the pT+2 position in the peptide sequence, HTGFLpTEpYVATRW. While both the FHAαERK1/2 variant and monoclonal antibody, mAbαERK1/2, are phosphorylation-dependent in binding this peptide ligand (Fig. 5A), only the FHAαERK1/2 variant was shown to be pThr-specific, as mAbαERK1/2 bound to peptides with pSer or pTyr residues at position 185/202 (Fig. 5B). To assess which phosphoresidue is important for phosphospecific reagent binding, variants of the cognate target containing either pThr or pTyr were created. The cognate target, the phosphorylated variants, and an unphosphorylated form of the cognate peptide served as targets in an ELISA (Fig. 5C). Interestingly, the FHAαERK1/2 variant bound the strongest to the monophosphorylated form of the peptide, HTGFLpTEYVATRW. In contrast, the mAbαERK1/2 bound to the doubly-phosphorylated peptide and nearly as well to the monophosphoryated pTyr peptide (HTGFLTEpYVATRW). Taken together, these data confirm the importance of the pThr and suggests that the pT+2 position contributes to binding to FHA domain for this variant, whereas the most important residue for the mAb-peptide interaction is the pTyr residue.
Figure 5. Comparison of phosphospecificαERK1/2 reagents.
A. The FHAαERK1/2 and mAbαERK1/2 were used in an ELISA to assess phosphorylation dependence. B. The FHAαERK1/2 and mAbαERK1/2 were used in an ELISA to assess pThr-specificity C. Binding of the FHAαERK1/2 and mAbαERK1/2 to ERK1/2 phosphopeptide variants targets. Binding to the preferred target peptide was set to 100% and the phosphopeptide variants were compared against it.
One can take advantage of the differing specificities of the two classes of affinity reagents to monitor phosphorylation of ERK1/2 in cells. The localization of the ERK1/2 when phosphorylated on Thr(185/202) and Tyr (187/204) is a well described event in the cell that has a range of physiological consequences including activation of transcription factors (30). Mass spectrometry has confirmed the three different isoforms of ERK1/2(31); however, there are currently no known biological consequences of these phosphorylated forms of ERK1/2.
The in vitro nature of phage display offers the ability to control epitope recognition, unlike immunization. In this way, it would be possible to continue to narrow the specificity of the FHAαERK1/2 through directed evolution experiments so that they only recognize the pThr-only variant target peptide and not the dual-phosphorylated target. Alternatively, it may be possible to evolve a FHA domain that discriminates between the mono- and doubly-phosphorylated targets. The availability of a set of recombinant affinity reagents with this narrow specificity may be useful in revealing a novel physiological aspect of this well-studied protein.
Conclusions
The FHA1 domain has been demonstrated to be an attractive alternative to commercially available antibodies. The domain has the innate ability to bind specifically to pThr, and not to pSer or pTyr, containing peptides. Accordingly, the FHA domain is very selective in binding certain phosphopeptides; our studies also confirms the pT+3 position contributes significantly to binding. It is conceivable that one could create a different FHA domain variant for every potential residue at this position. Thus, the FHA domain offers the potential to be used in a wide variety of biochemical and cellular applications that monitor phosphorylation of threonine residues.
Highlights.
The FHA1 library can yield reagents to various pThr-containing peptides.
FHA domain variants are phosphothreonine-specific.
The pT+3 residue on the peptide influences successful isolation of binders.
Acknowledgements
Financial support for the research was provided by a grant (U54 DK093444) from the Common Fund at the National Institutes of Health (NIH) and the USDA HSI Education Grant Program (NIFA Award 2010-38422-21223). We would like to thank the following for their review of the manuscript and suggestions: Dr. Margaret C. Costanzo, Ms. Imrose Kauser, Dr. Sujatha Koduvayur, Mrs. Malgorzata Kokoszka, Ms. Stacey Moreno, Dr. Donald A. Morrison, Mr. Julian Valdes, and Ms. Sarah Zinn. We appreciate Dr. Yury Polikanov for helping render the FHA1 image. Special thanks to Dr. Michael R. Kierny for his time in training, thoughtful discussions, suggestions, assistance, and review of the manuscript. We also thank members of the lab for their assistance and support.
Abbreviations
- FHA1
Forkhead-associate 1
- pThr, pT
Phosphothreonine
- pSer, pS
Phosphoserine
- pTyr, pY
Phosphotyrosine
- CaMKII
calmodulin-dependent protein kinase II
- ERK1/2
extracellular-signal regulated kinases 1 and 2
- pT+3
+3 position (C-terminal) to the pThr moiety
- SH2
Src Homology 2
- 10FnIII
domain, 10th fibronectin type III domain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Endicott JA, Noble ME, Johnson LN. The structural basis for control of eukaryotic protein kinases. Annual review of biochemistry. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. [DOI] [PubMed] [Google Scholar]
- 2.Ubersax JA, Ferrell JE., Jr. Mechanisms of specificity in protein phosphorylation. Nature reviews Molecular cell biology. 2007 Jul;8(7):530–41. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 3.Lin J, Xie Z, Zhu H, Qian J. Understanding protein phosphorylation on a systems level. Briefings in functional genomics. 2010 Jan;9(1):32–42. doi: 10.1093/bfgp/elp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann M, Ong SE, Gronborg M, Steen H, Jensen ON, Pandey A. Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends in biotechnology. 2002 Jun;20(6):261–8. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]
- 5.Nita-Lazar A, Saito-Benz H, White FM. Quantitative phosphoproteomics by mass spectrometry: past, present, and future. Proteomics. 2008 Nov;8(21):4433–43. doi: 10.1002/pmic.200800231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dephoure N, Gould KL, Gygi SP, Kellogg DR. Mapping and analysis of phosphorylation sites: a quick guide for cell biologists. Molecular biology of the cell. 2013 Mar;24(5):535–42. doi: 10.1091/mbc.E12-09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu P, Begley M, Michowski W, et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014 Apr 24;508(7497):541–5. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rardin MJ, Wiley SE, Naviaux RK, Murphy AN, Dixon JE. Monitoring phosphorylation of the pyruvate dehydrogenase complex. Analytical biochemistry. 2009 Jun 15;389(2):157–64. doi: 10.1016/j.ab.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker R, Glassy MS, Gude N, Sussman MA, Gottlieb RA, Glembotski CC. Kinetics of the translocation and phosphorylation of alphaB-crystallin in mouse heart mitochondria during ex vivo ischemia. American journal of physiology Heart and circulatory physiology. 2009 May;296(5):H1633–42. doi: 10.1152/ajpheart.01227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Z, Dolginov Y, Hanoch T, et al. Detection of partially phosphorylated forms of ERK by monoclonal antibodies reveals spatial regulation of ERK activity by phosphatases. FEBS Lett. 2000 Feb 18;468(1):37–42. doi: 10.1016/s0014-5793(00)01191-1. [DOI] [PubMed] [Google Scholar]
- 11.Bradbury AR, Sidhu S, Dubel S, McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol. 2011 Mar;29(3):245–54. doi: 10.1038/nbt.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimsey NL, Goodfellow CE, Scotter EL, Dowie MJ, Glass M, Graham ES. Specific detection of CB1 receptors; cannabinoid CB1 receptor antibodies are not all created equal! Journal of neuroscience methods. 2008 Jun 15;171(1):78–86. doi: 10.1016/j.jneumeth.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Bohmer T, Pfeiffer N, Gericke A. Three commercial antibodies against alpha1- adrenergic receptor subtypes lack specificity in paraffin-embedded sections of murine tissues. Naunyn-Schmiedeberg's archives of pharmacology. 2014 Jul;387(7):703–6. doi: 10.1007/s00210-014-0992-2. [DOI] [PubMed] [Google Scholar]
- 14.Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn-Schmiedeberg's archives of pharmacology. 2009 Apr;379(4):397–402. doi: 10.1007/s00210-009-0393-0. [DOI] [PubMed] [Google Scholar]
- 15.Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn-Schmiedeberg's archives of pharmacology. 2009 Apr;379(4):409–12. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couchman JR. Commercial antibodies: the good, bad, and really ugly. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2009 Jan;57(1):7–8. doi: 10.1369/jhc.2008.952820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kierny MR, Cunningham TD, Kay BK. Detection of biomarkers using recombinant antibodies coupled to nanostructured platforms. Nano reviews. 2012:3. doi: 10.3402/nano.v3i0.17240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidhu SS. Antibodies for all: The case for genome-wide affinity reagents. FEBS letters. 2012 Aug 14;586(17):2778–9. doi: 10.1016/j.febslet.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko T, Huang H, Cao X, et al. Superbinder SH2 domains act as antagonists of cell signaling. Science signaling. 2012 Sep;5(243):ra68. doi: 10.1126/scisignal.2003021. 25. [DOI] [PubMed] [Google Scholar]
- 20.Koerber JT, Thomsen ND, Hannigan BT, Degrado WF, Wells JA. Nature-inspired design of motif-specific antibody scaffolds. Nature biotechnology. 2013 Oct;31(10):916–21. doi: 10.1038/nbt.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson CA, Liao HI, Sun R, Roberts RW. mRNA display selection of a high-affinity, modification-specific phospho-IkappaBalpha-binding fibronectin. ACS chemical biology. 2008 Aug 15;3(8):480–5. doi: 10.1021/cb800069c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pershad K, Wypisniak K, Kay BK. Directed evolution of the forkhead-associated domain to generate anti-phosphospecific reagents by phage display. Journal of molecular biology. 2012 Nov 23;424(1-2):88–103. doi: 10.1016/j.jmb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durocher D, Taylor IA, Sarbassova D, et al. The molecular basis of FHA domain:phosphopeptide binding specificity and implications for phospho-dependent signaling mechanisms. Mol Cell. 2000 Nov;6(5):1169–82. doi: 10.1016/s1097-2765(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 24.Huang YM, Chang CE. Mechanism of PhosphoThreonine/Serine Recognition and Specificity for Modular Domains from All-atom Molecular Dynamics. BMC biophysics. 2011;4:12. doi: 10.1186/2046-1682-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kierny MR, Cunningham TD, Bouhenni RA, Edward DP, Kay BK. Generating Recombinant Antibodies against Putative Biomarkers of Retinal Injury. PloS one. 2015;10(4):e0124492. doi: 10.1371/journal.pone.0124492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. British journal of pharmacology. 2009 May;157(2):220–33. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz U, Versmold A, Kaufmann P, Frank HG. Phage display: a molecular tool for the generation of antibodies--a review. Placenta. 2000 Mar-Apr;21(Suppl A):S106–12. doi: 10.1053/plac.1999.0511. [DOI] [PubMed] [Google Scholar]
- 28.Pershad K, Kay BK. Generating thermal stable variants of protein domains through phage display. Methods. 2013 Mar 15;60(1):38–45. doi: 10.1016/j.ymeth.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Liao H, Yuan C, Su MI, et al. Structure of the FHA1 domain of yeast Rad53 and identification of binding sites for both FHA1 and its target protein Rad9. Journal of molecular biology. 2000 Dec 15;304(5):941–51. doi: 10.1006/jmbi.2000.4291. [DOI] [PubMed] [Google Scholar]
- 30.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell cycle. 2009 Apr 15;8(8):1168–75. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi T, Gao Y, Gaffrey MJ, et al. Sensitive targeted quantification of ERK phosphorylation dynamics and stoichiometry in human cells without affinity enrichment. Analytical chemistry. 2015 Jan 20;87(2):1103–10. doi: 10.1021/ac503797x. [DOI] [PMC free article] [PubMed] [Google Scholar]