Abstract
Mutations in the complement regulatory proteins are associated with several different diseases. Although these mutations cause dysregulated alternative pathway activation throughout the body, the kidneys are the most common site of injury. The susceptibility of the kidney to alternative pathway mediated injury may be due to limited expression of complement regulatory proteins on several tissue surfaces within the kidney. To examine the roles of the complement regulatory proteins factor H and Crry in protecting distinct renal surfaces from alternative pathway mediated injury, we generated mice with targeted deletions of the genes for both proteins. Surprisingly, mice with combined genetic deletions of factor H and Crry developed significantly milder renal injury than mice deficient in only factor H. Deficiency of both factor H and Crry was associated with C3 deposition at multiple locations within the kidney, but glomerular C3 deposition was lower than that in factor H alone deficient mice. Thus, factor H and Crry are critical for regulating complement activation at distinct anatomic sites within the kidney. However, widespread activation of the alternative pathway reduces injury by depleting the pool of C3 available at any one location.
Keywords: complement, glomerulonephritis, inflammation
Introduction
The complement system is an important part of the innate immune system. It defends the host against pathogens and also facilitates the clearance of injured cells and immune complexes.1 Uncontrolled activation of the complement system can cause inflammatory injury, however, and a group of regulatory proteins controls complement activation on host tissues.2 Every cell in the body expresses one or more of these regulatory proteins, and soluble regulatory proteins (factor H for the alternative pathway and C4 binding protein for the classical pathway) circulate at high concentrations in plasma. There is redundancy in the control of the complement cascade, and most tissue surfaces are protected by more than one regulatory protein. Nevertheless, a large number of diseases are associated with genetic mutations and variations in the complement regulators, highlighting the need for the full repertoire of these proteins to prevent pathologic complement activation and autologous injury. Interestingly, systemic defects in complement regulation often manifest with disease that is limited to specific organs, and the kidneys are particularly susceptible to injury in this setting.3
A key example of this is C3 glomerulopathy (C3G), a recently described form of glomerulonephritis in which complement proteins are deposited in the glomeruli in the relative absence of immunoglobulin.4 C3G is associated with numerous different molecular defects that cause systemic AP dysregulation, including mutations in the genes for complement regulatory proteins and autoantibodies that block alternative pathway (AP) regulation.5-9 Although these defects affect complement regulation throughout the body, patients typically present with isolated glomerulonephritis. Most patients with C3G have C3nef, an autoantibody that prevents degradation of the alternative pathway C3 convertase by factor H.10 Furthermore, the genetic mutations that have been identified in patients with C3G have most frequently involved the gene for factor H.5, 6, 11, 12 Mice with a targeted deletion of the gene for factor H (fH−/− mice) develop spontaneous glomerulonephritis with the histologic characteristics of C3G, including abundant C3 deposits along the glomerular basement membrane (GBM).13
It is not known why the kidney is so frequently and so uniquely affected in patients with systemic defects in regulation of the AP. Factor H controls AP activation both in the fluid phase and on tissue surfaces, and there is debate as to whether AP activation in C3G occurs in the plasma or directly on the GBM. Fluid phase complement activation could cause deposition of C3 fragments on the GBM as they are filtered through the glomeruli,14 and one of the genetic defects identified in patients with C3G was shown to impair regulation of the AP in the fluid phase.7 On the other hand, the GBM is an acellular surface that is exposed to plasma proteins through a fenestrated glomerular endothelium. The GBM matrix does not contain intrinsic complement regulatory proteins, and this may leave the GBM particularly vulnerable to AP-mediated inflammation in patients with factor H defects.
Mutations in the gene for MCP have been rarely identified in patients with C3G,5, 6 and MCP haplotypes also affect the risk of developing C3G.6 Abnormal MCP function would suggest that defective AP regulation on the surface of renal cells contributes to disease. It is also notable that complement regulation on tubular epithelial cells is critically dependent upon a single complement regulatory protein, the transmembrane protein Crry.15, 16 Mice with genetic deletion of Crry develop pathologic complement activation on the basolateral surface of the tubules.17, 18 Membrane cofactor protein (MCP) is expressed on human tubular epithelial cells, and probably functions on this surface in a fashion similar to Crry in rodents.15, 19 The complete absence of both Crry and factor H in mice would be predicted to cause more extensive injury than deficiency of either protein alone. The closest equivalent in humans, that of combined deficiency of FH and MCP, has not been reported.
The dependence of different renal surfaces on specific regulatory proteins may explain the unique susceptibility of the kidney to AP-mediated injury. Impaired function of factor H may be sufficient to permit AP activation on the GBM, for example, and impaired function of Crry may be sufficient to permit AP activation on tubular epithelial cells. Given the possibility that factor H and Crry protect distinct tissue sites within the kidney, we hypothesized that simultaneous deficiency of both proteins would cause renal injury that is more severe than that caused by either protein alone. To test this hypothesis we bred mice with targeted genetic deletions of the genes for both factor H and Crry (fH−/− Crry−/− mice) and compared them to mice with single targeted deletion of the gene for factor H (fH−/− mice). We also reconstituted fH−/− mice with purified factor H to determine whether factor H controls AP activation directly on the GBM. The goal of these studies was to identify the contribution of each complement regulatory protein to the control of AP activation on different surfaces within the kidney, and to improve our understanding of the mechanisms by which mutations in these proteins cause glomerular disease.
Results
Breeding of fH−/−Crry−/− mice
Homozygous deficiency of Crry in mice is lethal in utero due to uncontrolled alternative pathway activation on the placenta. This can be prevented by maternal C3 deficiency.20 Factor H deficiency leads to a secondary deficiency of C3 due to consumption of the C3.13 We intercrossed fH−/−Crry+/− mice and generated fH−/−Crry−/− pups. Once established, the fH−/−Crry−/− mice bred normally, indicating that the depletion of C3 in factor H deficient mothers was sufficient to prevent loss of loss Crry−/− embryos.
Deficiency of Crry reduces glomerular C3 deposits in fH−/− mice
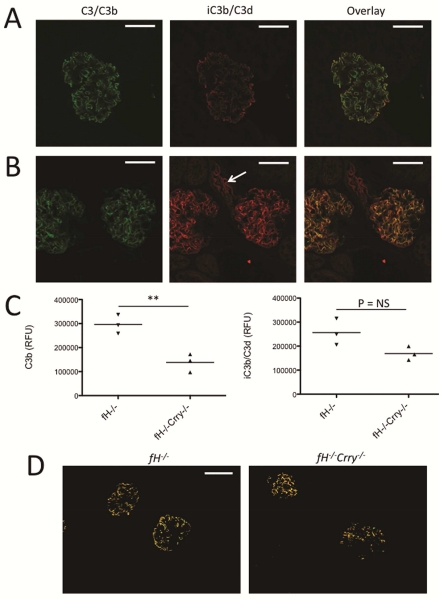
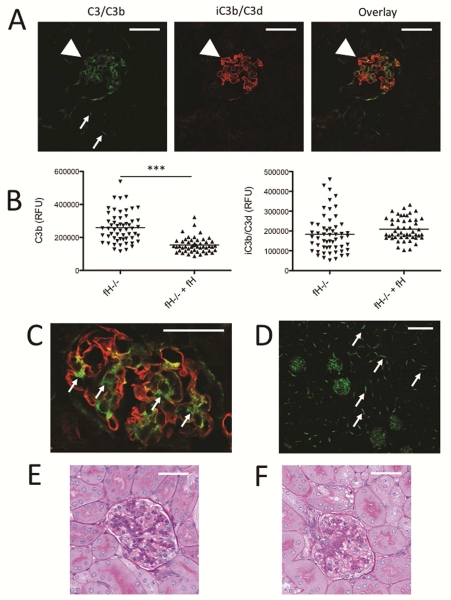
Complement activation on tissue surfaces causes covalent attachment of C3b.21 It has previously been reported that C3d (a downstream degradation fragment of C3b) is deposited in the glomeruli of fH−/− mice.22 To confirm this, we immunostained kidneys from 14 month-old fH−/− mice with an antibody that recognizes the iC3b and/or C3d forms of C3, but does not recognize intact C3 or C3b.23 We found that iC3b/C3d is deposited in the glomeruli of fH−/− mice in the same pattern as is C3b (Figure 1A), suggesting that the deposited C3b is metabolized to iC3b and/C3d in situ. We then examined C3 deposition in age-matched fH−/−Crry−/− mice (Figure 1B). We observed less overall C3b in fH−/−Crry−/− mice than in fH−/− mice (Figure 1C). Dual staining of kidney sections with antibodies for C3b and for a component of the GBM showed some areas of colocalization in fH−/− mice, although there were other areas where the proteins did not colocalize (Supplemental Figure S1).
Figure 1. C3 metabolism in the glomeruli of mice with targeted deletion of the genes for factor H and Crry.
We examined the deposition and metabolism of C3 in the glomeruli of fH−/− and fH−/−Crry−/− mice using confocal microscopy. A) Kidney sections of fH−/− mice were stained using antibodies to detect C3b (green) and iC3b/C3d (red). Both forms of C3 were detected along the capillary loops. Original magnification ×600. Bar = 50 μm. B) In fH−/−Crry−/− mice, C3b and iC3b/C3d co-localized within the mesangium. iC3b/C3d was also detected within the proximal tubules (arrow). Original magnification ×600. C) The intensities of C3b and iC3b/C3d were measured in the glomeruli of fH−/− and fH−/−Crry−/− mice. Less glomerular C3b was detected in the fH−/−Crry−/− mice. There was a trend towards lower levels of iC3b/C3d in the fH−/−Crry−/− mice, but some iC3b/C3d was seen in all of the glomeruli. D) To examine the sites of iC3b/C3d generation, the co-localization function of Olympus FV10-ASW software was used to isolate areas of C3b and iC3b/C3d co-localization in the kidneys of fH−/− and fH−/−Crry−/− mice. Co-localized C3b and iC3b/C3d deposits were seen in the capillary loops of fH−/− mice and in the mesangium of fH−/−Crry−/− mice. Original magnification ×200. Bar = 50 μm.
Both factor H and Crry serve as cofactors for the cleavage of C3b by factor I, so we anticipated that deficiency of both proteins would halt C3 metabolism at the level of C3b. There was a trend towards less iC3b/C3d in the glomeruli of fH−/−Crry−/− mice compared to fH−/− mice (Figure 1C). Nevertheless, some iC3b/C3d was detected in all of the glomeruli. To confirm these findings, C3 and C3d deposits were examined in fH−/− Crry−/− mice from a different animal colony (Supplemental Figure S2). C3b and C3d were significantly lower in the glomeruli of those mice. Extraction of images from kidneys double stained for C3b and iC3b/C3d showed a pattern of co-localization in the capillary loops of fH−/− mice and in the mesangium of fH−/−Crry−/− mice (Figure 1D). This indicates that cofactor function is available within the glomeruli, even in the absence of both factor H and Crry. In addition to the glomerular deposits, iC3b/C3d was also seen in the proximal tubules.
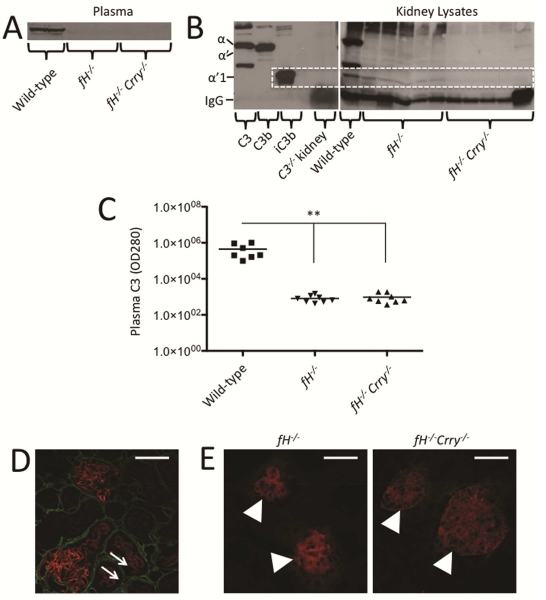
Intact C3 in the plasma of 14 month-old fH−/− and fH−/−Crry−/− mice was lower than that in wild-type mice by Western blot analysis, indicating that it was consumed in both strains of mice (Figure 2A). Lower levels of the α’1 C3 fragment were seen in kidney lysates from fH−/−Crry−/− mice than in fH−/− mice (Figure 2B), consistent with the confocal microscopy findings. Plasma C3 levels were also lower in both strains of mice compared to wild-type mice when measured by ELISA (Figure 2C). Dual staining of kidney sections for collagen IV and iC3b/C3d demonstrated that the tubulointerstitial iC3b/C3d in fH−/−Crry−/− mice was only detected on the apical surface of the proximal tubules (Figure 2D). Crry is expressed in the glomeruli and on the basolateral surface of tubular epithelial cells,15 so it is not clear why Crry deficiency is associated with deposition on the apical surface. Decay accelerating factor (DAF) was detected in the glomeruli of both fH−/− and fH−/−Crry−/− mice, but was not seen in the tubulointerstitium. The patterns of expression were not detectably different (Figure 2E). Together, these findings demonstrate that there is less overall C3 deposition in the kidneys of fH−/−Crry−/− mice than of fH−/− mice, although there is greater deposition in the mesangium and on the tubules.
Figure 2. Complement activation in mice with targeted deletions of the genes for both factor H and Crry.
A) Western blot analysis of C3 fragments in the plasma of fH−/− and fH−/−Crry−/− mice demonstrates that intact C3 levels are lower in both strains of mice than in wild-type mice. B) A western blot of kidney lysates demonstrates that the C3α’1 fragment of iC3b is more abundant in fH−/− mice than in fH−/−Crry−/− mice. Purified proteins were used to identify the different C3α chain fragments, and the C3α’1 band is indicated with the dashed line. C) Plasma C3 levels were measured by ELISA. C3 levels were significantly lower in the plasma of fH−/− and fH−/−Crry−/− mice than in that of wild-type mice. **P < 0.01. D) Kidney sections of fH−/−Crry−/− mice were stained using antibodies to detect collagen IV (green) and iC3b/C3d (red). Collagen IV was seen in the tubular basement membrane at the basolateral side of the tubules, demonstrating that iC3b/C3d was deposited on the apical surface of the proximal tubules (arrows). E) DAF was detected in the glomeruli (arrowheads) of both fH−/− and fH−/−Crry−/− mice. Original magnification ×600. Bar = 50 μm.
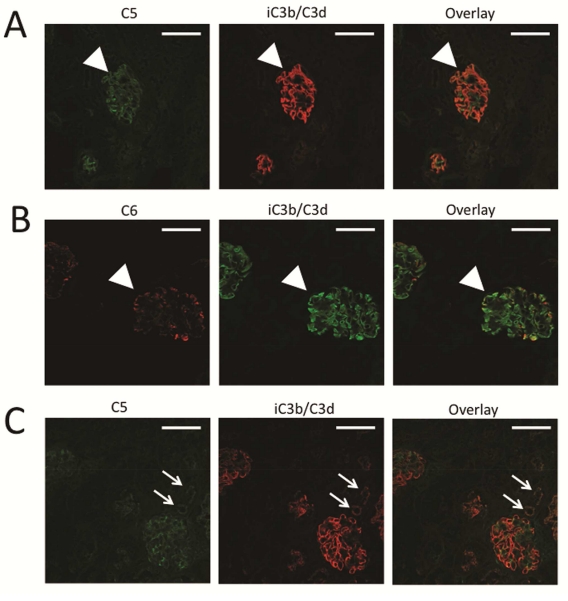
Fluid phase AP activation should not cause covalent fixation of C3 fragments to the GBM, and does not efficiently form the terminal complement complex (C5b-9).24 We immunostained the kidneys of fH−/− mice for C5 (Figure 3A) and C6 (Figure 3B), and both proteins were deposited within the glomeruli in a pattern similar to the iC3b/C3d deposits. The detection of these proteins in the glomeruli of fH−/− mice indicates that the terminal complement complex is generated, consistent with AP activation directly on this surface. A protein fragment at the expected molecular weight for C5b was also seen by Western blot analysis (Supplemental Figure S3). High-powered views of glomeruli stained for C5 and C6 demonstrate similar, although not identical patterns of deposition (Supplemental Figure S3). C5 deposition was also seen in the glomeruli of fH−/−Crry−/− mice, indicating complete activation of the complement cascade, not simply deposition of C3 fragments generated in the circulation (Figure 3C). C5 was also seen on the apical surface of the proximal tubules, similar to iC3b/C3d.
Figure 3. Terminal complement complex generation in factor H deficient mice.
To assess the degree of complement activation in the glomeruli of fH−/− and fH−/−Crry−/− mice, we examined the deposition of C5 and C6 using confocal microscopy. A) C5 (green) was detected in the glomeruli of fH−/− mice in a pattern similar to that of iC3b/C3d (red). B) C6 (red) and iC3b/C3d (green) co-localized in the glomeruli and in the proximal tubules of fH−/− mice, indicating that complement activation generated the terminal complement complex at this location. C) Segmental deposits of C5 (green) were also detected in the glomeruli of fH−/−Crry−/− mice in a pattern similar to iC3b/C3d (red). Original magnification ×600. Bar = 50 μm.
Deficiency of Crry protects fH−/− mice from glomerular injury
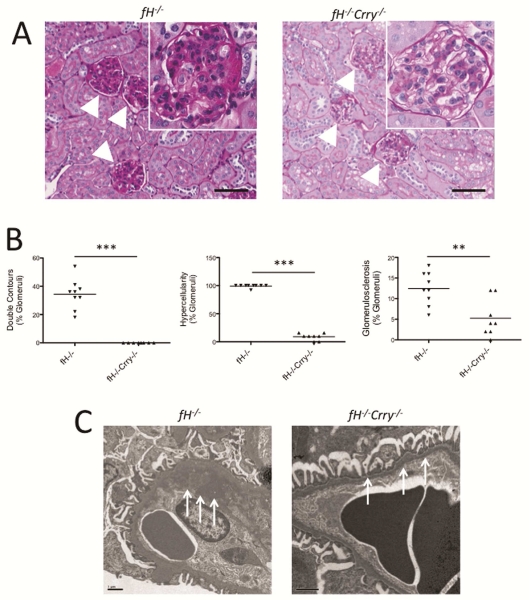
We examined kidneys from 14 month-old fH−/−Crry−/− mice by light microscopy and compared them to kidneys from age-matched fH−/− mice. The glomeruli of the fH−/− mice demonstrated extensive proliferation and hypercellularity in the mesangial and endocapillary compartments, double contours within the GBMs, and glomerulosclerosis (Figure 4A-B). These histologic markers of injury were significantly lower or absent in the glomeruli of fH−/− Crry−/− mice. Although mesangial hypercellularity and expansion were seen in the glomerulil of some fH−/−Crry−/− mice, no endocapillary proliferation was seen. By electron microscopy, electron dense deposits were seen in the GBM and in the subendothelial space of fH−/− mice, but these abnormalities were not detected in kidneys from fH−/−Crry−/− mice (Figure 4C).
Figure 4. Mice with targeted deletions of the genes for both factor H and Crry are protected from glomerular injury.
A) Kidney sections from 14 month-old fH−/− and fH−/−Crry−/− mice were stained with PAS and examined for histologic evidence of injury. Glomeruli in fH−/− mice demonstrated double contours of the GBM, hypercellularity, mesangial expansion, and glomerulosclerosis. Most of the glomeruli in the fH−/−Crry−/− mice were normal appearing. Expanded view of a representative glomerulus is shown in the inset. Original magnification ×200 (×400 for inset). Bar = 100 μm. B) Kidney sections from 14 month-old fH−/− and fH−/−Crry−/− mice were examined by a renal pathologist in a blinded fashion. Significantly fewer glomeruli in fH−/−Crry−/− mice demonstrated double contours of the GBM, hypercellularity, or glomerulosclerosis. All of the fH−/− mice had both endocapillary proliferation and mesangial expansion, whereas hypercellularity in the fH−/− mice was only seen in the mesangium. **P < 0.01, ***P < 0.001. C) Kidney sections from 14 month-old fH−/− and fH−/−Crry−/− mice were examined by electron microscopy. The GBMs of fH−/− mice were markedly thickened and contained electron dense material (arrows). The GBMs of fH−/−Crry−/− mice were normal appearing (arrows). The size bar represents 1 μm.
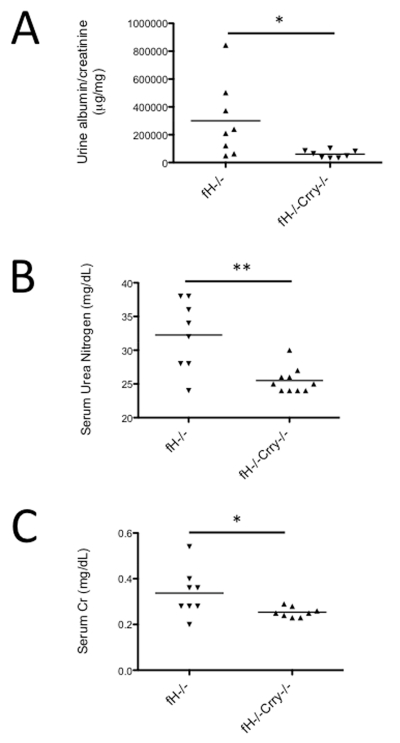
Urine albumin/creatinine was measured in the different mouse strains, and albumin/creatinine levels were significantly lower in urine from 14 month-old fH−/−Crry−/− mice compared to fH−/− mice (Figure 5A). Serum urea nitrogen (SUN) and serum creatinine levels were also significantly lower in the fH−/−Crry−/− mice (Figure 5B-C). These results indicate that simultaneous deficiency of both proteins protects mice from renal injury compared to fH−/− mice.
Figure 5. Mice with targeted deletions of the genes for both factor H and Crry are protected from development of albuminuria and renal failure.
A) Albumin and creatinine levels were measured in the urine from 14 month-old fH−/− and fH−/−Crry−/− mice. The albumin/creatinine content of fH−/−Crry−/− mice was significantly lower than that of fH−/− mice. B) Serum urea nitrogen and C) serum creatinine levels were measured in 14 month-old fH−/− and fH−/−Crry−/− mice. Serum urea nitrogen and creatinine were both significantly lower in fH−/−Crry−/− mice compared to fH−/− mice. *P < 0.05, **P < 0.01.
Factor H provides cofactor activity on the GBM
To investigate whether or not factor H regulates complement activity directly on the GBM, we injected ~3 month-old fH−/− mice intraperitoneally with 1 mg of purified factor H once per day for three successive days and the mice were sacrificed 24 hours after the last injection. As previously reported,22, 25 reconstitution of fH−/− mice with purified factor H resulted in a decrease in capillary loop C3b and the appearance of C3b deposits in the mesangium (Figure 6A-C). Injection of the factor H may reduce the abundance of glomerular C3b either by reducing the deposition of new C3b and/or providing cofactor activity for conversion of C3b to iC3b/C3d. We found that iC3b/C3d deposition in the glomerular capillary loops did not decrease (Figures 6B). In a prior study fH−/− mice were injected with human factor H. Five successive days of treatment caused a decrease in C3d deposits, indicating that C3d is eventually cleared from this location.22 Injection of the mice with factor H also caused deposition of C3b on the basolateral surface of tubular epithelial cells (Figure 6D), similar to what is seen in wild-type mice.26 By reducing the amount of C3 that is consumed on the GBM and/or in the fluid phase, reconstitution of the mice with factor H likely leaves more intact C3 available to support AP activation in the tubulointerstitium.
Figure 6. Factor H regulates complement activation on the GBM.
Factor H deficient mice were reconstituted with factor H purified from the plasma of wild-type mice, and complement proteins in the glomeruli were examined using confocal microscopy. A) Kidney sections of reconstituted fH−/− mice were stained using antibodies to detect C3b (green) and iC3b/C3d (red). The pattern of C3b deposits suggested deposition in the mesangium (arrowhead) and along the tubular basement membranes (small arrows). iC3b/C3d was heavily deposited along the GBM. Original magnification ×600. Bar = 50 μm. B) The intensities of C3b and iC3b/C3d were measured in the glomeruli of a fH−/− mouse injected with a vehicle control or with purified factor H. Injection with factor H reduced the intensity of glomerular C3b. C) A high-power view of a glomerulus from a fH−/− mouse injected with purified factor H demonstrates that areas of C3b without iC3b/C3d are seen in the mesangium, and iC3b/C3d is seen in the capillary loops. Original magnification ×600. Bar = 50 μm. D) A low power view of a kidney sections from a fH−/− mouse reconstituted with factor H shows that injection of factor H restored C3b deposition on the tubular basement membranes (arrows). Original magnification ×200. Bar = 100 μm. PAS stained kidneys from a representative fH−/− mouse injected with E) vehicle and F) factor H did are shown. Original magnification ×600. Bar = 50 μm.
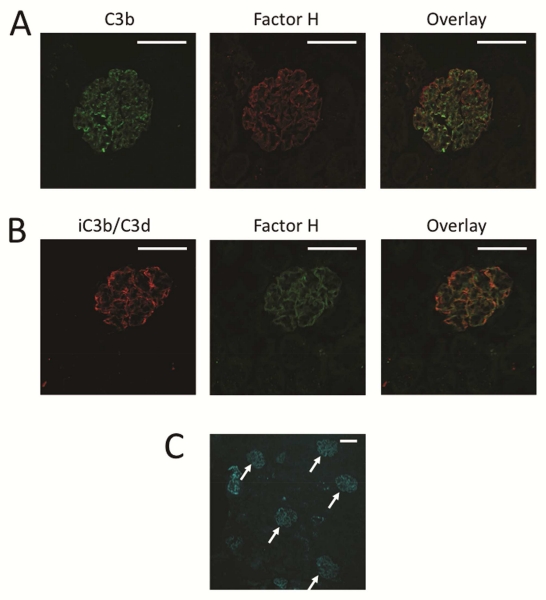
To determine whether the injected factor H binds directly to the GBM, we generated recombinant factor H and labeled it with DyLight 650. We injected ~175 μg of labeled recombinant factor H into fH−/− mice and sacrificed the mice 24 hours later. The unfixed kidneys were then analyzed by confocal microscopy. Labeled factor H was detectable in the capillary loops. Factor H did not co-localize with C3b in the mesangium (Figure 7A), whereas the factor H did co-localize with iC3b/C3d along the GBM (Figure 7B). This provides evidence that the factor H serves as a cofactor on the GBM, enabling the conversion of C3b to iC3b/C3d.
Figure 7. Factor H binds to the glomerular basement membrane of factor H deficient mice.
fH−/− mice were injected with DyLight 650-labeled recombinant factor H, and kidneys from these mice were examined by confocal microscopy. A) C3b was detected in the capillaries and the mesangium of injected mice, and factor H (shown as red in this panel) was deposited along the capillary walls. B) iC3b/C3d was detected along the capillary walls in a pattern similar to the pattern of the injected factor H deposition (shown as green in this panel). Original magnification ×600. Bar = 50 μm. C) A low power view of a kidney section demonstrates that factor H primarily localized to the glomeruli of fH−/− mice. Original magnification ×200. Bar = 50 μm.
Clearance of C3b in the glomerulus requires cofactor activity
We next treated the mice with 2 mg/day of an inhibitory mAb to mouse factor B to block AP activity. This antibody should reduce the deposition of new C3b, but it does not provide cofactor activity for cleavage of pre-existing C3b. Although treatment with the anti-factor B antibody reduced AP activation and increased plasma levels of intact C3, the glomerular C3b and iC3b/C3d deposits were unaffected (Supplemental Figure S4). This demonstrates that in the absence of factor H, limited cofactor activity is available for factor I mediated cleavage of preexisting C3b on the GBM.
Discussion
In the current study we examine the contributions of factor H and Crry to the regulation of complement activation within the kidney. We initially hypothesized that deficiency of factor H would permit AP activation on the GBM, and that concurrent deficiency of Crry would permit AP activation on tubular epithelial cells and exacerbate injury. Surprisingly, we found that co-deficiency of Crry ameliorated glomerulonephritis, and fH−/−Crry−/− mice were almost completely protected from glomerular injury. C3 fragments were deposited in the glomeruli and the apical surface of the renal tubules of fH−/−Crry−/− mice, but there was less overall glomerular C3 deposition than in fH−/− mice. Furthermore, co-deficiency of Crry and factor H protected the mice from fetal loss and tubulointerstitial injury as is seen in Crry−/− mice.17, 18, 20, 27 Further experiments demonstrated that purified factor H protein targeted and bound to the GBM after injection into fH−/− mice, suggesting that the injected factor H mediated conversion of C3b to iC3b/C3d along the GBM. In contrast, AP inhibition with a monoclonal antibody to factor B reduced fluid phase C3 consumption, but pre-existing C3b in the glomeruli of these mice did not change. This could be due to incomplete normalization of AP activity by the exogenous anti-factor B antibody. Alternatively, the anti-factor B antibody may not inhibit C3 activation long the GBM.
Many systemic defects of AP regulation manifest with isolated inflammation of the kidneys.5, 6, 8, 28-30 C3G is associated with genetic variations in factor H, MCP, factor I, C3, and the factor H related proteins (CFHRs).5, 6, 11, 28, 29, 31, 32 Furthermore, activation of the AP causes renal injury in a variety of diseases, including C3G,13 ischemic acute kidney injury,26, 33 atypical hemolytic uremic syndrome,34 focal segmental glomerulosclerosis,35 lupus nephritis,36 and ANCA associated vasculitis.37 In spite of these numerous associations, it is not known why dysregulated control of the AP so frequently and so uniquely affects the kidneys. Our data indicates that factor H can provide co-factor activity for the cleavage of C3b that is bound to the GBM. This local action may specifically render the GBM susceptible to complement activation in the setting of factor H abnormalities.
It has been noted that the ultrastructural localization of C3 and electron dense deposits in the kidney vary among patients with C3G.4, 5, 38 For example, patients can have subendothelial, mesangial, and/or epimembranous glomerular deposits.39 Based on our experimental findings, it is possible that the ultrastructural location of C3 deposits in the glomeruli reflects the underlying complement defect, and that different patterns of C3 deposition may be associated with varying degrees of disease severity. Our results also suggest that defective AP regulation on cell surfaces may lead to a milder phenotype than do factor H defects that permit activation directly on the GBM.
Our results also demonstrate that acquired C3 deficiency in factor H deficient individuals can be functionally similar to a primary C3 deficiency. Maternal alternative pathway activation within the placenta causes the demise of Crry−/− embryos, but deficiency of complement proteins (C3 or factor B) rescues the embryos.40 Our results demonstrate that factor H deficiency also rescues Crry−/− pups, likely due to acquired C3 deficiency in the fH−/−Crry+/− mothers. It is also notable that once Crry−/− mice are established they become functionally C3 deficient and breed normally, probably because of increased turnover of plasma C3 and reduced plasma levels of the protein.27, 41 In other words, widespread consumption of C3 in Crry−/− mothers reduces the availability of C3 for activation in the placenta. It is believed that AP activation on tissues surfaces (possibly the endothelium41) causes C3 consumption in Crry−/− mice.
There is evidence that C3 consumption in fH−/− mice is caused by uncontrolled AP activation in the fluid phase.13, 42 It has been proposed that glomerular C3 deposits are due to deposition of iC3b generated in the fluid phase onto the GBM, rather than direct AP activation on the GBM.42 However, our results indicate that AP activation may also occur directly on the GBM of fH−/− mice. Consistent with our findings, a recent study demonstrated that a recombinant form of factor H that contained the surface binding region of factor H (short consensus repeats 19 and 20) reduced the deposition of C3 fragments in the glomeruli of fH−/− mice, whereas a recombinant protein that did not contain this surface binding region and only contained the complement regulatory region (SCRs 1-5) did not reduce glomerular C3 deposition.43 The anatomic location at which complement activation occurs may have important therapeutic implications. There are recombinant proteins that can delivers the complement regulatory region of factor H to tissue-bound C3d.43, 44 On the other hand, agents that target complement proteins in solution have also been developed.45, 46 Our results do not exclude a role for fluid phase AP activation in glomerular injury, but they suggest that therapeutic complement inhibitors that are targeted to anatomic sites of complement inhibition will be protective in C3G.
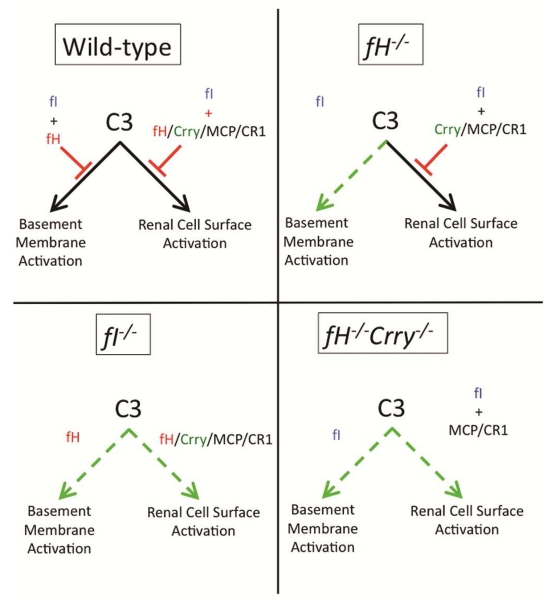
Factor I is a circulating protease that cleaves (inactivates) C3b, forming iC3b.47 The inactivation of C3b by factor I requires cofactor activity, which can be provided by factor H, Crry, CR1, and MCP.47 In this regard, fH−/−Crry−/− mice are functionally similar to factor I deficient (fI−/−) mice because they lack cofactor activity (and therefore factor I activity) in the plasma, on the GBM, and on cell surfaces. Rose et al. discovered that C3 fragments are not deposited in the glomerular capillaries of fI−/− mice.42 The authors conjectured that conversion of C3b to iC3b by factor I is necessary for the development of glomerular injury and that iC3b is a nephritogenic moiety.42 In view of the current study, however, an alternative explanation is that widespread complement activation in both the fI−/− and fH−/−Crry−/− mice reduces the amount of intact C3 available for activation on the GBM (Figure 8). AP dysregulation in both of these strains of mice is more widespread than it is in fH−/− mice, with the net result being less C3 deposition on the GBM and protection from glomerular injury.
Figure 8. Model of glomerular complement regulation.
Factor I and factor H together control AP activation on the GBM. Factor I also controls AP activation on renal cell surfaces. Cofactor function on cell surfaces is provided by factor H, MCP, CR1, and Crry (in rodents). Deficiency of factor H in fH−/− mice permits uncontrolled AP activation on the GBM. Factor I deficiency permits uncontrolled activation on the GBM and on cell surfaces. Combined factor H and Crry deficiency also permits activation on the GBM and on cell surfaces.
An unexpected finding of the current study is that iC3b/C3d fragments were generated in the mesangium and tubules of fH−/−Crry−/− mice. It is not clear what is serving as the cofactor in these mice. CR1 and MCP have cofactor activity, but are not expressed on the GBM or the apical surface of tubular epithelial calls.19 Other proteases can cleave C3b, including plasmin,48 thrombin, and cathepsin.49 These proteases may contribute to C3 metabolism in the kidneys of fH−/−Crry−/− mice. Nevertheless, reconstitution of fH−/− mice with factor H increased C3b degradation, confirming that factor H is the primary AP regulator on the GBM and that the other available cofactors and proteases do not fully compensate for factor H.
Complement-mediated injury occurs when regulatory proteins do not adequately protect host surfaces. We have shown that factor H directly inhibits complement activation on the GBM, and that deficiency of factor H renders this particular surface susceptible to complement activation. Cell surface complement regulatory proteins, such as Crry, do not function on the GBM, so deficiency of these proteins creates other anatomic sites that compete with the GBM for C3. Our results demonstrate that if complement activation is too widespread the depletion of intact C3 can reduce injury at particular tissue sites. A large number of complement defects have been discovered, including genetic variations, mutations, autoantibodies to complement proteins, as well as the identification of new proteins that interact with the complement system. Greater understanding of how these defects contribute to complement activation on particular tissues will improve our understanding of complement-mediated disease, and will help in the design of effective new complement inhibitory therapies.
Methods
Animals
Mice with targeted deletion of the genes for factor H13 and Crry20 were generated as previously described. The mice have been back-crossed onto a C57BL/6 background for more than 9 generations. The mice were intercrossed until we had established fH−/−Crry+/− breeding sets. Pups were genotyped by the polymerase chain reaction using the primers shown in Supplemental Figure S5, and a colony of fH−/−Crry−/− mice was then established. Eight male 14 month-old mice from two founder pairs were compared with an age-matched cohort of eight male fH−/− mice. In addition, a cohort of fH−/−Crry−/− mice was set up in the laboratory of Dr. Pickering and C3 deposition in the glomeruli of these mice was analyzed (Supplemental Figure S1). For experiments involving the reconstitution of fH−/− mice with purified factor H, three 3.5 month-old mice were used. Control C57BL/6 mice were obtained from Jackson Laboratories. Mice were housed and maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all experiments were approved by the University of Colorado Center for Laboratory Animal Care.
Reagents
The following antibodies were used in these studies: Unconjugated and FITC-conjugated goat IgG to mouse Complement C3 (MP Biomedicals, Santa Ana, CA), a mAb that only recognizes the iC3b and C3d fragments of C3 (mAb 3d29),23 a mAb that recognizes all of the C3 fragments by Western blot analysis (mAb 3d11),23 monoclonal murine anti-mouse C5,50 a mAb that recognizes the α3 chain of collagen IV (8D1),51 rabbit anti-mouse C6 (Hycult Biotech, Uden, the Netherlands), rabbit polyclonal antibody to collagen IV (EMD Millipore, Billerica, MA), Alexa-647-conjugated Armenian hamster anti-mouse CD55, clone RIKO-3 (Biolegend, San Diego, CA), HRP-conjugated goat IgG to mouse Complement C3 (MP Biomedicals), and dapi nuclear stain (Sigma Aldrich, St. Louis, MO). Secondary antibodies include Streptavidin conjugated with Alexa-555 and Alexa-488 (Life Technologies, Grand Island, NY), HRP, Cy3, and FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA), APC-conjugated goat anti-rabbit IgG, Alexa 594-conjugated donkey anti-rabbit IgG (Invitrogen, Grand Island, NY), and FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch). ChromePure Rabbit IgG, FITC-conjugated ChromePure Goat IgG (Jackson ImmunoResearch, West Grove, PA), and mouse anti-human factor I (Quidel, San Diego, CA) were used as isotype controls. The mAb 1379 was used to block mouse alternative pathway activity in vivo.52
Recombinant murine factor H was generated using the codon-optimized (Homo sapiens) DNA sequence for murine factor H (comprising residues 19-1234; Uniprot identifier: P06909). The gene was sub-cloned into the pDONR221 entry vector (Life Technologies; construct purchased from GeneArt, Grand Island, NY). The engineered sequence additionally contained DNA encoding an Ig kappa chain leader sequence to facilitate secretion of the target protein, a Gly-Ala-Gly-Ala-Gly-Ala linker region, a hexa-histidine (His6-) fusion tag, a second linker region (Asp-Tyr-Asp-Ile-Pro-Thr-Thr) and a Tobacco Etch Virus nuclear inclusion A endopeptidase (TEV) cleavage site (Glu-Asn-Leu-Tyr-Gln-Gly), all of which were located 5’ prime to the factor H gene. The synthetic gene was the re-combined into a pcDNA3.2/V5-Dest expression vector using Gateway LR clonase II enzyme mix (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. Chinese hamster ovary (CHO) cells were transfected with the vector using Lipofectamine 2000 (Invitrogen), and protein was produced under Geneticin 418 (ENZO Life Sciences, Farmingdale, NY) selection. The cells were grown in DMEM medium supplemented with fetal calf serum and Geneticin 418. Protein was purified using a HiTrap His column (GE Healthcare, Uppsala, Sweden) and purity of the protein was confirmed by electrophoresis on a 12.5% Tris-HCl polyacrylamide gel (Bio-Rad, Hercules, CA) followed by Coomassie staining and western blot analysis. In some experiments the recombinant protein was fluorescently labeled using a DyLight 650 Labeling Kit (Thermo Scientific) according to the manufacturer’s instructions.
Confocal microscopy
Kidneys were snap frozen in OCT compound (Sakura Finetek, Torrance, CA) at the time of harvest and stored at −80°C. Four μm sagittal sections were cut using a cryostat, warmed to room temperature, and fixed with acetone. Non-specific binding was blocked with 10% heat-inactivated goat serum. Primary antibodies were diluted in 2% heat-inactivated goat serum and incubated overnight at 4°C. Autofluorescence was blocked with 0.05% Sudan Black B in 70% ethanol for 20 minutes at room temperature.53 High-resolution images were obtained with an Olympus FV1000 FCS/RICS confocal microscope (Olympus Scientific Solutions Americas Corp, Waltham, MA). The localization of deposits in the mesangium or in peripheral capillary loops was assessed based on the pattern of deposition. The intensity and co-localization of proteins were measured by drawing regions of interest (ROIs) around glomeruli and analyzing the ROIs with Olympus FV10-ASW software. Staining using isotype control antibodies is shown in Supplemental Figure S6.
Western blot analysis
C3 and C5 were detected in plasma and kidney lysates by western blot analysis. Plasma samples diluted 1:10 in PBS or 200 μg of tissue lysate were reduced by boiling with 0.15 M dithiothreitol for 10 min. at 100° C. The proteins were resolved by electrophoresis with a 10% Tris-HCl gel (Bio-Rad, Hercules, CA), and transferred to a polyvinylidene fluoride membrane. C3 fragments were detected using mAb 3d11 diluted 1:1000 in 2.5% Milk in PBS. C5 fragments were detected using BB5.1 diluted 1:500 in 2.5% milk-PBS. In both cases the secondary antibody was HRP-conjugated goat anti-mouse IgG (H+L) (Jackson ImmunoResearch). The secondary antibody was detected using Pierce ECL Western Blotting Substrate (Thermo Scientific). Purified human C3, C3b, and iC3b (Comptech, Tyler, TX) were used as size controls.
C3 ELISA
Plasma C3 levels were compared using an ELISA. ELISA plates were coated in with goat IgG fraction to mouse Complement C3 (MP Bio) diluted 1:100 in carbonate-bicarbonate buffer as a capture antibody and incubated for 1 hour at 4° C. The plasma samples were diluted 1:3200 in carbonate-bicarbonate buffer and added to wells. Bound C3 was then detected with a HRP-conjugated Goat IgG fraction to mouse Complement C3 (MP Bio) diluted 1:800 in PBS with 0.1% Tween20. The ELISA plates were then developed using 1-Step Ultra TMB-ELISA (Thermo Scientific), and the optical density at 280 nm (OD280) was measured. All of the samples were included on the same plate, and OD280 values were compared.
Albumin, serum urea nitrogen, and creatinine measurements
Spontaneously voided urine samples were collected by placing mice onto ELISA plates or collected directly into eppendorf tubes. Urine albumin was determined by ELISA according to manufacturer’s instructions (Bethyl Laboratories, Inc, Montgomery, TX). Serum urea nitrogen and serum and urine creatinine were determined by automated analysis with the AlfaWassermann ACE® Clinical Chemistry Analyzer (Alfa Wassermann, West Caldwell, NJ) .
Renal histology
Kidney tissue was fixed in formalin, embedded in paraffin, cut into 4 μm sections, and stained with periodic acid-Schiff. Sections were assessed for the extent of hypercellularity, double contours, and glomerulosclerosis as previously described.54 Histologic analysis was performed by a renal pathologist blinded to the sample identity. Fifty glomeruli per animal per section were examined. The glomerular were assessed for hypercellularity and mesangial expansion as previously described.42, 54 The glomerular scores for each animal were averaged, and kidneys from 8-9 animals per group were included in the analysis. Additional kidney tissue for electron microscopy was fixed in 2% glutaraldehyde / 2% paraformaldehyde with 0.1 M cacodylate / 3% sucrose, embedded in resin, and cut into ultrathin sections. Five animals per group and 10-12 glomeruli per animal were analyzed.
Statistical analysis
Statistical analysis between groups was assessed by unpaired t-test using GraphPad Prism software (GraphPad Prism, La Jolla, CA). A P value of less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
We thank Dot Dill for assistance with electron micrographs and Hector Molina for providing Crry-deficient mice.
This work was supported by the KIDNEEDS Foundation (JMT), and National Institutes of Health Grants R01 DK076690 (JMT), R01 AR51749 (VMH), and P30CA046934.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary information is available at Kidney International’s website.
Disclosure.
JMT and VMH receive royalties from Alexion Pharmaceuticals, Inc.
References
- 1.Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipfel PF, Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 3.de Cordoba SR, de Jorge EG. Translational mini-review series on complement factor H: genetics and disease associations of human complement factor H. Clin Exp Immunol. 2008;151:1–13. doi: 10.1111/j.1365-2249.2007.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickering MC, D’Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Servais A, Fremeaux-Bacchi V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. Journal of medical genetics. 2007;44:193–199. doi: 10.1136/jmg.2006.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Servais A, Noel LH, Roumenina LT, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Barricarte R, Heurich M, Valdes-Canedo F, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120:3702–3712. doi: 10.1172/JCI43343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortajada A, Yebenes H, Abarrategui-Garrido C, et al. C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest. 2013;123:2434–2446. doi: 10.1172/JCI68280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Wiesener M, Eberhardt HU, et al. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest. 2014;124:145–155. doi: 10.1172/JCI71866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Meyer NC, Wang K, et al. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol. 2012;7:265–274. doi: 10.2215/CJN.07900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, et al. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15:787–795. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- 12.Habbig S, Mihatsch MJ, Heinen S, et al. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009;75:1230–1234. doi: 10.1038/ki.2008.354. [DOI] [PubMed] [Google Scholar]
- 13.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 14.Noris M, Remuzzi G. Glomerular Diseases Dependent on Complement Activation, Including Atypical Hemolytic Uremic Syndrome, Membranoproliferative Glomerulonephritis, and C3 Glomerulopathy: Core Curriculum 2015. Am J Kidney Dis. 2015;66:359–375. doi: 10.1053/j.ajkd.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurman JM, Ljubanovic D, Royer PA, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006;116:357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renner B, Coleman K, Goldberg R, et al. The complement inhibitors Crry and factor H are critical for preventing autologous complement activation on renal tubular epithelial cells. J Immunol. 2010;185:3086–3094. doi: 10.4049/jimmunol.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao L, Wang Y, Chang A, et al. Unrestricted C3 activation occurs in Crry-deficient kidneys and rapidly leads to chronic renal failure. J Am Soc Nephrol. 2007;18:811–822. doi: 10.1681/ASN.2006101176. [DOI] [PubMed] [Google Scholar]
- 18.Miao J, Lesher AM, Miwa T, et al. Tissue-specific deletion of Crry from mouse proximal tubular epithelial cells increases susceptibility to renal ischemia-reperfusion injury. Kidney Int. 2014;86:726–737. doi: 10.1038/ki.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichida S, Yuzawa Y, Okada H, et al. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 1994;46:89–96. doi: 10.1038/ki.1994.247. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Mao D, Holers VM, et al. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 21.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 22.Fakhouri F, de Jorge EG, Brune F, et al. Treatment with human complement factor H rapidly reverses renal complement deposition in factor H-deficient mice. Kidney Int. 2010;78:279–286. doi: 10.1038/ki.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thurman JM, Kulik L, Orth H, et al. Detection of complement activation using monoclonal antibodies against C3d. J Clin Invest. 2013;123:2218–2230. doi: 10.1172/JCI65861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pangburn MK, Rawal N. Structure and function of complement C5 convertase enzymes. Biochemical Society transactions. 2002;30:1006–1010. doi: 10.1042/bst0301006. [DOI] [PubMed] [Google Scholar]
- 25.Paixao-Cavalcante D, Hanson S, Botto M, et al. Factor H facilitates the clearance of GBM bound iC3b by controlling C3 activation in fluid phase. Mol Immunol. 2009;46:1942–1950. doi: 10.1016/j.molimm.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurman JM, Ljubanovic D, Edelstein CL, et al. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Spitzer D, Mao D, et al. Membrane protein Crry maintains homeostasis of the complement system. J Immunol. 2008;181:2732–2740. doi: 10.4049/jimmunol.181.4.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik TH, Lavin PJ, Goicoechea de Jorge E, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23:1155–1160. doi: 10.1681/ASN.2012020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gale DP, de Jorge EG, Cook HT, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strobel S, Zimmering M, Papp K, et al. Anti-factor B autoantibody in dense deposit disease. Mol Immunol. 2010;47:1476–1483. doi: 10.1016/j.molimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Athanasiou Y, Voskarides K, Gale DP, et al. Familial C3 glomerulopathy associated with CFHR5 mutations: clinical characteristics of 91 patients in 16 pedigrees. Clin J Am Soc Nephrol. 2011;6:1436–1446. doi: 10.2215/CJN.09541010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medjeral-Thomas N, Malik TH, Patel MP, et al. A novel CFHR5 fusion protein causes C3 glomerulopathy in a family without Cypriot ancestry. Kidney Int. 2014;85:933–937. doi: 10.1038/ki.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thurman JM, Royer PA, Ljubanovic D, et al. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol. 2006;17:707–715. doi: 10.1681/ASN.2005070698. [DOI] [PubMed] [Google Scholar]
- 34.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 35.Lenderink AM, Liegel K, Ljubanovic D, et al. The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. Am J Physiol Renal Physiol. 2007;293:F555–564. doi: 10.1152/ajprenal.00403.2006. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H, Garnier G, Circolo A, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164:786–794. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- 37.Xiao H, Schreiber A, Heeringa P, et al. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker PD, Ferrario F, Joh K, et al. Dense deposit disease is not a membranoproliferative glomerulonephritis. Mod Pathol. 2007;20:605–616. doi: 10.1038/modpathol.3800773. [DOI] [PubMed] [Google Scholar]
- 39.Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, et al. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9:46–53. doi: 10.2215/CJN.04700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao D, Wu X, Deppong C, et al. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 2003;19:813–822. doi: 10.1016/s1074-7613(03)00321-2. [DOI] [PubMed] [Google Scholar]
- 41.Ruseva MM, Hughes TR, Donev RM, et al. Crry deficiency in complement sufficient mice: C3 consumption occurs without associated renal injury. Mol Immunol. 2009;46:803–811. doi: 10.1016/j.molimm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nichols EM, Barbour TD, Pappworth IY, et al. An extended mini-complement factor H molecule ameliorates experimental C3 glomerulopathy. Kidney Int. 2015 doi: 10.1038/ki.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruseva MM, Peng T, Lasaro MA, et al. Efficacy of Targeted Complement Inhibition in Experimental C3 Glomerulopathy. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014121195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 46.Dilillo DJ, Pawluczkowycz AW, Peng W, et al. Selective and efficient inhibition of the alternative pathway of complement by a mAb that recognizes C3b/iC3b. Mol Immunol. 2005 doi: 10.1016/j.molimm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson SC, Sim RB, Lea SM, et al. Complement factor I in health and disease. Mol Immunol. 2011;48:1611–1620. doi: 10.1016/j.molimm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Barthel D, Schindler S, Zipfel PF. Plasminogen is a complement inhibitor. J Biol Chem. 2012 doi: 10.1074/jbc.M111.323287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markiewski MM, Nilsson B, Ekdahl KN, et al. Complement and coagulation: strangers or partners in crime? Trends in immunology. 2007;28:184–192. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Frei Y, Lambris JD, Stockinger B. Generation of a monoclonal antibody to mouse C5 application in an ELISA assay for detection of anti-C5 antibodies. Mol Cell Probes. 1987;1:141–149. doi: 10.1016/0890-8508(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 51.Summers SA, Steinmetz OM, Li M, et al. Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol. 2009;20:2518–2524. doi: 10.1681/ASN.2009030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurman JM, Kraus DM, Girardi G, et al. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol. 2005;42:87–97. doi: 10.1016/j.molimm.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Yu H, Zheng D, et al. Sudan black B reduces autofluorescence in murine renal tissue. Arch Pathol Lab Med. 2011;135:1335–1342. doi: 10.5858/arpa.2010-0549-OA. [DOI] [PubMed] [Google Scholar]
- 54.Panzer SE, Laskowski J, Renner B, et al. IgM exacerbates glomerular disease progression in complement-induced glomerulopathy. Kidney Int. 2015;88:528–537. doi: 10.1038/ki.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.