Abstract
Background
Varicella zoster virus (VZV) is a neurotropic exclusively human herpesvirus. Primary infection causes varicella (chickenpox), after which virus become latent in ganglionic neurons along the entire neuraxis. As cell-mediated immunity to VZV declines with advancing age and immunosuppression, VZV reactivates to produce zoster (shingles). One of the most serious complications of zoster is VZV vasculopathy.
Methods
We reviewed recent studies of stroke associated with varicella and zoster, how VZV vasculopathy is verified virologically, vaccination to prevent varicella and immunization to prevent zoster, and VZV in giant cell arteritis.
Findings
We report recent epidemiological studies revealing an increased risk of stroke after zoster; the clinical, laboratory and imaging features of VZV vasculopathy; that VZV vasculopathy is confirmed by the presence of either VZV DNA or anti-VZV IgG antibody in CSF; special features of VZV vasculopathy in children; vaccination to prevent varicella and immunization to prevent zoster; and the latest evidence linking VZV to giant cell arteritis.
Conclusion
In children and adults, VZV is a not uncommon cause of stroke.
Keywords: VZV, Vasculopathy, Stroke, Giant cell arteritis
Introduction
Varicella zoster virus (VZV) is a neurotropic alphaherpesvirus. Primary infection, usually in childhood, causes varicella (chickenpox), after which virus become latent in cranial nerve ganglia, dorsal root ganglia and autonomic ganglia along the entire neuraxis (1). As cell-mediated immunity to VZV declines with advancing age and immunosuppression, VZV reactivates to produce herpes zoster (shingles), frequently complicated by postherpetic neuralgia (radicular pain that persists long after the disappearance of rash). Zoster is also complicated by meningoencephalitis, myelitis, multiple serious ocular disorders and VZV vasculopathy. Importantly, all of the neurological and ocular complications of zoster may develop in the absence of rash. Diagnosis is confirmed either by the presence of VZV DNA or anti-VZV antibodies in CSF. Rapid virological verification and prompt treatment with antiviral agents can lead to complete recovery, even in patients with protracted disease.
Overview
VZV vasculopathy occurs in adults and children. Patients present with both transient ischemic attacks (TIAs) and stroke. Less often, patients present with subarachnoid or intracerebral hemorrhage secondary to ruptured aneurysm. Disease is often waxing and waning. Multiple cases of protracted disease that lasted for more than one year have been described. Both large and small arteries are affected. The characteristic pathology of VZV vasculopathy matches that of granulomatous arteritis. Virological analysis of intracerebral arteries of patients who died of VZV vasculopathy reveals Cowdry A inclusion bodies, multinucleated giant cells, herpes virions, VZV DNA and VZV antigen, indicating productive arterial infection by VZV. Interestingly, VZV is the only human virus that has been shown to replicate in cerebral arteries and produce disease.
Stroke after Zoster
In the past few years, multiple epidemiological studies from Taiwan, Europe, the U.K. and the U.S. have shown that the incidence of stroke after zoster is greater than in age-matched control patients. Analysis of Taiwanese National Health Research Institute records revealed a 30% increased risk within 1 year after zoster (2), increasing 4.5-fold with ophthalmic-distribution zoster (3). Similar analysis of the Danish National Registry revealed a 126% increased risk of stroke within 2 weeks after zoster, a 17% increased risk from 2 weeks to 1 year after zoster, and a 5% increased risk of stroke after the first year (4). Studies from the U.K. Health Improvement Network general practice database showed that not only was the risk of TIAs increased 1.15-fold, but also that myocardial infarctions (MIs) were increased 1.10-fold after zoster; and in zoster patients under 40 years of age, the risk for stroke, TIAs and MIs was significantly higher (1.74-, 2.42- and 1.49-fold, respectively) (5). A study from the U.K. Clinical Practice Research Datalink showed that the risk of stroke after zoster decreased over time in all dermatomes, with a statistically significant age-adjusted incidence of 1.63 at 1–4 weeks, 1.42 at 5–12 weeks, and 1.23 at 13–26 weeks after zoster, but no decrease at later times (6). In patients with ophthalmic-distribution zoster, the risk of stroke was increased 3-fold at 5–12 weeks after zoster. Finally, among 55% of zoster patients who received oral antiviral therapy, the stroke risk was reduced compared to that in untreated zoster patients, indicating the value of antiviral treatment in reducing stroke incidence after zoster.
More recently, a register-based cohort study in Sweden showed a 1.34-fold increased risk of stroke within 1 year after zoster in all age groups (7). As in the U.K. study, the risk of stroke in patients 39 years and younger was increased 10.3-fold within 1 year after zoster. Another U.K. study showed that the risk of stroke and MI increased 2.4- and 1.7-fold, respectively, within 2 weeks after zoster (8). Finally, in the first U.S. population-based study, the risk of stroke within 3 months of zoster was reportedly increased 1.53-fold (9). While stroke in the pediatric population is less common, approximately one-third of arterial ischemic stroke is associated with varicella (10), with 44% of transient cerebral arteriopathy preceded by varicella (11). Together, these studies show that varicella and zoster are risk factors for stroke, particularly in individuals who develop zoster under 40 years of age, and that antiviral therapy may decrease this risk.
Pathophysiology of VZV Vasculopathy
After zoster, most commonly after ophthalmic-distribution zoster or even in the absence of rash, intracerebral arteries likely become infected when virus that has reactivated from trigeminal or other cranial nerve ganglia spreads transaxonally. Decades ago, application of horseradish peroxidase to the external surface of the carotid and intracranial circulation, including the venous sinuses, of cats revealed that intracerebral arteries and veins receive a rich supply of trigeminal afferent fibers (12,13). After VZV reaches the arterial adventitia, virus spreads transmurally to infect all layers of the cerebral arteries, resulting in the characteristic pathology of granulomatous arteritis. Virological and immunological analyses of VZV-infected arteries have revealed disruption of the internal elastic lamina and progressive intimal thickening, with cells expressing smooth muscle actin but decreased smooth muscle cells in the media (14). The pathophysiology of VZV vasculopathy appears to be similar in children. Histopathology of the middle cerebral artery (MCA) of a 4 year-old girl who died of VZV vasculopathy revealed granulomatous arteritis with multinucleated giant cells, extensive lymphocytic infiltration, and VZV antigen, primarily in the smooth muscle layer (15).
Clinical, Laboratory and Imaging Features of VZV Vasculopathy
The onset of stroke or TIAs in elderly patients with a history of zoster or in children with varicella in recent months should alert the clinician to the possibility of VZV vasculopathy. While most VZV vasculopathies develop within 6 weeks after zoster, the median interval for stroke is 4 months after varicella (16). Besides stroke and TIAs, patients may develop severe headache, cognitive impairment/confusion or unsteadiness. Again, VZV vasculopathy in adults is often protracted. Cases that lasted one year before death (17) or that were confirmed virologically after 6 or 12 months of waxing and waning disease are well-documented, including a favorable response to antiviral treatment (18,19). CT or MRI scanning often reveals a single or multiple areas of ischemia/infarction in the distribution of large or small arteries and often both. In patients with multifocal VZV vasculopathy, lesions at gray-white matter junctions, along with deep-seated and cortical infarction, are common. Such a pattern leads the neuroradiologist to rightly propose metastatic carcinoma or embolization to explain the radiological abnormalities, while the possibility of VZV vasculopathy usually must be suggested by the clinician. Occlusion of the MCA with development of moyamoya collaterals has been reported in children (20). Vessel wall thickening and contrast-enhanced vessel walls on imaging have been seen in VZV vasculopathy (21). In 5/6 patients with virologically-verified VZV vasculopathy confirmed by the presence of anti-VZV IgG antibody in CSF, vessel wall enhancement on high-resolution MRI resolved and arterial stenosis improved or stabilized after treatment with intravenous acyclovir (22). In 2/3 of patients with virologically-verified VZV vasculopathy, angiography reveals evidence of narrowing or beading in cerebral arteries, and the CSF contains a mononuclear pleocytosis. Thus, the absence of cells in the CSF does not rule out VZV vasculopathy. Finally, while VZV vasculopathy in more frequent in immunocompromised individuals, it is also common in immunocompetent children and adults.
Virologic Confirmation of VZV Vasculopathy
When the characteristic rash of varicella or zoster is present at the time of vasculopathy or other neurological complications, the diagnosis of VZV-induced neurological disease is straightforward. When rash is absent, CSF examination is necessary. The presence of amplifiable VZV DNA or anti-VZV antibody in CSF verifies the presence of VZV. Importantly, many cases of VZV vasculopathy are protracted, and the optimal virological test is detection of anti-VZV IgG in CSF or anti-VZV IgM in serum or CSF. Among 30 patients with virologically-verified VZV vasculopathy, PCR of CSF for VZV DNA was positive in 30%, and anti-VZV IgG antibody was present in 93%; the 2 patients who did not have anti-VZV IgG antibodies in their CSF were children who developed vasculopathy after varicella and whose CSF was examined within the first week after stroke (23).
Treatment
As noted above, while VZV vasculopathy is often chronic, disease has been halted or cured even when antiviral therapy was initiated many months and, in one instance, years after the onset of disease (19). Thus, in a patient with suspected VZV vasculopathy, it is never too late to search for VZV DNA or anti-VZV IgG in CSF. Because VZV vasculopathy is due to productive virus infection, antiviral treatment with intravenous acyclovir is recommended. Doses of 10–15 mg/kg q 8 hours for 7–10 days have been used. An insufficient number of cases have been studied to know whether corticosteroids confer additional benefit. Nevertheless, because the pathology of intracerebral VZV vasculopathy is characterized by granulomatous arteritis, we administer a short 5- to 7-day course of oral prednisone, 1 mg/kg. On the other hand, we have encountered many patients with VZV vasculopathy who had been misdiagnosed with “CNS vasculitis” and who worsened on long-term steroids or other immunomodulatory drugs. These patients should be treated immediately with intravenous acyclovir while tapering and eventually discontinuing steroids or other immunomodulatory drugs.
Finally, the condition of some patients who improve with intravenous acyclovir may worsen when antiviral therapy is discontinued. Many of these patients have been immunocompromised by long-term steroids or other drugs, and others are HIV+. If these patients deteriorate clinically or develop new lesions on MRI, a second course of intravenous acyclovir may be necessary. If administration of steroids or other immunomodulatory drugs cannot be discontinued before completion of a 2-week course of intravenous acyclovir, then the patient should remain on oral antiviral treatment (e.g., valacyclovir, one gram 3 times daily for adults for 6–8 weeks) after steroids have been stopped.
VZV Vasculopathy in Children
Varicella is strongly associated with ischemic stroke in childhood (10,24–28). Although varicella may increase the risk of stroke due to nonspecific inflammation and transient thrombophilia, most notably protein S deficiency (29,30), the predominant pathophysiology of varicella-associated stroke is likely VZV vasculopathy, similar to that in adults.
The term “post-varicella arteriopathy of childhood” (PVA) is operationally defined as cerebral arteriopathy affecting the supraclinoid internal cerebral artery (ICA), the A1 or A2 segments of the anterior cerebral artery (ACA), or the M1 or M2 segments of the MCA manifesting as TIA or stroke within 12 months of chickenpox (24). PVA usually occurs in otherwise healthy, immunocompetent children and is usually monophasic, although progressive arteriopathy with recurrent TIA and stroke has been reported (11,16,24). As in adults, the CSF in children with VZV vasculopathy contains amplifiable VZV DNA or anti-VZV antibody, indicative of active infection (Table 1) and consistent with the detection of VZV antigen in granulomatous arteritis lesions of the left MCA of a 4-year-old girl who developed TIAs 8 months after varicella and died of malignant left hemispheric infarction 5 months later (15). Radiologically, PVA and VZV vasculopathy in childhood resemble the VZV vasculopathy described in immunocompromised patients and older adults (31), with large-vessel arteriopathy involving the anterior circulation reported most often in virologically-confirmed VZV vasculopathy in childhood (Table 1).
Table 1.
Virologically confirmed VZV vasculopathy in childhood
| Age | Gender | Interval from varicella to presentation of vasculopathy | Cerebral arteriopathy on imaging | CSF VZV DNA | CSF anti-VZV IgG | Reference |
|---|---|---|---|---|---|---|
| 3 | Boy | 3 months | ICA, MCA, ACA stenosis | + | NR | 32 |
| 5 | Girl | 4 years; thoracic zoster 3 months earlier | MCA stenosis | + | NR | 33 |
| 4 | Boy | 5 months | ICA stenosis | + | NR | 34 |
| 15 months | Boy | 6 months | MCA stenosis | + | + | 35 |
| 18 months | Boy | 5 months | MCA stenosis | + | + | 35 |
| 13 months | Girl | 6 weeks | ICA, MCA stenosis | + | − | 35 |
| 8 | Girl with AIDS | NR, zoster at presentation | MCA, bilateral ACA stenosis | + | NR | 36 |
| 2 | Boy | 3 months (diagnosed 8 months later) | MCA stenosis | − | + | 37 |
| 11 | Girl with HIV with reconstituti on syndrome | 7 years | ICA occlusion | + | NR | 38 |
| 9 | Girl | 1 month | ACA, MCA stenosis; ACOM aneurysm 3 years later | − | + | 39 |
| 2 | Boy | 8 months | Bilateral MCA occlusion and stenosis | + | + | 40 |
| 6 | Boy with DOCK8 | No history of varicella; received VZV vaccination at 1 year; history of herpes zoster 1 year earlier | Bilateral MCA, ACA, ACOM stenosis | +* | + | 41 |
| 15 | Boy | 2 months | Vertebral stenosis | NR | + | 42 |
| 4 | Boy | 6 months | MCA occlusion | − | + | 20 |
Abbreviations: ACA, anterior cerebral artery; ACOM, anterior communicating artery; ICA, internal carotid artery; MCA, middle cerebral artery; NR, not recorded.
Vaccine strain VZV.
VZV Vaccination to Prevent Varicella and Immunization to Prevent Zoster
Varivax
The development of the live attenuated Oka varicella vaccine Varivax has led to major clinical advances, making varicella and zoster the only vaccine-preventable human herpesvirus infection to date. In 1974, Takahashi and colleagues generated the first varicella vaccine, inspired by Takahashi’s observations of a family member with severe chickenpox and fever, wherein he harvested the “Oka strain” of vaccine virus from a 3-year-old boy and propagated it 11 times in human embryonic lung cells and in guinea pig embryo cells (43). Numerous clinical trials throughout the U. S. and Japan demonstrated the long-term efficacy of VZV vaccine in healthy children and adults, including the elderly. In 1995, varicella vaccine became available in the U. S. The CDC recommends immunization at 1 year of age and a second dose at age 4–6 years. Contraindications to the vaccine include: life-threatening allergy to previous administration, gelatin or neomycin; pregnancy; severe illness or immunosuppression; and life-threatening allergic reactions. Among states that accurately reported the incidence of varicella, disease declined by 79% overall between 2000 and 2010, likely due to vaccine (44).
Importantly, Varivax vaccine contains live attenuated virus that becomes latent and can reactivate to cause zoster and other neurological disorders, including stroke. Several reports have described zoster meningitis in vaccinated individuals (45–48). Multifocal large artery vasculopathy in a 6-year-old boy immunocompromised by DOCK8 deficiency who received the Varivax vaccine was found to be due to vaccine strain VZV based on sequence analysis of PCR-amplified VZV DNA in his CSF (41). Two cases of ischemic stroke have been reported in children 5 days and 3 weeks, respectively, after Varivax vaccination; however viral isolation was not attempted (28).
Zostavax
In 2005, the Shingles Prevention Study (SPS), a randomized placebo-controlled trial, provided evidence that the herpes zoster vaccine reduced the incidence of zoster and post-herpetic neuralgia (PHN) in the elderly (49). In that study, 38,546 healthy adults ≥ 60 years of age (median 69 years) were randomly assigned to receive a single dose of attenuated VZV with 14-fold higher potency than varicella vaccine or placebo. The higher-potency vaccine was required in order to increase VZV-specific cell-mediated immunity in latently infected older adults. The incidence of zoster as well as the burden of illness due to zoster (total pain and discomfort) and the incidence of PHN were determined. Average follow-up of 3.13 years in a total of 19,270 adults who received zoster vaccine and 19,276 who received placebo revealed 957 confirmed cases of zoster (315 in vaccine recipients; 642 in placebo recipients). In both groups, >93% of the subjects with zoster were positive for wild-type VZV DNA by PCR; none had vaccine strain Oka (vOka) DNA. Zoster vaccine reduced the incidence of zoster by 51.3% (63.9% in people aged 60–69 years, but only 37.6% in people aged ≥ 70 years). The burden of disease was reduced by 61.1% (65.5% in people aged 60–69 years and 55.4% in people age ≥ 70 years), and the duration of pain and discomfort among subjects with zoster was shorter in vaccinated candidates compared with placebo recipients. PHN was reduced by >65% for both age groups, with most benefit in the ≥ 70 years group. SPS also showed that vaccine reduced the adverse impact of zoster on patients’ daily life activities and on health-related quality-of-life.
Zoster vaccine is safe. Rates of serious adverse events, systemic adverse events, hospitalizations and deaths were low in vaccine recipients in the SPS and comparable to those in placebo recipients. During the first 42 days after vaccination, there were 24 cases of zoster in placebo recipients and 7 cases in the vaccination group, but none were caused by vOka. Unlike prophylactic vaccines such as those against varicella and measles, zoster vaccine is a therapeutic vaccine aimed at preventing reactivation of latent VZV in humans who have been infected before vaccination and already have substantial immunity.
The CDC recommends Zostavax for healthy adults age ≥ 60 years to prevent herpes zoster (http://www.cdc.gov/vaccines/vpd-vac/shingles/). Post-licensure studies have confirmed the vaccine’s safety and efficacy (50,51). Unfortunately, participation in the zoster vaccine program has been low, likely due to cost and failure to recognize the importance of preventing infectious diseases in older adults. Zoster vaccine has now been shown to be safe and effective in healthy individuals 50–59 years of age. Thus, the FDA has approved its administration in healthy adults ≥ 50 years of age, with contraindications including anaphylaxis to gelatin or neomycin, immunosuppression, immunodeficiency and pregnancy. The SPS demonstrated efficacy for 4 years after vaccination, while subsequent studies (52,53) indicated efficacy for 8 years (54). Currently, CDC does not recommend booster doses of zoster vaccine, but may in the future.
GSK Vaccine (HZ/su)
A recent advance in zoster prevention has been the development of a liposome-based subunit vaccine (HZ/su) containing the VZV glycoprotein E and the adjuvant ASO1B. Studies revealed that 2 doses of HZ/su containing 50 μg of recombinant VZV glycoprotein E administered at 1- or 2- month intervals were well-tolerated and induced much more robust VZV-specific and VZV glycoprotein E-specific CD4+T cell and antibody responses than did vOka (55,56). A randomized placebo-controlled study of 15,411 subjects ≥ 50 years of age revealed a remarkable 97.2% efficacy in preventing zoster for a 3.2-year period that did not diminish with increasing age (57). Compared to the placebo group, systemic adverse reactions were 2.2-fold greater in the vaccine group. Because HZ/su does not replicate, it will be safe for immunosuppressed patients. The duration of its effect is unknown. HZ/su vaccine is not yet FDA-approved.
VZV and Giant Cell Arteritis
An exciting finding in the past few years is that productive VZV infection and vascular disease is not limited to the intracranial circulation; indeed, VZV infects extracranial temporal arteries (TAs) and leads to giant cell arteritis (GCA). The search for VZV in GCA was motivated by virtually identical pathological changes seen in the arteries of patients with both intracerebral VZV vasculopathy and GCA. In both conditions, the pathology is characterized by granulomatous arteritis, in which inflammation, often transmural, is seen with necrosis, usually in the arterial media, accompanied by multinucleated giant cells, epithelioid macrophages or both. These findings have prompted examination of TA biopsies for VZV from patients with pathologically-verified GCA as well as from patients with clinical features and laboratory abnormalities of GCA whose TA biopsies were pathologically-negative for VZV. Thus, formalin-fixed, paraffin-embedded GCA-positive TA biopsies (50 sections/TA), including adjacent skeletal muscle, and normal TA biopsies from subjects >50 years of age were examined for the presence and distribution of VZV antigen by immunohistochemistry and ultrastructurally for virions; adjacent regions were examined by hematoxylin-eosin staining. VZV antigen-positive slides were also analyzed by PCR for VZV DNA.
Immunohistochemical analyses have to date revealed VZV antigen in 73/104 (70%) GCA-positive and 58/100 (58%) GCA-Bx-negative TAs compared to 11/61 (18%) normal TAs (58). Overall, VZV antigen was 3.89-fold more likely to be present in GCA-positive TAs than in normal TAs (95% CI = 2.3819, 7.2384, p<0.0001) and 3.22 times more likely to be present in GCA-Bx-negative TAs than in normal TAs (95% CI = 1.9391, 6.0303, p<0.0001). All TAs contained viral antigen in multiple arterial layers. In GCA-positive and GCA-Bx-negative subjects, viral antigen was seen in the adventitia (86% and 95%, respectively), media (67% and 53%, respectively) and intima (52% and 45%, respectively); in normal TAs, viral antigen was seen in the adventitia (91%) and equally in media (82%) and intima (82%).
Examination of 58 GCA-positive, VZV antigen-positive TAs for VZV DNA showed that all contained cellular DNA and 23 (40%) contained the viral DNA. Of 58 patients with GCA who were Bx-negative, VZV antigen-positive TAs were examined. Fifty-one contained cellular DNA, of which 9 (18%) contained VZV DNA. Nine of 11 normal VZV antigen-positive TAs contained cellular DNA, of which 3 (33%) contained VZV DNA. Adventitial inflammation was seen adjacent to viral antigen in 26 (52%) of 58 GCA-Bx-negative subjects whose TAs contained VZV antigen. No inflammation was seen in normal control TAs containing VZV antigen.
Overall, the detection of VZV mostly in the adventitia of GCA-positive TAs, together with the presence of VZV in the inflamed adventitia of GCA-Bx-negative TAs, indicates that inflammation follows VZV reactivation from ganglia and transaxonal transport to arterial adventitia. More detailed steps in the evolution of GCA (transmural inflammation and necrosis with giant and/or epithelioid cells) after virus infection of the adventitia and adventitial inflammation remain to be determined.
An important finding is the presence of VZV DNA and VZV antigen in 18% of control TAs without inflammation, indicating that VZV reactivates subclinically in some people over age 50 years. VZV DNA is found in latently infected human ganglia, but VZV expression is limited to the immediate-early VZV gene 63 RNA (59). If VZV were latent in TAs, only VZV DNA would be found, not VZV late glycoproteins and VZ virions.
The prevalence of VZV in the TAs of patients with clinically-suspected GCA is similar, independent of whether biopsy is negative or positive pathologically. Detection of adventitial inflammation adjacent to VZV antigen in 52% of GCA-Bx-negative TAs for the first time connects the presence of VZV with pathology in GCA-Bx-negative TAs. Inflammation restricted to the adventitia may represent a milder form of GCA.
As for treatment of GCA, no trials have yet been conducted to determine whether antivirals and steroids confer additional benefit to steroids alone. Although many GCA patients improve with steroids, reports are legion of GCA patients who relapse with corticosteroid withdrawal and may also develop more disseminated VZV vasculopathy and die (60,61). Because VZV triggers the immunopathology of GCA, antiviral treatment is likely to confer additional benefit to corticosteroids. The optimal antiviral regimen remains to be determined. We currently treat GCA with prednisone, 1 mg/kg, along with valacyclovir, 1 gm 3 times daily. If the patient improves after 4–6 weeks, we recommend tapering prednisone while continuing administration of antiviral agents for another 4–6 weeks. Long-term antiviral drugs are far less risky than long-term corticosteroids. Furthermore, if during a prednisone taper, patients’ symptoms recur or worsen, along with increases in ESR or CRP, oral antivirals should be added rather than increasing the prednisone dose. In our experience, antiviral treatment has successfully normalized symptoms and inflammatory markers. The value of our approach awaits confirmation in large prospective studies.
Conclusions
In children and adults, VZV is a not uncommon cause of stroke. VZV vasculopathy in children appears to affect large vessels primarily, while stroke after zoster in adults mostly involves large and small arteries. VZV vasculopathy is confirmed by the detection of either VZV DNA or anti-VZV antibody in CSF. Vaccination effectively prevents varicella in children. While immunization with the current FDA-approved Zostavax vaccine reduces the incidence of zoster and postherpetic neuralgia in adults, a newer subunit VZV vaccine has a remarkable 97.2% efficacy in preventing zoster for 3.2 years, although the duration of its effect is unknown. Finally, VZV appears to trigger the immunopathology of giant cell arteritis.
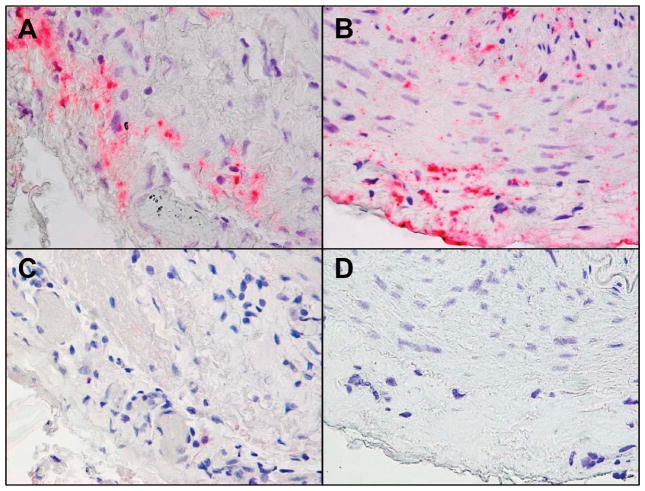
Figure 1.
Varicella zoster virus (VZV) antigen in giant cell arteritis (GCA)-positive and GCA-Bx-negative temporal arteries (TAs). (A) Immunohistochemical analysis with mouse anti-VZV gE IgG1 antibody shows VZV antigen in the adventitia of a GCA-positive TA and (B) in the adventitia, media and intima of a GCA-Bx-negative TA. (C,D) No staining was seen when mouse anti-IgG1 isotype control antibody was substituted for the primary antibody. Magnification 600×.
Acknowledgments
Grant support: This work was supported by a grant from the National Institutes of Health (grant no. AG032958 to D.G.).
The authors thank Marina Hoffman for editorial review and Cathy Allen for word processing and formatting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahalingam R, Wellish M, Wolf W, et al. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323:627–631. doi: 10.1056/NEJM199009063231002. [DOI] [PubMed] [Google Scholar]
- 2.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 3.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 4.Sreenivasan N, Basit S, Wohlfahrt J, et al. The short- and long-term risk of stroke after herpes zoster - a nationwide population-based cohort study. PLoS One. 2013;8:e69156. doi: 10.1371/journal.pone.0069156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breuer J, Pacou M, Gautier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;83:e27–33. doi: 10.1212/WNL.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 6.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58:1497–1503. doi: 10.1093/cid/ciu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundstrom K, Weibull CE, Soderberg-Lofdal K, et al. Incidence of herpes zoster and associated events including stroke--a population-based cohort study. BMC Infect Dis. 2015;15:488. doi: 10.1186/s12879-015-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minassian C, Thomas SL, Smeeth L, et al. Acute cardiovascular events after herpes zoster: A self-controlled case series analysis in vaccinated and unvaccinated older residents of the United States. PLoS Med. 2015;12:e1001919. doi: 10.1371/journal.pmed.1001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yawn BP, Wollan PC, Nagel MA, Gilden D. Risk of stroke and myocardial infarction after herpes zoster in older adults in a US community population. Mayo Clin Proc. 2016;91:33–44. doi: 10.1016/j.mayocp.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askalan R, Laughlin S, Mayank S, et al. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2001;32:1257–1262. doi: 10.1161/01.str.32.6.1257. [DOI] [PubMed] [Google Scholar]
- 11.Braun KP, Bulder MM, Chabrier S, et al. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain. 2009;132:544–557. doi: 10.1093/brain/awn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg MR, Zervas NT, Moskowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984;223:46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- 14.Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger TM, Caduff JH, Gebbers JO. Fatal varicella-zoster virus antigen-positive giant cell arteritis of the central nervous system. Pediatr Infect Dis J. 2000;19:653–656. doi: 10.1097/00006454-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Miravet E, Danchaivijitr N, Basu H, et al. Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev Med Child Neurol. 2007;49:417–422. doi: 10.1111/j.1469-8749.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 17.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, et al. Varicella zoster virus, a cause of waxing and waning vasculitis: The New England Journal of Medicine case 5–1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 18.Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, et al. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- 19.Silver B, Nagel MA, Mahalingam R, et al. Varicella zoster virus vasculopathy: a treatable form of rapidly progressive multi-infarct dementia after 2 years’ duration. J Neurol Sci. 2012;323:245–247. doi: 10.1016/j.jns.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno M, Oka A, Koeda T, et al. Unilateral occlusion of the middle cerebral artery after varicella-zoster virus infection. Brain Dev. 2002;24:106–108. doi: 10.1016/s0387-7604(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 21.Katchanov J, Siebert E, Klingebiel R, Endres M. Infectious vasculopathy of intracranial large- and medium-sized vessels in neurological intensive care unit: a clinico-radiological study. Neurocrit Care. 2010;12:369–374. doi: 10.1007/s12028-010-9335-4. [DOI] [PubMed] [Google Scholar]
- 22.Cheng-Ching E, Jones S, Hui FK, et al. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci. 2015;351:168–173. doi: 10.1016/j.jns.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanthier S, Armstrong D, Domi T, de Veber G. Post-varicella arteriopathy of childhood: natural history of vascular stenosis. Neurology. 2005;64:660–663. doi: 10.1212/01.WNL.0000151851.66154.27. [DOI] [PubMed] [Google Scholar]
- 25.Losurdo G, Giacchino R, Castagnola E, et al. Cerebrovascular disease and varicella in children. Brain Dev. 2006;28:366–370. doi: 10.1016/j.braindev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Moriuchi H, Rodriguez W. Role of varicella-zoster virus in stroke syndromes. The Pediatr Infect Dis J. 2000;19:648–653. doi: 10.1097/00006454-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Sebire G, Meyer L, Chabrier S. Varicella as a risk factor for cerebral infarction in childhood: a case-control study. Ann Neurol. 1999;45:679–680. doi: 10.1002/1531-8249(199905)45:5<679::aid-ana22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Thomas SL, Minassian C, Ganesan V, et al. Chickenpox and risk of stroke: a self-controlled case series analysis. Clin Infect Dis. 2014;58:61–68. doi: 10.1093/cid/cit659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen P, Reynaud J, Pouzol P, et al. Varicella and thrombotic complications associated with transient protein C and protein S deficiencies in children. Eur J Pediatr. 1994;153:646–649. doi: 10.1007/BF02190684. [DOI] [PubMed] [Google Scholar]
- 30.Manco-Johnson MJ, Nuss R, Key N, et al. Lupus anticoagulant and protein S deficiency in children with postvaricella purpura fulminans or thrombosis. J Pediatr. 1996;128:319–323. doi: 10.1016/s0022-3476(96)70274-3. [DOI] [PubMed] [Google Scholar]
- 31.Gilden DH, Mahalingam R, Cohrs RJ, et al. The protean manifestations of varicella-zoster virus vasculopathy. J Neurovirol. 2002;8(Suppl 2):75–79. doi: 10.1080/13550280290167902. [DOI] [PubMed] [Google Scholar]
- 32.Bulder MM, ten Houten R, Klijn CJ, Braun KP. Unilateral movement disorder as a presenting sign of paediatric post-varicella angiopathy. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-009437. pii: bcr2013009437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciccone S, Faggioli R, Calzolari F, et al. Stroke after varicella-zoster infection: report of a case and review of the literature. Pediatr Infect Dis J. 2010;29:864–867. doi: 10.1097/inf.0b013e3181ddefb6. [DOI] [PubMed] [Google Scholar]
- 34.Darteyre S, Hubert A, Chabrier S, et al. In vivo evidence of arterial wall inflammation in childhood varicella-zoster virus cerebral vasculopathy. Dev Med Child Neurol. 2014;56:1219–1220. doi: 10.1111/dmcn.12329. [DOI] [PubMed] [Google Scholar]
- 35.Dunkhase-Heinl U, Stausbol-Gron B, Christensen J, Ostergaard JR. Post-varicella angiopathy: a series of 4 patients with focus on virologic and neuroimaging findings. Pediatr Neurol. 2014;50:581–585. doi: 10.1016/j.pediatrneurol.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Frank Y, Lu D, Pavlakis S, Black K, et al. Childhood AIDS, varicella zoster, and cerebral vasculopathy. J Child Neurol. 1997;12:464–466. doi: 10.1177/088307389701200710. [DOI] [PubMed] [Google Scholar]
- 37.Hattori H, Higuchi Y, Tsuji M. Recurrent strokes after varicella. Ann Neurol. 2000;47:136. [PubMed] [Google Scholar]
- 38.Iro MA, Kirkham FJ, Macdonald JH, et al. Varicella zoster virus central nervous system immune reconstitution inflammatory syndrome presenting in a child. Pediatr Infect Dis J. 2013;32:1283–1284. doi: 10.1097/INF.0b013e31829aa4fc. [DOI] [PubMed] [Google Scholar]
- 39.Kawatani M, Nakai A, Okuno T, et al. A case of intracranial saccular aneurysm after primary varicella zoster virus infection. Brain Dev. 2012;34:80–82. doi: 10.1016/j.braindev.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Okanishi T, Kondo A, Inoue T, et al. Bilateral middle cerebral artery infarctions following mild varicella infection: a case report. Brain Dev. 2009;31:86–89. doi: 10.1016/j.braindev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Sabry A, Hauk PJ, Jing H, et al. Vaccine strain varicella-zoster virus-induced central nervous system vasculopathy as the presenting feature of DOCK8 deficiency. J Allergy Clin Immunol. 2014;133:1225–1227. doi: 10.1016/j.jaci.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selvakumar CJ, Justin C, Gnaneswaran TR, Chandrasekaran M. Post varicella angiopathy. J Assoc Physicians India. 2010;58:572–574. [PubMed] [Google Scholar]
- 43.Takahashi M, Otsuka T, Okuno Y, et al. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–90. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention (CDC) Evolution of varicella surveillance--selected states, 2000–2010. MMWR Morb Mortal Wkly Rep. 2012;61:609–612. [PubMed] [Google Scholar]
- 45.Wise RP, Salive ME, Braun MM, et al. Postlicensure safety surveillance for varicella vaccine. JAMA. 2000;284:1271–1279. doi: 10.1001/jama.284.10.1271. [DOI] [PubMed] [Google Scholar]
- 46.Levin MJ, DeBiasi RL, Bostik V, Schmid DS. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444–1447. doi: 10.1086/592452. [DOI] [PubMed] [Google Scholar]
- 47.Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792–795. doi: 10.1016/j.annemergmed.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266–268. doi: 10.1097/INF.0b013e3181f63cf9. [DOI] [PubMed] [Google Scholar]
- 49.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 50.Oxman MN, Levin MJ Shingles Prevention Study Group. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008;197(Suppl 2):228–236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levin MJ, Schmader KE, Gnann JW, et al. Varicella-zoster virus-specific antibody responses in 50–59-year-old recipients of zoster vaccine. J Infect Dis. 2013;208:1386–1390. doi: 10.1093/infdis/jit342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harpaz R, Ortega-Sanchez IR, Seward JF, et al. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. quiz CE2-4. [PubMed] [Google Scholar]
- 53.Simberkoff MS, Arbeit RD, Johnson GR, et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152:545–454. doi: 10.7326/0003-4819-152-9-201005040-00004. [DOI] [PubMed] [Google Scholar]
- 54.Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–909. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tseng HF, Smith N, Harpaz R, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–166. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 56.Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420. doi: 10.1371/journal.pmed.1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 58.Gilden D, White T, Khmeleva N, et al. VZV in biopsy-positive and -negative giant cell arteritis: analysis of 100+ temporal arteries. Neurol Neuroimmunol Neuroinflamm. 2016;3:e216. doi: 10.1212/NXI.0000000000000216. doi:10/1212/NXI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ouwendijk WJD, Choe A, Nagel MA, et al. Restricted varicella zoster virus transcription in human trigeminal ganglia obtained soon after death. J Virol. 2012;86:10203–10206. doi: 10.1128/JVI.01331-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagel MA, Lenggenhager D, White T, et al. Disseminated VZV infection and asymptomatic VZV vasculopathy after steroid abuse. J Clin Virol. 2015;66:72–75. doi: 10.1016/j.jcv.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilden D, White T, Galetta SL, et al. Widespread arterial infection by varicella zoster virus explains refractory giant cell arteritis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e125. doi: 10.1212/NXI.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]