Abstract
Myocardial infarction, atherosclerosis and hypertension are the most common heart-related diseases that affect both the heart and the blood vessels. Multiple independent risk factors have been shown to be responsible for cardiovascular diseases. The combination of a healthy diet, exercise and smoking cessation keeps these risk factors in check and helps maintain homeostasis. The dynamic monolayer endothelial cell integrity and cell-cell communication are the fundamental mechanisms in maintaining homeostasis. Recently, it has been revealed that small non-coding RNAs (ncRNAs) play a critical role in regulation of genes involved in either posttranscriptional or pretranslational modifications. They also control diverse biological functions like development, differentiation, growth, and metabolism. Among ncRNAs, the short interfering RNAs (siRNAs) and microRNAs (miRNAs) have been extensively studied, but their specific functions remain largely unknown. In recent years, miRNAs are efficiently studied as one of the important candidates for involvement in most biological processes and have been implicated in many human diseases. Thus, the identification and the respective targets of miRNAs may provide novel molecular insight and new therapeutic strategies to treat diseases. This review summarizes the recent developments and insight on the role of miRNAs in cardiovascular disease prognosis, diagnostic and clinical applications.
Keywords: MicroRNAs, Endothelial cells, Cardiac fibrosis, Atherosclerosis, Myocardial infarction, Stroke, Hypertension
1. Introduction
Heart diseases are the leading cause of death in the United States and around the world [30]. Hypertension, tobacco exposure, high cholesterol, obesity, diabetes, unhealthy diets, and alcohol seem to have an additive effect for the causation of cardiac diseases. Myocardial infarction (heart attacks), cerebrovascular disease (stroke), atherosclerosis and hypertensions (raised blood pressure) are the most common cardiovascular diseases, which involve heart and blood vessels. Number of recent studies has shown that miRNAs are essential for the normal development and physiology of various organs, including the heart. Studies have also started to characterize the link between micro RNAs (miRNAs) and different aspects of cardiac pathogenesis such as chamber morphogenesis, conduction, and contraction. Moreover, congenital anomalies of the heart can be associated with the dysregulation of specific miRNAs [50].
Recently, investigators have demonstrated that RNA functions not only as an intermediate molecule between DNA and protein, but is also involved in the complicated process of gene regulation and expression. Some of the RNAs are the functional molecules that are not capable of translating into proteins. Hence, these RNAs are called noncoding RNAs (ncRNAs). Among the several classes of ncRNAs, miRNA is the most extensively studied and has gained prominence in current research. These ncRNAs are found within intergenic or intragenic regions of host genes, which make up approximately 10% of the human genome [14]. Recently, a rapid progress has been made in profiling miRNA and reported it in various diseases and cell types. MiRNAs are involved in a number of biological processes, including cell proliferation, apoptosis, stress response, hematopoiesis, and oncogenesis. Studies have highlighted that miRNA could be a potential molecular therapeutic strategy for various diseases, including heart diseases [88]. MiRNAs are tissue and lineage specific, and many more to be discovered. The recently developed high-throughput approaches revealed the miRNA size, and their target, and the connectivity of the miRNA-dependent regulatory network. One step further, the expression levels of miRNAs and their decay rates have been identified in individual cell types. These works together help us to understand miRNA-dependent gene regulation to study the response of the entire network. Studies have shown that miRNAs stably regulate many developmental and cellular processes, including numerous eukaryotic plants, and mammals with vary in expression at extracellular and intracellular fractions [48]. During the past decade, numerous research articles have shown a wide knowledge about the basic mechanisms of miRNAs, biogenesis and its functions in the circulatory system. This review will discuss the biosynthesis of the miRNA and its functional role in cardiovascular diseases, as well as the challenges in miRNA-based therapy.
2. Noncoding RNAs
The ncRNAs are the functional entity of a cell in regulating the gene expression. These regulatory RNAs have function but do not encode proteins. In 1950s, discoveries of ribosomal RNA (rRNA) and transfer RNA (tRNA) are known as the principle RNA molecules that participate in gene expression. In the early 1980s, the existence of small nuclear RNAs (snRNAs) was discovered. Recently, the other ncRNAs, such as small cajal body specific RNAs (scaRNAs), small nucleolar RNAs (snoRNAs), long noncoding RNAs (lncRNAs), piwi-interacting RNAs (piRNAs) and circular RNAs (circRNAs) were discovered for the labile genes. The discovery of miRNAs was a huge revolution because it depicted their importance of posttranscriptional events in gene expression, particularly in eukaryotic organisms [11].
2.1. SiRNAs
The siRNAs are small double stranded RNAs and well studied among the ncRNAs of approximately 20-25 base pair (bp) in length. The major role of siRNA is to involve in RNA interference (RNAi) pathway in regulating the gene expression. This RNAi-mediated gene regulation can be executed by either siRNA or miRNA, but there are subtle differences between the two. However, siRNA based strategies have some disadvantages, mostly RNase susceptibility. The use of RNAi-based therapeutics from a clinical standpoint, is a new platform that is steadily developing [74].
2.2. PiRNAs
Comprising a length of approximately 24-30 bp. PiRNAs are the Dicer-independent ncRNAs that associated with PIWI subfamily proteins. PiRNAs are highly abundant in germ cells. Some are involved in the formation of heterochromatin or RNA destabilization, which mediates gene silencing [9].
2.3. SnoRNAs
SnoRNAs are intermediate-sized ncRNAs (60–300 bp) responsible for post-transcriptional modifications and assist in folding and stability of rRNA [46].
2.4. Sca-RNAs
The scaRNAs (60-425 bp) are a subset of snoRNA family, which is large family of conserved ncRNAs that primarily guide biochemical modifications of particular nucleotides (e.g., methylation and pseudouridylation) of rRNAs and snoRNAs. Without the specific modifications controlled by the scaRNAs, the spliceosome fails to function properly [59]. Dysregulation of alternative splicing has been associated with the regulation of heart development and cardiovascular diseases [49]. It has been already reported that 125 snoRNAs including 12 scaRNAs were down regulated in the right ventricle from 16 infants with tetralogy of Fallot [65]. These suggest that scaRNAs playing an important role in heart development and cardiovascular diseases.
2.5. LncRNAs
LncRNAs are a heterogeneous group of non-coding transcripts more than 200 bp long that plays an important role in many biological processes. These constitute the largest portion of the mammalian non-coding transcriptome [62]. Mostly, lncRNAs are known to mediate epigenetic modifications of DNA by recruiting chromatin remodeling complexes to specific loci [62]. These transcripts are identified by searching for chromatin signatures that associated with active transcription in the regions across which transcriptional elongation takes place. Recent studies have shown that some lncRNAs are regulated during acute myocardial infarction (eg, Novlnc6) and heart failure (eg, Mhrt), whereas others control hypertrophy, mitochondrial function and apoptosis of cardiomyocytes and therefore, considered as a powerful foe in cardiac functions [87].
2.6. CircRNAs
The circRNAs are one among the ncRNAs and have most recently been identified and characterized, and predominantly present in the cytoplasm [31]. The circRNAs, unlike the other known RNAs, the 5’ and 3’ ends present in the RNA molecules are covalently joined together and forms a circular loop. Even though, circRNAs arise from protein-coding genes in the cell but have not been shown to code for proteins. These circRNAs are more stable than other ncRNAs because of its circular nature and do not have 5’ or 3’ ends for endonuclease-mediated degradation. A study has shown that the circRNAs can act as antagonists of miRNAs [61].
2.7. MiRNAs
MiRNAs are a new class of highly conserved RNAs of approximately 22 bp in length, genetically encoded, endogenous, noncoding single-stranded RNAs. It has been shown that the first evidence for small regulatory RNAs called the miRNAs at the genetic loci of lin-4 and let-7, which regulate the timing of Caenorhabditis elegans during development [51]. MiRNAs mainly down regulate the gene expression and in rare instances, miRNAs have been reported to up regulate target gene expression [16, 90]. The siRNAs and miRNAs are a class of small, non-coding RNAs that are processed inside the cell by an enzyme called Dicer and incorporated into an RNA inducing silencing complex (RISC). However, siRNA is considered a double-stranded whereas, miRNA is a single-stranded RNA and possesses a small hairpin-like structure [98]. Based on their chemical composition and/or functions, these two classes of RNAs cannot be distinguished easily because of their equal size with 5’-phosphate and 3’hydroxy termini and functionally interchangeable [45]. While both are involved in translational repression or mRNA cleavage, they differ on the degree of complementary between the small RNA and its target. SiRNAs cause degradation of a target mRNA molecule in a sequence specific manner. On the other hand, miRNAs typically suppress the translation of many different mRNA sequences because of its partial complementary pairing. Interestingly, both are important for therapeutic use because of the roles they play in controlling gene expression.
3. MiRNAs biosynthesis and function
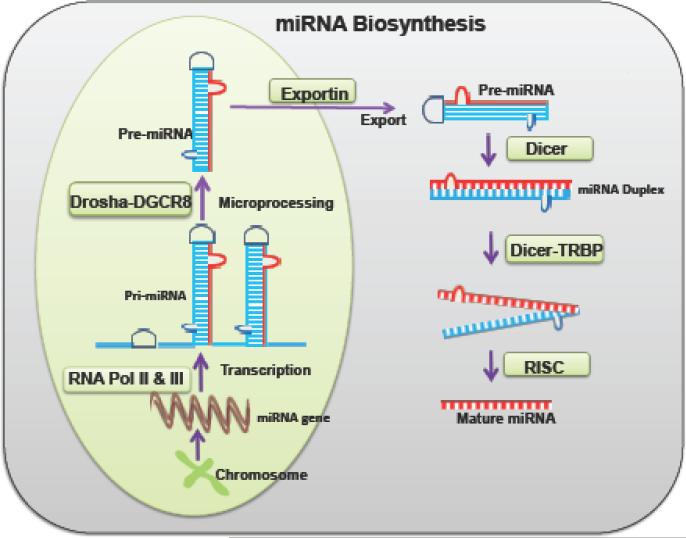
The biosynthesis of miRNAs is a complex, multi-step process, which begins in the nucleus and the maturation process ends in the cytoplasm of the cell (Fig 1). The synthesis process starts from the miRNA genes that are transcribed either by RNA polymerase II or III arising out of introns of protein-coding genes or from independent genes of long primary miRNA (pri-miRNA) transcripts [48]. These gene transcripts have their own promoting sites to express independently and are organized in clusters for the transcriptional regulation. The resulting pri-miRNAs are cleaved by the microprocessor enzyme complex DROSHA and DGCR8 (DiGeorge Critical Region 8), which are present in the nucleus. The DGCR8 determines the precise cleavage site at the pri-miRNA [34]. The cleaved precursor miRNA (pre-miRNA) molecules contain a local hairpin structure and are approximately 100 bp in length [44]. The maturation process of miRNA is first carried out by DROSHA, a RNAse III family protein in the nucleus. Then, the pre-miRNAs are exported from the nucleus to the cytoplasm through nuclear pores via a GTP-GDP gradient by the enzyme exportin-5 [44]. In the cytoplasm, DICER, derived from an RNA III family interact with another protein partner transactivation response RNA binding protein (TRBP) together cleaves the pre-miRNAs into short double-stranded RNA. This ultimately forms the mature miRNAs. The length of an average mature human miRNA contains a hairpin loop of 33 base pairs [98]. Multiple overlapping proteins and RNA interactions involve and play a great role in the miRNAs biogenesis regulation [48]. There are three major pathways involved in miRNA biosynthesis.
Figure 1. The common miRNA biosynthesis pathway.
pri-miRNA- primary miRNA; pol II & III- polymerase II or III; DGCR8 -DiGeorge Critical Region 8; TRBP – Transactivation response RNA binding protein; RISC- RNA-induced silencing complex.
The mature miRNAs gene expression was regulated according to the direction of RISC along with argonaute protein (Ago) assembly [33] to the target mRNA. This leads to the imperfect or perfect complementary of miRNA: mRNA interaction at the 3’ UTR region, resulting in either translational inhibition or transcript degradation, respectively [16, 90]. Studies have shown that p53/p73/p63 functions as transcription factors, but it also regulates miRNAs processing machinery DROSHA-DGCR-8, DICER-TRBP2 and argonaute proteins. In particular, these transcription factors that regulate the processing of miRNAs are let-7, miRNA-200c, miRNA-143, miRNA-107, miRNA-16, miRNA-145, miRNA-134, miRNA-449a, miRNA-503, and miRNA-21 [7]. Since the discovery of miRNAs, number of biological, clinical research is performed on numerous aspects, including cardiovascular diseases. Over expression and suppression of miRNAs revealed the importance of miRNAs in pathophysiology.
4. MiRNAs mimics and inhibitors
The individual miRNA mimics and inhibitors are the useful tools in modulating the specific cell phenotype. MiRNA mimics would be a small and chemically modified double-stranded RNAs that imitate the mature miRNAs. These miRNA mimics enhance the function of endogenous miRNA, leading to a decrease in protein expression. Studies have shown that miRNA mimics could be a useful tool in modulating the failing human heart [77]. MiRNA inhibitors are single stranded oligonucleotides that bind irreversibly to the endogenous miRNAs and inactivate its function. In contrast to miRNA mimics, miRNA inhibitors are also known as an antagomir that suppresses the function of endogenous miRNA and lead to an increase in protein expression. Antagomirs are also served as potential tools for preventing cancer [85] and therefore, could be used in the clinical practice to repress tumor growth. Moreover, the use of anti-sense miRNA treatment is an important strategy to overcome other pathological states like spinal cord injury [71] and ischemic stroke [92]. Thus, RNA mimics and inhibitors are useful in identifying and confirming the pairs of miRNA/target genes that are predicted by computational tools.
5. MiRNAs in cardiovascular diseases
Recently, several studies have reported that the dysregulation of miRNAs expression in tissues is linked to cardiovascular diseases. In addition, studies demonstrated that miRNAs also existed in blood, in the form of circulating miRNAs and are resistant to endogenous ribonuclease activity and can be present in a remarkably stable form during pathological conditions. More importantly, circulating miRNAs are currently explored as biomarkers in a wide range of cardiovascular conditions, including atherosclerotic disease. In the following sections, we are discussing an overview on the role of miRNAs and its association with various cardiovascular diseases.
5.1. MiRNAs: Atherosclerosis
Atherosclerosis (ATH) or arteriosclerosis is an inflammatory disease in which hypercholesterolemia plays a major role in the origin and development of the pathology. During ATH, the artery wall thickens as a result of accumulation of calcium and fatty materials such as cholesterol and triglycerides [56]. Early stages of ATH are characterized by hyperplasia of vascular smooth muscle cells (VSMCs), which forms a multilayer compartment internally to tunica media called as neointima and the infiltration of low-density lipoproteins (LDLs) in the arterial wall. Accumulation of LDL, monocytes and macrophages forms a vascular plaque. This atherosclerotic plaque becomes fragile and may rupture or erode from either immature or advanced lesions associated with thin, collagen-poor fibrous caps. Formation of a lesion in the neointima compromises the elasticity of the arterial walls and leads to angina. This results in flow-limiting stenosis and therefore, causes cardiovascular diseases such as peripheral artery diseases (PAD), coronary artery diseases (CAD), myocardial infarction, and aneurysms [99].
Animal and human evidence suggests that initiation of ATH is from the dysfunction of ECs of the arterial wall, which is subjected to different noxious stimuli and injuries [79]. The major stimuli/injuries that cause EC-dysfunction includes diabetes mellitus, dyslipidemia, hypertension, smoking, and aging. This injuries-mediated oxidative stress and proinflammatory mediators become predominant in causing ATH. An understanding of the role of miRNAs in ATH may provide novel targets and therapeutic opportunities for controlling disease and other chronic inflammatory disease states, and is depicted in Fig.2. Knowledge of miRNA expression pattern and function in distinctive cell types in human is limited because of difficulty in obtaining samples. The currently available information about the patterns of miRNAs observed in different cell types is discussed below.
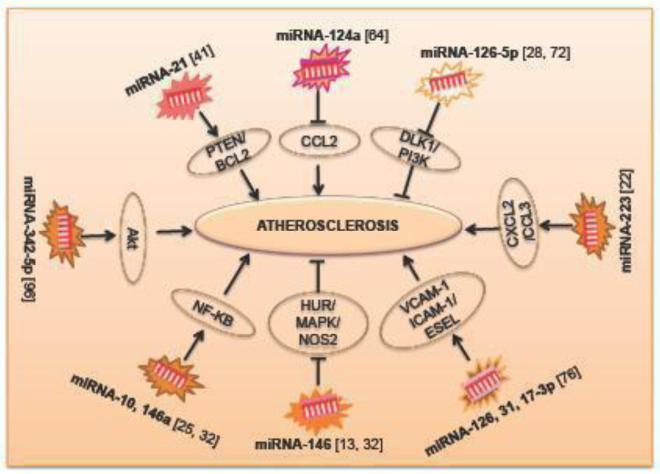
Figure 2. The role of miRNAs involved in atherosclerosis.
PTEN - Phosphatase and tensin homolog; Bcl2 - B-cell lymphoma; CCL2 - Chemokine (C-C motif) ligand 2; DLK1- Delta-like 1 homology; PI3K- phosphatidylinositol-3-kinase; CCL2- Chemokine (C-C motif) ligand 2; CXCL2- Chemokine (C-X-C) ligand 2; VCAM - Vascular cell adhesion molecule; ICAM – Inter-cellular adhesion molecule; HUR – Human antigen R; MAPK - Mitogen-activated protein kinases; NOS2 - Nitric oxide synthase 2; NF-KB – Nuclear factor kappa-B.
5.1.1. Endothelial cells
Emerging evidence has revealed that miRNAs constitute a new class of intra- and intercellular signaling molecules to modulate inflammation in endothelial cells (ECs). The miRNA expression profile revealed that the association of a group of miRNAs shows an important role in ATH and the role of individual miRNAs in ECs is poorly understood. Many miRNAs such as MiRNA-126, miRNA-31 and miRNA-17-3p regulate vascular inflammation by controlling the expression of the adhesion molecules VCAM-1, ICAM-1 and E-SEL [76]. It has been established that miR-146a has a potential role in plaque destabilization and the onset of acute coronary syndrome and a novel regulator of inflammation during ATH. This effect is partly by activating the NF-κB signal-transduction pathway [32]. Studies have also shown that miRNA-10 is playing an important role in the regulation of ATH, specifically governing the NF-κB signaling pathway [25]. Recently, it has been found that endothelial miRNA-126-5p maintains a proliferative reserve in ECs through suppression of the Notch1 inhibitor delta-like 1 homolog (Dlk1) and thereby prevent atherosclerotic lesion formation [72]. Moreover, mRNA-126 promotes angiogenesis by the inhibition of suppressors of the phosphatidylinositol kinase (PI3K) pathway resulting in VEGF and FGF induced endothelial cell proliferation and tube formation [28].
Furthermore, miRNA- 146 represses the pro- inflammatory NF- κB pathway as well as the MAP kinase pathway and downstream early growth response transcription factors. This study also demonstrates that HuR, an RNA binding protein that promotes EC activation by suppressing expression of endothelial nitric oxide synthase (eNOS or NOS2), is a novel miRNA- 146 target [13]. Recent studies have illustrated the role of miRNA-223 in the regulation of high-density lipoprotein-cholesterol (HDL-C) uptake and in the inhibition of cholesterol biosynthesis. Moreover, miRNA-223 plays an important role in cholesterol metabolism [91]. Recently, the novel role of miRNA-26a and its therapeutic potential for atherosclerosis associated with apoptotic cell death has been reported [102]. Thus, miRNAs from ECs are contributing significantly during ATH pathogenesis.
5.1.2. Smooth muscle cells (SMCs)
Many reports have shown that miRNAs undergo specific fate, phenotypic changes and differentiation in VSMCs [57]. It has been shown that miRNA-21 can be up regulated during an injury and be participated also in cell proliferation and survival by targeting PTEN and Bcl2 in smooth muscle cells from blood vessels. Depletion of miRNA-21 resulted in decreased cell proliferation [41]. Studies have further supported the role of miRNA-221 and miRNA-222 in the SMCs proliferation and differentiation [57]. Recently, researchers reported that miRNA-145 is essential for differentiation [15] but later the in vivo miRNA-143/145 KO mice studies revealed that these miRNAs are not the only essential component for the VSMCs development [6]. A study also shown that the family of miRNA-130/301 targets TGF-BMP pathway members SMAD4 and SMAD5 as well as peroxisome proliferator-activated receptor (PPARγ), to control pulmonary VSMCs proliferation during PH [5]. These studies suggest that VSMCs are important for the miRNA-mediated regulation of ATH pathogenesis.
5.1.3. Monocytes and macrophages
Monocytes are the precursors of tissue macrophages and myeloid-derived dendritic cells that influence plaque development followed by recruitment into the intima and differentiation to foam cells [2]. Foam cell macrophages play a crucial role in plaque destabilization and rupture, which makes them the centerpiece in the study of atherogenesis for a long time. Until now, the infiltrated macrophages have been one of the focal points of ATH research and are believed to be critical in regulating changes in the local environment [12]. MiRNAs can also affect monocyte infiltration via several mechanisms. For instance, miR-124a modulates CCL2 expression to facilitate the rolling and attachment of monocytes to the vessel wall [64]. Several miRNAs such as miRNA-17, miRNA-20a and miRNA-106a also regulate macrophage infiltration through the direct suppression of signal-regulatory protein-α [104]. Moreover, high levels of miRNA-145 expressed by VSMCs can decrease macrophage infiltration and, thus, could be a potential target in developing new therapeutic strategy to alleviate ATH [58]. MiRNA-223 directly targets numerous chemo-attractants, such as chemokine C-X-C motif ligand 2 (CXCL2) and CCL3 and regulates the infiltration of myeloid cells. [22]. There is an increased expression of miRNA-155 and a decreased expression of the colony-stimulating factor 1 receptor (CSF1R) and B-cell lymphoma 6 (BCL6) was observed by the inflammatory macrophages present in the atherosclerotic lesions [97]. MiRNA-342-5p derived from macrophages is responsible for the up regulation of atherogenesis, leading to the increase of nitro-oxidative stress during atherosclerotic lesion formation. This may be due to the up regulation of NOS2 in proinflammatory macrophages, which removes the repression of miRNA-155. MiRNA-143, miRNA-145 and miRNA-365 were down regulated, and miRNA-21, miRNA-146, miRNA-214, and miRNA-352 were up regulated in neointimal formation models [41]. Moreover, miRNA-342-5p promotes inflammatory macrophage activation through Akt pathway during ATH. Thus, targeting miRNA-342-5p in activated macrophages during atherogenesis may be a promising therapeutic strategy because it prevents the initiation of a cascade of molecular events that sensitize macrophages to inflammatory stimulation [96].
5.2. MiRNAs: Hypertension
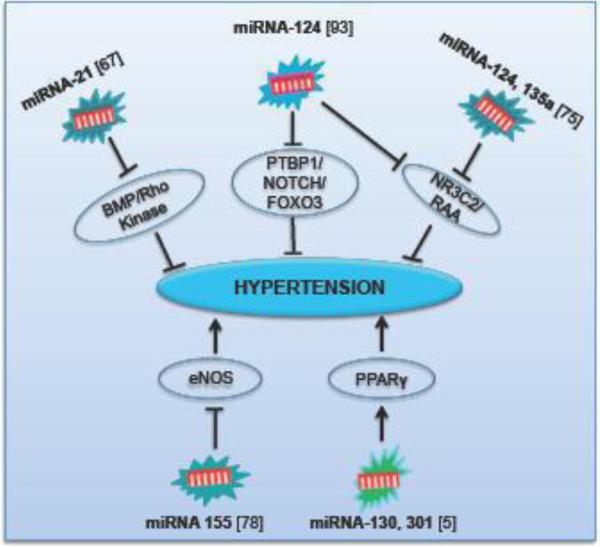
Hypertension is the persistent elevation of systemic blood pressure, which is one of the most common medical conditions involving the cardiovascular diseases such as MI and stroke as well as the leading cause of kidney failure [53]. Despite continuous advancement in treatment options, it is an increasingly important health problem, affecting as many as one billion people worldwide with high morbidity and mortality. The role of miRNAs in the regulation of hypertension is summarized in Fig. 3. Recently, miRNAs have been considered as potential biomarkers for the regulation of PH. It has been shown that there is a down regulation of miRNA-204 in PH associated pulmonary arterial smooth muscles’ cells both in animal and human samples [37]. MiRNA-21 is predicted to be a PH-modifying miRNA in regulating targets integral to the bone morphogenetic protein (BMP) and Rho/Rho kinase signaling. Therefore, miRNA-21 considered as an important regulator to control PH pathogenic signaling [67]. Another study has unveiled that miRNA-124 controls the proliferative, migratory and inflammatory phenotype of pulmonary vascular fibroblast. Additionally, it regulates fibroblast proliferation via direct binding to the 3' UTR of polypyrimidine tract-binding protein 1 (PTBP1) and subsequent regulation of Notch1/PTEN/FOXO3/p21Cip1 and p27Kip1 signaling [93]. MiRNA124 and miRNA-135a are also associated with mineralocorticoid receptor (alternative name: nuclear receptor subfamily 3 group C member 2-NR3C2) regulation. It is a ligand-dependent transcription factor that regulates the expression of ionic and water transporters in response to steroid hormones, primarily aldosterone. MiRNA-124 and miRNA-135a also attenuate signaling in the renin–angiotensin–aldosterone (RAA) system and thus participate in the regulation of blood pressure [75]. Sun and coworkers also studied the complexity of RAA system, exercise and miRNAs in the development of PH. They observed that miR-155 was an essential regulator of endothelial nitric oxide synthase (eNOS) expression and endothelium-dependent vasorelaxation while mediating inflammation in PH. Nitric oxide generated by the eNOS plays a significant role in maintaining cardiovascular homeostasis. Therefore, inhibition of miRNA-155 may be a new therapeutic approach in improving endothelial dysfunction during the development of cardiovascular diseases [78]. A recent molecular network analysis study forecasted that the miRNA-130/301 family is a master regulator in controlling the cellular proliferation in PH by unexpectedly interacts with each other by targeting PPARγ [5]. These studies suggest that miRNAs are the important regulator for PH.
Figure 3. The miRNA-mediated pathways involved during hypertension.
PPARγ – Peroxisome proliferator-activated receptor gamma; eNOS- Endothelial nitric oxide synthase; BMP - Bone morphogenetic protein; Rho – Ras homology gene family; PTBP1 – Polypyrimidine tract-binding protein 1; FOXO3 – Forkhead box O-3; NR3C2 – Nuclear receptor subfamily 3 group C member 2; RAA - Renin-angiotensin-aldosterone.
5.3. MiRNAs: Myocardial Infarction (MI)
Ischemic heart disease resulting in loss or dysfunction of cardiomyocytes is the leading cause of death worldwide. The survivors of acute MI eventually develop chronic heart failure, with over 5 million cases in the United States alone [30]. The cause may be due to a variety of factors, including MI, hypertension or genetic mutation, which overall result in lack or poor flow of oxygen to heart, heart tissues. This results to coronary artery occlusion, cardiac arrest and subsequently death happen. This phenomenon is called acute MI, and more commonly known as heart attack. Role of tissue-specific miRNAs has been documented in both the physiological and pathological conditions of myocardial infarction. Circulating miRNAs in patients with MI have been examined very recently. It was reported that miRNAs served as a critical modulating regulator, which participates in almost all aspects of cardiovascular diseases and vascular biology [1].
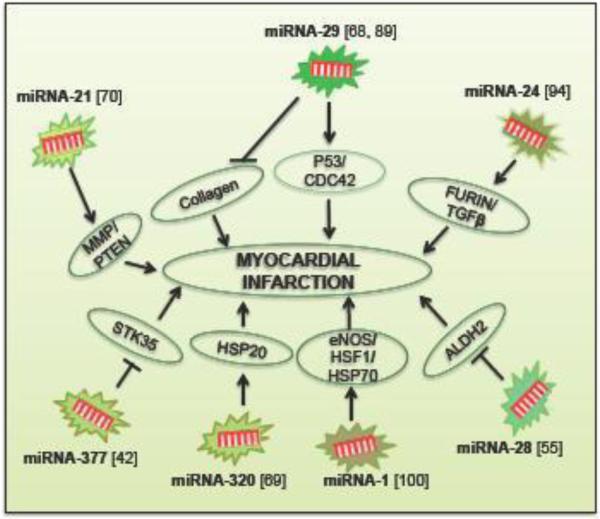
The expression levels of miRNAs vary between tissues. MiRNA-1, miRNA-133a and -133b are expressed strongly in heart and skeletal muscle. However, after acute MI, there was no significant difference in the expression of miRNA-1, miRNA-133a and miRNA-133b, which normally exhibits at high levels in the heart [1, 17]. Studies also show that after ischemic preconditioning in the heart, miRNA-1, miRNA-21and miRNA-24 were significantly increased. This increased miRNAs further triggers cardio-protection by up regulating eNOS, heat shock protein-70 (HSP-70), and heat-shock factor 1 (HSF-1) [100]. MiRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting HSP 20. Therefore, miRNA-320 could be a new therapeutic target for ischemic heart diseases [69]. It has also been studied that miRNA-21 regulates matrix metalloproteinase 2 (MMP-2) expression at the fibrosis area of the infarcted zone via a PTEN pathway [70]. Another study has shown that miRNA-133a of adult cardiac progenitor cells (CPCs) increases cardiac function after MI by the reduction of fibrosis, hypertrophy coupled to an increase in vascularization and cardiomyocyte proliferation [40]. A study has shown that MiRNA-24 is playing a critical role in cardiac fibroblast function and cardiac fibrosis after MI through a furin-TGF-β signaling pathway [94].
An emerging role of miRNA-26 family and their implications for different cell types during cardiovascular disease condition was reviewed recently [38]. The miRNA-29 family is another important miRNA that acts as a negative regulator of gene expression by targeting the mRNAs that encode proteins involved in fibrosis, including multiple collagens, fibrillins, and elastin. Down-regulation of miRNA-29 with anti-miRNAs in vitro and in vivo induces the expression of collagens, whereas over-expression of miRNA-29 in fibroblasts reduces collagen expression and thereby protects the heart from MI [89] as explained in Fig.4. Meanwhile, some other studies have shown that miRNA-29 family induces apoptosis by activating p53 and targeting p85alpha and cell division control protein 42 (Cdc42) [68]. In addition, a recent report has declared that miRNA-28 promotes myocardial ischemia through the inhibition of Aldehyde dehydrogenase 2 (ALDH2) expression in Mus muculus cardiomyocytes and thus can be served as a potential target for therapy [55]. Recently, a study reported that patients with ischemic heart disease have been substantial up regulation of miRNA-377 in biopsy samples from myocardial tissues. Further more, transplantation of miRNA-377 knockdown human CD34 cells into the ischemic mouse myocardium promoted cardiac repair and attenuated ventricular function. This study also identified that STK35 kinase is the direct target of miRNA 377 during MI [42]. These studies documented that miRNAs are important in regulating the pathological conditions of MI.
Figure 4. The role of miRNAs in pathogenesis of MI.
ALDH2 – Aldehyde dehydrogenase 2; eNOS- Endothelial nitric oxide synthase; HSF1 – Heat shock factor 1; HSP70 – Heat shock protein 70; STK35 – Serine/threonine-protein kinase 35; MMP - Matrix metalloproteinase; PTEN - Phosphatase and tensin homolog; CDC42 – Cell division cycle 42; TGFβ - Transforming growth factor beta.
5.4. MiRNAs: Cardiac Hypertrophy
Cardiac hypertrophy or hypertrophic cardiomyopathy is an inheritable disease and also called the disease of the sarcomere, where the heart muscle becomes thicker in some parts of the heart. This thickening of the muscle forces the heart to work harder to pump blood to circulate different parts of the body [29]. Hypertrophic cardiomyopathy exists as physiological and pathological forms. In the physiological form, there is an enlargement of the heart in healthy individuals due to heavy exercise and is not associated with any kind of cardiac disorder or damage. In the pathological form, the size of the heart initially increases to compensate for the damage to cardiac tissue, but later leads to a decline in left ventricular function [52]. Pathological hypertrophy has been extensively studied and reviewed in relation to miRNAs that are present in the heart [18, 43]. To understand the specific role of miRNAs in cardiac hypertrophy, van Rooij et al. have reported the first microarray analysis data using two different animal models of pathological cardiac hypertrophy. They found that miRNA expression patterns in the two models were similar, indicating the presence of similar miRNA-controlled hypertrophic mechanisms [88]. Dysregulation of IGF-1 signaling has also been involved in pathological hypertrophy, which is regulated by miRNAs. MiRNA-378 and is mostly expressed in cardiac myocytes, during cardiac hypertrophy [19, 63]. The comprehensive role miRNAs in hypertrophic cardiomyopathy have summarized in Fig. 5.
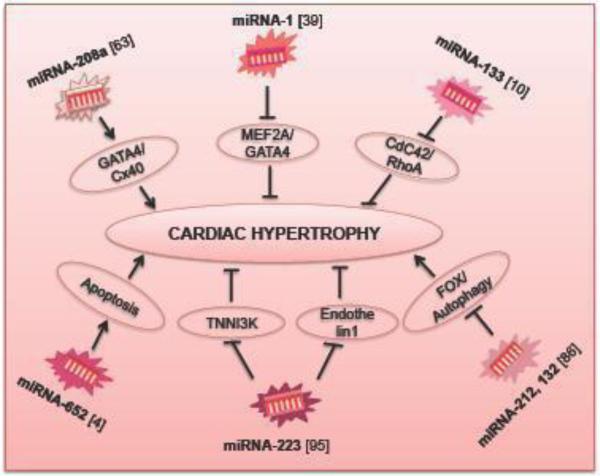
Figure 5. The role of miRNAs in cardiac hypertrophy.
TNNI3K – Cardiac troponin-I3 interacting kinase 3; Cx40 – Connexin 40; MEF2A – Myocyte enhancer factor 2A; CDC42 – Cell division cycle 42; RhoA – Ras homology gene family member A; FOX - Forkhead box O.
MiRNA-1 and miRNA-133 have shown to be down regulated in exercised trained rats and cardiac-specific Akt transgenic mice, which are models of physiological cardiac hypertrophy. MiRNA-133 is one of the key regulators of cardiac hypertrophy and can be targeted in therapy. Moreover, MiRNA133 works negatively on RhoA/CDC42 signaling and therefore, regulated hypertrophy [10]. Recent study has shown that miRNA-1 also negatively regulates hypertrophy by acting on Mef2a and Gata4, two transcription factors in calcium signaling [39]. Furthermore, a recent study determined that expression of miRNA-652 increases in the hypertrophic heart. The suppression of miRNA-652 improved the cardiac function, reduced fibrosis and fewer apoptosis in the hypertrophic heart. Thus, silencing of miRNA-652 protects the hypertophic heart and has the therapeutic potential against cardiac dysfunction [4]. Another study showed miRNA-223 was down regulated in endothelin-1 induced hypertrophic cardiomyocytes and in the hypertrophic heart. Therefore, over expression of this miRNA will rescue the hypertrophy by up regulating TNNI3K, a novel cardiotroponin I (CTI)-interacting and cardiac hypertrophy related kinase [95]. Studies have shown that miRNA-212 and miRNA-132 families have major roles in both cardiac hypertrophy and cardiomyocyte autophagy by regulating Fox signaling pathway [86]. Furthermore, a study reveals that miRNA-208a is required for proper cardiac conduction and expression of the cardiac transcription factors homeo domain-only protein, GATA4 and the gap junction protein connexin 40 (Cx40). Therefore, miRNA-208a modulates cardiac hypertrophy and electrical conduction [63]. These studies suggest that miRNAs have a specific role in cardiac hypertrophic disease remodeling.
5.5. MiRNAs: Cardiac fibrosis
Cardiac fibrosis is characterized by excessive accumulation of extracellular matrix (ECM), collagens and α-smooth muscle actin that ultimately destroys tissue architecture and eventually abolishes normal function [81]. During cardiac disease, there are fibroblast activation and proliferation that lead to inappropriate secretion of ECM proteins. Both an increased synthesis and decreased degradation of ECM components can lead to fibrosis. Fibrosis causes imbalance in the turnover of ECM proteins and impairment in cardiac contractility. This alters the electromechanical properties of the myocardium, often leading to arrhythmias, which is a major cause of mortality in heart disease. MMPs are a family of proteolytic enzymes that degrade ECM components and therefore, up regulate matrix remodeling, whereas a tissue inhibitor of metalloproteinase is down regulated in cardiac fibrosis [60]. Collagens are the major ECM protein, but other important ECM proteins such as fibronectins, elastin and fibrillins also play a significant role during the development of fibrosis. The release of profibrotic mediators after tissue injuries, such as TGF-β and platelet-derived growth factor (PDGF) are caused fibroblasts remodeling by differentiating into myofibroblasts [35]. It has been shown that MiRNA-7a/b protects cardiomyocytes form cell death during ischemia/reperfusion injury by effectively inhibiting MMPs expression and cardiac fibroblast migration suggesting its therapeutic value in treating cardiac diseases [54]. Although, the mechanism of the development of fibrosis has been recently uncovered, and the therapeutic options are yet to emerge as an effective antifibrotic agent.
Studies have shown that TGFβ mediated down regulation of the miRNA-29 family members (miRNA-29a, 29b and 29c) and their target genes, including ECM proteins during myocardial infarction play a significant role in cardiac fibrosis. Although the functional consequences of the change in the miRNAs during cardiac fibrosis are unknown, it has been shown that there is a down-regulation of miRNA-149 and an increased expression of miRNA-21, miRNA-214 and miRNA-223 in cardiac fibrosis [35]. The role of miRNAs in cardiac fibrosis is explained in Fig. 6. A recent report demonstrates that miRNA-24 regulates TGF-β1 pathway and thereby has a functional involvement MI fibrosis remodeling. It has also been observed that miRNA-24 could decrease the synthesis of ECM proteins and hence, the differentiation of fibroblasts to myofibroblast. MiRNA-24 also reduces TGF-β secretion in cardiac fibrosis, which decreases by smad2/3 phosphorylation [94].
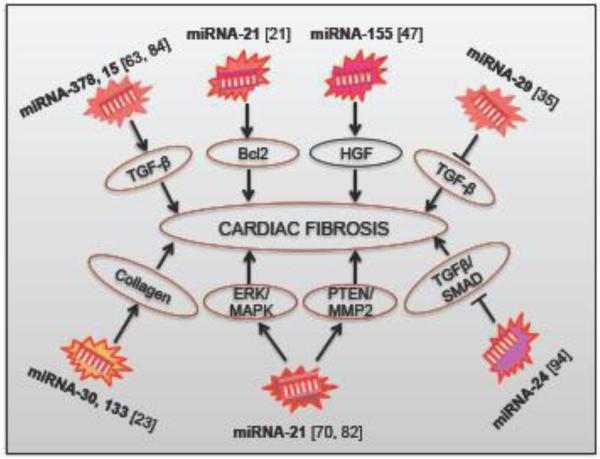
Figure 6. The role of miRNAs in the development of cardiac fibrosis.
HGF - Hepatocyte growth factor; Bcl2 - B-cell lymphoma; TGFβ - Transforming growth factor beta; Erk – Extracellular signal- regulated kinase; MAPK - Mitogen-activated protein kinases; - Matrix metalloproteinase; PTEN - Phosphatase and tensin homolog.
In addition to all these miRNAs, during cardiac fibrosis, miRNA-21 becomes predominant in cardiac fibroblasts in response to cardiac hypertrophy and failure. MiRNA-21 also regulates the extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase (MAPK) signaling pathway through inhibition of sprouty-1. Therefore, it promotes fibroblast survival and growth factor secretion. Silencing of miRNA-21 with an antagomir results in the reduction of cardiac ERK-MAPK activity, inhibition of interstitial fibrosis, and improvement of cardiac dysfunction [82]. MiRNA-21 also regulates cardiac fibrosis of the infarct zone via Bcl2 signaling [21] and MMP2-PTEN signaling pathways [70]. There is a down regulation of miRNA-133a in angiotensin II-mediated hypertension and cardiac fibrosis, leading to a derepression of its target collagen, collagen type 1A1. Additionally, another study found that the expression of connective tissue growth factor, one of the major fibrosis-promoting factors, is regulated by miRNA-30 and miRNA-133 in human and animal cardiac tissue [23]. It was also displayed recently that the down-regulation of miRNA-122 in heart might be involved in myocardial fibrosis in aortic valve stenosis, probably through TGF-β1 up-regulation [3]. MiRNA-15 and miRNA-378 families are another novel regulator of cardiac hypertrophy and fibrosis acting by the inhibition of the TGFβ [63, 84], and thus show the link between miRNA and TGFβ-pathway. A study has shown that miRNA-155 also plays an important role in regulating cardiac fibrosis in the diabetic heart [47]. Recently, it has been shown that MiRNA-503 promotes angiotensin-II induced cardiac fibrosis, and it would be a promising therapeutic target for reducing cardiac fibrosis [103].
5.6. MiRNAs; Cerebral ischemia (CI) and Stroke
Stroke targets approximately 2-4% of the total healthcare worldwide and is the second most common cause of death. In stroke etiology, miRNAs have distinct expression patterns that modulate pathogenic processes. MiRNAs regulate 30% of protein-coding genes in the gene expression-governing neuronal network. Three different subtypes of stroke are unveiled through miRNA micro array analysis like large artery, small artery and cardio embolic stroke. There are 836 miRNAs have listed in stroke patient's blood samples. Out of these, let-7f, miRNA-126, miRNA-1259, miRNA-142-3p, miRNA-15b, miRNA-186, miRNA-519e and miRNA-786-5p were expressed poorly, but let-7e, miRNA-1184, miRNA-1246, miRNA-1261, miRNA-1275, miRNA-1285, miRNA-1290, miRNA-181a, miRNA-25*, miRNA-513a-5p, miRNA-550, miRNA-602, miRNA-665, miRNA-891a, miRNA-933, miRNA-923 and miRNA-939 miRNAs are expressed high [80] .
The down regulation of miRNA-29c leads to de-repression of its target DNA methyltransferase 3a is promoting ischemic brain damage. Highly expressing miRNA-29c in PC12 cells are down regulating in adult rats after focal ischemia and oxygen-glucose deprivation (OGD) respectively. Pandi and his group have been well documented that pre miRNA-29c prevents OGD-induced cell death [66]. MiRNA-497 works as a proapoptotic regulator and targets the expression of anti-apoptotic genes like Bcl-2 [101]. Knockdown of miRNAs, miRNA-497 and miRNA-15a are considered to be beneficial as they contribute to the post ischemic brain death by targeting BcL-2. Whereas miRNA-21 up-regulation prevents the translation of Fas ligand and thereby decrease post ischemic apoptosis [66]. MiRNA-29c is considered a neuroprotector because it plays a vital role in adult brain and cellular functions. MiRNA-9, miRNA-124a, miRNA-124b, miRNA-135, miRNA-153, miRNA-183 and miRNA-219 are brain specific miRNAs in mouse and affect the mammalian neuronal process too [73]. Both in vivo and in vitro experiments displayed that miRNA-29b-2, miRNA-339-5p, miRNA-19b and 341 are up-regulated in response to CI [20]. These studies clearly show that during CI or stroke, the miRNAs are significantly contributed in disease pathogenesis.
6. MiRNA in diagnosis and their Clinical applications
Recent studies have shown that circulating miRNAs have the potential of early detection and prognosis of many diseases, including cardiovascular diseases, cancer, diabetes, rheumatoid arthritis, and kidney diseases. The currently available proteins based biomarkers for identifying cardiac diseases are providing false-positive results that lead to wrong diagnosis and treatment. Therefore, early detections of cardiac functional or damage-related biomarkers are needed for proper diagnosis and to reduce the death rates in humans. Studies from clinical samples raised the possibility of considering miRNAs as a useful biomarker to define disease states. Studies have shown that miRNAs appear to be the most important biomarker for acute and prognostic disease assessment as well as therapeutic agents for cardiovascular diseases. It is believed that miRNAs could be used as highly sensitive early diagnostic biomarkers and therapeutic targets for many cardiovascular diseases such as atherosclerosis, acute myocardial infarction, heart failure, hypertension and stroke as well as cancer, and was reviewed and published recently [26, 71]. In heart failure patients, miRNA-423-5p is highly expressed in the blood irrespective of age and gender and might be a sensitive biomarker for heart diseases [83]. Moreover, plasma miRNA-423-5p is elevated in dilated cardiomyopathy patients and has a positive correlation with the N-terminal pro-brain natriuretic peptide [24]. A recent study was shown that the serum of coronary artery disease patients had reduced circulating miRNA-126 and miRNA-145 [27]. It has been shown that miRNA-208 up regulated and miRNA-1 and miRNA-133a/b were down regulated in cardiac tissue samples from MI patients when compared to normal controls [8]. Therefore, miRNAs represent important biomarker for the identification and prognosis of different diseases. And the use of miRNAs in the translational field is increasing as they can be detected easily by quantitative polymerase chain reaction (qPCR), which is relatively inexpensive and sensitive to even low amounts due to robust amplification of signal.
7. Challenges in miRNA therapy:
It is important to identify the gene targets and the signaling pathways responsible for their cardiovascular effects in order to develop new miRNA based therapeutics. Also, inhibition of miRNAs by antisense strategies or pharmacological approaches can serve as an alternate, safe method. The preclinical and clinical studies we discussed above indicate that miRNAs playing an important role in disease prognosis and pathogenesis. MiRNA mimics and antagomir can target these mRNAs that are responsible for disease phenotype can have profound effects on different diseases, supporting enthusiasm for further discovery of miRNAs as novel drug candidates. However, in general, several challenges and questions remain to be answered in the developments of miRNA-based therapy. To data, most of the in vivo miRNA studies have been focused on site-specific phenotypic effects, which might ignore the off-target effects in other tissues. Thus, studies are needed to focus on the in vivo miRNA effects on systemic approach instead of site-specific approaches. Another important challenge is to identify appropriate translational dosing regiments in order to get the lowest doses with highest efficiency and minimal side effects.
8. Summary
The role of miRNAs in regulating the major genes of different cardiovascular disorders is summarized in Fig. 2-6 to make us understand the key target molecules for each miRNA that regulates. With this information, one can easily formulate a better therapeutic strategy for cardiovascular diseases. Taken together, these recent pieces of evidence show that miRNAs play powerful roles in cardiovascular systems and are sure to open the door to be previously unappreciated medical therapies.
9. Future perspectives
It is now known that miRNAs have some role in the context of diseases. It regulates many valuable genes in the pathways and is also involved in the regulation of epigenetic modifications. But it is important to understand the exact mechanisms how they are involved in the initiation and in the progression of the diseases. Moreover, it is necessary to identify the early phase and late phase miRNAs during the disease progression. The early phase miRNAs could serve as the important marker specific for the disease that can be targeted in the therapy. However, since late phase miRNAs remain in the body for a longer time, they may not be disease specific. Also, miRNAs could be more readily used as therapy in case of chronic diseases like diabetes or neurological disorders than acute diseases like MI or stroke because the availability of the specific miRNAs will be higher in the chronic diseases. Additionally, the use of miRNA in therapy will be more successful if it is used in systemic disorders rather than organ specific diseases. The miRNA serve as an important biomarker for diseases as it has some advantages over other candidates like proteins and metabolites [36].
10. Conclusion
The research of miRNAs in cardiovascular diseases is a fairly recent area of study with a promising future. MiRNAs role in identifying the prognosis, diagnosis and changes associated with different types of diseases, including cardiovascular disease is truly exciting. Even though encouraging outcomes in the miRNA research from animal models, still several technical and practical difficulties need to be addressed before translation to clinical practice. Once the challenges and the problems are solved in the field of miRNA-based therapy, it will be a potential candidate to compete with the protein inhibitors in the market.
Acknowledgements
This work was supported, in part, by American Heart Association Grant - Jon Holden DeHaan Foundation 10SDG2630181 and National Institutes of Health grant R21HL97349 (to JR).
Abbreviations Used
- ATH
Atherosclerosis
- ALDH2
Aldehyde dehydrogenase 2
- BMP
Bone morphogenetic protein
- Bcl2
B-cell lymphoma
- CAD
Coronary artery diseases
- CDC42
Cell division cycle 42
- CPCs
Cardiac progenitor cells
- CM
Cardiomyopathy
- CCL2
Chemokine (C-C motif) ligand 2
- CircRNAs
Circular RNAs
- CTGF
Connective tissue growth factor
- Cx40
Connexin 40
- DCM
Diabetic cardiomyopathy
- DGCR8
DiGeorge Critical Region 8)
- DLK1
Delta-like 1 homology
- Dyrk
Dual specificity Tyrosine Regulated Kinase
- EC
Endothelial cell
- ECM
Extra cellular matrix
- Erk
Extracellar signal- regulated kinase
- eNOS
Endothelial nitric oxide synthase
- FGF
Fibroblast growth factor
- FOXO3
Forkhead box O-3
- Grb2
Growth factor receptor-bound protein 2
- GATA4
Gata binding protein 4
- HGF
Hepatocyte growth factor
- HSP70
Heat shock protein 70
- HSF1
Heat shock factor 1
- HUR
Human antigen R
- IGFR1
Insulin growth factor 1 receptor
- ICAM
Intercellular adhesion molecule
- LDLs
Low-density lipoproteins
- MMP
Matrix metalloproteinase
- mRNA
messenger RNA
- MiRNA
Micro RNA
- MAPK
Mitogen-activated protein kinases
- MCP1
Monocyte chemo attractant protein-1
- MEF2A
Myocyte enhancer factor 2A
- MI
Myocardial infarction
- NOS2
Nitric oxide synthase 2
- ncRNA
Noncoding RNA
- NF-KB
Nuclear factor kappa-B
- NR3C2
Nuclear receptor subfamily 3 group C member 2
- PAD
Peripheral artery diseases
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PTBP1
Polypyrimidine tract-binding protein 1
- PTEN
Phosphatase and tensin homolog
- PiRNA
Piwi-interacting RNAs
- PI3K
Phosphatidylinositol-3-kinase
- PH
Pulmonary hypertension
- RAA
Renin-angiotensin-aldosterone
- rRNA
Ribosomal RNA
- Rho
Ras homology gene family
- RISC
RNA inducing silencing complex
- scaRNAs
Small cajal body specific RNAs
- SMA-α
Smooth muscle actin-alpha
- snoRNAs
Small nucleolar RNAs
- STK35
Serine/threonine-protein kinase 35
- tRNA
Transfer RNA
- TRBP
Transactivation response RNA binding protein
- TGFβ
Transforming growth factor beta
- TNNI3K
Cardiac troponin-I3 interacting kinase 3
- VCAM
Vascular cell adhesion molecule
- VEGF
Vascular endothelial growth factor
- VSMCs
Vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ai J, Zhang R, Li Y, Pu J, Lu Y, Jiao J, et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73–7. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Angelovich TA, Hearps AC, Jaworowski A. Inflammation-induced foam cell formation in chronic inflammatory disease. Immunol Cell Biol. 2015 doi: 10.1038/icb.2015.26. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont J, Lopez B, Hermida N, Schroen B, San Jose G, Heymans S, et al. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-beta1 up-regulation. Clin Sci (Lond) 2014;126:497–506. doi: 10.1042/CS20130538. [DOI] [PubMed] [Google Scholar]
- 4.Bernardo BC, Nguyen SS, Winbanks CE, Gao XM, Boey EJ, Tham YK, et al. Therapeutic silencing of miR-652 restores heart function and attenuates adverse remodeling in a setting of established pathological hypertrophy. Faseb j. 2014;28:5097–110. doi: 10.1096/fj.14-253856. [DOI] [PubMed] [Google Scholar]
- 5.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124:3514–28. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–47. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boominathan L. The tumor suppressors p53, p63, and p73 are regulators of microRNA processing complex. PLoS One. 2010;5:e10615. doi: 10.1371/journal.pone.0010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bostjancic E, Zidar N, Stajer D, Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology. 2010;115:163–9. doi: 10.1159/000268088. [DOI] [PubMed] [Google Scholar]
- 9.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–8. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 11.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Chang RC, Ying W, Bazer FW, Zhou B. MicroRNAs Control Macrophage Formation and Activation: The Inflammatory Link between Obesity and Cardiovascular Diseases. Cells. 2014;3:702–12. doi: 10.3390/cells3030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HS, Sivachandran N, Lau A, Boudreau E, Zhao JL, Baltimore D, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol Med. 2013;5:949–66. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claverie JM. Fewer genes, more noncoding RNA. Science. 2005;309:1529–30. doi: 10.1126/science.1116800. [DOI] [PubMed] [Google Scholar]
- 15.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–10. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordes KR, Srivastava D, Ivey KN. MicroRNAs in cardiac development. Pediatr Cardiol. 2010;31:349–56. doi: 10.1007/s00246-010-9639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG, et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765–73. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Costa Martins PA, De Windt LJ. MicroRNAs in control of cardiac hypertrophy. Cardiovasc Res. 2012;93:563–72. doi: 10.1093/cvr/cvs013. [DOI] [PubMed] [Google Scholar]
- 19.Devaux Y, Stammet P, Friberg H, Hassager C, Kuiper MA, Wise MP, et al. MicroRNAs: new biomarkers and therapeutic targets after cardiac arrest? Crit Care. 2015;19:767. doi: 10.1186/s13054-015-0767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhiraj DK, Chrysanthou E, Mallucci GR, Bushell M. miRNAs-19b, -29b-2* and -339-5p show an early and sustained up-regulation in ischemic models of stroke. PLoS One. 2013;8:e83717. doi: 10.1371/journal.pone.0083717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong S, Ma W, Hao B, Hu F, Yan L, Yan X, et al. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int J Clin Exp Pathol. 2014;7:565–74. [PMC free article] [PubMed] [Google Scholar]
- 22.Dorhoi A, Iannaccone M, Farinacci M, Fae KC, Schreiber J, Moura-Alves P, et al. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest. 2013;123:4836–48. doi: 10.1172/JCI67604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–8. doi: 10.1161/CIRCRESAHA.108.182535. 6p following 8. [DOI] [PubMed] [Google Scholar]
- 24.Fan KL, Zhang HF, Shen J, Zhang Q, Li XL. Circulating microRNAs levels in Chinese heart failure patients caused by dilated cardiomyopathy. Indian Heart J. 2013;65:12–6. doi: 10.1016/j.ihj.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107:13450–5. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faruq O, Vecchione A. microRNA: Diagnostic Perspective. Front Med (Lausanne) 2015;2:51. doi: 10.3389/fmed.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107:677–84. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 28.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–84. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Force T, Bonow RO, Houser SR, Solaro RJ, Hershberger RE, Adhikari B, et al. Research priorities in hypertrophic cardiomyopathy: report of a Working Group of the National Heart, Lung, and Blood Institute. Circulation. 2010;122:1130–3. doi: 10.1161/CIRCULATIONAHA.110.950089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 31.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo M, Mao X, Ji Q, Lang M, Li S, Peng Y, et al. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol. 2010;88:555–64. doi: 10.1038/icb.2010.16. [DOI] [PubMed] [Google Scholar]
- 33.Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–50. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 34.Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Huang C, Lin X, Li J. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie. 2013;95:1355–9. doi: 10.1016/j.biochi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Hewitt KJ, Garlick JA. Cellular reprogramming to reset epigenetic signatures. Mol Aspects Med. 2012 doi: 10.1016/j.mam.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Huang JB, Liang J, Zhao XF, Wu WS, Zhang F. Epigenetics: novel mechanism of pulmonary hypertension. Lung. 2013;191:601–10. doi: 10.1007/s00408-013-9505-1. [DOI] [PubMed] [Google Scholar]
- 38.Icli B, Dorbala P, Feinberg MW. An emerging role for the miR-26 family in cardiovascular disease. Trends Cardiovasc Med. 2014;24:241–8. doi: 10.1016/j.tcm.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikeda S, He A, Kong SW, Lu J, Bejar R, Bodyak N, et al. MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes. Mol Cell Biol. 2009;29:2193–204. doi: 10.1128/MCB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izarra A, Moscoso I, Levent E, Canon S, Cerrada I, Diez-Juan A, et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Reports. 2014;3:1029–42. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–88. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 42.Joladarashi D, Srikanth Garikipati VN, Thandavarayan RA, Verma SK, Mackie AR, Khan M, et al. Enhanced Cardiac Regenerative Ability of Stem Cells After Ischemia-Reperfusion Injury: Role of Human CD34(+) Cells Deficient in MicroRNA-377. J Am Coll Cardiol. 2015;66:2214–26. doi: 10.1016/j.jacc.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joladarashi D, Thandavarayan RA, Babu SS, Krishnamurthy P. Small engine, big power: microRNAs as regulators of cardiac diseases and regeneration. Int J Mol Sci. 2014;15:15891–911. doi: 10.3390/ijms150915891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim VN. MicroRNA precursors in motion: exportin-5 mediates their nuclear export. Trends Cell Biol. 2004;14:156–9. doi: 10.1016/j.tcb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Kim VN. Small RNAs: classification, biogenesis, and function. Mol Cells. 2005;19:1–15. [PubMed] [Google Scholar]
- 46.King TH, Liu B, McCully RR, Fournier MJ. Ribosome structure and activity are altered in cells lacking snoRNPs that form pseudouridines in the peptidyl transferase center. Mol Cell. 2003;11:425–35. doi: 10.1016/s1097-2765(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 47.Kishore R, Verma SK, Mackie AR, Vaughan EE, Abramova TV, Aiko I, et al. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS One. 2013;8:e60161. doi: 10.1371/journal.pone.0060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 49.Lara-Pezzi E, Dopazo A, Manzanares M. Understanding cardiovascular disease: a journey through the genome (and what we found there). Dis Model Mech. 2012;5:434–43. doi: 10.1242/dmm.009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Latronico MV, Catalucci D, Condorelli G. MicroRNA and cardiac pathologies. Physiol Genomics. 2008;34:239–42. doi: 10.1152/physiolgenomics.90254.2008. [DOI] [PubMed] [Google Scholar]
- 51.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 52.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 53.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 54.Li R, Xiao J, Qing X, Xing J, Xia Y, Qi J, et al. Sp1 Mediates a Therapeutic Role of MiR-7a/b in Angiotensin II-Induced Cardiac Fibrosis via Mechanism Involving the TGF-beta and MAPKs Pathways in Cardiac Fibroblasts. PLoS One. 2015;10:e0125513. doi: 10.1371/journal.pone.0125513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li SP, Liu B, Song B, Wang CX, Zhou YC. miR-28 promotes cardiac ischemia by targeting mitochondrial aldehyde dehydrogenase 2 (ALDH2) in mus musculus cardiac myocytes. Eur Rev Med Pharmacol Sci. 2015;19:752–8. [PubMed] [Google Scholar]
- 56.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–87. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012;126:S81–90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 59.Marz M, Gruber AR, Honer Zu Siederdissen C, Amman F, Badelt S, Bartschat S, et al. Animal snoRNAs and scaRNAs with exceptional structures. RNA Biol. 2011;8:938–46. doi: 10.4161/rna.8.6.16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meiners S, Hocher B, Weller A, Laule M, Stangl V, Guenther C, et al. Downregulation of matrix metalloproteinases and collagens and suppression of cardiac fibrosis by inhibition of the proteasome. Hypertension. 2004;44:471–7. doi: 10.1161/01.HYP.0000142772.71367.65. [DOI] [PubMed] [Google Scholar]
- 61.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 62.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 63.Nagalingam RS, Sundaresan NR, Noor M, Gupta MP, Solaro RJ, Gupta M. Deficiency of cardiomyocyte-specific microRNA-378 contributes to the development of cardiac fibrosis involving a transforming growth factor beta (TGFbeta1)-dependent paracrine mechanism. J Biol Chem. 2014;289:27199–214. doi: 10.1074/jbc.M114.580977. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Nakamachi Y, Kawano S, Takenokuchi M, Nishimura K, Sakai Y, Chin T, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009;60:1294–304. doi: 10.1002/art.24475. [DOI] [PubMed] [Google Scholar]
- 65.O'Brien JE, Jr., Kibiryeva N, Zhou XG, Marshall JA, Lofland GK, Artman M, et al. Noncoding RNA expression in myocardium from infants with tetralogy of Fallot. Circ Cardiovasc Genet. 2012;5:279–86. doi: 10.1161/CIRCGENETICS.111.961474. [DOI] [PubMed] [Google Scholar]
- 66.Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R. MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage. PLoS One. 2013;8:e58039. doi: 10.1371/journal.pone.0058039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–32. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–9. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 69.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–66. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–9. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sayed AS, Xia K, Salma U, Yang T, Peng J. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ. 2014;23:503–10. doi: 10.1016/j.hlc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–76. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snead NM, Wu X, Li A, Cui Q, Sakurai K, Burnett JC, et al. Molecular basis for improved gene silencing by Dicer substrate interfering RNA compared with other siRNA variants. Nucleic Acids Research. 2013;41:6209–21. doi: 10.1093/nar/gkt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sober S, Laan M, Annilo T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem Biophys Res Commun. 2010;391:727–32. doi: 10.1016/j.bbrc.2009.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Staszel T, Zapala B, Polus A, Sadakierska-Chudy A, Kiec-Wilk B, Stepien E, et al. Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn. 2011;121:361–6. [PubMed] [Google Scholar]
- 77.Sucharov C, Bristow MR, Port JD. miRNA expression in the failing human heart: functional correlates. J Mol Cell Cardiol. 2008;45:185–92. doi: 10.1016/j.yjmcc.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun HX, Zeng DY, Li RT, Pang RP, Yang H, Hu YL, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–14. doi: 10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 79.Sun X, Belkin N, Feinberg MW. Endothelial microRNAs and atherosclerosis. Curr Atheroscler Rep. 2013;15:372. doi: 10.1007/s11883-013-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, et al. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tao H, Shi KH, Yang JJ, Huang C, Liu LP, Li J. Epigenetic regulation of cardiac fibrosis. Cell Signal. 2013;25:1932–8. doi: 10.1016/j.cellsig.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 82.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 83.Tijsen AJ, Creemers EE, Moerland PD, de Windt LJ, van der Wal AC, Kok WE, et al. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010;106:1035–9. doi: 10.1161/CIRCRESAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 84.Tijsen AJ, van der Made I, van den Hoogenhof MM, Wijnen WJ, van Deel ED, de Groot NE, et al. The microRNA-15 family inhibits the TGFbeta-pathway in the heart. Cardiovasc Res. 2014;104:61–71. doi: 10.1093/cvr/cvu184. [DOI] [PubMed] [Google Scholar]
- 85.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–22. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun. 2012;3:1078. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uchida S, Dimmeler S. Long Noncoding RNAs in Cardiovascular Diseases. Circ Res. 2015;116:737–50. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 88.van Rooij E, Olson EN. MicroRNA therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Discov. 2012;11:860–72. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–32. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–4. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 91.Vickers KC, Landstreet SR, Levin MG, Shoucri BM, Toth CL, Taylor RC, et al. MicroRNA-223 coordinates cholesterol homeostasis. Proc Natl Acad Sci U S A. 2014;111:14518–23. doi: 10.1073/pnas.1215767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Volny O, Kasickova L, Coufalova D, Cimflova P, Novak J. microRNAs in Cerebrovascular Disease. Adv Exp Med Biol. 2015;888:155–95. doi: 10.1007/978-3-319-22671-2_9. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Huang W, Xu R, Nie Y, Cao X, Meng J, et al. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J Cell Mol Med. 2012;16:2150–60. doi: 10.1111/j.1582-4934.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang YS, Zhou J, Hong K, Cheng XS, Li YG. MicroRNA-223 Displays a Protective Role Against Cardiomyocyte Hypertrophy by Targeting Cardiac Troponin I-Interacting Kinase. Cell Physiol Biochem. 2015;35:1546–56. doi: 10.1159/000373970. [DOI] [PubMed] [Google Scholar]
- 96.Wei Y, Nazari-Jahantigh M, Chan L, Zhu M, Heyll K, Corbalan-Campos J, et al. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–19. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 97.Wei Y, Zhu M, Corbalan-Campos J, Heyll K, Weber C, Schober A. Regulation of Csf1r and Bcl6 in macrophages mediates the stage-specific effects of microRNA-155 on atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:796–803. doi: 10.1161/ATVBAHA.114.304723. [DOI] [PubMed] [Google Scholar]
- 98.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 99.Yamakuchi M. MicroRNAs in Vascular Biology. Int J Vasc Med. 2012;2012:794898. doi: 10.1155/2012/794898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. 2009;104:572–5. doi: 10.1161/CIRCRESAHA.108.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, et al. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, et al. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep. 2015;5:9401. doi: 10.1038/srep09401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou Y, Deng L, Zhao D, Chen L, Yao Z, Guo X, et al. MicroRNA-503 promotes angiotensin II-induced cardiac fibrosis by targeting Apelin-13. J Cell Mol Med. 2016 doi: 10.1111/jcmm.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu D, Pan C, Li L, Bian Z, Lv Z, Shi L, et al. MicroRNA-17/20a/106a modulate macrophage inflammatory responses through targeting signal-regulatory protein alpha. J Allergy Clin Immunol. 2013;132:426–36. e8. doi: 10.1016/j.jaci.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]