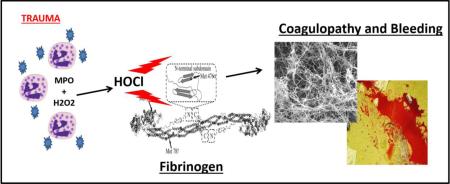
Abstract
Victims of trauma often develop impaired blood clot formation (coagulopathy) that contributes to bleeding and mortality. Fibrin polymerization is one critical component of clot formation that can be impacted by post-translational oxidative modifications of fibrinogen after exposure to oxidants. In vitro evidence suggests that Aα-C domain methionine sulfoxide formation, in particular, can induce conformational changes that prevent lateral aggregation of fibrin protofibrils during polymerization. We used mass spectrometry of plasma from trauma patients to find that fibrinogen Aα-C domain methionine sulfoxide content was selectively-increased in patients with coagulopathy vs. those without coagulopathy. This evidence supports a novel linkage between oxidative stress, coagulopathy, and bleeding after injury.
Keywords: Oxidative stress, fibrinogen, coagulopathy, methionine sulfoxide, hemorrhage, trauma
Graphical Abstract
INTRODUCTION
Hemostasis is often impaired after severe traumatic injury leading to pathological bleeding that is known as trauma-induced coagulopathy (TIC). TIC is present in one-quarter to one-third of severely injured patients almost immediately after injury, carries a 4-6 times increased risk of mortality, and is associated with increased incidence of multi-organ failure, intensive care utilization, and increased need for blood transfusion [1-4]. Early hallmarks of TIC include extrinsic coagulation pathway dysfunction identified by a prolongation of prothrombin time (PT) ratio and/or International normalized ratio (INR) and decreased viscoelastic clot strength [1-3]. Those patients with abnormally elevated PT ratio's recorded in the Emergency Department are twice as likely to die compared to those with normal PT ratios [4]. The cause of TIC is felt to be multifactorial as a result tissue hypoperfusion and hypoxia, activation of the anticoagulant Protein C (aPC), thrombin deficiency, and fibrinogen proteolysis and dysfunction [5].
Perhaps the most significant component of TIC is increased enzymatic clot breakdown, or hyperfibrinolysis. After injury with blood loss producing hemorrhagic shock, the concentration of tissue plasminogen activator (tPA) is increased in blood, which increases activation of plasminogen to plasmin [5-7]. Plasmin is the primary proteolytic enzyme for fibrin, causing rapid degradation of fibrin clots by cleaving fibrin strands at lysine residues. Impaired fibrin polymerization can also directly contribute to hyperfibrinolysis by increasing susceptibility to enzymatic degradation [8,9]. Hyperfibrinolysis is strongly associated with trauma patient mortality and when significant, often predicts imminent death [10]. In addition, antifibrinolytic therapy using the lysine analogue tranexamic acid is the only therapy found to reduce mortality when given early after injury in a large randomized controlled trial [11].
The hemostatic plasma protein fibrinogen is a key determinant of bleeding after injury. Fibrinogen becomes fibrin clot by cleavage of short peptides by thrombin from the Aα (FpA) and Bβ (FpB) chains. Fibrin polymerization and subsequent fibrin clot structure is then governed by specific interaction of complementary binding sites exposed after fibrinopeptide cleavage. Loss of fibrinogen to hemorrhage, consumption, and hyperfibrinolysis are associated with decreased survival after trauma [12]. Fibrinogen is also the first hemostatic protein to decrease to critically low functional concentrations in surgical patients with significant blood loss [13]. Therefore, fibrinogen is an important and labile hemostatic protein that is affected by trauma.
Fibrinogen is also extremely sensitive to oxidation relative to other plasma proteins [14]. We have previously demonstrated in vitro that exposure of fibrinogen to hypochlorous acid is associated with preferential oxidation of Methionine 476 in the Aα-C domain to methionine sulfoxide (Aα-Met476(SO), inhibition of lateral aggregation of fibrin protofibrils during fibrin polymerization, and formation of thin-fibered fibrin clots that are mechanically weak [15]. In silico modelling has also revealed that specific Aα-Met476 oxdiation to Aα-Met476(SO) plausibly mediates the inhibition of fibrin lateral aggregation by disruption of a key beta hairpin structure involved in the fibrin lateral aggregation mechanism [16]. HOCl is predominantly generated in the plasma as a product of neutrophil lysozomal myeloperoxidase, chloride, and hydrogen peroxide and is a key leukocyte-specific host-defense mechanism mediating bacterial killing by the formation of methionine sulfoxide in bacterial membrane proteins [17]. Leukocytes upregulate the same oxidative enzymes after blunt trauma and oxidation has been implicated in vascular responses to hemorrhagic shock [18-20].
Despite evidence that oxidants are generated after trauma and fibrinogen is highly sensitive to these oxidants, fibrinogen oxidation has not been detected in trauma patients with coagulopathy. Having established a plausible in vitro link between Aα-Met476(SO) content and altered fibrin clot formation, the purpose of this study was to test the hypothesis that the same methionine residue of fibrinogen is selectively-oxidized in trauma patients with coagulopathy.
MATERIALS AND METHODS
Trauma Patients
Plasma was obtained from three de-identified human Emergency Department trauma repositories. Human subjects approvals were obtained from local institutional review boards for repository and medical records access according to the guidelines set forth by the declaration of Helsinki. The first cohort was used to identify selectively-vulnerable methionine residues on fibrinogen that were oxidized to methionine sulfoxide in coagulopathic trauma patients. Nano-liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS) approach was used to identify these residues in this cohort from glycine-purified trauma patient fibrinogen For the second cohort, we used an ultra-performance LC (UPLC)-MS/MS with Multiple Reaction Monitoring (MRM) mode to confirm increased fibrinogen Aα-Met476(SO) content directly in plasma without purification. We then used LC (UPLC)-MS/MS and viscoelastic clotting measurements to examine associations between fibrinogen Aα-Met476(SO)% and clot formation after injury in a third cohort.
Trauma Derivation Cohort
Samples of platelet-poor plasma were obtained directly from a trauma plasma biorepository. Blood samples were drawn from trauma patients at Harborview Medical Center, a U.S. Level-I trauma center in Seattle WA, on arrival to the Emergency Department. Leftover citrated plasma was obtained from these samples after all clinical tests were performed. Leftover plasma was immediately frozen at −80 deg C and given a unique study number by the clinical laboratory for inclusion in the biorepository. Patient medical records were then reviewed and vital signs, laboratory data, injury patterns, and clinical outcomes were abstracted and matched to plasma samples after removal of all protected health information. To detect fibrinogen oxidation, fibrinogen was first purified from plasma by 4 rounds of glycine purification yielding highly-purified fibrinogen. Purified fibrinogen was then subjected to nanoLC-MS/MS to identify sites of increased methionine sulfoxide content. Samples were grouped for comparison by the presence or absence of coagulopathy as defined by an INR >1.2 reported by the hospital laboratory [4].
Trauma Confirmation Cohort
The specific MS signatures identified in the first cohort were then used to quantify fibrinogen methionine sulfoxide content directly in plasma without purification using UPLC-MS/MS-MRM. Blood was sampled from trauma patients presenting to Virginia Commonwealth University Medical Center, a U.S. Level I trauma center in Richmond VA. Blood was obtained in the Emergency Department and any subjects known to have received blood product transfusions prior to the blood sample were excluded. A subset of N=25 de-identified plasma samples with matched clinical laboratory data were submitted for UPLC-MS/MS-MRM for specific AαMet476(SO) signatures as detected directly in plasma without purification. MS personnel were blinded during measurement and analysis. Samples were again grouped for comparison by the presence or absence of coagulopathy as defined by an INR >1.2 reported by the hospital laboratory. To examine for selectivity of oxidation of fibrinogen over other plasma proteins, the M353(SO) content of albumin, a similar solvent-exposed methionine residue, was simultaneously measured in the same plasma.
Clot Formation Cohort
Given the importance of clot formation and fibrinolysis to trauma patient outcomes, we then examined for a direct association between fibrinogen Aα-M476(SO) content and viscoelastic clot formation in a third cohort of trauma patients. This cohort was obtained from a trauma biorepository of citrated plasma from Emergency Department trauma patients sampled on arrival to Memorial-Hermann Medical Center, a U.S. Level I trauma center in Houston TX. These patients also underwent Emergency Department point of care rapid thrombelastography of whole blood (rTEG, Haemonetics, Braintree MA, USA) at the time of initial blood draw as part of their clinical care. rTEG is a viscoelastic measurement of clot formation using tissue factor is useful to guide bleeding management after trauma [21]. rTEG reports the activated clotting time (ACT) and onset time (R), or time required to achieve detectable clot formation, representing coagulation factor activity; clot formation time (K) and alpha angle which represent fibrin polymerization and platelet activity; maximal amplitude of deflection (MA) which represents fibrin clot integrity and platelet-induced clot contraction; and clot durability (LY30%) reported as percent clot breakdown, or lysis, at 30 minutes after MA, representing the activity of fibrinolytic enzymes. We examined n=50 citrated plasma samples, half of which demonstrated deficient clot formation by rTEG as indicated by increased clot breakdown of at least 3% LY30% to ensure a range of coagulopathy and clot formation phenotypes in the cohort [22-23]. Fibrinogen Aα-M476(SO) content was again measured directly in these plasma samples using UPLC-MS/MS- MRM. Corresponding clinical data regarding injury severity, shock severity, and relevant outcomes were also abstracted from the repository.
Glycine Purification of Fibrinogen from Plasma
Fibrinogen was purified from plasma using glycine precipitation. Plasma was incubated with glycine in a ratio of 0.1ml plasma to 15.6 mg glycine for one hour on ice and the precipitate collected by microcentrifugation for 10 min at 4°C. The supernatant was removed and the pellet suspended in phosphate buffer at pH 6.4. This process was repeated 3 additional times for a total of 4 rounds of glycine precipitation and fibrinogen protein yield measured by BCA colorimetric assay.
Thrombin and Reptilase time Assays
The START-4 steel ball coagulation analyzer (Diagnostica Stago, Asnières, France) was used to measure fibrin polymerization by thrombin time and reptilase time. Thrombin time measures the time to clot formation for plasma at 37°C after addition of an excess of thrombin which activates fibrinogen to fibrin by cleavage of fibrinopeptides A and B. Reptilase time uses batroxobin, a viper venom, to measure fibrin polymerization induced by cleavage of fibrinopeptide A only. Thrombin time is sensitive to the presence of antithrombin anticoagulants such as heparin, while the Reptilase time is not.
Protein Digestion
Purified fibrinogen samples from patients (5 μg) or patient plasma (20 μg total protein) were reduced with 5 mM dithiothreitol (DTT) at 70°C for 20 min, alkylated with 12.5 mM iodoacetamide at room temperature for 15 min, and then digested with sequencing grade modified trypsin (Promega) (1:10 wt/wt, trypsin/protein) in 50 mM ammonium bicarbonate containing 5% acetonitrile at 37°C overnight. Digestion was halted by acidification with trifluoroaceticacid. The digested mixtures were either concentrated and desalted with C18 extraction disk cartridges (3M) or submitted directly for LC-MS analysis.
NanoLC-MS/MS Analysis
Proteolytic peptides derived from purified fibrinogen samples from patients (5 μg) were analyzed by nanoLC-MS/MS with a Thermo Scientific LTQ Orbitrap Velos mass spectrometer coupled to a Waters nanoAcquity Ultra Performance LC system. Peptides were separated at a flow rate of 300 nL/min on a nanoUPLC BEH130 C18 column (100 × 0.075 mm, 1.7 μm, Waters), using solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). Peptides were eluted using a linear gradient of 5%–35% solvent B over 90 min. The spray voltage was 3.0 kV, and the collision energy for MS/MS was 35%. Percent oxidation of individual Met residues was determined by dividing the peak area of the peptide containing methionine sulfoxide by the sum of the peak areas of both oxidized and non-oxidized peptides.
UPLC-MS/MS-MRM Analysis
UPLC-MS/MS-MRM analyses of tryptical peptides obtained directly from patient plasma were performed with the QTRAP® 6500 mass spectrometer (AB SCIEX Inc, Framingham, MA USA) in positive electrospray ionization mode (ESI+) with multiple reaction monitoring (MRM) coupled to an ACQUITY UPLC I-Class system (Waters Corp, Milford, MA. USA). Peptides were separated at a flow rate of 300 μL/min on a CORTECS C18 UPLC column (2.1 × 100 mm, 1.6 μm, Waters Corp. Milford, MA USA), using solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile). Peptides were eluted using a linear gradient of 5%–35% solvent B over 12 min.
Statistical Analysis
Normally-distributed variables were described using mean and standard deviation or standard error of the mean (SEM) and were compared using standard T-test. Those variables having significantly skewed distributions by normal quantile plot were described using median and interquartile range and were compared using the nonparametric Wilcoxon Rank Sum test. Simple bivariate associations were tested using Pearson Product Moment Correlation, and linear regression was used to test for multivariate associations. Categorical outcomes were tested using Chi Square analysis. A retrospective post-hoc power analysis was performed using the results of the derivation cohort to prospectively calculate the number of samples needed to achieve adequate power to detect differences between coagulopathic and noncoagulopathic groups by INR in the second cohort, and differences in thrombelastography clot formation parameters according to Aα-Met476(SO) quartile in the final cohort. All differences were considered significant at p<0.05. Statistical analysis was performed using JMP 9.0 (SAS Corp, Cary, NC USA).
RESULTS
The mean (SD) age of subjects enrolled was 51.5 (20.4) years for Seattle, 39.7 (19.0) years for Richmond, and 48.8 (20.8) years for Houston cohorts. The majority of subjects were male in Seattle (67%), Richmond (83%), and Houston (68%). In Seattle 90% were injured by blunt vs. penetrating trauma, while in Richmond 57% were injured by blunt trauma and 43% by penetrating injury. In Houston 68% were injured by a blunt mechanism, 16% were injured by penetrating injury, and 16% sustained burns. In Seattle, 43% of subjects were transfused with blood products within the first 24 hours of hospitalization and hospital mortality was 33%. In Richmond, 13% of subjects received blood products and all subjects survived to hospital discharge (0% mortality). In Houston, 46% of subjects received blood transfusions acutely and in-hospital mortality was 32%. Emergency Department blood pressure, Glasgow Coma Score as a clinical estimate of brain injury, blood hemoglobin concentration as an estimate of blood loss, and base excess as an estimate of metabolic shock severity measured at the time of blood sample are given in Table 1.
Table 1.
Physiological parameters of trauma subjects in the Emergency Department at the time of blood sample collection.
| Seattle, WA | Richmond, VA | Houston, TX | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Std Dev | N | Mean | Std Dev | N | Mean | Std Dev | |
| Systolic Blood Pressure (mmHg) | 21 | 136.0 | 39.2 | 23 | 135.5 | 29.7 | 50 | 129.8 | 35.2 |
| Diastolic Blood Pressure (mmHg) | 21 | 96.9 | 27.0 | 23 | 83.3 | 21.2 | 50 | 76.4 | 21.8 |
| Glasgow Coma Score* | 16 | 8 | 6 | 22 | 12 | 5 | 50 | 7 | 5 |
| Blood Hemoglobin conc. (g/dl) | 21 | 12.1 | 2.6 | 23 | 12.3 | 2.9 | 49 | 12.9 | 2.2 |
| Base Excess (meq/L)* | 11 | −5.5 | 4.1 | 21 | −2.1 | 5.6 | 48 | −3.8 | 6.9 |
Glasgow Coma Score is an aggregate score of neurological function after injury that predicts neurological outcomes. The maximum score is 15, and points are deducted for verbal, eye opening, and movement deficits elicited during the clinical exam. Lower scores indicate increasingly severe brain injury. Base excess represents the number of miliequivalents of base required to return the blood to normal pH=7.4 and is an indicator of acidosis incurred by hemorrhagic shock. More negative numbers indicate greater blood loss and more severe hemorrhagic shock.
Derivation Cohort
In the first Seattle cohort, samples from 22 hospitalized trauma patients were selected from the plasma biorepository. One-half of these samples (N=11) were selected from trauma patients meeting clinical criteria for coagulopathy (INR > 1.2)) for comparison to a randomly selected non-coagulopathic control group with having INR≤1.2 (N=11).
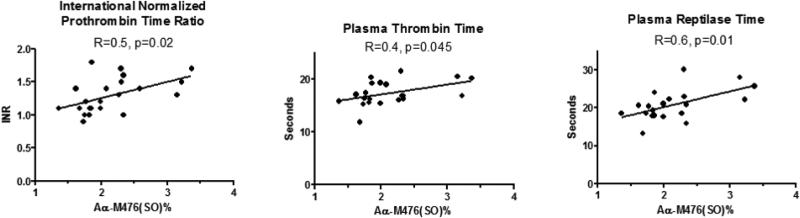
Methionine sulfoxide content of fibrinogen was quantified in glycine-purified fibrinogen using nanoLC-MS/MS. Methionine sulfoxide was detected in 7 different methionine positions was subtly-increased in coagulopathic subjects at positions Bβ-M314 and Aα-M476 compared to controls. (Table 2.) Aα-M476(SO)% was significantly increased in the coagulpathy group and was the only modification associated with INR, thrombin time, and Reptilase time. (Figure 1.) Multivariate linear regression was used to evaluate for a possible confounding effect of resuscitation fluids and blood transfusions received prior to sampling. Aα-M476(SO)% remained significantly associated with INR after adjusting for either total volume of crystalloid fluid administered at the time of sample draw (whole model R2=0.59, p=0.0005; AαM476(SO) effect p=0.042), or total units of blood products transfused (sum of packed red blood cells, fresh frozen plasma, platelets) prior to the time of sampling (whole model R2=0.72, p=0.0001; Aα-M476(SO) effect p=0.046). A significant effect of Aα-M476(SO)% on reptilase time remained after adjusting for the possible confounders D-dimer concentration, fibrinogen concentration, and volume of plasma transfused at the time of measurement (whole model R2=0.77, p=0.003; AαM476(SO)% effect p=0.035). Post-hoc power analysis revealed that only N=17 total samples were required to detect the same absolute 0.63% (SD=0.43) difference in Aα-M476(SO) content with alpha=0.05 and 80% power to detect differences. Therefore, the sample size of N=22 was adequate and we prospectively recruited the same number of samples for comparison in the second confirmation cohort assuming a similar absolute difference in Aα-M476(SO) content.
Table 2.
Fibrinogen Methionine Sulfoxide Content (% of Total)
| Control (INR≤1.2) | Coagulopathy (INR>1.2) | ||||||
|---|---|---|---|---|---|---|---|
| Methionine Position(Chain) | N | Mean | SEM | N | Mean | SEM | |
| T test P value | |||||||
| 422(β) | 11 | 0.22% | 0.02% | 11 | 0.18% | 0.02% | 0.199 |
| 305(β) | 11 | 1.45% | 0.12% | 11 | 1.78% | 0.12% | 0.063 |
| 314(β) | 11 | 0.73% | 0.14% | 11 | 1.15% | 0.14% | 0.046 |
| 367(β) | 11 | 1.67% | 0.20% | 11 | 1.64% | 0.20% | 0.917 |
| 373(β) | 11 | 0.36% | 0.03% | 11 | 0.39% | 0.03% | 0.496 |
| 78(γ) | 11 | 1.08% | 0.08% | 11 | 1.21% | 0.08% | 0.228 |
| 476(α) | 11 | 1.83% | 0.13% | 11 | 2.46% | 0.13% | 0.003 |
INR, International Normalized Ratio
Figure 1.
Associations between fibrinogen Aα-M476(SO)% and international normalized prothrombin time ratio (INR), thrombin time, and Reptilase time measured in plasma. R=Pearson product moment correlation coefficient.
Effect of low-level Aα-M476(SO)% on fibrin polymerization
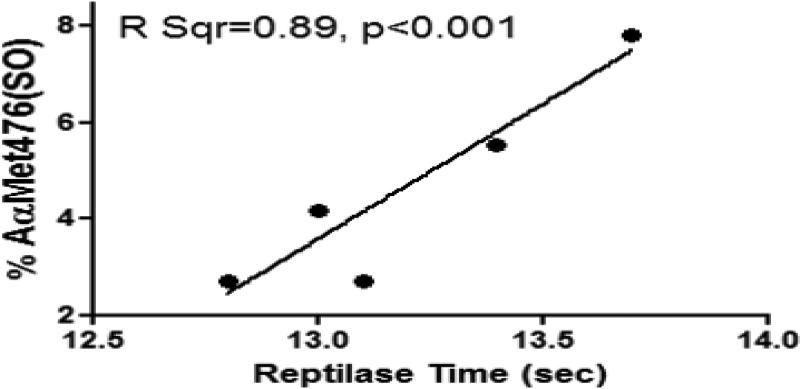
We then confirmed experimentally that the Aα-M476(SO) content found in coagulopathic trauma patients could affect fibrin polymerization when initiated by Reptilase. Fibrinogen was first purified by glycine precipitation from pooled normal human plasma and incubated with HOCL to induce maximal Aα-M476(SO)% formation according to previously published methods [14]. Oxidized fibrinogen was then mixed at low concentrations with non-oxidized fibrinogen purified from the same plasma ranging from 2-8% of the total fibrinogen, at a ratio similar to those found in coagulopathic trauma patients. Reptilase time was then determined for each concentration. LC/MS/MS was used to confirm the fibrinogen Aα-M476(SO)% in the final preparations. We found that even at very low Aα-M476(SO)%, there remained a significant positive linear association between Aα-M476(SO)% and Reptilase time. (Figure 2).
Figure 2.
Increased Aα-MSO476 sulfoxide content is associated with fibrin polymerization impairment by reptilase activation at low levels corresponding to those found in trauma patients. Other oxidative modifications of fibrinogen are also possible after exposure to HOCL, but were not investigated.
Confirmation Cohort
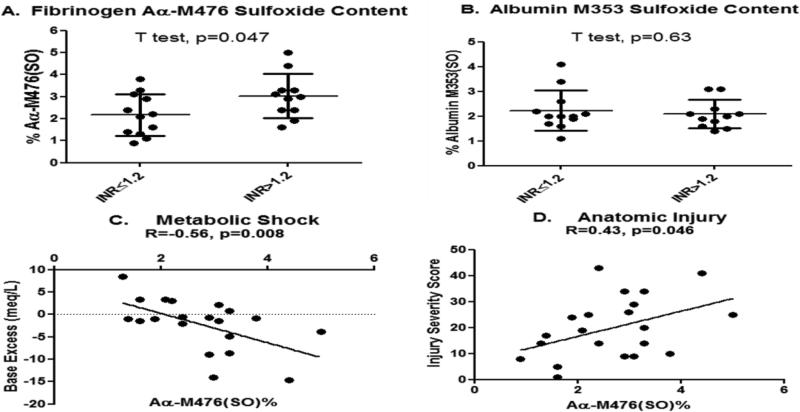
The Richmond cohort was used to confirm that Aα-M476(SO)% was increased in coagulopathic trauma patients. N=23 plasma samples were submitted for MS analysis in a blinded fashion and underwent post-hoc analysis after stratifying into two groups by the same INR>1.2 coagulopathy criteria. Trauma subjects (N=12) were assigned to the control group (INR ≤1.2) and were compared to subjects assigned to the coagulopathy group (N=11) (INR> 1.2). Mean (SD) Aα-M476(SO)% was again subtly, but significantly, increased in the group with coagulopathy compared to control. (Figure 3.) In contrast, albumin M353(SO)% present in the same plasma was not increased with coagulopathy.
Figure 3. Fibrinogen methionine sulfoxide content is preferentially-increased in coagulopathic trauma patients and is associated with important clinical variables.
(A) Mean Aα-M476(SO)% is increased under coagulopathic conditions (INR>1.2) in trauma patients when detected directly in plasma. (B) Albumin M353(SO)% is not increased in the same plasma sample. Fibrinogen Aα-M476(SO)% was also negatively associated with base excess as a clinical marker of metabolic shock severity (C), and positively associated with injury severity measured using the Injury Severity Score (D) Bars= Mean and StdDev. R=Pearson product moment correlation coefficient. (Richmond Cohort)
Fibrinogen Aα-M476(SO)% was also associated with increasing anatomical injury determined by Injury Severity Score (ISS), and base excess as a marker of metabolic shock.
Fibrinogen oxidation and clot formation
We examined changes in multiple TEG clot formation parameters when stratified by Aα-Met476(SO) quartile in the third trauma patient cohort from Houston TX. Based upon the derivation cohort power analysis, and assuming that TEG clot formation parameters would behave somewhat similarly to INR, N=11 samples were obtained for each Aα-Met476(SO) quartile (a total of N≥44 required) so that individual quartiles could be compared with adequate power to detect differences.
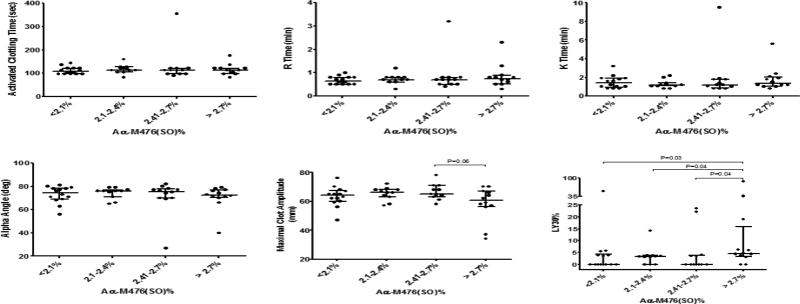
We obtained 50 plasma samples and N=49 plasma samples had adequate volume for full analysis. Median (IQR) fibrinogen Aα-M476(SO)% for the entire cohort was 2.4% (2.1, 2.7) ranging from a minimum of 1.4% to a maximum of 5.1%. The median Albumin M353(SO)% content was 4.1% (3.4, 5.4) ranging from a minimum of 2.7% to a maximum of 9.2%. rTEG parameters were grouped and compared for differences according to fibrinogen Aα-M476(SO)% quartile. (Figure 4.) There was a strong trend towards decreased MA in the highest fibrinogen Aα-Met476(SO) quartile compared to the next highest quartile (Wilcoxon p=0.06). LY30% was significantly increased in the highest Aα-M476(SO)% quartile compared to all other quartiles (Wilcoxon p≤0.041 for all comparisons). There were no significant differences in rTEG parameters when stratified by Albumin M353(SO)% (data not shown). There were also a limited number of INR values measured for these samples, as INR is not routinely measured in this trauma center. Of 12 available measurements, there was a significant positive linear association between fibrinogen Aα-M476(SO)% and INR (Pearson R=0.7, p=0.01). There was no significant association between Albumin M353(SO)% and INR (Pearson R=0.5, p=0.12).
Figure 4. Viscoelastic clot formation parameters by Aα-M476(SO)% in Emergency Department trauma patients.
Whole blood viscoelastic clot formation measured in the Emergency Department using rapid TEG are compared by Aα-M476(SO)% quartile measured by UPLC-MS/MS-MRM. Percent clot lysis (LY30%) was significantly increased in the highest Aα-M476(SO)% quartile compared to all other quartiles. Points are individual measurements and bars represent median and interquartile range. P values= nonparametric Wilcoxon Rank Sums test.
DISCUSSION
This is the first report of selective oxidation of fibrinogen being associated with impaired blood coagulation after traumatic injury. This result suggests that oxidation of fibrinogen may contribute to coagulopathy, specifically a reduction in clot strength and increase in fibrinolysis, after trauma.
Fibrinogen Oxidation
There is evidence for similar post-translational modifications of fibrinogen in response to acute diseases when measuring Advanced Oxidative Protein Products (AOPP), which is predominantly made up of undifferentiated oxidized albumin and fibrinogen [24]. AOPP's become elevated in diseases where oxidative stress is acutely increased including; acute renal dysfunction, acute on chronic liver dysfunction, and in critical illness [25,26]. AOPP's are also increased in plasma exposed in vitro to HOCl and can be significantly elevated and associated with MPO and plasma NADPH oxidase concentration and activity in hemodialysis patients [27,28]. Fibrinogen is also localized with leukocytes in vivo via integrin αMβ2/Mac-1 binding to the Ɣ-chain (N390RLSIGE396) binding sequence and its binding supports inflammatory host responses to infection [29]. Such close spatial association may facilitate direct oxidation of fibrinogen sulfhydryl groups by leukocyte-derived HOCL in vivo.
Formation of methionine sulfoxide is not exclusive to HOCl. However, reactions of HOCl with methionine residues and other sulfur containing side chains are the fastest known, with 2nd order rate constants of up to 3.8 ×107/M/sec at physiologic pH [30]. Sulfhydryl groups of human plasma proteins are the main target for PMN-derived HOCL oxidation, followed by thioether groups and amino groups [31]. Antioxidant mechanisms reversing fibrinogen oxidation are much-less efficient and may also become impaired after trauma. The rate of oxidation of methionine by HOCl is considerably faster than the rate constant of 37.0/M/sec reported for its subsequent reduction by its primary antioxidant enzyme methionine sulfoxide reductase [32]. The mechanism of reduction of methionine sulfoxide by methionine sulfoxide reductase is a three-step process of which, reduction by thioredoxin is the rate-limiting step, and is therefore dependent on the thioredoxin recycling process [33]. Thioredoxin cycling can also become inhibited during hemorrhagic shock by expression of a family of thioredoxin-interacting proteins that can directly inhibit the expression and reducing activity of thioredoxin [33,34]. Additional support for an unbalancing of blood redox balance after trauma is provided by Rael and colleagues who found that plasma oxidation-reduction potential was significantly elevated in trauma patients and could be used to discriminate between mild and severe injury profiles [35]. Therefore, our observation of subtly-increased methionine sulfoxide content of fibrinogen may suggest disruption of local sulfonyl redox balance either from inflammatory leukocyte activation (a common feature of trauma), a reduced antioxidant capacity, or both.
Relevance to Coagulopathy
Dissociation and interaction of fibrinogen αC domains from the E region enables fibrin protofibril lateral aggregation during clot formation [36-38]. Alpha C double hairpin structures interact with adjacent and complementary hairpin structures on adjacent fibrin monomers to form large beta sheet structures, eventually coalescing into fibers [39]. We have previously characterized the effect of Aα-Met476(SO)% on local double hairpin structural conformations using molecular dynamics simulation [16]. The simulations suggest that oxidation of the single Aα-Met476 site causes a hinge-like transition of the hairpin structure to a more linear and extended configuration. The functional consequences appear to be inhibition of fibrin protofibril lateral aggregation during fibrin polymerization producing thinner fibrin fibers and mechanically weak clots that are paradoxically less susceptible to fibrinolysis in vitro due to decreased clot porosity [15].
Our results suggest that fibrin polymerization may become impaired in trauma patients with increased fibrinogen Aα-Met476(SO)%, manifesting as decreased clot strength and increased fibrinolysis after injury as determined by viscoelastometry. A similar disruption of the carboxyl-terminal region of the Aa chain can be found in the case of congenital fibrinogen Caracas II. Fibrinogen Caracas II is characterized by prolonged thrombin time and translucent fibrin clots [40]. The mutation is described as a unique N-glycosylated Asn substitution for a Ser at position 434 of the mutant Aa chain that disrupts folding of the carboxyl-terminal region of the Aa chain back upon the central region of the molecule, disrupting its opportunity to participate in lateral aggregation events [40]. Woodhead and Weisel et al., used electron microscopy of individual molecules to find that most of the alpha C domains of fibrinogen Caracas II fail to interact with each other or with the central domain [41]. Caracas II fibrin was made up of thinner fibers that were less-ordered than normal fibers. However, whole clots contained large pores bounded by local fiber networks made up of thin fibers and permeation experiments suggested increased pore diameter, both of which are in direct conflict with decreased pore size and permeation measured after Aα-Met476(SO) formation in vitro [15]. The viscoelastic properties of the Caracas II clot were also similar to control clots, leading the authors to conclude that this dysfibrinogenemia is relatively asymptomatic. Clearly, altering the Aα-carboxy terminal region of fibrinogen can have important effects on fibrin clot structure and function that is sensitive to both pre and post-translational modification.
Relevance to Trauma Care
Fibrinogen oxidation might promote blood loss after trauma by directly inducing changes in fibrin clot structure via altered fibrin polymerization. The earliest documented change in clot formation in trauma patients is a reduction of clot strength or elastic modulus when measured using viscoelastic methods [3, 42]. Our previous work suggests a similar reduction in elastic and storage modulus of fibrin clot when fibrinogen was exposed to HOCl in vitro [15]. We found that rTEG maximal clot amplitude tended to decrease and fibrinolysis was significantly increased in the highest fibrinogen Aα-Met476(SO)% quartile. In addition, a strong positive correlation between INR and Aα-Met476(SO)% was preserved across three different trauma cohorts. These results suggest that oxidants present in plasma may also affect fibrinogen polymerization, clot strength, and resulting susceptibility to fibrinolysis. Further confirmatory work is needed to determine the contribution of fibrinogen oxidation to clot formation, bleeding, and outcomes after trauma.
Our results are also important because other plasma coagulation proteins are also susceptible to oxidation. Vulnerable methionine residues on both thrombomodulin and protein C have been identified that, when oxidized, result in drastic decreases of functional activity of these proteins [43,44]. The favored mechanisms of coagulopathy after trauma hinge upon increased thrombomodulin-thrombin interactions and activation of protein C in response to shock and tissue injury. Increased soluble thrombomodulin sequesters circulating thrombin, shifting its affinity from fibrinogen to protein C, thus forming activated protein C. Increased concentration of activated protein C then inhibits the activity of Factors V and VIII on the platelet surface and causes disinhibition of tissue plasminogen activator via inhibition of plasminogen activator inhibitor, resulting in both anticoagulation and hyperfibrinolysis [5]. Oxidation of a single methionine within the catalytic thrombin-binding pocket of thrombomodulin (Met388) decreases thrombin binding affinity in vitro [44]. The specific site of oxidation site of protein C has not yet been identified, but exposure to HOCl in vitro also severely reduces its activity [43]. Therefore, if significant in vivo methionine sulfoxide formation is found on thrombomodulin and protein C after injury, their function may be altered from that expected and refinement of our current understanding of the traumatic coagulopathy mechanism would be required.
Recognizing the need for proper fibrinogen function to stop bleeding, some clinical guidelines are now prioritizing liberal fibrinogen replacement using fibrinogen concentrates guided by viscoelastic testing during severe bleeding management [45]. Our results suggest that antioxidant therapies may also be useful to improve other important outcomes after trauma. Thomson, and Ischiropoulos, et al. found that immunoglobulins recognizing the 3-nitrotyrosine post-translational modification were significantly higher in trauma patients who subsequently developed acute lung injury compared to control trauma patients [46]. Furthermore, antioxidant supplementation in critically-ill trauma and surgical patients has been associated with less multi-organ failure and acute respiratory distress syndrome [47]. However, it remains to be determined how antioxidant therapies might specifically impact the early course of coagulopathy after trauma.
Limitations
Our conclusions are limited only to associations between fibrinogen function, one specific methionine oxidation, and clot formation after trauma. Further focused study in experimental models is required to reveal mechanisms of action. Our results in no way indicate that Aα-Met476(SO) content is the only oxidative modification of fibrinogen after trauma, or is solely responsible for the witnessed changes in coagulation function. Given the ease in which fibrinogen is oxidized by multiple oxidative and nitrative stressors, it is likely that other relevant modifications are also associated with coagulopathy. The human samples that we examined were also limited in number. We were also unable to obtain plasma prior to any clinical therapy. Therefore, we could not fully separate fibrinogen oxidation produced purely from injury and blood loss from the effects of treatment in the human cohorts. The majority of oxidized fibrinogen may also have been converted to fibrin at wound sites or converted to fibrin degradation products by increase of fibrinolysis during TIC. These alternative oxidized fibrin products would have been lost during glycine purification of fibrinogen from plasma and undetectable by our specific MS-based detection methods. Oxidized fibrinogen is also found in the plasma in two compartments, free and bound to apo(a) of Lp(a) which may have been lost during purification [48]. Therefore, even the subtle increase of Aα-Met476(SO)% we found in intact fibrinogen sampled from the systemic circulation may represent even more significant oxidation taking place more locally at wounds.
CONCLUSION
Selectively-increased methionine sulfoxide content of fibrinogen in vivo after trauma may signal coagulopathy after injury. Definitive mechanistic study of fibrinogen oxidation after trauma, including identification of additional oxidative modifications, is warranted.
Highlights.
Fibrinogen methionine sulfoxide is increased in coagulopathic trauma patients.
Methionine sulfoxide is preferentially formed in the fibrinogen Aα-C domain.
Oxidation by inflammation may modulate blood coagulation responses to injury.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the valuable contributions of Junmei Chen, and Scott Parsons of Bloodworks Northwest for their thoughtful assistance and guidance. We also acknowledge the valuable efforts of Jason Newton at the Virginia Commonwealth University Coagulation Advancement Laboratory and key personnel, Lisa Baer and Jeanette Podbielski, in the Center for Translational Injury Research team. This work was supported by NIH postdoctoral training grant GM008695-09 (N.J.W), NHLBI 1F32HL120549-01A1 (J.C.C) and by Grant Number KL2 TR000421 from the National Center for Advancing Translational Sciences (NCATS) (N.J.W), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH. N.J.W., X.F., and Y.W. declare an invention disclosure related to detection of oxidized fibrinogen in plasma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORSHIP
Contribution: N.J.W., X.W., A.E.S.J., E.B.L., K.R.W., X.F., Y.W., and D.C., performed experiments; N.J.W analyzed results, made the figures, and wrote the paper; N.J.W, J.C., C.W., S.A.S., J.L., E.J.M, D.F.B and D.C., designed the research and made critical paper revisions.
Potential Conflicts of Interest
N.J. White, Y. Wang, X. Fu, J.A. López, and D. Chung hold intellectual property regarding detection methods for oxidative modifications of fibrinogen. J.C. Cardenas, E.J. Martin, D.F. Brophy, C.E. Wade, X.Wang, A.E. St. John, E.B. Lim, and S.A. Stern declare no conflicts of interest associated with this manuscript. K.R. Ward has a number of invention disclosures and patents pending for coagulation monitoring technologies through the University of Michigan and no direct conflicts with the information contained in this manuscript.
All other authors declare no competing financial interests.
REFERENCES
- 1.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. Journal of Trauma-Injury Infection and Critical Care. 2003;54(6):1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 2.Peltan ID, Vande Vusse LK, Maier RV, Watkins TR. An International Normalized Ratio-Based Definition of Acute Traumatic Coagulopathy Is Associated With Mortality, Venous Thromboembolism, and Multiple Organ Failure After Injury. Crit Care Med. Jul. 2015;43(7):1429–38. doi: 10.1097/CCM.0000000000000981. doi: 10.1097/CCM.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport R, Manson J, De'Ath H, Platton S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, MacCallum P, Stanworth S, Brohi K. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–8. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, Johansson PI, Stanworth S, Thiemermann C, Brohi K. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8(9):1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet J. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of Surgery. 2007;245(5):812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–21. doi: 10.1097/SHK.0000000000000161. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa M, Sawamura A, Gando S, Kubota N, Uegaki S, Shimojima H, Sugano M, Ieko M. Disseminated intravascular coagulation at an early phase of trauma is associated with consumption coagulopathy and excessive fibrinolysis both by plasmin and neutrophil elastase. Surgery. 2011;149(2):221–30. doi: 10.1016/j.surg.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6(3):161–80. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- 9.Carr ME, Alving BM. Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coagul Fibrinolysis. 1995;6(6):567–73. doi: 10.1097/00001721-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Schochl H, Frietsch T, Pavelka M, Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67(1):125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 11.CRASH-2 trial collaborators. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, Herrera J, Hunt B, Iribhogbe P, Izurieta M, Khamis H, Komolafe E, Marrero MA, Mejía-Mantilla J, Miranda J, Morales C, Olaomi O, Olldashi F, Perel P, Peto R, Ramana PV, Ravi RR, Yutthakasemsunt S. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;3376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 12.Rourke C, Curry N, Khan S, Taylor R, Raza I, Davenport I, Stanworth S, Brohi K. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10(7):1342–51. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 13.Hiippala ST, Myllyki GJ, Vahtera EM. Hemostatic Factors and Replacement of Major Blood Loss with Plasma-Poor Red Cell Concentrates. Anesth Analg. 1995;81:360–5. doi: 10.1097/00000539-199508000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Shacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radical Biology & Medicine. 1994;17(5):429–37. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 15.Weigandt KM, White N, Chung D, Ellingson E, Wang Y, Fu X, Pozzo DC. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys J. 2012;103(11):2399–407. doi: 10.1016/j.bpj.2012.10.036. doi: 10.1016/j.bpj.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burney PR, White N, Pfaendtner J. Structural effects of methionine oxidation on isolated subdomains of human fibrin D and αC regions. PLoS One. Jan 27. 2014;9(1):e86981. doi: 10.1371/journal.pone.0086981. doi: 10.1371/journal.pone.0086981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc Natl Acad Sci U S A. 2009;106(44):18686–91. doi: 10.1073/pnas.0909464106. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandfellner HM, Ruparel SB, Gelfond JA, Hargreaves KM. Major blunt trauma evokes selective upregulation of oxidative enzymes in circulating leukocytes. Shock. 2013;40(3):182–7. doi: 10.1097/SHK.0b013e31829de02f. doi: 10.1097/SHK.0b013e31829de02f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laplace C, Huet O, Vicaut E, Ract C, Martin L, Benhamou D, Duranteau J. Endothelial oxidative stress induced by serum from patients with severe trauma hemorrhage. Intensive Care Med. 2005;31(9):1174–80. doi: 10.1007/s00134-005-2737-7. [DOI] [PubMed] [Google Scholar]
- 20.Szabó C, Módis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010;34(Suppl 1):4–14. doi: 10.1097/SHK.0b013e3181e7e9ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holcomb JB, Minei KM, Scerbo ML, Radwan ZA, Wade CE, Kozar RA, Gill BS, Albarado R, McNutt MK, Khan S, Adams PR, McCarthy JJ, Cotton BA. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012. 256(3):476–86. doi: 10.1097/SLA.0b013e3182658180. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 22.Chapman MP, Moore EE, Ramos CR, Ghasabyan A, Harr JN, Chin TL, Stringham JR, Sauaia A, Silliman CC, Banerjee A. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J Trauma Acute Care Surg. 2013;75(6):961–7. doi: 10.1097/TA.0b013e3182aa9c9f. discussion 967. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotton BA, Harvin JA, Kostousouv V, Minei KM, Radwan ZA, Schockl H, Wade CE, Holcomb JB, Matijevic N. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 24.Selmeci L, Székely M, Soós P, Seres L, Klinga N, Geiger A, Acsády G. Human blood plasma advanced oxidation protein products (AOPP) correlates with fibrinogen levels. Free Radic Res. 2006;40(9):952–8. doi: 10.1080/10715760600818789. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Han T, Tian J, Zhu ZY, Liu Y, Li Y, Xiao SX, Li Y, Feng YY. Monitoring oxidative stress in acute-on-chronic liver failure by advanced oxidation protein products. Hepatol Res. 2012;42(2):171–80. doi: 10.1111/j.1872-034X.2011.00911.x. doi: 10.1111/j.1872-034X.2011.00911.x. [DOI] [PubMed] [Google Scholar]
- 26.Selmeci L, Seres L, Antal M, Lukács J, Regöly-Mérei A, Acsády G. Advanced oxidation protein products (AOPP) for monitoring oxidative stress in critically ill patients: a simple, fast and inexpensive automated technique. Clin Chem Lab Med. 2005;43(3):294–7. doi: 10.1515/CCLM.2005.050. [DOI] [PubMed] [Google Scholar]
- 27.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–13. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 28.Capeillère-Blandin C, Gausson V, Nguyen AT, Descamps-Latscha B, Drüeke T, Witko-Sarsat V. Respective role of uraemic toxins and myeloperoxidase in the uraemic state. Nephrol Dial Transplant. 2006;21(6):1555–63. doi: 10.1093/ndt/gfl007. [DOI] [PubMed] [Google Scholar]
- 29.Flick MJ, Du X, Witte DP, Jirousková M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113(11):1596–606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side-chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 31.Arnhold J, Hammerschmidt S, Arnold K. Role of functional groups of human plasma and luminol in scavenging of NaOCl and neutrophil-derived hypochlorous acid. Biochim Biophys Acta. 1991;231097(2):145–51. doi: 10.1016/0925-4439(91)90099-u. [DOI] [PubMed] [Google Scholar]
- 32.Branlant G. The enzymology and biochemistry of methionine sulfoxide reductases. Biochimica et biophysica acta. 2005;1703(2):231–238. doi: 10.1016/j.bbapap.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life. 2001;52(1-2):29–33. doi: 10.1080/15216540252774739. [DOI] [PubMed] [Google Scholar]
- 34.Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G, Scott MJ, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol. 2011;187(9):4809–1. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rael LT, Bar-Or R, Salottolo K, Mains CW, Slone DS, Offner PJ, Bar-Or D. Injury severity and serum amyloid A correlate with plasma oxidation-reduction potential in multi-trauma patients: a retrospective analysis. Scand J Trauma Resusc Emerg Med. 2009;1917:57. doi: 10.1186/1757-7241-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorkun OV, Veklich YI, Medved LV, Henschen AH, Weisel JW. Role of the alpha C domains of fibrin in clot formation. Biochemistry. 1994;33:6986–6997. doi: 10.1021/bi00188a031. [DOI] [PubMed] [Google Scholar]
- 37.Weisel JW, Medved L. The structure and function of the alpha C domains of fibrinogen. Ann. N.Y. Acad. Sci. 2001;936:312–327. doi: 10.1111/j.1749-6632.2001.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 38.Ping L, Huang L, Cardinali B, Profumo A, Gorkun OV, Lord ST. Substitution of the human αC region with the analogous chicken domain generates a fibrinogen with severely impaired lateral aggregation: fibrin monomers assemble into protofibrils but protofibrils do not assemble into fibers. Biochemistry. 50(42):9066–75. doi: 10.1021/bi201094v. 201 doi: 10.1021/bi201094v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsurupa G, Pechik I, Litvinov RI, Hantgan RR, Tjandra N, Weisel JW, Medved L. On the mechanism of αC polymer formation in fibrin. Biochemistry. 2012;51(12):2526–38. doi: 10.1021/bi2017848. doi: 10.1021/bi2017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maekawa H, Yamazumi K, Muramatsu S, Kaneko M, Hirata H, Takahashi N, de Bosch NB, Carvajal Z, Ojeda A, Arocha-Piñango CL. An A alpha Ser-434 to N-glycosylated Asn substitution in a dysfibrinogen, fibrinogen Caracas II, characterized by impaired fibrin gel formation. J Biol Chem. 1991;266(18):11575–8. [PubMed] [Google Scholar]
- 41.Woodhead JL, Nagaswami C, Matsuda M, Arocha-Piñango CL, Weisel JW. The ultrastructure of fibrinogen Caracas II molecules, fibers, and clots. J Biol Chem. 1996 Mar 1;271(9):4946–53. doi: 10.1074/jbc.271.9.4946. [DOI] [PubMed] [Google Scholar]
- 42.White NJ, Newton JC, Martin EJ, Mohammed BM, Contaifer D, Jr., Bostic JL, Brophy GM, Spiess BD, Pusateri AE, Ward KR, Brophy DF. Clot formation is associated with fibrinogen and platelet forces in a cohort of severely-injured Emergency Department trauma patients. Shock. 2015;44(Suppl 1):39–44. doi: 10.1097/SHK.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nalian A, Iakhiaev AV. Possible mechanisms contributing to oxidative inactivation of activated protein C: molecular dynamics study. Thromb Haemost. 2008;100(1):18–25. doi: 10.1160/TH07-12-0750. [DOI] [PubMed] [Google Scholar]
- 44.Glaser CB, Morser J, Clarke JH, Blasko E, McLean K, Kuhn I, Chang RJ, Lin JH, Vilander L, William H, Andrews WH, Light DR. Oxidation of a Specific Methionine in Thrombomodulin by Activated Neutrophil Products Blocks Cofactor Activity. J. Clin. Invest. 1992;90:2565–2573. doi: 10.1172/JCI116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schöchl H1, Voelckel W, Grassetto A, Schlimp CJ. Practical application of point-of-care coagulation testing to guide treatment decisions in trauma. J Trauma Acute Care Surg. 2013;74(6):1587–98. doi: 10.1097/TA.0b013e31828c3171. doi: 10.1097/TA.0b013e31828c3171. [DOI] [PubMed] [Google Scholar]
- 46.Thomson L, Christie J, Vadseth C, Lanken PN, Fu X, Hazen SL, Ischiropoulos H. Identification of immunoglobulins that recognize 3-nitrotyrosine in patients with acute lung injury after major trauma. Am J Respir Cell Mol Biol. 2007;36(2):152–7. doi: 10.1165/rcmb.2006-0288SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236(6):814–22. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selmeci L, Seres L, Székely M, Soós P, Acsády G. Assay of oxidized fibrinogen reactivity (OFR) as a biomarker of oxidative stress in human plasma: the role of lysine analogs. Clin Chem Lab Med. 2010;48(3):379–82. doi: 10.1515/CCLM.2010.076. doi: 10.1515/CCLM.2010.076. [DOI] [PubMed] [Google Scholar]