Abstract
Atlantic killifish (Fundulus heteroclitus) inhabiting the Atlantic Wood Industries region of the Elizabeth River, Virginia have passed polycyclic aromatic hydrocarbon (PAH)-resistance to their offspring as evidenced by early life stage-testing of developmental toxicity after exposure to specific PAHs. Our study focused on environmentally relevant PAH mixtures in the form of Elizabeth River Sediment Extract (ERSE). Juvenile (5-mo) F1 progeny of pollution-adapted Atlantic Wood (AW) parents and of reference site (King's Creek/KC) parents were exposed as embryos to ERSE. Liver alterations, including non-neoplastic lesions and microvesicular vacuolation, were observed in both populations. ERSE-exposed KC fish developed significantly more alterations than unexposed KC fish. Interestingly, unexposed AW killifish developed significantly more alterations than unexposed KC individuals, suggesting that AW juveniles are not fully protected from liver disease; rapid growth of juvenile fish may also be an accelerating factor for tumorigenesis. Because recent reports show hepatic tumor formation in adult AW fish, the differing responses from the two populations provided a way to determine whether embryo toxicity protection extends to juveniles. Future investigations will analyze older life stages of killifish to determine differences in responses related to chronic disease.
Keywords: Fundulus heteroclitus, polycyclic aromatic hydrocarbons (PAHs), creosote-contaminated site, Elizabeth River, adaptation, hepatic lesions, developmental exposure
Introduction
The Elizabeth River, located in southeastern Virginia, USA, has been the focus of ecologists, toxicologists, and developmental biologists for decades (Vogelbein et al., 1990; Di Giulio and Clark, 2015). Of particular interest is the Atlantic Wood Industries (AWI) Superfund site (hereafter referred to as Atlantic Wood/AW), on the southern branch of the river, where Atlantic Wood Industries, Inc. operated a wood-treatment facility from 1926 to 1992 (Walker and Dickhut, 2001; Jung et al., 2011). Over 20 years after the plant closure and until recent remediation sequestered the most contaminated sediment, this polluted area reflected the adverse consequences of creosote contamination (Di Giulio and Clark, 2015). Creosote, the major mixture used by this operation, is comprised of numerous organic molecules, including polycyclic aromatic hydrocarbons (PAHs) (Wills et al., 2010; Van Veld and Nacci, 2008). In the 1960s, creosote spills of 20,000 and 30,000 gallons contaminated the river, resulting in the accumulation of PAHs in river sediment (Walker and Dickhut, 2001; Vogelbein and Unger, 2006). Native organisms including the Atlantic killifish (Fundulus heteroclitus) have been exposed to this pollution since wood-treatment facilities began production (Vogelbein and Unger, 2006).
Some PAHs act as agonists of the aryl hydrocarbon receptor (AhR) pathway in teleost fish, resulting in the up-regulation of certain genes including cytochrome P450 enzymes that can transform some PAHs into products that bind to DNA and cause alterations (Rotchell et al., 2008). If not repaired or repaired wrongly, this can lead to cancer (Rotchell et al., 2008). Some PAHs also result in developmental abnormalities similar to that seen with dioxin-like compounds such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Spitsbergen et al., 1991; Helder, 1981; Di Giulio and Clark, 2015). Elizabeth River killifish developed resistance to the teratogenicity of these contaminants (Meyer and Di Giulio, 2002) — an inherited resistance in first- and second-generation laboratory raised fish (Meyer and Di Giulio, 2003) from AW populations. However, this resistance comes with fitness costs in the form of susceptibility to other stressors (e.g., hypoxia) (Meyer and Di Giulio, 2003).
AW killifish larvae may genetically inherit adaptations that prevent cardiac abnormalities observed in non-PAH-adapted populations (Clark et al., 2014; Meyer and Di Giulio, 2003; Ownby et al., 2002; Wills et al., 2010; Clark et al., 2013), and Wills et al. (2010) reported that 9-mo old AW killifish appear to inherit resistance to hepatic lesions caused by benzo[a]pyrene (BaP); however, few laboratory studies have analyzed the toxic effects of PAH mixtures. Use of sediment extract from the AW site coupled with controlled laboratory experiments on progeny of differing killifish populations provides environmentally relevant approaches to examine the toxicity of PAH mixtures.
Previous research on killifish primarily involved adults of unknown age captured at specific sites (Cooper et al., 1999; Frederick et al., 2007; Jung et al., 2011; Pinkney and Harshbarger, 2006; Schmalz Jr. et al., 2002; Stine et al., 2004; Vogelbein et al., 1999; Vogelbein et al., 1990; Vogelbein and Unger, 2006); and, after spawning captured adults, embryo progeny were analyzed (Clark et al., 2014; Meyer and Di Giulio, 2003; Ownby et al., 2002; Wills et al., 2010; Weis and Weis, 1989; Clark et al., 2013). In field-collected AW killifish, tumors were found in 33% of adults (Vogelbein et al., 1990) but could not be exclusively linked to PAH-exposure since the animals were taken directly from the Elizabeth River where they were likely exposed to a complex mixture of creosote and industrial wastes. Such reports have been compared to other sites such as Lake Erie where PAHs were associated with effluents from a steel plant and associated coking facility (Baumann et al., 1990).
The AW site has undergone remediation by the United States Environmental Protection Agency that has cleared the site of sediments, covered it with new soils, and separated it from the Elizabeth River by an impermeable wall barrier (Di Giulio and Clark, 2015). Strategically, prior to these actions, sediment samples and killifish were collected for laboratory studies. Our study focuses on a juvenile F1 generation of these fish, addressing a current gap in the literature for this life stage. This life stage is important not only for body growth and development but, given the maintenance of a liver to body weight ratio, there is a potential for populations of mutated hepatocytes to undergo uncontrolled cell proliferation (Ames et al., 1993), leading to advanced steps in the tumorigenic process.
The killifish is an ideal organism for this analysis because it is currently among the few species of fish that both exhibit pollution-induced hepatic lesions in the wild and can be reared in the laboratory (Vogelbein and Unger, 2006) where the full complement of modern tools are available (Burnett et al., 2007). Due to the relative non-migratory nature of killifish (Vogelbeinet al., 1990; Meyer and Di Giulio, 2003; Pinkney and Harshbarger, 2006; Vogelbein and Unger, 2006), the identification of carcinogenic precursor lesions in juvenile PAH-exposed killifish can be used to draw conclusions about the condition of the animals’ native environment as well as their susceptibility or resistance to chronic disease (Wills et al., 2010; Vogelbein et al., 1990; Vogelbein and Unger, 2006).
Our study focused on the occurrence of hepatic alterations among PAH-adapted and non-adapted juvenile killifish after embryonic exposure to Elizabeth River Sediment Extract (ERSE), with the goal of linking early life stage exposure to later life consequences.
Materials and Methods
Site Characteristics & Sediment Extraction
Sediment samples from Atlantic Wood Industries, Inc. (36°48′ 27.2″N, 76°17′ 38.1″W) were collected in November 2011, before remediation of the site. Sample preparation and chemical analysis of ERSE was described by Fang et al. (2014). Previous research suggests that porewater (ERSE), a mixture of water and suspended solids, is a more accurate representation of environmentally relevant compounds for aquatic species than whole-sediment (Fang et al., 2014). For fish exposures, ERSE was diluted to 0.1% and 1.0%, corresponding to 5.04 and 50.45 μg/L of total PAHs, respectively (Table S1).
Fish Collection & Exposure
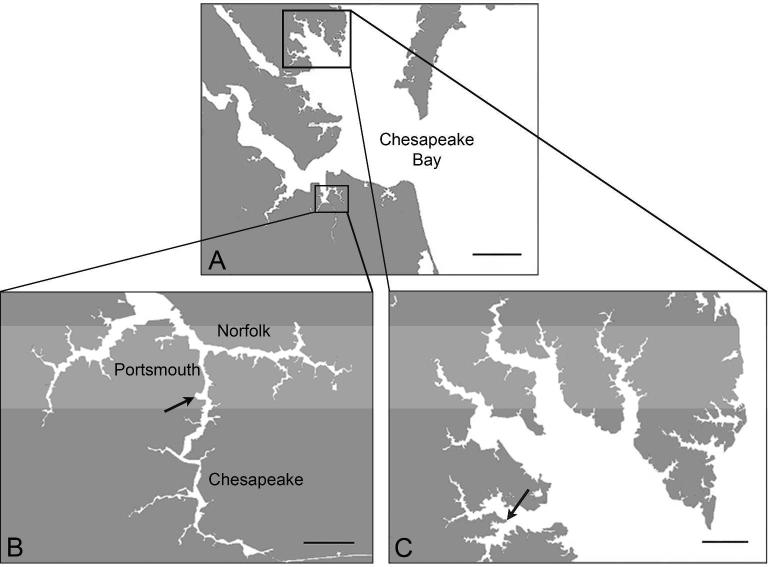
Adult killifish were collected from AW and a King's Creek (KC) reference site (37°18′ 6.2″ N, 76°24′ 58.9″ W) (Figure 1) in Virginia (April-September 2012-2013) using wire mesh minnow traps. They were maintained in labs at Duke University, Durham, NC in 30- or 40-L tanks in a flow-through system with artificial seawater (15 ppt, Instant Ocean, Foster and Smith, Rhinelander, WI, USA) at 25-28°C and a 14:10 hr light:dark cycle. Adults were fed ad libitum with pelleted feed (Aquamax® Fingerling Starter 300; PMI Nutritional International, LLC, Brentwood, MO, USA).
Figure 1.
The Chesapeake Bay map (A, scale bar 10 mi) includes locations for Atlantic Wood (B, arrow, scale bar 2 mi) and King's Creek (C, arrow, scale bar 2 mi) killifish collection sites.
In vitro fertilization, as per Brown et al. (2016), produced F1 killifish embryos for this study. At 24 hpf, embryos, confirmed to show normal development (Oppenheimer, 1937; Armstrong and Child, 1965), were exposed to 10-ml of either 0% (control), 0.1%, or 1.0% ERSE in 20-ml glass scintillation vials (VWR International, Wheaton WorldWide, Millville, NJ, USA) for a total period of 120 hrs and then transferred to Petri dishes lined with absorbent filter paper and maintained in an incubator at 27°C for up to 14 days post-fertilization (dpf). Next, following filter paper removal, artificial seawater was added and dishes were transferred to an orbital shaker until hatching was complete.
After hatching, larvae (eleutheroembryos) were maintained in 2-L beakers at 27°C in an incubator for 1 week and fed a diet of Artemia nauplii (Brine Shrimp Direct, Aquatic Habitats, Apopka, FL, USA). Next, larvae were evenly distributed, with 3 fish per L, in 10-L tanks among two recirculating AHAB systems (Aquatic Habitats, Apopka, FL, USA) at 28°C on a 14:10 hr light:dark cycle for 5 months grow-out. For the initial 2 weeks, larvae were fed Artemia nauplii, after which they were fed Ziegler's Adult Zebrafish Complete Diet (Aquatic Habitats, Apopka, FL, USA) and Cyclop-eeze (Argent Chemical Laboratories, Redmond, WA, USA). All care and maintenance was non-invasive and followed a protocol reviewed and approved by the Duke University Institutional Animal Care & Use Committee.
Fixation & Histological Staining
At 5-mo of age, killifish were euthanized and fixed in 10% neutral buffered formalin (VWR). Fixative entry was ensured by a midline incision through the ventral body wall extending from anus to near pectoral girdle. Next, a disposable transfer pipet with extended fine tip was used to introduce fixative to all abdominal organs, after which the pipet was introduced to the buccal cavity and fixative flushed into the pharynx and proximal digestive tract. Processing, embedment, sectioning, and staining were completed at the Histology Laboratory, Department of Population, Health and Pathobiology, North Carolina State University College of Veterinary Medicine, Raleigh. Fish were decalcified in 10% formic acid for 48 h, washed and placed in an automated tissue processor (Thermo Shandon PathCentre, Grand Island, NY, USA) for ethanol dehydration followed by clearing and embedment in paraffin, with each individual fish oriented in left lateral recumbency. A Leica 2135 rotary microtome (Leica Biosystems Inc., Buffalo Grove, IL) was used to section individuals. Planes of section were from the retina of the left eye to the middle of the contralateral eye. Step sections were made until the liver was encountered, then 5-μm thick serial sections were made through the entire organ. Slides were routinely stained by hematoxylin and eosin (H&E). Based on the body axis of Fundulus heteroclitus, parasagittal sections in this orientation provided thorough representations of the liver, adjacent extrahepatic biliary system and pancreas. A total of 41 progeny from AW parents and 40 from KC parents were included.
Initial Qualitative Screening, Slide Selection & Blinding
All slides were screened with a 2X objective lens using a Nikon Eclipse E600 compound microscope (Nikon Instruments Inc., Melville, NY) for sections along or near the midline that provided ample liver area. One slide (7 sections) per fish was selected for further analysis. Gonads were visible and yielded sex of individuals in all but 6 fish (Table S3). Selected slides were then randomized and blind-labeled by a second party placing opaque stickers numbered 1-81 over slide labels, which were maintained until all evaluations were completed.
Liver Evaluation & Image Processing
On each slide, three non-overlapping sections (at least 10-μm apart) were selected and within each, three non-overlapping fields of the liver (two for smaller individuals) were imaged with a 20X objective lens. Microscopic fields were restricted to the following liver regions: 1) rostral apex, 2) dorsal, adjacent to the gut, and 3) adjacent to the ventral body wall. This sub-sampling methodology allowed for later measurements of area fraction to have reduced sampling and systematic biases (Howard and Reed, 2004). Images were captured using a Nikon Eclipse E600 microscope with a Nikon DMX1200 digital camera and NIS-elements 3.20.01 software (Nikon Instruments Inc., Melville, NY). Across the 81 slides, 243 sections were analyzed and 699 images were generated.
In addition, whole livers on each of the selected sections were evaluated semi-quantitatively for the occurrence (presence/absence) of lesions (basophilic foci, clear cell foci, mixed foci, and adenomas). In addition to these counts, qualitative observations were recorded for microvesicular vacuolation, apparent lipid accumulation and staining properties of the liver. Foci and adenoma identifications were based on Vogelbein et al. (1990), Boorman et al. (1997), Vethaak and Wester (1996), and Blazer et al. (2006). Microvesicular vacuolation identification was based on Tandra et al. (2011), and areas of lymphocyte accumulation were compared to images and descriptions in Boorman et al. (1997). As in other histopathological studies, the identification of lesions can be challenging due to stain variation, sex differences, and fixation inconsistencies (Boorman et al., 1997), and as such, normal parenchyma was diagnosed based on the staining within each individual liver.
In conjunction with these counts, all livers were scored using a severity index for degree of vacuolation based on a scale from 0-4 (Table S2) modified from Köhler et al. (1992) and Shackelford et al. (2002). The ethanol dehydration process extracted lipids, leaving vacuoles with smooth margins as apparent sites of former lipid. Therefore, lipid vacuoles were identified based on their shape (circular) and precise margins (van Dyk et al., 2007; Wills et al., 2010; Wolf et al., 2015; Wolf and Wolfe, 2005).
To supplement severity scoring, percent area of vacuolation for each image was measured in ImageJ 1.48 (Rasband, 2014, Bethesda, Maryland, USA) using the color deconvolution plugin set to the pre-defined H&E 2 vectors (Landini, 2015; Ruifrok and Johnston, 2001). If computer-generated percent area of vacuolation did not correspond to recorded assessments (Table S2), then scores were determined based on visual evaluation. Additional details on this method are provided in Supplementary Materials.
To calculate percent area of the liver occupied by alterations, lesions were traced in ImageJ. Because the structures of interest were within the hepatocellular compartment of the parenchyma, removal of other structures/components was necessary. For this, area occupied by hepatopancreas, blood vessels and bile ducts ≥ 1.25-μm diameter and sectioning artifacts were measured; smaller ducts and vessels were disregarded (Fournie and Vogelbein, 1994). These measurements were averaged across sections on each slide and subtracted from the total image area to generate total parenchymal area. Percent area of alteration(s) was computed by comparing average lesion area to parenchymal area.
Statistical Analysis
After slides were unblinded, all statistical tests were run in R (R Core Team, 2015, Vienna, Austria) and significance was declared if p≤0.05. Count data were tested for normality with a Shapiro-Wilk test and were then square root transformed. These transformed data passed Bartlett's test for equality of variances and allowed us to use a nested ANOVA with a post-hoc Tukey test.
Data comparing basophilic foci counts and total alterations to the sex of each fish were also square root transformed, passed Bartlett's test, and used for one-way ANOVA with a post-hoc Tukey test (Table S3). In this analysis, six individuals were excluded because the sex of these fish was unclear due to inadequate gonadal tissue in the sections. In addition, numbers of individuals with alterations were compared within and between AW and KC populations using a chi-squared goodness of fit test. Lastly, severity scores were compared within and between AW and KC populations using a Kruskal-Wallis test and compared to occurrence of microvesicular vacuolation using a Spearman rank correlation coefficient.
One AW control individual displayed numerous (N=47) eosinophilic foci, a noteworthy phenotype. These foci were irregularly shaped with sharp borders, had cleft-like spaces between enlarged hepatocytes, contained bi-nucleated hepatocytes, but had no mitotic figures (Vogelbeinet al., 1990; Okihiro and Hinton, 2000; Boorman et al., 1997; Blazer et al., 2006; Braunbeck et al., 1992) (Figure S2). However, while this single occurrence may indicate that AW killifish are not fully protected from liver disease, the extreme number of foci in this individual categorized it as an outlier in the data set and therefore it was removed from all statistical analyses.
Results
Within 2-mo after ERSE exposure, AW fish in all groups, including the control, had higher mortality relative to KC fish, and only KC fish exposed to 1.0% ERSE had significantly higher mortality relative to other KC groups (Brown et al., 2016). By 5-mo, no differences in mortality between any exposure groups was seen (Brown et al., 2016).
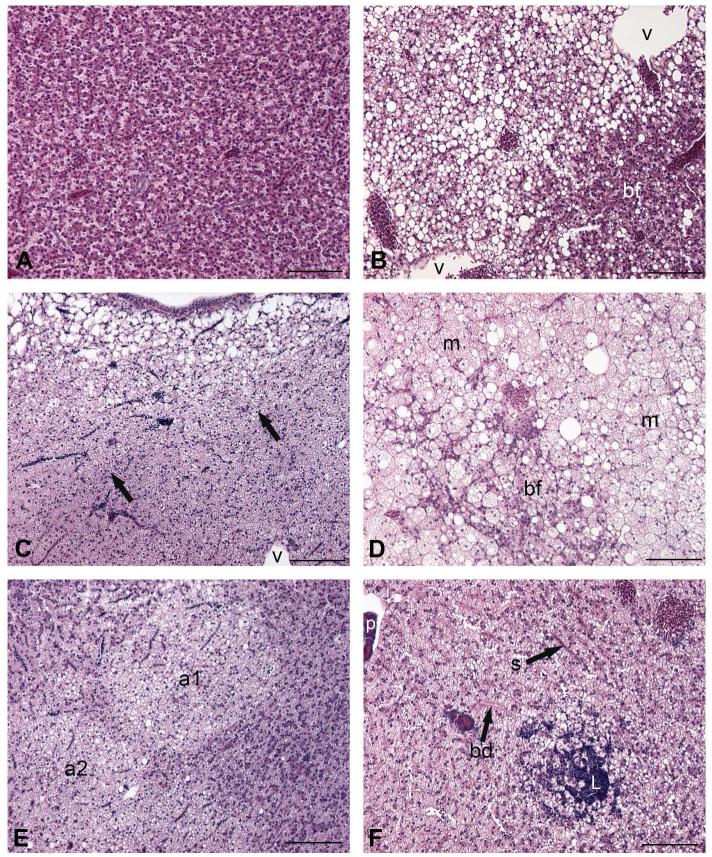
Histological evaluation of control and ERSE-exposed KC and AW livers yielded the following hepatic alterations: basophilic foci, clear cell foci, mixed foci, adenomas, and lymphocyte aggregates (Figure 2). Uniform staining characterized normal parenchyma (Figure 2A). Classifications of basophilic foci (Figure 2B, 2D), clear cell foci (Figure 2C), and adenomas (Figure 2E) were consistent with descriptions from Vogelbein et al. (1990), Boorman et al. (1997), and Vethaak and Wester (1996). Non-neoplastic lesions, predominantly clear cell and basophilic foci, were the most commonly identified alterations (Table 1, Figure 3B, Figure S1). Slightly more than half of adenomas (57%) were classified as clear cell lesions as shown in Figure 2E. Additionally, one basophilic adenoma was identified due to its sharp border and dark staining.
Figure 2.
Morphology of liver and hepatocellular alterations in Fundulus heteroclitus. A, normal parenchyma, note uniformity of hepatocytes and their staining. B-F altered liver. B, basophilic focus (bf); v, sections through veins. Due to the absence of overt hepatopancreas, venous profiles cannot be identified as portal (afferent) or hepatic (efferent). C, clear cell focus; arrows point to border of lesion; v, vein. D, mixed focus composed of basophilic focus (bf) and microvesicular vacuolation (m) surrounding the focus. E, clear cell adenomas (a1 and a2) are separated by a band of normal-appearing tissue. F, lymphocyte aggregate (L) in hepatic parenchyma near bile ductules (bd); s, sinusoid; venous profile at upper left of field shows portion of exocrine pancreatic tissue (p), likely indicating portal vein. All scale bars 10 μm. 20X, H&E.
TABLE 1.
Summary of raw counts of alterations and transformed averages of lesions per fish in each exposure group. Gray line denotes split cells; the top values of which represent raw counts and bottom values transformed means.
| King's Creek Control (N = 18) | King's Creek 0.1% ERSE (N = 11) | King's Creek 1.0% ERSE (N = 11) | Atlantic Woods Control (N = 13) | Atlantic Woods 0.1% ERSE (N = 11) | Atlantic Woods 1.0% ERSE (N = 16) | |
|---|---|---|---|---|---|---|
| Basophilic Foci | 11 | 33 | 20 | 18 | 13 | 42 |
| 0.51a | 1.41b | 1.24b | 1.01ab | 0.96ab | 1.46b | |
| Clear Cell Foci | 1 | 6 | 7 | 7 | 4 | 16 |
| 0.056b | 0.49ab | 0.58ab | 0.49ab | 0.22b | 0.85a | |
| Mixed Foci | 1 | 1 | 0 | 2 | 0 | 1 |
| Adenoma | 0 | 3 | 1 | 0 | 1 | 3 |
| Lymphocyte Aggregation | 0 | 3 | 0 | 0 | 0 | 0 |
| Total Foci | 13 | 40 | 27 | 27 | 17 | 59 |
| 0.58b | 1.67a | 1.46a | 1.37a | 1.05ab | 1.79a | |
| Total Alterations | 13 | 46 | 28 | 27 | 18 | 62 |
| 0.587b | 1.9a | 1.48a | 1.37a | 1.08ab | 1.83a |
Different letters indicate statistical differences in each row. Nested ANOVA with post-hoc Tukey test (p≤0.05).
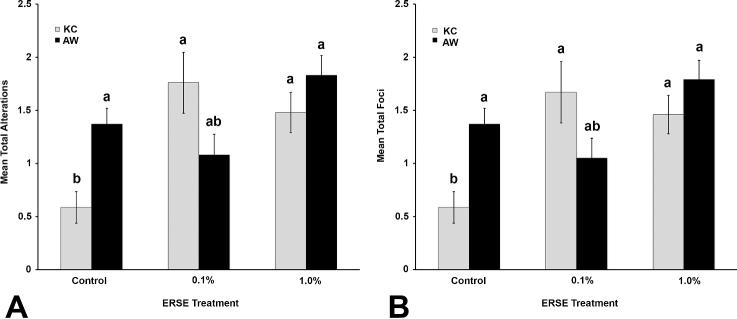
FIGURE 3.
Histogram representing total alterations (A) and total foci (B) in King's Creek (gray bars) and Atlantic Wood (black bars) populations across all ERSE treatment groups. Bars represent means of transformed data with error bars representing standard error of the mean. Different letters indicate statistical differences. Nested ANOVA with post-hoc Tukey test (p≤0.05).
Overall, AW fish (control and exposed) had significantly more total alterations (p=0.042), total foci (p=0.037), and clear cell foci (p=0.035) than did KC fish (control and exposed). More specifically, AW control fish had significantly more total alterations (p=0.031) and total foci (p=0.027) than did KC control individuals (Figure 3). The 0.1% ERSE-exposed KC fish had significantly more total alterations (p<0.001), total foci (p=0.001), and basophilic foci (p=0.014) than did KC control individuals (Table 1, Figure 3, Figure S1A). The 1.0% ERSE-exposed AW fish had significantly more clear cell foci than the 0.1% exposed AW individuals (p = 0.023; Table 1, Figure S1B). Interestingly, lymphocyte aggregates were restricted to 0.1% ERSE-exposed KC fish, and nearly 27% of individuals in this group showed this condition (Figure 2F, Table 1).
Individuals in each control and treatment group were analyzed for lesion prevalence (as percent of individuals in each treatment displaying at least one alteration) (Table 2) and lesion area. Despite robust testing, no significant differences were observed, but trends were noted with respect to basophilic and clear cell foci in each population (Table 2) that were similar to that of count data above. For example, ERSE exposure increased the number of individuals with basophilic foci in a dose-dependent manner in the KC population. However, a near doubling of individuals in the AW control contained basophilic foci and exposure to ERSE did not appreciably affect this number. In addition, out of the 36 occurrences of microvesicular vacuolation, 25 (69%) were observed in fish that scored a 2, 3, or 4 on the vacuolation severity index. The correlation between these two observations was not determined to be statistically significant but could also be indicative of a trend.
TABLE 2.
Hepatic lesion prevalence. Percentages of individuals exhibiting a specific alteration calculated as the number of individuals with at least one occurrence of that alteration within a treatment.
| Population | ERSE Treatment | Basophilic Foci (%) | Clear Cell Foci (%) | Mixed Foci (%) | Adenomas (%) | Microvesicular Vacuolation (%) |
|---|---|---|---|---|---|---|
| KC | 0% (Control) | 44 | 5.6 | 5.6 | 0 | 39 |
| KC | 0.1% | 82 | 45 | 9.1 | 27 | 45 |
| KC | 1.0% | 91 | 55 | 0 | 9.1 | 55 |
| AW | 0% (Control) | 85 | 46 | 15 | 0 | 31 |
| AW | 0.1% | 82 | 18 | 0 | 9.1 | 36 |
| AW | 1.0% | 88 | 75 | 0 | 19 | 25 |
Regarding the 80 fish assessed in the vacuolation severity index (Table S2), the quantitative ImageJ methodology matched severity scores in 44 (55%) of fish examined. The remaining individuals were evaluated semi-qualitatively to assign a severity score (see additional discussion in Supplementary Materials). Comparisons of assigned severity scores did not yield statistically significant differences. Based on the relative compatibility of the quantitative, semi-quantitative and qualitative methods for evaluating vacuolation, we believe that lipids were identified to the best of our ability given that a lipid-specific stain was not performed.
Discussion
PAH adaptation in Fundulus heteroclitus from creosote-contaminated areas of the Elizabeth River is a currently evolving area of research. Early studies by Vogelbein et al. (1990) demonstrated that field-collected, creosote-exposed killifish developed 93% more hepatocellular lesions than reference site fish. Work on AW embryos suggests that offspring may be resistant to the effects of PAHs (Clark et al., 2014; Meyer and Di Giulio, 2003; Ownby et al., 2002; Wills et al., 2010; Clark et al., 2013), but post-hatch stages do not appear to reflect this protection (Vogelbein and Unger, 2006). Although Wills et al. (2010) reported that 9-mo old fish may be protected from hepatic lesion development, their study was based on killifish exposed to only BaP, whereas our study exposed fish to contaminated sediment extract, which contained a mixture of PAHs and other compounds (Fang et al., 2014).
Our use of sediment extract as opposed to single PAHs may partially explain the differences we observed. Similarly, Braunbeck (1998) found that exposing zebrafish to a mixture of 3,4-dichloroaniline and lindane induced different hepatocellular alterations compared to individual exposures. Our study supports previous findings from Vogelbein et al. (1990), Pinkney and Harshbarger (2006), Schmalz Jr. et al. (2002), Vogelbein and Unger (2006), and Wills et al. (2010) confirming that PAH exposure induces hepatic lesions in killifish. As we expected, ERSE exposure induced hepatic lesion development in KC fish, and significant differences were observed between control and exposed populations for lesion prevalence. PAH-adapted killifish from the AW site are able to pass these features to their offspring (Clark et al., 2014; Meyer and Di Giulio, 2003; Ownby et al., 2002; Wills et al., 2010), yet our study found somewhat different results. Our results support a summary from Weis and Weis (1989) on the tradeoffs of pollution tolerance and stress. They reported that pollution-adapted killifish began life as tolerant embryos, but when adapted fish that had been exposed to methylmercury and inorganic mercury as embryos were evaluated as adults, they showed more signs of stress (slower regeneration rate, reduced feeding, etc.) compared to reference killifish (Weis and Weis, 1989). Our findings agree that the AW adaptation may protect the fish until sexual maturity, but our results also suggest that this protection from liver disease may not be conferred to later life stages. Furthermore, AW control individuals appeared different from KC control (i.e., more alterations in the former than the latter). Taken together, AW killifish may not be completely protected from the development of hepatic lesions, and the protection related to the heart may not extend to the liver. As Weis and Weis (1989) discuss, this is evolutionarily logical because acute effects—such as cardiac abnormalities—can drive adaptations at a population level whereas chronic conditions such as liver neoplasia are not often associated with population-wide genetic changes.
Meyer and Di Giulio (2003) found that F1 AW larvae showed reduced survivorship under clean laboratory conditions compared to offspring of reference site individuals. These findings suggest that pollution-adapted fish may undergo a “reverse” selection when grown in the laboratory (i.e., removed from a polluted environment) (Meyer and Di Giulio, 2003). Additionally, our use of a single exposure may not be sufficient to result in hepatic tumor formation. Accordingly, laboratory studies of longer duration and repeated exposures may be needed for us to determine whether tumors result from ERSE exposure. Continued field studies should allow us to determine over time whether the recent remediation of the AW site will affect tumor abundance in neighboring native killifish.
Wills et al. (2010) showed that lesion incidence in killifish increased with increasing concentrations of BaP — a potent carcinogenic PAH. Our data support this and suggest that exposure to ERSE was related to higher hepatic lesion prevalence in the KC population. Such was not the case in the AW population, whose rate of alterations remained fairly constant across all treatment groups. In our study, differences between KC 0.1% ERSE-exposed fish and KC control were most pronounced in terms of lesion development. The 0.1%-exposed KC population was the only cohort that showed all alterations and the only group to have lymphocyte aggregates, indicating that it could be the most adversely affected group in our study. Because no other groups showed these aggregates, protection/alteration may involve the immune system. While this is potentially of importance, it remains conjecture at this point. Perhaps more metabolic transformation of PAHs occurred among the 0.1% ERSE-exposed KC population. With more host metabolism, procarcinogens in PAHs could have been activated to carcinogenic forms. PAH metabolites could be one of the main drivers of cancer (Gupta, 2012; Stalker et al., 1991; Aas et al., 2001), resulting in more hepatocellular changes including neoplastic alterations (Hinton et al., 2008). Likewise, Hendricks et al. (1985) found increased hepatocellular alterations, specifically basophilic foci and carcinomas, in rainbow trout (Salmo gairdneri) exposed to BaP by diet or intraperitoneal injection. The lack of carcinomas in our study was not surprising given the age of the fish, for advanced neoplastic lesions are rarely observed in young individuals (Wills et al., 2010). During the initial screening process, we noted that numerous females showed advanced stages of oocyte development, but other individuals had not yet achieved sexual maturation. At 5-mo of age, some individuals are juveniles while others are young adults. Although not quantified, the observation that numerous 5-mo old killifish were sexually mature suggests that PAH adaptation may allow the organism to reach sexual maturity and reproduce, but the fish is highly prone to developing liver disease later in life. As such, this adaptation may not be sustainable for the life of the organism.
Although exposed KC fish developed more basophilic foci, no advanced neoplastic lesions such as carcinomas of liver or pancreas were observed in our study. It is possible that these advanced lesions will occur in some older fish or that single early life exposure is not enough of a perturbation to result in appreciable tumor prevalence. Foci and adenomas like we observed are known to play important roles in the pathogenesis of teleost liver tumors. Work by Vethaak and Wester (1996) on flounder (Platichthys flesus) from a polluted Dutch estuary found that a decrease in foci diameter corresponded to an increase in neoplasm diameter; furthermore, foci prevalence decreased with age while neoplasm prevalence increased. These findings suggest that, over time, foci develop into neoplasms (Vethaak and Wester, 1996). While very interesting, similarities to Vethaak and Wester (1996) will have to await our evaluation of individuals older than 5-mo of age. However, our findings with individuals of known origin and age will be important. Will the lesions seen at 5-mo develop into overt liver neoplasms? To address this question, our observation period in laboratory studies will need to be extended to give us a better understanding of host response along the continuum between early life exposure and later life effects.
Throughout the course of our study, attention was drawn to clear cell foci with vacuolated cells along the border of the liver. This was observed in 17 fish, 13 of which were exposed to ERSE. With the large number of individuals in our study, special stains were not possible, but our experience in processing killifish livers employing osmium tetroxide fixation helped to illustrate the size, shape, and abundance of lipid in Fundulus hepatocytes (Blickley, 2010). Vacuoles were also compared to work by Wills et al. (2010) and van Dyk et al. (2007) in paraffin-embedded livers processed by graded ethanol dehydration that did not include frozen sections and special fat stains. There, putative lipid-storing cells were similarly identified based on empty vacuole characteristics such as: round, spherical shape and sharp borders (van Dyk et al., 2007; Wills et al., 2010; Wolf et al., 2015; Wolf and Wolfe, 2005). Vacuolated cells of clear cell foci in our evaluation did not appear to fit the description of fatty cysts from Boorman et al. (1997). Our observations could represent hepatocytes that have lost volume regulatory control and are retaining large amounts of fluids in cytoplasmic spaces (i.e., endoplasmic reticulum). A certain degree of lipid accumulation appears to be normal for killifish, and additional descriptions of non-alcoholic fatty liver disease (NAFLD) versus lipid storage in these teleosts are needed for further differentiation.
By storing PAHs in lipid droplets, animals facilitate sequestration of harmful chemicals (Gupta, 2012). If the PAH is catabolized, it becomes metabolically available for the organism, which can lead to lesion development (Gupta, 2012). Au (2004) cites several examples of PAH exposure increasing hepatocellular lipid content in fish. Schmalz Jr. et al. (2002) found that killifish in a PAH-contaminated environment had more than twice the amount of lipid as a non-adapted reference population, but our study found no correlation between lipid content and ERSE exposure. In their overview of toxicity indicators of fish liver, Wolf and Wolfe (2005) noted that the lipid content of laboratory reared flounder (Paralichthys olivaceus) decreased dramatically after return to the wild, possibly due to artificial diet and/or decreased physical activity under laboratory conditions. Similarly, liver lipid content in killifish of our study may have increased due to laboratory conditions.
The improved resolution provided by glycol methacrylate embedment and/or transmission electron microscopy in future studies could address cellular alterations that may be confused as lipid. For example, Howarth et al. (2010) used these methods to show enlarged and damaged hepatocyte mitochondria and small bile ductular alterations that could be considered as lipid vacuoles in standard H&E survey morphology. Du et al. (2015) addressed BaP toxicity mechanistically and showed it to affect mitochondrial function in adult KC killifish compared to AW. They showed that BaP disrupted mitochondrial membrane potential and ion flow (Du et al., 2015); and they posited that excess water may be crossing altered membranes and accumulating within organelles, causing them to appear as cytoplasmic vacuoles in light microscopic preparations.
Although the microvesicular vacuolation we observed resembles steatosis, additional staining and resolution are needed to resolve the differences between these phenotypes. In mammals, mitochondrial dysfunction has been linked to non-alcoholic steatohepatitis (NASH) and drug-induced liver injury (Vickers, 2009; Pessayre, 2007). Köhler et al. (1992) suggested that steatosis—fatty liver disease—can be a precursor for the development of basophilic and clear cell foci, and there is a correlation between microvesicular steatosis and markers of cellular injury including “megamitochondria,” advanced fibrosis and ballooning (Tandra et al., 2011). Although we did not explicitly identify these markers, we observed microvesicular vacuolation to contrast with neighboring parenchyma and noted that some of the empty vacuoles could be distended mitochondria. Our observation of microvesicular vacuolation across all populations suggests that AW and KC killifish may develop metabolic diseases whether or not they are exposed to ERSE. More definitive analysis will be needed to address similarities and differences with respect to PAH toxicity, genetic adaptation and metabolic alteration emanating from exposure to multiple agents in sediment such as those in Cooper et al. (1999).
In summary, by examining the response of juvenile killifish to ERSE, our study provides new insight on liver alterations in this life stage and suggests that PAH-adapted killifish may not be protected from liver disease. Although the AW adaptation may promote embryo survival, the health of the organism in later stages could be negatively affected. The AW adaptation also seems to be highly dependent on the precise components of the pollutant mixture (i.e., ERSE). AW killifish may be protected from certain PAHs while exposure to multiple compounds does not seem to protect fish from deleterious effects. PAH adaptation is known to have a strong genetic component (Meyer and Di Giulio, 2003; Clark et al., 2014; Ownby et al., 2002; Wills et al., 2010; Clark et al., 2013), but our study demonstrates that environmental aspects should also be considered.
Future work will include evaluations of the immune system, expression of genes at the juvenile life stage, effects of sex and reproductive status of the individual, and a detailed histopathological evaluation of other organs because studies to date have focused on heart, large veins and the liver. In addition, effects of repeated or longer term exposures on the rate of tumorigenesis could elucidate important environmental factors. Finally, future studies could provide more insight on the regulation of genetic adaptations as well as the long- and short-term effects of PAH exposure on the health and survival of aquatic organisms.
Supplementary Material
Acknowledgements
This work was funded by the NIH Superfund Research Center (NIEHS P42-ES010356) and completed as part of Center-supported research for undergraduates program. We would like to thank the Analytical Chemistry Core at Duke University, specifically Ellen Cooper, for their assistance with ERSE characterization. Any findings, conclusions, opinions, and recommendations are those of the authors and do not necessarily reflect the view of the NIH.
Abbreviations
- AW
Atlantic Wood
- AWI
Atlantic Wood Industries
- BaP
benzo[a]pyrene
- dpf
days post-fertilization
- ERSE
Elizabeth River Sediment Extract
- H&E
hematoxylin & eosin
- hpf
hours post-fertilization
- KC
King's Creek
- PAHs
polycyclic aromatic hydrocarbons
REFERENCES
- Aas E, Beyer J, Jonsson G, Reichert WL, Andersen OK. Evidence of uptake, biotransformation and DNA binding of polyaromatic hydrocarbons in Atlantic cod and corkwing wrasse caught in the vicinity of an aluminium works. Marine Environmental Research. 2001;52:213–229. doi: 10.1016/s0141-1136(00)00269-5. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Gold LS. DNA lesions, inducible DNA repair, and cell division: three key factors in mutagenesis and carcinogenesis. Environmental Health Perspectives. 1993;101:35–44. doi: 10.1289/ehp.93101s535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong PB, Child JS. Stages in the normal development of Fundulus heteroclitus. Biological Bulletin. 1965;128:143–168. [Google Scholar]
- Au DWT. The application of histo-cytopathological biomarkers in marine pollution monitoring: a review. Marine Pollution Bulletin. 2004;48:817–834. doi: 10.1016/j.marpolbul.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Baumann PC, Harshbarger JC, Hartman KJ. Relationship between liver tumors and age in brown bullhead populations from two Lake Erie tributaries. Science of the Total Environment. 1990;94:71–87. doi: 10.1016/0048-9697(90)90365-2. [DOI] [PubMed] [Google Scholar]
- Blazer VS, Fournie JW, Wolf JC, Wolfe MJ. Diagnostic criteria for proliferative hepatic lesions in brown bullhead Ameiurus nebulosus. Diseases of Aquatic Organisms. 2006;72:19–30. doi: 10.3354/dao072019. [DOI] [PubMed] [Google Scholar]
- Blickley TM. The toxicological effects of engineered nanoparticles, quantum dots, in estuarine fish. Ph.D. Dissertation. Duke University, Nicholas School of the Environment; Durham, NC.: 2010. [Google Scholar]
- Boorman GA, Botts S, Dunton TE, Fournie JW, Harshbarger JC, Hawkins WE, Hinton DE, Jokinen MP, Okihiro MS, Wolfe MJ. Diagnostic criteria for degenerative, inflammatory, proliferative nonneoplastic and neoplastic liver lesions in medaka (Oryzias lapites): consensus of a National Noxicology Program Pathology Working Group. Toxicologic Pathology. 1997;25:202–210. doi: 10.1177/019262339702500210. [DOI] [PubMed] [Google Scholar]
- Braunbeck T. In: Cytological alterations in fish hepatocytes following in vivo and in vitro sublethal exposure to xenobiotics--structural biomarkers of environmental contamination In Fish Ecotoxicology. Braunbeck T, et al., editors. Birkhauser Verlag; Basel, Switzerland: 1998. pp. 61–140. [Google Scholar]
- Braunbeck TA, Teh SJ, Lester SM, Hinton DE. Ultrastructural alterations in liver of medaka (Oryzias latipes) exposed to diethylnitrosamine. Toxicologic Pathology. 1992;20:179–196. doi: 10.1177/019262339202000205. [DOI] [PubMed] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicology and Teratology. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gómez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, MacLatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL. Fundulus as the premier teleost model in environmental biology: Opportunities for new insights using genomics. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2007;2:257–286. doi: 10.1016/j.cbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Bone AJ, Di Giulio RT. Resistance to teratogenesis by F1 and F2 embryos of PAH-adapted Fundulus heteroclitus is strongly inherited despite reduced recalcitrance of the AHR pathway. Environ Sci Pollut Res. 2014;21:13898–13908. doi: 10.1007/s11356-013-2446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound- and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River Estuary (Virginia, USA). Environmental Science & Technology. 2013;47:10556–10566. doi: 10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PS, Vogelbein WK, Van Veld PA. Altered expression of the xenobiotic transporter P-glycoprotein in liver and liver tumours of mummichog Fundulus heteroclitus from a creosote-contaminated environment. Biomarkers. 1999;4:48–58. doi: 10.1080/135475099230994. [DOI] [PubMed] [Google Scholar]
- Di Giulio RT, Clark BW. The Elizabeth River story: a case study in evolutionary toxicology. Journal of Toxicology and Environmental Health, Part B. 2015;18:259–298. doi: 10.1080/15320383.2015.1074841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Crawford DL, Oleksiak MF. Effects of anthropogenic pollution on the oxidative phosphorylation pathway of hepatocytes from natural populations of Fundulus heteroclitus. Aquatic Toxicology. 2015;165:231–240. doi: 10.1016/j.aquatox.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Fang M, Getzinger GJ, Cooper EM, Clark BW, Garner LVT, Di Giulio RT, Ferguson PL, Stapleton HM. Effect-directed analysis of Elizabeth River porewater: developmental toxicity in zebrafish (Danio rerio). Environmental Toxicology and Chemistry. 2014;33:2767–2774. doi: 10.1002/etc.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie JW, Vogelbein WK. Exocrine pancreatic neoplasms in the mummichog (Fundulus heteroclitus) from a creosote-contaminated site. Toxicologic Pathology. 1994;22:237–247. doi: 10.1177/019262339402200302. [DOI] [PubMed] [Google Scholar]
- Frederick LA, Van Veld PA, Rice CD. Bioindicators of immune function in creosote-adapted estuarine killifish, Fundulus heteroclitus. Journal of Toxicology and Environmental Health, Part A. 2007;70:1433–1442. doi: 10.1080/15287390701382910. [DOI] [PubMed] [Google Scholar]
- Gupta RC. Veterinary toxicology: basic and clinical principles. Elsevier Academic Press; San Diego, CA.: 2012. [Google Scholar]
- Helder T. Effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) on early life stages of rainbow trout (Salmo gairdneri, Richardson). Toxicology. 1981;19:101–112. doi: 10.1016/0300-483x(81)90092-5. [DOI] [PubMed] [Google Scholar]
- Hendricks JD, Meyers TR, Shelton DW, Casteel JL, Bailey GS. Hepatocarcinogenicity of benzo[a]pyrene to rainbow trout by dietary exposure and intraperitoneal injection. Journal of the National Cancer Institute. 1985;74:839–851. [PubMed] [Google Scholar]
- Hinton DE, Segner H, Au DWT, Kullman SW, Hardman RC. In: Liver toxicity In The Toxicology of Fishes. DiGiulio RT, Hinton DE, editors. CRC Press - Taylor & Francis Group; Boca Raton, FL.: 2008. pp. 327–400. [Google Scholar]
- Howard CV, Reed MG. Unbiased stereology: three-dimensional measurement in microscopy. Garland Science; New York, NY.: 2004. [Google Scholar]
- Howarth DL, Law SHW, Law JM, Mondon JA, Kullman SW, Hinton DE. Exposure to the synthetic FXR agonist GW4064 causes alterations in gene expression and sublethal hepatotoxicity in eleutheroembryo medaka (Oryzias latipes). Toxicology and Applied Pharmacology. 2010;243:111–121. doi: 10.1016/j.taap.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung DW, Matson CW, Collins LB, Laban G, Stapleton HM, Bickham JW, Swenberg JA, Di Giulio RT. Genotoxicity in Atlantic killifish (Fundulus heteroclitus) from a PAH-contaminated Superfund site on the Elizabeth River, Virginia. Ecotoxicology (London, England) 2011;20:1890–1899. doi: 10.1007/s10646-011-0727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler A, Deisemann H, Lauritzen B. Histological and cytochemical indices of toxic injury in the liver of dab Limanda limanda. Marine Ecology Progress Series. 1992;91:141–153. [Google Scholar]
- Landini G. Color Deconvolution plugin In NIH ImageJ 1.48. 2015 Website: http://www.mecourse.com/landinig/software/cdeconv/cdeconv.html.
- Meyer J, Di Giulio R. Patterns of heritability of decreased EROD activity and resistance to PCB 126-induced teratogenesis in laboratory-reared offspring of killifish (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, VA, USA. Marine Environmental Research. 2002;54:621–626. doi: 10.1016/s0141-1136(02)00170-8. [DOI] [PubMed] [Google Scholar]
- Meyer JN, Di Giulio RT. Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecological Applications. 2003;13:490–503. [Google Scholar]
- Okihiro MS, Hinton DE. Partial hepatectomy and bile duct ligation in rainbow trout (Oncorhynchus mykiss): histologic, immunohistochemical and enzyme histochemical characterization of hepatic regeneration and biliary hyperplasia. Toxicologic Pathology. 2000;28:342–356. doi: 10.1177/019262330002800215. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JM. The normal stages of Fundulus heteroclitus. The Anatomical Record. 1937;68:1–15. [Google Scholar]
- Ownby DR, Newman MC, Mulvey M, Vogelbein WK, Unger MA, Arzayus LF. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environmental Toxicology and Chemistry. 2002;21:1897–1902. [PubMed] [Google Scholar]
- Pessayre D. Role of mitochondria in non-alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology. 2007;22:S20–S27. doi: 10.1111/j.1440-1746.2006.04640.x. [DOI] [PubMed] [Google Scholar]
- Pinkney AE, Harshbarger JC. Tumor prevalence in mummichogs from the Delaware Estuary watershed. J. Aquat. Anim. Health. 2006;18:244–251. doi: 10.1577/H05-053.1. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing. Vienna, Austria: 2015. Web Site: http://www.R-project.org/ [Google Scholar]
- Rasband WS. ImageJ 1.46. U. S. National Institutes of Health, Bethesda, Maryland, USA; 2014. Web Site: http://imagej.nih.gov/ij/ [Google Scholar]
- Rotchell JM, Miller MR, Hinton DE, Di Giulio RT, Ostrander GK. In: Chemical carcinogenesis in fishes In The Toxicology of Fishes. Di Giulio RT, Hinton DE, editors. CRC Press - Taylor & Francis Group; Boca Raton, FL.: 2008. pp. 531–596. [Google Scholar]
- Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Analytical and Quantitative Cytology and Histology. 2001;23:291–299. [PubMed] [Google Scholar]
- Schmalz WF, Jr., Hernandez AD, Weis P. Hepatic histopathology in two populations of the mummichog, Fundulus heteroclitus. Marine Environmental Research. 2002;54:539–542. doi: 10.1016/s0141-1136(02)00132-0. [DOI] [PubMed] [Google Scholar]
- Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicologic Pathology. 2002;30:93–96. doi: 10.1080/01926230252824761. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquatic Toxicology. 1991;19:41–71. [Google Scholar]
- Stalker MJ, Kirby GM, Kocal TE, Smith IR, Hayes MA. Loss of glutathione S-transferases in pollution-associated liver neoplasms in thite suckers (Catostomus commerson) from Lake Ontario. Carcinogenesis. 1991;12:2221–2226. doi: 10.1093/carcin/12.12.2221. [DOI] [PubMed] [Google Scholar]
- Stine CB, Smith DL, Vogelbein WK, Harshbarger JC, Gudla PR, Lipsky MM, Kane AS. Morphometry of hepatic neoplasms and altered foci in the mummichog, Fundulus heteroclitus. Toxicologic Pathology. 2004;32:375–383. doi: 10.1080/01926230490440899. [DOI] [PubMed] [Google Scholar]
- Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Ünalp-Arida A, Wilson LA, Chalasani N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. Journal of Hepatology. 2011;55:654–659. doi: 10.1016/j.jhep.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA . Peer consultation workshop on approaches to polycyclic aromatic hydrocarbon (PAH) health assessment, 40CFR Part 261. US Government Printing Office; Washington, DC.: 2002. [Google Scholar]
- van Dyk JC, Pieterse GM, van Vuren JHJ. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicology and Environmental Safety. 2007;66:432–440. doi: 10.1016/j.ecoenv.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Van Veld PA, Nacci DE. In: Toxicity resistance In Toxicology of Fishes. DiGiulio RT, Hinton DE, editors. CRC Press - Taylor & Francis Group; Boca Raton, FL.: 2008. pp. 597–641. [Google Scholar]
- Vethaak AD, Wester PW. Diseases of flounder Platichthys flesus in Dutch coastal and estuarine waters, with particular reference to environmental stress factors. II. Liver histopathology. Diseases of Aquatic Organisms. 1996;26:99–116. [Google Scholar]
- Vickers AEM. Characterization of hepatic mitochondrial injury induced by fatty acid oxidation inhibitors. Toxicologic Pathology. 2009;37:78–88. doi: 10.1177/0192623308329285. [DOI] [PubMed] [Google Scholar]
- Vogelbein WK, Fournie JW, Cooper PS, Van Veld PA. Hepatoblastomas in the mummichog, Fundulus heteroclitus (L.), from a creosote-contaminated environment: a histologic, ultrastructural and immunohistochemical study. Journal of Fish Diseases. 1999;22:419–431. [Google Scholar]
- Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Research. 1990;50:5978–5986. [PubMed] [Google Scholar]
- Vogelbein WK, Unger MA. Liver carcinogenesis in a non-migratory fish: the association with polycyclic aromatic hydrocarbon exposure. Bull. Eur. Assoc. Fish Pathol. 2006;26:11–20. [Google Scholar]
- Walker SE, Dickhut RM. Sources of PAHs to sediments of the Elizabeth River, VA. Soil. Sediment. Contam. 2001;10:611–632. [Google Scholar]
- Weis JS, Weis P. Tolerance and stress in a polluted environment. Bioscience. 1989;39:89–95. [Google Scholar]
- Wills LP, Jung D, Koehrn K, Zhu S, Willett KL, Hinton DE, Di Giulio RT. Comparative chronic liver toxicity of benzo[a]pyrene in two populations of the Atlantic killifish (Fundulus heteroclitus) with different exposure histories. Environmental Health Perspectives. 2010;118:1376–1381. doi: 10.1289/ehp.0901799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JC, Baumgartner WA, Blazer VS, Camus AC, Engelhardt JA, Fournie JW, Frasca S, Groman DB, Kent ML, Khoo LH, Law JM, Lombardini ED, Ruehl-Fehlert C, Segner HE, Smith SA, Spitsbergen JM, Weber K, Wolfe MJ. Nonlesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies: a guide for investigators, authors, reviewers, and readers. Toxicologic Pathology. 2015;43:297–325. doi: 10.1177/0192623314540229. [DOI] [PubMed] [Google Scholar]
- Wolf JC, Wolfe MJ. A brief overview of nonneoplastic hepatic toxicity in fish. Toxicologic Pathology. 2005;33:75–85. doi: 10.1080/01926230590890187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.