Abstract
Creativity relies on a diverse set of cognitive processes associated with distinct neural correlates, and one important aspect of creativity, divergent thinking, has been associated with the hippocampus. However, hippocampal contributions to another important aspect of creativity, convergent problem solving, have not been investigated. We tested the necessity of hippocampus for convergent problem solving using a neuropsychological method. Participants with amnesia due to hippocampal damage (N=5) and healthy normal comparison participants (N=5) were tested using a task that promoted solutions based on existing knowledge (Bowden and Jung-Beeman, 2003). During each trial, participants were given a list of three words (e.g., fly, man, place) and asked to respond with a word that could be combined with each of the three words (e.g., fire). The amnesic group produced significantly fewer correct responses than the healthy comparison group. These findings indicate that the hippocampus is necessary for normal convergent problem solving and that changes in the status of the hippocampus should affect convergent problem solving in the context of creative problem-solving across short intervals. This proposed contribution of the hippocampus to convergent problem solving is consistent with an expanded perspective on hippocampal function that acknowledges its role in cognitive processes beyond declarative memory.
Keywords: hippocampus, amnesia, MTL, convergent problem solving, insight, creativity
Main Text
Creativity can describe many kinds of cognition including open-ended generation of novel ideas (i.e., divergent thinking) or deliberate focus on solving a specific problem (i.e., convergent thinking or convergent problem solving). For example, divergent thinking processes might be engaged by imagining many potential uses for a paperclip; convergent thinking processes might be used to solve a specific problem such as how to use a paperclip to snag a cable that has slipped behind your desk. Creative thinking of either type must integrate many distinct cognitive processes, and similar diversity would therefore be expected of creativity's neural correlates (Abraham, 2013; Fink et al., 2007; Sawyer, 2011; Simonov, 1997). That diversity is reflected in recent findings from neuropsychological and neuroimaging investigations suggesting that the hippocampus — a structure often thought to be solely associated with long-term declarative memory (Squire et al., 2004) — contributes to creative thinking (Chávez-Eakle et al., 2007; Duff et al., 2013; Ellamil et al., 2012; Luo and Niki, 2003). These hippocampal contributions appear to be necessary for some forms of creative thinking because patients with bilateral hippocampal damage show deficits in divergent thinking (Duff et al., 2013). This finding is consistent with the predictions of relational memory theory which suggests that the hippocampus is necessary for the rapid binding of arbitrarily-related information (Davachi and Dobbins, 2008; Eichenbaum and Cohen, 2001; Eichenbaum and Cohen, 2014; Ranganath, 2010). The necessity of hippocampal relational processing for normal divergent thinking may also hold for convergent problem solving, but this relationship has not been examined neuropsychologically. Evaluating the contributions of the hippocampus to a second major component of creative thought would have important implications for theories addressing the brain bases of creativity.
Evidence indicating that the hippocampus contributes to one aspect of creativity, divergent thinking, has come from research using the Torrance Tests of Creative Thinking (TTCT). The TCTT is a commonly used, multipart test of divergent thinking that has been employed in neuroimaging and neuropsychological investigations. For example, increased hippocampal activation at rest has been positively related to performance on the TTCT . Paralleling these results, a neuropsychological investigation using the TTCT reported that patients with bilateral hippocampal damage performed less well on the TCTT's subcomponents than healthy normal comparison participants . These converging findings indicate that the hippocampus contributes to divergent thinking and appears to be necessary for normal creative performance, but do not address whether the deficit extends to other aspects of creativity.
The necessity of the hippocampus for convergent problem solving has not been directly evaluated but prior work is suggestive. Unilateral temporal lobectomy (including hippocampus) has been shown to reduce performance on a temporally extended convergent problem solving task (Sheldon et al., 2011), and hippocampal activation has been associated with convergent problem solving when solving simple riddles (Luo and Niki, 2003). These findings could be interpreted as evidence that hippocampal-dependent declarative memory is necessary for normal convergent problem solving. A trivial interpretation of this proposition is clearly true because problem solving must be supported by existing knowledge (Addis et al., 2014). However, there is also empirical support for the more nuanced interpretation that declarative memory processes are actively used during problem solving, as shown by studies which exercise episodic declarative memory and report subsequent enhancement of divergent problem solving (although the effects did not extend to convergent problem solving) (Madore et al., 2015; Madore and Schacter, 2014). Beyond declarative memory, recent findings have shown that the hippocampus contributes to many different forms of cognition more rapidly than previously understood (Duff and Brown-Schmidt, 2012; Voss et al., 2011; Warren et al., 2014; Warren et al., 2012; Watson et al., 2013). The proposition that the hippocampus supports convergent problem solving could be tested by examining the performance of patients with hippocampal damage on a task that does not explicitly require declarative memory or impose a significant short-term memory load (Jeneson and Squire, 2012).
In the current project, we investigated the necessity of hippocampus for normal convergent problem solving using a neuropsychological methodology and an established task of convergent problem solving ability (Bowden and Jung-Beeman, 2003) that has been linked to creativity (Aiello et al., 2012; Ansburg, 2000; Storm et al., 2011) (but see Lee et al., 2014). We enrolled human patients with bilateral hippocampal damage and severe amnesia (“amnesic group”; N=5; 1F, 4M) and healthy comparison participants (“NC group”; N=5; 1F, 4M) case-matched to the patients on sex, age, education, and handedness. The amnesic group included the same participants reported by Duff et al. (2013) to have deficits in divergent thinking. There were no significant between-group differences on any demographic variable, each T(8) ≤ 1, each p > 0.3. Neuropsychologically, amnesia was defined as a difference of 25 or more points between a patient's WAIS-3/4 full-scale IQ and WMS-3 General Memory Index. All amnesic patients met this criterion and had relatively preserved cognition outside of memory functions (Table 1). Among the patients, neuroanatomy and etiology co-varied: 3 patients had relatively focal, bilateral hippocampal atrophy subsequent to anoxic events (1846, 2363, and 2563); 2 patients had very large lesions encompassing the medial temporal lobes, substantial portions of the remaining temporal lobes, and additional regions as a result of herpes simplex encephalitis (1951 and 2308). All participants provided informed consent, their participation was remunerated, and all research was conducted according to the Declaration of Helsinki.
Table 1. Demographic and neuropsychological data characterizing participants.
| Group | ID | Age | Sex | Hand | Edu. | Eti. | Chr. | FSIQ | VIQ | PIQ | DS | Inf. | GMI | AVLT | CFT C/R | COWA | HcV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amn | 1846 | 51 | F | 100 | 14 | An./SE | 21 | 84 | 89 | 79 | 10 | 8 | 57 | 7/3 | 28/6 | 24 | -4.23* |
| 1951 | 62 | M | 100 | 16 | HSE | 34 | 106 | 105 | 106 | 9 | 11 | 57 | 9/2 | 32/4 | 40 | ≫ | |
| 2308 | 58 | M | -100 | 16 | HSE | 15 | 98 | 95 | 92 | 9 | 8 | 45 | 5/0 | 32/0 | 29 | ≫ | |
| 2363 | 58 | M | 100 | 18 | An. | 16 | 98 | 112 | 83 | 8 | 13 | 73 | 8/0 | 26/5 | 26 | -2.64* | |
| 2563 | 59 | M | -80 | 16 | An. | 14 | 94 | 91 | 98 | 14 | 12 | 63 | 10/4 | 36/7 | 38 | NA | |
| Mean | 57.6 | — | — | 16.0 | — | 20.0 | 96.0 | 98.4 | 91.6 | 10.0 | 10.4 | 59.0 | 7.8/1.8 | 30.8/4.4 | 31.4 | — | |
| SD | 4.0 | — | — | 1.4 | — | 8.3 | 8.0 | 9.8 | 11.0 | 2.3 | 2.3 | 10.2 | 1.9/1.8 | 3.9/2.7 | 7.2 | — | |
| NC | Mean | 58.8 | — | — | 16.8 | — | — | — | — | — | — | — | — | — | — | — | — |
| SD | 3.3 | — | — | 1.1 | — | — | — | — | — | — | — | — | — | — | — | — |
Data are presented for each participant with amnesia, followed by amnesic (Amn) group means and healthy normal comparison (NC) group means (and standard deviations). Abbreviations: Age, years; Hand, handedness (+100=fully right handed, -100=fully left handed); Edu., education, years; Eti., Etiology; Anoxia/An., anoxic/ischemic episode, SE, status epilepticus, HSE, herpes simplex encephalitis; Chr., Chronicity, years since injury; FSIQ, WAIS 3/4 full-scale IQ; VIQ, verbal IQ; PIQ, performance IQ; DS, digit span; GMI, WMS-III general memory index; AVLT, Rey Auditory Verbal Learning Task, trial 5/30-min. delay; CFT, Complex Figure Task copy/recall; COWA, Controlled Oral Word Association task; HcV, bilateral hippocampal volumes per Allen et al. (2006). Volumes expressed in Studentized residuals vs. normative expectations:
from Allen et al.; ≫, near-complete bilateral hippocampal lesion; NA, no volume available due to contraindications for MRI.
Materials were compound remote associate problems implemented as 3-word lists (henceforth, “triads”). Each triad was composed of three words that were related to a solution or “target word” (e.g., “cream”, “skate”, and “water” are all related to “ice”). In each problem, the target word could be combined with each of the triad words to form a compound word or common two-word phrase. Triads, target words, and normative data describing solution frequency for each triad were obtained from a previous report (Bowden and Jung-Beeman, 2003). 74 triads were used in the task, and triads were always presented in the same order.
The task was to report the target word related to a triad during each trial. A practice phase consisting of 4 trials was administered prior to a main test phase which consisted of 70 trials. Task duration was 1 hour. Instructions and the task were presented visually on a computer display using Microsoft PowerPoint. Task instructions were as follows: “You will be presented a list of 3 words. Try to think of a word which relates to each of the 3 words.” The experimenter confirmed that participants understood these instructions and continued with verbal instructions during the practice phase (e.g., noting that the solution word could occur before or after each triad member). One triad was displayed for the entire duration of each trial. Participants had up to 30 sec. to report a word that they thought was related to the triad. Each trial continued until time had elapsed or participants indicated completion. Responses were scored immediately by the experimenter. Following Bowden & Jung-Beeman (2003) response times were binned by interval: 0-2 sec.; 2-7 sec.; 7-15 sec.; 15-30 sec.
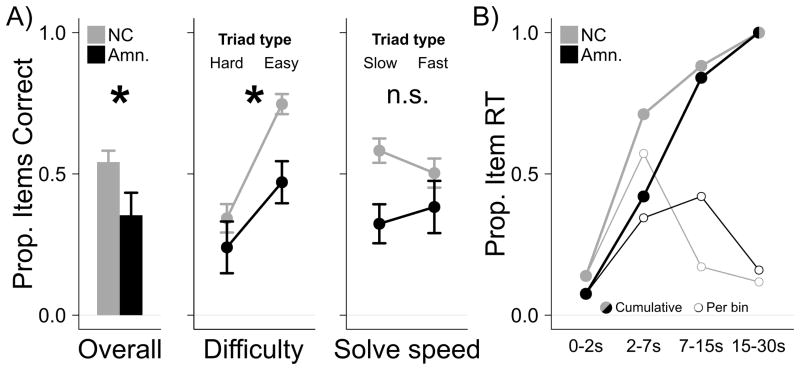
The amnesic group provided fewer correct responses than the NC group overall, Wilcox Z = 1.781, p=0.044, Cohen's d = 1.363 (Figure 1A, left). Also, while the NC group performed no differently than previously-reported normative data (NC group > norm. for 28 of 69 triads, p=0.148) the amnesic group was significantly impaired relative to the normative sample (amnesic group > norm. for 8 of 69 triads, p<0.001). This pattern of impaired task performance by the amnesic group was confirmed and extended with a generalized hierarchical logistic regression model (see Detailed Methods). According to the model analysis, the amnesic group performed less well than the NC group, Z=2.401, p=0.016, and one other factor also significantly influenced performance. Specifically, a component that varied with normative triad difficulty was significantly related to correct responses, Z=6.535, p<0.001 (Figure 1A, center). Another component that varied with normative speed to answer triads was not significantly related to correct responses, Z=1.145, p=0.252 (Figure 1A, right). Intriguingly, a significant interaction between the triad difficulty factor and group, Z=2.525, p=0.012, indicated that the amnesic group was more impaired when solving normatively easy items and less impaired when solving normatively difficult items (Figure 1A, center). We cannot fully account for this effect, but it may be partly attributable to floor effects related to difficult items.
Figure 1. Convergent problem solving performance and response time (RT).
A) Left, the amnesic group (black) was impaired relative to the NC group (gray) overall, responding correctly to a smaller proportion of triads. Middle, a significant interaction in a regression analysis indicated that the amnesic group was relatively more impaired for normatively easy items. Right, the regression analysis revealed no interaction of group with normative solution speed. Whiskers indicate SEM; *, p < 0.05 for relevant contrast or interaction, see main text. B) RT distributions show that the amnesic group responded more slowly than the NC group on average, but both groups responded in <15 sec. (of the 30 sec. response window) during at least 80% of trials. Closed circles show cumulative proportion of response time (RT) and open circles show proportion of response time by bin.
Response time (RT) was not a dependent variable of primary interest, but potential group differences in the distribution of per-trial RT across the coded timebins was analyzed using Fisher's exact test. There was evidence that the two groups responded at different speeds, Fisher's exact test p<0.001, with the NC group responding more quickly. Inspection of the RT distribution across the four time bins (Figure 1B) revealed that both groups made more than 80% of their responses in 15 sec. or less, suggesting that the 30 sec. trial duration we employed did not selectively reduce either group's measured performance.
We hypothesized that the hippocampus is necessary for normal convergent problem solving, and we observed that patients with hippocampal damage and severe amnesia (but without other cognitive deficits according to standard neuropsychological tests) showed impaired performance relative to healthy comparison participants on a task requiring convergent problem solving. This impairment suggests that the hippocampus normally contributes to convergent problem solving and parallels a previously reported deficit in the same group of patients on a measure of divergent thinking ability as well as correlational evidence of hippocampal activation with measures of creativity (Chávez-Eakle et al., 2007; Ellamil et al., 2012; Luo and Niki, 2003). Our findings suggest that deficits in convergent problem solving may contribute to broader impairment of creativity, while deficits in other aspects of creativity or on-line processing that our study did not measure may also be important.
A role for the hippocampus in convergent problem solving and creativity is consistent with an emerging perspective that this structure contributes to cognition beyond declarative memory (Barense et al., 2007; Duff and Brown-Schmidt, 2012; Voss et al., 2011; Warren et al., 2012; Watson et al., 2013). Two common themes run through these findings. First, the hippocampus is necessary for processing and storing information about arbitrary relations among items. Second, hippocampus contributes to cognition much more rapidly than expected and in contexts that do not explicitly require long-term memory processes such as maintaining information over very short intervals (e.g., < 1 sec.). These fundamental deficits in relational processing and maintenance of information may address why damage to the hippocampus has such wide-ranging effects on cognitive processes including memory, language, social judgments, and expert performance. Importantly, our implementation of the convergent problem solving task was intended to limit memory load and task-related maintenance demands — by presenting the three words of each triad continuously throughout each trial — in order to address concerns that recruitment of long-term memory processes might be necessary for normal performance (Jeneson and Squire, 2012). Regarding convergent problem solving, we suggest that this ability requires binding together a problem space and existing knowledge flexibly across short intervals, and we hypothesize that damage to the hippocampus would be expected to cause impairment to the extent that relational binding contributes to convergent problem solving. This account is consistent with our findings but must remain speculative because our study did not explicitly include a non-relational condition.
Our findings indicate that the hippocampus is necessary for normal convergent problem solving, and they are consistent with other investigations implicating the hippocampus in these processes. There is correlational evidence from neuroimaging, as with Luo & Niki (2003) who observed right hippocampal activation related to solving riddles. Notably, Luo & Niki reported that hippocampal activation was accompanied by activations in a wide array of other brain areas including frontal, cingulate, temporal, parietal, and subcortical regions; this widespread pattern of activation reinforces other reports suggesting that convergent problem solving and creativity recruit many cognitive processes relying on diverse neural correlates including portions of the prefrontal cortex (Abraham et al., 2012; Reverberi et al., 2005; Seeley et al., 2008; Shamay-Tsoory et al., 2011). Neuropsychological methodology has also contributed, as when Warren et al. (2012) showed that patients with hippocampal damage had deficits in identifying common objects based on partial visual information (Experiment 3). Although not described as such by those authors, the perceptual and semantic constraints imposed by the partial information may have engaged convergent problem solving processes (but perhaps not creativity). Finally, a recent study using the tools of cognitive psychology showed that exercising episodic memory processes associated with the hippocampus enhanced subsequent creativity as indexed by a test of divergent thinking (Madore et al., 2015). Interestingly, those authors also reported a small numerical (but non-significant) increase in convergent problem solving performance as measured using triad stimuli from Bowden and Jung-Beeman (2003). In our study, we observed an unexpected inverse relationship between item difficulty and the impairment of the amnesic group. Should the findings of our post hoc item difficulty analysis be confirmed by subsequent neuropsychological research, studies adapting the methods of Madore et al. might conditionally vary item difficulty to evaluate a potential relationship between item difficulty and hippocampal involvement.
Our study had some limitations. As in many neuropsychological studies, our sample sizes were not large. However, our sample proved sufficient to observe statistically significant reductions in the performance of amnesic patients, and we were able to rely on previously-published normative data from larger samples to support certain analyses. Despite our robust findings, it is possible that the deficits we observed in convergent problem solving could be attributable to deficits in some precursor process; for example, it is possible that amnesic patients may have deficits in generating any solutions to problems rather than producing correct solutions. We suggest that this is not the case for two reasons: first, the amnesic group was not normatively impaired on neuropsychological tests requiring generation; second, their non-mnemonic cognitive abilities were generally normal. Future research might investigate this issue by requiring on-line verbalization of convergent problem solving, and we speculate that deficits in generation will not be observed.
Alternatively, recently reported deficits in semantic memory accompanying hippocampal damage (Klooster and Duff, 2015) may bear on the current findings. We believe the same underlying deficits in hippocampal dependent processing (e.g., relational binding and processing, information maintenance, representational [re]compositionality and flexibility) give rise to a range of observed behavioral disruptions including to remote semantic memory and convergent problem solving as well as to perception, language use, and social cognition as reported elsewhere. However, there are notable differences in demand characteristics between studies that suggest semantic processes played distinct roles here and in the work of Klooster & Duff (2015). Specifically, the earlier report relied predominantly on tasks requiring the open-ended generation of features or senses related to a word (i.e., probing depth of knowledge) while the current task required participants to discover the relationship between a given triad and a non-presented associate (i.e., probing more distal associations). Thus, while disruptions in remote semantic memory reported by Klooster & Duff (2015) and those in convergent problem solving reported here may share an underlying mechanism, we suggest that the current results extend the reach of hippocampal contributions to novel aspects of cognitive processing and behavior.
Lastly and regarding construct validity, we adopted an inclusive definition of convergent problem solving and operationalized it as the ability to solve triads over a short period of time (30 sec. or less). Our design did not measure subjective feelings of insight by participants (i.e., “Aha!” moments), so we cannot address whether the observed deficit in convergent problem solving by the amnesic group was accompanied by reduced subjective feelings of insight. At most, we can observe that the slightly increased response latency of the amnesic group may indicate a reduction in rapid insight, but we cannot draw strong conclusions because nearly all forms of brain damage cause some increase in response latency. Future research could address this issue by focusing on insight processes a priori.
In conclusion, our findings are consistent with the perspective that the hippocampus is necessary for normal convergent problem solving. The deficit in convergent problem solving that we observed in patients with hippocampal damage aligns with evidence from other studies that have found deficits in creativity among similar patients (Duff et al., 2009; Duff et al., 2013). These deficits in quickly and creatively combining new and old knowledge in response to task demands are consistent with the role of hippocampus described by relational memory theory (Davachi and Dobbins, 2008; Eichenbaum and Cohen, 2001; Eichenbaum and Cohen, 2014; Ranganath, 2010). The contributions of hippocampus to convergent problem solving have significant clinical implications for patients with MTL damage and other neurological disorders affecting the hippocampus.
Detailed Methods
Our test materials were selected from a published set of triad items that included data summarizing normative performance (Bowden and Jung-Beeman, 2003). In order to avoid floor effects by our participants, we selected the 74 triads that were solved most often within 15 sec. by the normative sample. Several tested items were excluded from later analysis: four triads that were used as practice items; and one triad that was entered incorrectly into our stimulus set (see SI Table S1).
Data analysis was conducted using R software (version 3.2.3) and all analyses used α=0.05. The proportion of correct responses (PC) to the sixty-nine tested triads was the primary dependent variable of interest. Our prediction that the NC group would perform better than the amnesic group was first evaluated using a non-parametric one-tailed Wilcox test, and a non-parametric variant of Cohen's d was calculated (Ivarsson et al., 2013). We also tested whether the groups performed similarly to normative data for each item. We used a binomial test: number of successes was number of triads in which the group (NC or amnesic) PC was greater than the normative sample (Bowden and Jung-Beeman, 2003); number of observations was number of triads that elicited non-floor and non-ceiling PC across the entire sample (i.e., 1.0 > sample PC > 0.0); and the null hypothesis was that successes would be distributed evenly across experimental and normative groups (p=0.5).
To supplement the preceding analysis, a generalized hierarchical logistic regression analysis was conducted to characterize the relationship between several independent variables (predictors) and trial-level accuracy (outcomes). The predictors included fixed effects for group and two continuous triad descriptors (as well as interactions of group with each descriptor) along with random effects for triad and participant. The two triad-level fixed effects were the first two orthogonal components from a principal components analysis (PCA) of normalized triad accuracy rates at each of the four intervals (2, 7, 15, and 30 sec.) from the normative data of Bowden & Jung-Beeman (2003). PCA was used to isolate orthogonal sources of variance in accuracy because normative performance at all intervals was highly correlated. We described the first and second components as (1) triad difficulty and (2) solve speed. See the Table S2 caption for more information about our PCA approach. For model terms, Z-scaled scores and corresponding p values are reported.
Supplementary Material
Acknowledgments
We would like to acknowledge our funding sources: NIH R01-DC011755 (MCD) & NIH R01-MH062500 (DEW). We would like to thank Roxanne Calderwood for testing participants and the patients for participating in our research.
Footnotes
Author contributions: D.E.W., J.K., and M.C.D. designed research; D.E.W., J.K., and M.C.D. performed research; D.E.W. analyzed data; D.E.W., J.K., and M.C.D. wrote the paper.
Conflicts of interest: The authors declare that they have no competing financial interests.
References
- Abraham A. The promises and perils of the neuroscience of creativity. Frontiers in human neuroscience. 2013;7(246):1–9. doi: 10.3389/fnhum.2013.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Beudt S, Ott DVM, Yves Von Cramon D. Creative cognition and the brain: Dissociations between frontal, parietal-temporal and basal ganglia groups. Brain Research. 2012;1482:55–70. doi: 10.1016/j.brainres.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Musicaro R, Schacter DL. Divergent thinking and constructing episodic simulations. Memory. 2014;24(1):89–97. doi: 10.1080/09658211.2014.985591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello DA, Jarosz AF, Cushen PJ, Wiley J. Firing the executive: when an analytic approach to problem solving helps and hurts. The Journal of Problem Solving. 2012;4(2) [Google Scholar]
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. Journal of Clinical and Experimental Neuropsychology. 2006;28(4):457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Ansburg PI. Individual differences in problem solving via insight. Current Psychology. 2000;19(2):143–146. [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Bowden EM, Jung-Beeman M. Normative data for 144 compound remote associate problems. Behavior research methods, instruments, & computers. 2003;35(4):634–639. doi: 10.3758/bf03195543. [DOI] [PubMed] [Google Scholar]
- Chávez-Eakle RA, Graff-Guerrero A, García-Reyna JC, Vaugier V, Cruz-Fuentes C. Cerebral blood flow associated with creative performance: A comparative study. NeuroImage. 2007;38(3):519–528. doi: 10.1016/j.neuroimage.2007.07.059. [DOI] [PubMed] [Google Scholar]
- Davachi L, Dobbins IG. Declarative memory. Current directions in psychological science. 2008;17(2):112–118. doi: 10.1111/j.1467-8721.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Frontiers in Human Neuroscience. 2012;6(69):1–11. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst JA, Tranel D, Cohen NJ. Hippocampal amnesia disrupts verbal play and the creative use of language in social interaction. Aphasiology. 2009;23(7-8):926–939. doi: 10.1080/02687030802533748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Kurczek J, Rubin R, Cohen NJ, Tranel D. Hippocampal amnesia disrupts creative thinking. Hippocampus. 2013;23(12):1143–1149. doi: 10.1002/hipo.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. The hippocampal memory system From conditioning to conscious recollection: Memory systems of the brain. New York, New York: Oxford University Press; 2001. pp. 305–343. [Google Scholar]
- Eichenbaum H, Cohen NJ. Can we reconcile the declarative memory and spatial navigation views on hippocampal function? Neuron. 2014;83(4):764–70. doi: 10.1016/j.neuron.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. NeuroImage. 2012;59(2):1783–1794. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Fink A, Benedek M, Grabner RH, Staudt B, Neubauer AC. Creativity meets neuroscience: Experimental tasks for the neuroscientific study of creative thinking. Methods. 2007;42(1):68–76. doi: 10.1016/j.ymeth.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Ivarsson A, Andersen MB, Johnson U, Lindwall M. To adjust or not adjust: Nonparametric effect sizes, confidence intervals, and real-world meaning. Psychology of Sport and Exercise. 2013;14(1):97–102. [Google Scholar]
- Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learning & Memory. 2012;19(1):15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster NB, Duff MC. Remote semantic memory is impoverished in hippocampal amnesia. Neuropsychologia. 2015;79:42–52. doi: 10.1016/j.neuropsychologia.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Huggins AC, Therriault DJ. A measure of creativity or intelligence? Examining internal and external structure validity evidence of the Remote Associates Test. Psychology of Aesthetics, Creativity, and the Arts. 2014;8(4):446–460. [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. Oxford University Press; USA: 2012. [Google Scholar]
- Luo J, Niki K. Function of hippocampus in “insight” of problem solving. Hippocampus. 2003;13(3):316–323. doi: 10.1002/hipo.10069. [DOI] [PubMed] [Google Scholar]
- Madore KP, Addis DR, Schacter DL. Creativity and memory: Effects of an episodic-specificity induction on divergent thinking. Psychological Science. 20152014:1461–1468. doi: 10.1177/0956797615591863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychology and aging. 2014;29(4):913–24. doi: 10.1037/a0038209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Reverberi C, Toraldo A, D'Agostini S, Skrap M. Better without (lateral) frontal cortex? Insight problems solved by frontal patients. Brain. 2005;128(12):2882–2890. doi: 10.1093/brain/awh577. [DOI] [PubMed] [Google Scholar]
- Sawyer K. The cognitive neuroscience of creativity: A critical review. Creativity Research Journal. 2011;23(2):137–154. [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, Miller BL. Unravelling Boléro: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131(1):39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Adler N, Aharon-Peretz J, Perry D, Mayseless N. The origins of originality: The neural bases of creative thinking and originality. Neuropsychologia. 2011;49(2):178–185. doi: 10.1016/j.neuropsychologia.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Sheldon S, McAndrews MP, Moscovitch M. Episodic memory processes mediated by the medial temporal lobes contribute to open-ended problem solving. Neuropsychologia. 2011;49(9):2439–2447. doi: 10.1016/j.neuropsychologia.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Simonov PV. Neurobiological basis of creativity. Neuroscience and behavioral physiology. 1997;27(5):585–591. doi: 10.1007/BF02463907. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Storm BC, Angello G, Bjork EL. Thinking can cause forgetting: Memory dynamics in creative problem solving. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37(5):1287–1293. doi: 10.1037/a0023921. [DOI] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):E402–9. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Cohen NJ, Tranel D. Hippocampus contributes to the maintenance but not the quality of visual information over time. Learning & Memory. 2014;22(1):6–10. doi: 10.1101/lm.037127.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ. Hiding in plain view: lesions of the medial temporal lobe impair online representation. Hippocampus. 2012;22(7):1577–1588. doi: 10.1002/hipo.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013;23(7):570–580. doi: 10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.