Abstract
Background
The prevalence of tranexamic acid (TXA) use for trauma and other conditions in children is unknown.
Objective
The objective of this study was to describe the use of TXA in US children’s hospitals for children in general, and specifically for trauma.
Methods
We conducted a secondary analysis of a large, administrative database of 36 US children’s hospitals. We included children younger than 18 years who received TXA (based on pharmacy charge codes) from 2009 to 2013. Patients were grouped into the following diagnostic categories: trauma, congenital heart surgery, scoliosis surgery, craniosynostosis/craniofacial surgery, and other, based on ICD-9 principle procedure and diagnostic codes. TXA administration and dosage, in-hospital clinical variables, and diagnostic and procedure codes were documented.
Results
A total of 35,478 pediatric encounters with a TXA charge were included in the study cohort. The proportions of children who received TXA were similar across the years 2009–2013. Only 110 encounters (0.31%) were for traumatic conditions. Congenital heart surgery accounted for more than one-half of the encounters (22,863, 64%).. Overall the median estimated weight-based dose of TXA was 22.4 mg/kg (IQR 7.3 to 84.9 mg/kg).
Conclusions
We identified a wide frequency of use and range of doses of TXA for several diagnostic conditions in children. The use of TXA among injured children, however, appears to be rare despite its common use and efficacy among injured adults. Further work is needed to recommend appropriate indications for TXA, and provide dosage guidelines among children with a variety of conditions, including trauma.
Keywords: Wounds and Injuries, Child, Tranexamic Acid
INTRODUCTION
The antifibrinolytic agent, tranexamic acid (TXA), is a lysine analogue that blocks the conversion of plasminogen to plasmin, thus decreasing fibrin clot breakdown and bleeding.1 TXA is a generic drug and is approved by the US Food and Drug Administration for the treatment of bleeding in patients with hemophilia and in patients with severe menorrhagia.2 The potential for TXA to decrease fibrinolysis, reduce bleeding and decrease blood transfusions has led to prophylactic, off-label use in major surgical procedures in both adults and children.3–5
In adults with hemorrhagic trauma, the use of TXA is arguably considered the standard of care.6,7 The use of TXA in adult trauma is based on the results of a large, multicenter, randomized placebo controlled trial (The CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Hemorrhage 2), which demonstrated that early administration of TXA could improve survival in trauma patients. This was potentially explained by the adequate inhibition of fibrinolytic activity obtained after the administration of 2 g TXA over 8 hours. In a sub-analysis, the authors also demonstrated that TXA should be administered as soon as possible after injury, because they observed a significant reduction in all-cause mortality when treatment was administered within the first 3 hours following injury, while late administration (> 3 hours after injury) seemed to increase mortality due to bleeding.8 Despite the increasing evidence of the efficacy of TXA in demonstrating reductions in surgical blood loss and blood transfusion requirements in pediatric cardiac 4, orthopedic 3, and craniofacial surgeries 5, the use of TXA has been less studied in traumatized children. A single retrospective cohort study of 766 injured children in Afghanistan reported that TXA was only used in 10% of pediatric trauma admissions, although its administration was independently associated with a 27% reduction in mortality (p=0.03).9
TXA is usually administered with a loading dose followed by a continuous infusion, although a wide variability in dosing schemes has been reported.4 In a systematic review of pediatric cardiac and non-cardiac surgery trials, dosing schemes varied from single to multiple boluses with or without maintenance infusions. Loading doses of TXA ranged from 10 to 100 mg/kg and maintenance doses ranged from 1 to 15 mg/kg/hr.3,4
Given the use of TXA in adults with hemorrhagic injuries and its efficacy in pediatric surgery, the question arises as to whether TXA should be used in children with hemorrhagic injuries. Some experts advocate for the use of TXA in severely injured children,10 and others recommend specific doses of TXA for injured children.11 However, it is unknown how frequently and at what dose TXA is used in injured children in the US.
The objective of this study was to describe the use of TXA among children in US children’s hospitals, and to compare the prevalence of its use among different clinical settings, including trauma. In addition, we sought to estimate the variability in doses, and identify the areas for further study.
MATERIALS AND METHODS
Study Design
Data for this study were obtained from the Pediatric Health Information System (PHIS), an administrative database that contains inpatient, emergency department (ED), ambulatory surgery and observation encounter-level data from over 45 not-for-profit, tertiary care pediatric hospitals in the United States. These hospitals are affiliated with the Children’s Hospital Association (Overland Park, KS). Data quality and reliability are assured through a joint effort between the Children’s Hospital Association and participating hospitals. Portions of the data submission and data quality processes for the PHIS database are managed by Truven Health Analytics (Ann Arbor, MI). For the purposes of external benchmarking, participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. Nearly all of these hospitals also submit resource utilization data (e.g. pharmaceuticals, imaging, and laboratory) into PHIS. Data are de-identified at the time of data submission, and data are subjected to a number of reliability and validity checks before being included in the database. The data in PHIS represents approximately 13% of the national volume of US hospitalized pediatric patients. IRB approval was obtained from study sites.
Study Setting and Population
All children in the PHIS database younger than 18 years who received TXA during ED or in-patient care from January 1, 2009 to December 31, 2013 were included. A total of 36 free-standing children’s hospitals with complete data during all quarters of the study period were included. Of these hospitals, 26 were American College of Surgery verified Level I Pediatric trauma centers and 5 were Level II trauma centers.
Patients were grouped into five categories based on ICD-9 diagnostic or procedural codes: congenital heart surgery, scoliosis surgery, craniosynostosis/craniofacial surgery, traumatic injuries, and other (i.e., use of TXA among children not grouped into one of the other four categories). We selected the first three categories of surgical procedures based on a review of the literature suggesting that these were the most common indications for TXA use in children, while the use of TXA for children with traumatic injuries was a specific focus of our research question. Patients were grouped into the congenital heart, scoliosis, and craniosynostosis/craniofacial surgery categories based on lists of ICD-9 principle procedure codes derived and modified from prior studies (see eTable for detailed criteria).12–14 For the traumatic injuries category, patients were included if they had ICD-9 principle diagnostic codes consistent with a previously defined coding scheme for trauma.15 The ICD-9 principle diagnostic codes were used rather than the ICD-9 procedure codes because it is possible that these patients received TXA without receiving a surgical procedure. Moreover, a wide range of surgical procedures may be performed after acute injuries, making classification by principle procedure code difficult. However, we used a combination of diagnostic and procedural coding to help determine if children with trauma received TXA for their injuries or for surgeries performed as a consequence of their injuries. Patients who received TXA but were not grouped into the first four categories were grouped into the “other” category. Categories were mutually exclusive. All patients with ICD-9 diagnostic codes for hemophilia (V8301 and V8302) and other factor deficiencies (286.x) were excluded as the indication for TXA use would likely be due to the underlying pre-existing bleeding disorder.
Study Protocol and Measurements
We abstracted the following variables from eligible patient encounters: ICD-9 diagnostic and procedural code, patient age, sex, TXA dose, disposition, hospital length of stay, ICU admission, ED and operating room service use, blood product transfusion, and in-hospital mortality.
In the PHIS database, TXA use is limited to the dose and hospital day of each administration. Patient weight and whether drug administration was a bolus or maintenance dose was not available. Therefore we used the World Health Organization (0 to 2 years of age) and Centers for Disease Control (2 years and older) growth charts to estimate weights based on age and sex to obtain weight-based dosing estimates.16
Data Analysis
We formatted the data and recoded the variables using STATA 13.1 statistical software (STATA Corp, College Station, TX). We characterized the study population overall and within each indication category using descriptive statistics. We reported non-normal interval data with medians and interquartile ranges, and presented proportions with 95% confidence intervals (CIs). We searched for the most frequent ICD-9 diagnostic and procedure codes within the trauma category and the “other” categories to evaluate the indications for TXA use within these categories.
RESULTS
We identified 39,086 encounters during the five-year study period across the 36 children’s hospitals that had a TXA charge included and met other study criteria. Of these encounters, 3,608 (9.2%) had diagnostic codes for hemophilia or other factor deficiencies and were excluded, leaving 35,478 encounters for further analysis.
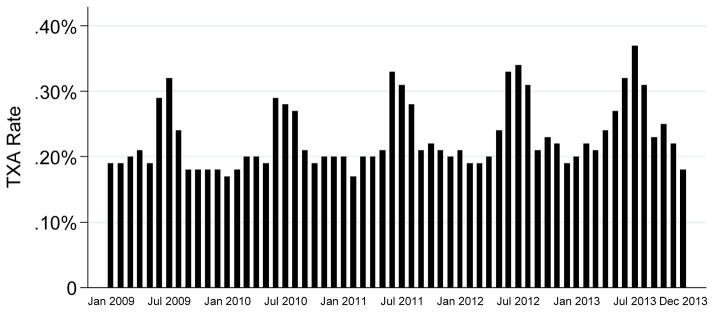
The proportions of children who received TXA were similar across each year (range 0.21% in 2009 to 0.25% in 2013) (Figure). An increase in TXA use occurred during summer months (June to August) in all study years. Congenital heart surgery accounted for 22,863 (64%) of TXA use, scoliosis surgery for 6,322 (18%), craniosynostosis/craniofacial surgery for 1,275 (3.6%), and other 4,908 (14%) (Table 1). Trauma accounted for only 110 of the TXA encounters (0.31%). The most frequent diagnostic and procedure codes for the trauma and other categories are listed in Table 2 and 3.
Figure.
Proportion of children receiving tranexamic acid (TXA) over time across 36 academic pediatric children’s hospitals, 2009–2013
Table 1.
Demographic and clinical characteristics of children receiving tranexamic acid across 36 children’s hospitals, 2009–2013, n (%)
| Demographic/Clinical Characteristics | Total Sample (n=35,478) | Congenital Heart Surgery (n=22,863) | Scoliosis Surgery (n=6,322) | Craniosynostosis / Craniofacial Surgery (n=1,275) | Trauma (n=110) | Other (n=4,908) |
|---|---|---|---|---|---|---|
| Age | ||||||
| 0 to 1 year | 14,017 (40) | 12,565 (55) | 4 (<1) | 456 (36) | 7 (6) | 985 (20) |
| >1 to 5 years | 6,343 (18) | 4,938 (22) | 97 (2) | 353 (28) | 41 (37) | 914 (19) |
| >5 years | 15,118 (43) | 5,360 (23) | 6,221 (98) | 466 (37) | 62 (56) | 3,009 (61) |
| Male | 18,138 (51) | 12,609 (55) | 2,187 (35) | 761 (60) | 73 (66) | 2,508 (51) |
| Evaluated in the emergency department | 2,746 (8) | 909 (4) | 34 (1) | 175 (14) | 93 (85) | 1,535 (31) |
| Used operating room services | 32,231 (91) | 21,655 (95) | 6,272 (99) | 1,243 (97) | 37 (34) | 3,024 (62) |
| Intensive care unit admission | 28,143 (79) | 21,554 (94) | 3,665 (58) | 726 (57) | 21 (19) | 2,177 (44) |
| Hospital length of stay, median (IQR), days | 6 [4, 15] | 8 [5, 19] | 5 [4, 6] | 3 [1, 4] | 1 [1, 5] | 5 [1, 18] |
| In-hospital mortality | 979 (3) | 748 (3) | 6 (<1) | 3 (<1) | 0 (0) | 222 (5) |
| Mechanical ventilation | 19,217 (58) | 16,674 (79) | 930 (16) | 109 (9) | 14 (14) | 1,490 (32) |
| Packed red blood cell transfusion | 16,980 (48) | 11,183 (49) | 3,590 (57) | 241 (19) | 21 (19) | 1,945 (40) |
| Other blood product transfusion | 7,780 (22) | 6,296 (28) | 350 (6) | 82 (6) | 10 (9) | 1,042 (21) |
Table 2.
Five most frequent ICD-9 principal diagnoses and procedures in the trauma category
| ICD-9 Principal Diagnosis | n |
|---|---|
| Open wound of tongue and floor of mouth, without mention of complication | 11 |
| Injury of face and neck | 7 |
| Head injury, unspecified | 6 |
| Ankle sprain | 5 |
| Secondary and recurrent hemorrhage | 4 |
| Open wound of tooth (broken) (fractured) (due to trauma), without mention of complication | 4 |
| ICD-9 Principal Procedure | n |
| Extraction of other tooth | 2 |
| Other repair and plastic operations on bronchus | 2 |
| Esophagogastroduodenoscopy [EGD] with closed biopsy | 2 |
| Closed reduction of fracture without internal fixation, radius and ulna | 2 |
| Open reduction of fracture with internal fixation, radius and ulna | 2 |
| Open reduction of fracture with internal fixation, femur | 2 |
| Closure of skin and subcutaneous tissue of other sites | 2 |
| Transfusion of platelets | 3 |
Table 3.
Ten most frequent ICD-9 principal diagnoses and procedures in the Other category
| ICD-9 Principal Diagnosis | n |
|---|---|
| Immune thrombocytopenic purpura | 252 |
| Epistaxis | 195 |
| Anomalies of skull and face bones | 130 |
| Hemorrhage complicating a procedure | 127 |
| Qualitative platelet defects | 111 |
| Scoliosis [and kyphoscoliosis], idiopathic | 100 |
| Encounter for antineoplastic chemotherapy | 96 |
| Excessive or frequent menstruation | 89 |
| Dental caries, unspecified | 79 |
| Other anomalies of larynx, trachea, and bronchus | 74 |
| ICD-9 Principal Procedure | n |
| Transfusion of packed cells | 330 |
| Injection or infusion of immunoglobulin | 110 |
| Bilateral lung transplantation | 99 |
| Internal fixation of bone without fracture reduction, other bones | 93 |
| Transfusion of platelets | 91 |
| Graft of muscle or fascia | 83 |
| Continuous invasive mechanical ventilation for 96 consecutive hours or more | 82 |
| Other repair and plastic operations on trachea | 81 |
| Injection or infusion of cancer chemotherapeutic substance | 69 |
| Biopsy of bone marrow | 68 |
Overall, the median age of patients receiving TXA was 3.1 years (IQR 0.4 to 12.4 years), and 51% were male. A total of 28,143 (79%) patients were admitted to the intensive care unit (ICU), 32,231 (89%) underwent surgery, and 979 (2.8%) died during hospitalization. Overall, 16,980 (48%) patients received packed red blood cell (PRBC) transfusions, while 7,780 (22%) patients received other blood product transfusions.
TXA was first administered on the first day of hospitalization in 65% of the encounters. The median maximum dose was highest for the congenital heart surgery category (30 mg/kg, IQR 11–104 mg/kg) and lowest for the scoliosis surgery category (7.3 mg/kg, IQR 2.2 to 68 mg/kg) (Table 4).
Table 4.
Dose characteristics of children receiving tranexamic acid across 36 children’s hospitals, 2009–2013, n (%)
| Dose Characteristics | Total Sample (n=35,478) | Congenital Heart Surgery (n=22,863) | Scoliosis Surgery (n=6,322) | Craniosynostosis / Craniofacial Surgery (n=1,275) | Trauma (n=110) | Other (n=4,908) |
|---|---|---|---|---|---|---|
| Maximum dose, median (IQR), mg/kg a | 22.4 [7.3, 84.9] | 29.9 [10.7, 104.2] | 7.3 [2.2, 67.9] | 20.2 [11.6, 101.7] | 19.4 [9.7, 45.1] | 19.2 [8.1, 67.9] |
| First TXA administration | ||||||
| Day 1 of encounter | 21,354 (65) | 12,014 (57) | 5,631 (95) | 1,089 (89) | 66 (65) | 2,554 (55) |
| Day 2 of encounter | 3,036 (9) | 2,152 (10) | 158 (3) | 89 (7) | 16 (16) | 621 (13) |
| Day 3 or later | 8,409 (26) | 6,753 (32) | 149 (3) | 42 (3) | 19 (19) | 1,446 (31) |
- Weight estimated from WHO/CDC median weight for age (in months) and sex
Within the trauma category, 66 (65%) encounters received TXA on the first hospital day. Of all patients in the trauma category, 93 (85%) were evaluated initially in the ED, 37 (34%) underwent surgery, and 21 (19%) received PRBC transfusions.
DISCUSSION
In this large study, we described the prevalence of TXA use in US children’s hospitals, quantifying TXA use across the most frequent indications. We found that TXA use was similar across all years of the study. While congenital heart surgery was associated with the majority of TXA use, there were many different principle procedure codes associated with TXA use. Of particular note, TXA was used rarely for children with traumatic injuries.
We were particularly interested in the use of TXA in injured children. In severely injured adults, TXA is the only drug demonstrated to improve mortality.17 The CRASH-2 (Clinical Randomization of an Antifibrinolytic in Significant Haemorrhage-2) randomized controlled trial, which enrolled 20,211 adults with hemorrhagic trauma in 40 countries, demonstrated that TXA decreased mortality at 28 days compared to placebo (risk of death 0.91; 95%CI 0.85 to 0.97) and had an even greater impact on death due to hemorrhage (risk of death due to hemorrhage OR, 95% CI).17 Based on the results of this adult trial and that of an observational pediatric study that also demonstrated improved survival associated with TXA use,9 some experts have advocated for the use of TXA in injured children with potentially hemorrhagic injuries.10 In the UK, the Royal College of Paediatrics and Child Health and the Neonatal and Paediatric Pharmacists Group issued an evidence statement recommending a pragmatic dosage of TXA for children with major trauma.11 Furthermore in “Tranexamic acid in pediatric trauma: why not?”, Beno and colleagues from Toronto, Canada suggest that TXA should be used in pediatric trauma when the immediate need for transfusion is accompanied by severe shock.10
Our study demonstrated that in fact TXA was rarely used in injured children at US children’s hospitals during the study time period. TXA is most effective if given within the first 3 hours after trauma and may have detrimental effects in adults if given more than 3 hours after the injury.8 In our study, 34% of children categorized as trauma received TXA after the first hospital day. Thus it is possible that these children received TXA as a part of subsequent procedures rather than during the initial resuscitation in the ED. We anticipated that if children received TXA after trauma, it would be for significant trauma such as intra-abdominal injuries associated with hemorrhage. However, the most frequent diagnostic and procedure codes within the trauma category (Table 2) suggest that TXA was not often given for significant hemorrhage in the acute setting but rather for patients with preexisting bleeding disorders with minor procedures, or for surgical procedures after trauma. While we excluded patients with diagnostic codes consistent with preexisting factor deficiencies, it is possible that these codes were at times not listed in the database.
Due to the limitations of the PHIS database, we were unable to describe dosing schemes – bolus versus maintenance – and total dosages. We estimated weight-based dosing using the highest dose of TXA given during hospitalization and the age and sex of the patient, and our results suggest significant variability of TXA doses used between and within indications. This is consistent with prior studies which have also demonstrated wide dosing variability of TXA.4
TXA is generally considered to have an excellent safety profile. Prior studies evaluating TXA use across a range of indications have consistently reported low rates of acute thrombosis (0–4.4%) and pooled analyses have shown no increased risk compared to placebo or no TXA.9,17–21 Seizures has also been reported after TXA use.22 In one study of adult patients undergoing cardiopulmonary bypass, an increased risk for seizures was seen with very high doses of TXA.23 In general, administrative databases should not be used, and are not designed to assess the relationship between a treatment and adverse events.24 Because we were not able to differentiate between pre-existing and in-hospital diagnoses of these adverse events, or if the adverse event occurred before or after TXA administration, we elected not to report the prevalence of adverse events.
LIMITATIONS
Our results should be interpreted in the context of other limitations as well. The PHIS database is an administrative database used for billing purposes and thus lacks specific clinical data. Therefore, we were not able not investigate causal effects TXA may have on clinical outcomes such as blood product requirements, adverse events, and mortality. We inferred that TXA was given for the principle procedure or diagnostic code provided for that hospital encounter. It is possible, however, that patients received TXA for other procedures or diagnoses not listed as the principle procedure or diagnostic code. We were also unable to differentiate between bolus or maintenance doses of TXA. Finally, the hospitals reporting data to PHIS may not be representative of all hospitals providing care to ill and injured children in the US.
CONCLUSION
In conclusion, TXA use for children at 36 US children’s hospitals was similar between 2009 and 2013. More than one-half of the encounters with TXA use were children undergoing congenital heart surgery. However, we identified a wide range of clinical indications, and wide range of dosing, for TXA in these children. The use of TXA for children with acute trauma was rare.
Supplementary Material
ARTICLE SUMMARY.
1. Why is this topic important?
Tranexamic acid (TXA) is an antifibrinolytic agent that reduces bleeding and decreases blood transfusions in pediatric surgery. It also improves survival in adults with hemorrhagic trauma. The prevalence of TXA use for trauma and other conditions in children however is unknown.
2. What does this study attempt to show?
We conducted a secondary analysis of a large, administrative database of 36 US children’s hospitals from 2009 to 2013 to describe the use of TXA in US hospitals for children in general, and specifically for trauma.
3. What are the key findings?
TXA is used across several diagnostic conditions in children with congenital heart surgery accounting for more than one-half of the encounters. Only 110 encounters (0.31%) were for traumatic conditions.
4. How is patient care impacted?
Despite the common use of TXA in injured adults, the use of TXA among injured children appears to be rare. Further work is needed to recommend appropriate indications for TXA, and provide dosage guidelines among children with a variety of conditions, including trauma.
Acknowledgments
This work was supported by a grant from the Clinical and Translational Science Center at the University of California at Davis. Dr. Nishijima was supported through Mentored Clinical Research Training Program Award (Grant Number UL1TR000002 and linked award KL2TR000134) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel K. Nishijima, Email: dnishijima@ucdavis.edu.
Michael C. Monuteaux, Email: michael.monuteaux@childrens.harvard.edu.
David Faraoni, Email: david.faraoni@childrens.harvard.edu.
Susan M. Goobie, Email: susan.goobie@childrens.harvard.edu.
Lois Lee, Email: lois.lee@childrens.harvard.edu.
Joseph Galante, Email: jmgalante@ucdavis.edu.
James F. Holmes, Email: jfholmes@ucdavis.edu.
Nathan Kuppermann, Email: nkuppermann@ucdavis.edu.
References
- 1.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356(22):2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 2.Tengborn L, Blomback M, Berntorp E. Tranexamic acid--an old drug still going strong and making a revival. Thromb Res. 2015;135(2):231–42. doi: 10.1016/j.thromres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Faraoni D, Goobie SM. The efficacy of antifibrinolytic drugs in children undergoing noncardiac surgery: a systematic review of the literature. Anesth Anal. 2014;118(3):628–36. doi: 10.1213/ANE.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 4.Faraoni D, Willems A, Melot C, De Hert S, Van der Linden P. Efficacy of tranexamic acid in paediatric cardiac surgery: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2012;42(5):781–6. doi: 10.1093/ejcts/ezs127. [DOI] [PubMed] [Google Scholar]
- 5.White N, Bayliss S, Moore D. Systematic review of interventions for minimizing perioperative blood transfusion for surgery for craniosynostosis. J Craniofac Surg. 2015;26(1):26–36. doi: 10.1097/SCS.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 6.Napolitano LM, Cohen MJ, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: how should we use it? J Trauma Acute Care Surg. 2013;74(6):1575–86. doi: 10.1097/TA.0b013e318292cc54. [DOI] [PubMed] [Google Scholar]
- 7.Pusateri AE, Weiskopf RB, Bebarta V, et al. Tranexamic acid and trauma: current status and knowledge gaps with recommended research priorities. Shock. 2013;39(2):121–6. doi: 10.1097/SHK.0b013e318280409a. [DOI] [PubMed] [Google Scholar]
- 8.CRASH-2 collaborators. Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377(9771):1096–101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 9.Eckert MJ, Wertin TM, Tyner SD, Nelson DW, Izenberg S, Martin MJ. Tranexamic acid administration to pediatric trauma patients in a combat setting: The pediatric trauma and tranexamic acid study (PED-TRAX) J Trauma Acute Care Surg. 2014;77(6):852–8. doi: 10.1097/TA.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 10.Beno S, Ackery AD, Callum J, Rizoli S. Tranexamic acid in pediatric trauma: why not? Crit Care. 2014;18(4):313. doi: 10.1186/cc13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Royal College of Paediatrics and Child Health. [Accessed Oct 4, 2014];Major Trauma and the Use of Tranexamic Acid in Children. http://www.rcpch.ac.uk/node/1//system/files/protected/page/121112_TXA%20evidence%20statement_final%20v2.pdf.
- 12.Gupta P, Beam BW, Noel TR, et al. Impact of preoperative location on outcomes in congenital heart surgery. Ann Thorac Surg. 2014;98(3):896–903. doi: 10.1016/j.athoracsur.2014.04.123. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen C, Hernandez-Boussard T, Khosla RK, Curtin CM. A national study on craniosynostosis surgical repair. Cleft Palate Craniofac J. 2013;50(5):555–60. doi: 10.1597/11-324. [DOI] [PubMed] [Google Scholar]
- 14.McLeod LM, French B, Flynn JM, Dormans JP, Keren R. Antifibrinolytic Use and Blood Transfusions in Pediatric Scoliosis Surgeries Performed at US Children’s Hospitals. J Spinal Disord Tech. 2013 doi: 10.1097/BSD.0b013e3182a22a54. forthcoming. [DOI] [PubMed] [Google Scholar]
- 15.Consensus Recommendations for Injury Surveillance in State Health Departments. [Accessed June 11, 2015];Report from the Planning Comprehensive Injury Surveillance in State Health Departments Working Group. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/Injury/isw1.pdf.
- 16.WHO and CDC growth charts. Centers for Disease Control and Prevention; [Accessed June 11, 2015]. http://www.cdc.gov/growthcharts/ [Google Scholar]
- 17.CRASH-2 collaborators. Shakur H, Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 18.Martin K, Breuer T, Gertler R, et al. Tranexamic acid versus varepsilon-aminocaproic acid: efficacy and safety in paediatric cardiac surgery. Eur J Cardiothorac Surg. 2011;39(6):892–7. doi: 10.1016/j.ejcts.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147(2):113–9. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 20.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts I, Shakur H, Ker K, Coats T CRASH-2 Trial Collaborators. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2012;12:CD004896. doi: 10.1002/14651858.CD004896.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Breuer T, Martin K, Wilhelm M, et al. The blood sparing effect and the safety of aprotinin compared to tranexamic acid in paediatric cardiac surgery. Eur J Cardiothorac Surg. 2009;35(1):167–71. doi: 10.1016/j.ejcts.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic Acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350–3. doi: 10.1213/ANE.0b013e3181c92b23. [DOI] [PubMed] [Google Scholar]
- 24.Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi: 10.2106/JBJS.M.01490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.