Abstract
Background
High insulin-like growth factor-1 (IGF-1), measured once during acute-stroke, is associated with greater survival rates and lower stroke severity. However, information is lacking regarding how IGF-1 availability, determined by IGF-1’s ratio to insulin-like growth factor binding protein-3 (IGFBP-3), relates to recovery and how the response of IGF-1 during the first week of stroke relates to outcomes. The purpose of this study was to determine: 1) the relationship between percent-change in IGF-1 and IGF-1 ratio during the first week of stroke and stroke outcomes; and 2) the difference in percent-change in IGF-1 and IGF-1 ratio in individuals who discharged home and individuals who discharged to inpatient facilities.
Methods
IGF-1 and IGFBP-3 were quantified from blood sampled twice (<72 hours of admission; 1-week post-stroke) in fifteen individuals with acute-stroke. Length of stay, modified Rankin Scale at one-month, and discharge destination were obtained from electronic medical records.
Results
Percent-change in IGF-1 ratio was related to length of stay (r=.54; p=.04). Modified Rankin Scale (n=10) was related to percent-change in IGF-1 (r=.90; p<.001) and IGF-1 ratio (r=.75 p=.01). Those who went home (n=7) had decreases in IGF-1 (−24±25%) and IGF-1 ratio (−36±50%), while those who went to inpatient facilities (n=8) had increases in IGF-1 (37±46%) and IGF-1 ratio (30±40%). These differences were significant (IGF-1: p=.008; IGF-1 ratio p=.01).
Conclusion
Our findings suggest that a decrease in IGF-1 and IGF-1 ratio during the first week of stroke is associated with favorable outcomes: shorter length of stay, greater independence at one-month on the modified Rankin Scale, and discharging home.
Keywords: acute stroke, insulin-like growth factor-1, discharge destination, length of stay
INTRODUCTION
In humans, low levels of insulin-like growth factor-1 (IGF-1) are associated with stroke risk factors such as ischemic heart disease, myocardial infarction, diabetes, and coronary artery disease, while high insulin-like growth factor binding protein-3 (IGFBP-3) levels are associated with ischemic heart disease (1–3). Moreover, low IGF-1 has been directly correlated to greater stroke risk in humans (4–6). Higher levels of IGF-1 with simultaneously low levels of IGFBP-3 allow for greater amounts of free, unbound IGF-1 in circulation, which, in turn, elicits IGF-1 to trigger cascades in neuroprotective pathways. In individuals with stroke, many studies have agreed that high baseline levels of IGF-1 in circulation, measured once within the first few weeks of stroke, are linked to lower stroke severity and greater rates of survival at one-month post stroke (6–11).
While some studies have examined IGFBP-3 after stroke (8, 10), there still remains a disparity in knowledge regarding the relationship of IGF-1 ratio (IGF-1:IGFBP-3) to stroke recovery. Because IGF-1 ratio reflects the amount of free IGF-1 available to reach its receptor, it is important to consider. Furthermore, while high baseline IGF-1 levels have been shown to be related to positive outcomes, the response of IGF-1 during the first week is not yet known and may provide further insight into IGF-1’s potential neuroprotective benefit to stroke outcomes.
Outcomes such as length of stay (LOS) in the hospital, modified Rankin Scale (mRS), and discharge destination have been used in individuals with stroke to evaluate the relationship between easily-assessable and short-term outcomes, functional status, and other measures of stroke recovery. One study showed that motor recovery was improved after the first year of stroke in people with a shorter LOS during their first hospital admission for stroke (12). LOS was also negatively related to improvements on the Functional Independence Measure (FIM), such that individuals who stayed in the hospital longer had poorer scores on the FIM (13). Moreover, discharge destination has been associated with acute FIM measures, mortality rates, and global disability at 3 months post-stroke (14–16). However, the relationship between these stroke outcomes and IGF-1 and IGF-1 are yet to be determined.
Therefore, the purpose of this study was to: 1) determine the relationship between percent change in IGF-1 and IGF-1 ratio during the first week of stroke and stroke outcomes (LOS in the hospital during acute stroke and mRS at one-month post-stroke); and 2) determine the difference in percent change in IGF-1 and IGF-1 ratio in discharge destination (i.e. individuals who discharged home vs. individuals who discharged to inpatient facilities). We hypothesized that: 1) an increase in IGF-1 and IGF-1 ratio during the first week of stroke would be associated with fewer days spent in the hospital (LOS) and more functional independence at one-month post-stroke (mRS); and 2) individuals who discharge home will have a significantly greater percent change in IGF-1 and IGF-1 ratio compared to individuals who discharge to inpatient facilities.
METHODS
Study Design
This study used a sample of convenience from University of Kansas Hospital’s certified Comprehensive Stroke neuro-progressive and neuro-intensive care units. Institution approval from the Human Subjects Committee at KU Medical Center was obtained before commencement of the study (HSC #: 12490). Written informed consent was obtained by the participant or their surrogate decision maker prior to study participation.
Participants
Individuals with stroke were eligible for participation if they were admitted to the KU Hospital with a diagnosis of acute ischemic or hemorrhagic stroke and were between 25 and 85 years of age. Individuals were excluded from the study if they: 1) had acute renal failure; 2) had a history of ischemic or hemorrhagic stroke, ischemic cardiovascular event, or coronary artery bypass graft (CABG) surgery less than 3 months prior to their current hospital admission for stroke; 3) had a diagnosis of congestive heart failure; or 4) had severe peripheral artery disease.
General Methods
Individuals were enrolled into the study and had baseline blood samples collected within 72 hours of hospital admission for acute stroke. Blood at baseline was sampled between 7:30 and 9:00 am after an overnight fast. One week following stroke, blood was sampled again during similar hours, after an overnight fast. Demographic data (age, height, weight, and body mass index), results of laboratory chemistry testing on admission (hemoglobin A1c), enzyme analysis of liver function on admission (aspartate aminotransferase (AST) and alanine aminotransferare (ALT)), and other stroke related information (admitting National Institutes of Health Stroke Score (NIHSS) and lesion volume) were obtained from each participants’ electronic medical record (EMR). Demographics of enrolled participants can be found in Table 1.
Table 1.
Participant Demographics
| Characteristics, n = 15 | Number or Sample Mean (SD) | Range | |
|---|---|---|---|
|
| |||
| Male | 8 | ||
| Age (years) | 61.1 (10.7) | (38–75) | |
| Body Mass Index | 30.3 (5.7) | (22.8–45.7) | |
| National Institutes of Health Stroke Scale | 8.5 (8.0) | (1–22) | |
| Modified Rankin Scale | 2 (1.4) | (1–5) | |
| HbA1C | 5.9 (1.3) | (5.1–9.8) | |
| Race/Ethnicity | |||
| White/Caucasian | 13 | ||
| African American | 2 | ||
| Marital Status | |||
| Married/Partner | 7 | ||
| Divorced | 5 | ||
| Single | 3 | ||
| Side of Impairment | |||
| Left | 7 | ||
| Right | 6 | ||
| Neither | 2 | ||
| Type of Stroke | |||
| Ischemic | 13 | ||
| Hemorrhagic | 2 | ||
| Received tPA | 4 | ||
| Comorbidities | |||
| Smoker | 4 | ||
| Atrial Fibrillation | 2 | ||
| Seizures | 1 | ||
| Medicines | |||
| Anti-Hypertensive | 8 | ||
| Blood Thinners | 4 | ||
| Cholesterol | 7 | ||
Stroke Outcomes
Calendar days spent in the hospital (LOS), discharge destination (i.e. home vs. inpatient facility), and functional independence at 1-month post-stroke, assessed by the mRS, was also obtained from each participant’s EMR.
Quantification of Biomarkers
One, ten-milliliter (mL) sodium heparinized tube was used for obtaining blood from each participant. Gloves were worn by study team members during blood sampling, blood processing, and protein quantification procedures. Drawing needles were not recapped and immediately placed into nearby sharps containers to avoid injury. Collection tubes were immediately put on ice and transferred to the laboratory for processing. Within an hour of collection, blood samples were centrifuged to separate and obtain plasma. Plasma was then aliquotted into 3, 1.5-mL polypropylene PP tubes and stored frozen at −80 degrees Celsius until assaying. Freeze/thaw cycles were limited by using a new, once-thawed aliquot for each assay.
After all samples were obtained from each participant, enzyme-linked immunosorbent assays (ELISAs) were used to quantify total circulating IGF-1 (Alpco: Salem, NJ; cat#22-IGFHU-E01) and IGFBP-3 (Alpco; Salem, NJ; cat#22-BP3HU-E01). All samples from one participant were quantified on the same ELISA plate. Samples were slowly thawed to room temperate using ice before assaying in order to avoid protein-shock. Procedures were performed exactly to the manufacturer’s recommendations. Six standards were made in order to create a standard curve. Two internal controls of known concentrations were provided by each kit. Observed values of both the standard curve and the known concentrations were determined to be within acceptable ranges before interpreting the data.
Statistical Analysis
Baseline demographics were calculated using descriptive statistics. IGF-1 ratio (the ratio of IGF-1 to IGFBP-3) was calculated by dividing the amount of total circulating IGF-1 by the amount of total circulating IGFBP-3. Percent change in IGF-1 and IGF-1 ratio during the first week of stroke was calculated as [(1 week – baseline)/(baseline)*100]. Pearson correlations were performed to determine the relationship between percent change in IGF-1 and IGF-1 ratio and stroke outcomes (LOS and mRS). Independent t-tests were performed to determine the difference in percent change in IGF-1 and IGF-1 ratio between those who discharged home and those who discharged to an inpatient facility. All analyses were performed using IBM® SPSS® Statistics Version 22 (SPSS, Inc, Chicago, IL) and alpha levels were set at p < .05.
RESULTS
Fifteen individuals (8 males, 61.1 ± 10.7 years) were enrolled into the study and started data collection within 72 hours of hospital admission. Table 2 outlines IGF-1 and IGF-1 ratio levels at each time point and percent changes in IGF-1 and IGF-1 ratio from baseline to 1-week post-stroke. Additionally, since the majority of circulating IGF-1 is synthesized in the liver, enzymes to assess liver function (AST and ALT) were analyzed. Liver enzymes, AST and ALT, were within normal ranges for all subjects (Table 3).
Table 2.
Blood Levels
| n = 15 | Visit 1 (<72 Hours) |
Visit 2 (1 Week) |
% Change |
|---|---|---|---|
|
| |||
| IGF-1 (ng/mL) | 123 (57) | 129 (89) | 8.5 (47.7) |
| IGFBP-3 (ng/mL) | 2220 (675) | 2536 (589) | 41.2 (109.9) |
| IGF-1 Ratio | .07 (.06) | .05 (.03) | −.67 (55.2) |
Table 3.
Characteristics of Individuals Who Discharged Home vs. Individuals Who Discharged to Inpatient Facilities
| n = 15 | Home | Inpatient | p-value |
|---|---|---|---|
|
| |||
| Age (years) | 62 (10) | 60 (11) | .75 |
| Body Mass Index | 28 (4) | 32 (6) | .17 |
| Lesion Volume (cm3) | 2 (3) | 25 (29) | .06 |
| Length of Stay (days) | 6 (5) | 10 (6) | .27 |
| Baseline IGF-1 (ng/mL) | 145 (48) | 103 (60) | .17 |
| 1 Week IGF-1 (ng/mL) | 112 (59) | 144 (110) | .51 |
| Baseline IGFBP-3 (ng/mL) | 2036 (906) | 2380 (380) | .38 |
| 1 Week IGFBP-3 (ng/mL) | 2610 (829) | 2471 (308) | .67 |
| Baseline IGF-1 Ratio | .04 (.02) | .1 (.08) | .11 |
| 1 Week IGF-1 Ratio | .01 (.03) | .05 (.01) | .38 |
| Aspartate Aminotransferase (U/L) | 14 (4) | 19 (8) | .68 |
| Alanine Aminotransferare (U/L) | 19 (8) | 24 (12) | .46 |
Length of Stay
Our results showed that percent change in IGF-1 ratio was significantly related to LOS (r = .54; p = .04) (Figure 1), suggesting that as the percent change in IGF-1 ratio during the first week of stroke increases, individuals are staying in the hospital longer. However, percent change in IGF-1 alone was not significantly related to LOS.
Figure 1. Relationship of Percent Change in IGF-1 Ratio and Length of Stay.
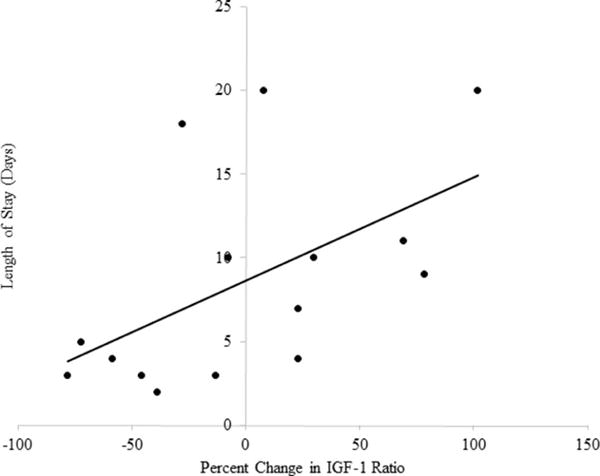
Individuals with a higher percent change in IGF-1 Ratio during the first week of stroke stay more days in the hospital.
Modified Rankin Scale
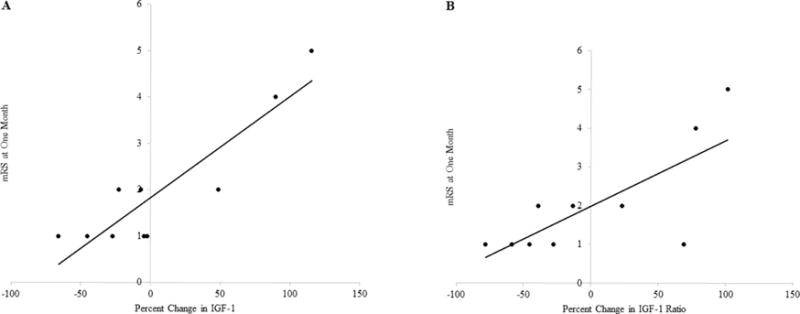
For individuals with a mRS at 1 month post-stroke (n = 10), our results showed that there was a significant relationship between mRS and percent change in both IGF-1 (r = .901; p < .001) (Figure 3A) and IGF-1 ratio (r = .750; p = .012) (Figure 3B) during the first week of stroke. As individuals had larger increases in IGF-1 and IGF-1 ratio during the first week of stroke, they also had greater scores on the mRS (lower functional independence) at one-month post-stroke. These results corroborate the observation of the relationship of IGF-1 ratio to LOS and provide additional evidence to suggest that a decrease in circulating IGF-1 and IGF-1 ratio is associated with positive, or more favorable, outcomes.
Figure 3.
(A) Correlation Between Percent Change in IGF-1 and mRS at One Month
Individuals with a greater precent changes in IGF-1 during the first week of have a poorer outcome on the mRS at one month post-stroke.
(B) Correlation Between Percent Change in IGF-1 Ratio and mRS at One Month
Individuals with a greater percent change in IGF-1 Ratio during the first week of have a poorer outcome on the mRS at one month post-stroke.
Discharge Destination
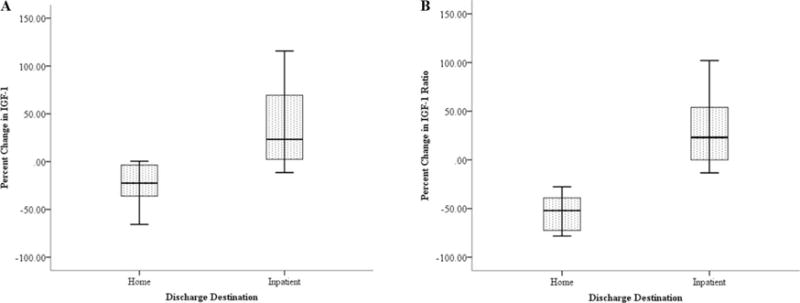
Finally, those who went home (n = 7) had negative percent changes (decreases) in IGF-1 (−24 ± 25%) (Figure 2A) and IGF-1 ratio (−36 ± 50%) (Figure 2B) during the first week of stroke. Contrarily, those who went to inpatient facilities (n = 8) had positive percent changes (increases) in IGF-1 (37 ± 46%) and IGF-1 ratio (30 ± 40%) during the first week of stroke. The difference in percent change of IGF-1 (p = .008) and IGF-1 ratio (p = .014) between discharge destinations (home vs. inpatient) was significant.
Figure 2.
(A) Change in IGF-1 and Discharge Destination
Individuals who discharged home from the hospital had decreases in IGF-1 levels during the first week of stroke, while individuals who discharged to inpatient facilities had increases in IGF-1 levels (p = .008).
(B) Change in IGF-1 Ratio and Discharge Destination
Individuals who discharged home from the hospital had decreases in IGF-1 Ratio during the first week of stroke, while individuals who discharged to inpatient facilities had increases in IGF-1 Ratio (p = .014).
Baseline demographics were not significantly different between individuals who went home and individuals who discharged to impatient facilities (Table 3). Further, because both groups did not have a significant difference in either liver enzymes, we cannot contribute the differences in IGF-1 and IGF-1 ratio to impaired liver function.
DISCUSSION
Our hypothesis that an increase in IGF-1 and IGF-1 ratio during the first week of stroke would result in fewer days spent in the hospital, lower mRS scores (more functional independence) at one month, and discharging home was not supported. In fact, our results showed the opposite in some measures of IGF-1: a decrease in IGF-1 and/or IGF-1 ratio during the first week of stroke was related to positive outcomes (i.e. discharging home and lower mRS at one month). Although the results of the current study were not as expected, the findings have important implications for stroke recovery in humans and raise additional questions for which future work should be based.
Insulin-Like Growth Factor-1
In humans, studies have been performed for which their results indicate that high baseline measurements of IGF-1 are related to better stroke recovery and greater survival rates. Aberg and colleagues measured IGF-1 levels from the serum of stroke patients between 1 and 19 days post-stroke(7). Baseline serum IGF-1 levels in individuals with acute stroke were significantly associated with improvements in the mRS and Scandinavian Stroke Scale tested at 3 and 24 months post stroke. However, the widespread variation of sampling may have impeded their ability to draw conclusions. Individuals who had their blood sampled from Day 0–2 showed statistically different IGF-1 levels compared to those who had their blood sampled on Days 9–19. This indicates that IGF-1 levels during the first two weeks of stroke are not stable (show either significant decreases or increases) and that regulation of IGF-1 has the potential to differ from person to person. No studies have examined how this response, during the first few weeks of stroke, relates to recovery or stroke outcomes. The current study seeks to fill this gap and provides novel, preliminary evidence to suggest that decreases in IGF-1 and IGF-1 ratio during the first week of stroke may be beneficial to recovery.
Experimental studies may provide insight into why the results of the current study were opposite to our original hypotheses. Chang and colleagues showed important evidence of IGF-1’s ability to exert neuroprotective benefits and that during this time, brain levels of IGF-1 increase while peripheral levels decrease, suggesting uptake of IGF-1 into the brain from circulation (17). IGF-1 levels were measured 14 days after MCAO from the affected motor cortex, striatum, and plasma. IGF-1 was significantly decreased in the plasma, while significantly increased in the motor cortex and striatum. Although we are not able to measure IGF-1 in the brain of humans pre-mortem, the findings of Chang’s study are important for the interpretation for the current study in that the decreases in IGF-1 seen in the periphery in humans may underlie increases in IGF-1 in the brain. It is important for future studies in acute stroke to examine the change in both peripheral circulating IGF-1 and CSF in order to determine whether individuals who have decreases in IGF-1 during the first week are more efficient at IGF-1 uptake into the CSF and brain and whether these measures are related to functional stroke outcomes.
Stroke Outcomes
Many studies have used stroke outcomes such as LOS in the hospital, mRS, and discharge destination to assess recovery. These outcomes are extremely easy to collect and are many times automatically tracked and calculated by electronic medical record systems. LOS and discharge destination can be used as markers of recovery in the investigations of meaningful relationships to other recovery outcomes and neuroprotective agents.
A study performed by Kuptniratsaikul and colleagues examined motor recovery in 192 individuals with stroke across nine hospitals (12). Motor recovery at 6 and 12 months after stroke was assessed by the Brunnstrom motor recovery stages (BMRS). The BMRS is broken into 6 stages of recovery with each level (Levels 1–6) describing progressive improvements in movement patterns in the hand, arm and leg, separately. Their results showed that improvements in the BMRS were associated with a shorter length of stay (12). Another study, which investigated 481 individuals with acute stroke, determined the relationship between Functional Independence Measure (FIM) scores on admission and discharge destination (14). They observed that individuals who discharged to the community, rather than to inpatient rehabilitation, had significantly better FIM scores upon admission (14). Other studies have shown significant relationships between discharge destination and LOS with global disability at three months and having a fall during hospitalization (16, 18).
These studies indicate that easily obtainable outcome measures such as LOS and discharge destination can give valuable information into stroke recovery and physical function in individuals with stroke. However, their relationship to the response of IGF-1 and IGF-1 ratio after stroke has been understudied in humans. Our results were novel in that we showed that decreases in IGF-1 and IGF-1 were associated with a shorter LOS, better mRS at one-month post-stroke, and discharging home. Large clinical trials examining the relationship between the response of IGF-1 and IGF-1 ratio during the first week and objective functional outcomes can provide important insight into neuroprotection during stroke recovery.
Limitations
This study provides novel evidence regarding the potential importance of the percent change in IGF-1 and IGF-1 ratio during the first week of stroke in understanding recovery. However, a limitation of the current study is that circulating concentrations of IGF-1 were not supplemented with the levels of IGF-1 in the cerebral spinal fluid (CSF). Quantifying IGF-1 and IGF-1 ratio in the CSF can provide information regarding levels in the brain. IGF-1 is known to cross the blood-brain barrier (BBB) and measuring it in CSF can be beneficial. An experimental study showed evidence of IGF-1 crossing the BBB by inducing a middle cerebral artery occlusion (MCAO) in rats and administering radio-labeled IGF-1 into periphery immediately after. Within 30 minutes of IGF-1 administration, the majority of IGF-1 proteins were seen to be associated with neurons of the infarcted hemisphere (19).
Another limitation of the current study is the small sample size and lack of objective functional measurements. Larger clinical trials need to be performed in order to corroborate these findings, while also assessing functional ambulation and performance of activities of daily living. Although used in previous literature, the outcomes in this study (LOS, mRS, and discharge destination) limit our ability to understand how percent change in IGF-1 and IGF-1 ratio relate to objective measurements of functional recovery such as walking endurance and walking speed. Finally, other factors, unaccounted for in the current study, may influence the outcome of LOS and discharge destination. One study suggests that decisions of discharge destinations may be influenced by not only the condition of the individual with stroke, but also by social, economic, and policy factors (20). However, another suggests that discharge destination is among the strongest of variables to predict global disability at 3 months (16). The current study provides support in favor of undergoing larger, clinical trials that use objective, measurable assessments of physical function to assess the potential benefits of IGF-1 and IGF-1 ratio.
CONCLUSIONS
Despite the suggestion of current literature regarding high baseline levels of IGF-1, our novel findings suggest that a decrease in IGF-1 and IGF-1 ratio during the first week of stroke may be related to positive, or more favorable, outcomes (i.e. shorter LOS, discharging home vs inpatient facilities, and improved functional independence at one-month post-stroke). When studying IGF-1 and its relationship to stroke recovery in humans, it may be important to consider multiple measurements of IGF-1 instead of single, baseline time points. Moreover, multiple measurements of IGF-1 ratio may also deepen our understanding of how IGF-1 is regulated after stroke and how this relates to functional recovery. The percent change in IGF-1 and IGF-1 ratio may be a meaningful biomarker of stroke recovery at one month. Further work is warranted in a larger clinical trial and in experimental models to determine why decreases in IGF-1 and IGF-1 ratio are beneficial and their relationship to objective measurements of function during the recovery of stroke.
Acknowledgments
SAB was supported in part by award number K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. AEM was supported in part by award number T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and in part by award number 14PRE20040026 from the American Heart Association. REACH laboratory space is supported by the Georgia Holland Endowment Fund. We would also like to thank the KU hospital stroke units for their support, participants, and their families for their time and dedication to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Juul A. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: A population-based case-control study. Circulation. 2002;106(8):939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Sullivan LM, D’Agostino RB, et al. Serum insulin-like growth factor 1 and risk for heart failure in elderly individuals without a previous myocardial infarction - the framingham heart study. Ann Intern Med. 2003;39:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 3.Rajpathak SN, He M, Sun Q, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. 2012;61(9):2248–2254. doi: 10.2337/db11-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnsen SP. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90(11):5937–5941. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- 5.Dong X, Chang G, Ji XF, et al. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One. 2014;9(4):e94845. doi: 10.1371/journal.pone.0094845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Denti L, Annoni V, Cattadori E, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117(5):312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 7.Aberg D, Jood K, Blomstrand C, et al. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J Clin Endocrinol Metab. 2011;96(7):e1055–1064. doi: 10.1210/jc.2010-2802. [DOI] [PubMed] [Google Scholar]
- 8.De Smedt A, Brouns R, Uyttenboogaart M, et al. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42(8):2180–2185. doi: 10.1161/STROKEAHA.110.600783. [DOI] [PubMed] [Google Scholar]
- 9.Bondanelli M, Ambrosio MR, Onofri A, et al. Predictive value of circulating insulin-like growth factor I levels in ischemic stroke outcome. J Clin Endocrinol Metab. 2006;91(10):3928–3934. doi: 10.1210/jc.2006-1040. [DOI] [PubMed] [Google Scholar]
- 10.Ebinger M, Ipsen N, Leonards CO, et al. Circulating insulin-like growth factor binding protein-3 predicts one-year outcome after ischemic stroke. Exp Clin Endocrinol Diabetes. 2015;123(8):461–465. doi: 10.1055/s-0035-1554632. [DOI] [PubMed] [Google Scholar]
- 11.Tang JH, Ma LL, Yu TX, et al. Insulin-like growth factor-1 as a prognostic marker in patients with acute ischemic stroke. PLoS One. 2014;9(6):99186. doi: 10.1371/journal.pone.0099186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Kuptniratsaikul V, Kovindha A, Suethanapornkul S, et al. Motor recovery of stroke patients after rehabilitation: 1-year follow-up study. Int J Neurosci. 2016:1–17. doi: 10.3109/00207454.2016.1138474. [DOI] [PubMed] [Google Scholar]
- 13.Ifejika N, Vahidy F, Aramburo-Maldonado L, et al. Acute intravenous tissue plasminogen activator therapy does not impact community discharge after inpatient rehabilitation. Int J Neurorehabil. 2015;2(4):183. [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts P, Mix J, Rupp K, et al. Using functional status in the acute hospital to predict discharge destination for stroke patients. Am J Phys Med Rehabil. 2015 doi: 10.1097/PHM.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 15.Bekelis K, Missios S, Coy S, et al. Comparison of outcomes of patients with inpatient or outpatient onset ischemic stroke. J Neurointerv Surg. 2016 doi: 10.1136/neurintsurg-2015-012145. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Yang Y, Saver J. Discharge destination after acute hospitalization strongly predicts three month disability outcome in ischemic stroke. Restor Neurol Neurosci. 2015;33(5):771–775. doi: 10.3233/RNN-150531. [DOI] [PubMed] [Google Scholar]
- 17.Schabitz WR, Hoffmann TT, Heiland S, et al. Delayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRI. Stroke. 2001;32(5):1226–1233. doi: 10.1161/01.str.32.5.1226. [DOI] [PubMed] [Google Scholar]
- 18.Wong JS, Brooks D, Mansfield A. Do falls experienced during in-patient stroke rehabilitation affect length of stay, functional status, and discharge destination? Arch Phys Med Rehabil. 2015 doi: 10.1016/j.apmr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XF, Fawcett JR, Thorne RG, et al. Intranasal administration of insulin-like growth factor-I bypasses blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 20.Johnson J, Smith G, Wilkinson A. Factors that influence the decision-making of an interdischiplinary rehabilitation team when choosing a discharge destination for stroke survivors. Can J Neurosci Nurs. 2015;37(2):26–32. [PubMed] [Google Scholar]


