Abstract
Patient-derived stem cells enable promising regenerative strategies but display heterogenous cardiac reparative proficiency, leading to unpredictable therapeutic outcomes impeding practice adoption. Means to establish and certify the regenerative potency of emerging biotherapies are thus warranted. In this era of clinomics, deconvolution of variant cytoreparative performance in clinical trials offers an unprecedented opportunity to map pathways that segregate regenerative from non-regenerative states informing the evolution of cardioregenerative quality systems. A maiden example of this approach is cardiopoiesis-mediated lineage-specification developed to ensure regenerative performance. Successfully tested in pre-clinical and early clinical studies, the safety and efficacy of the cardiopoietic stem cell phenotype is undergoing validation in pivotal trials for chronic ischemic cardiomyopathy offering the prospect of a next-generation regenerative solution for heart failure.
Keywords: cardiovascular disease, cardiopoiesis, cardiopoietic stem cell, clinical trial, clinomics, epidemic, healthcare, myocardial infarction, next generation, regenerative medicine, quality, system
Heart failure paradox
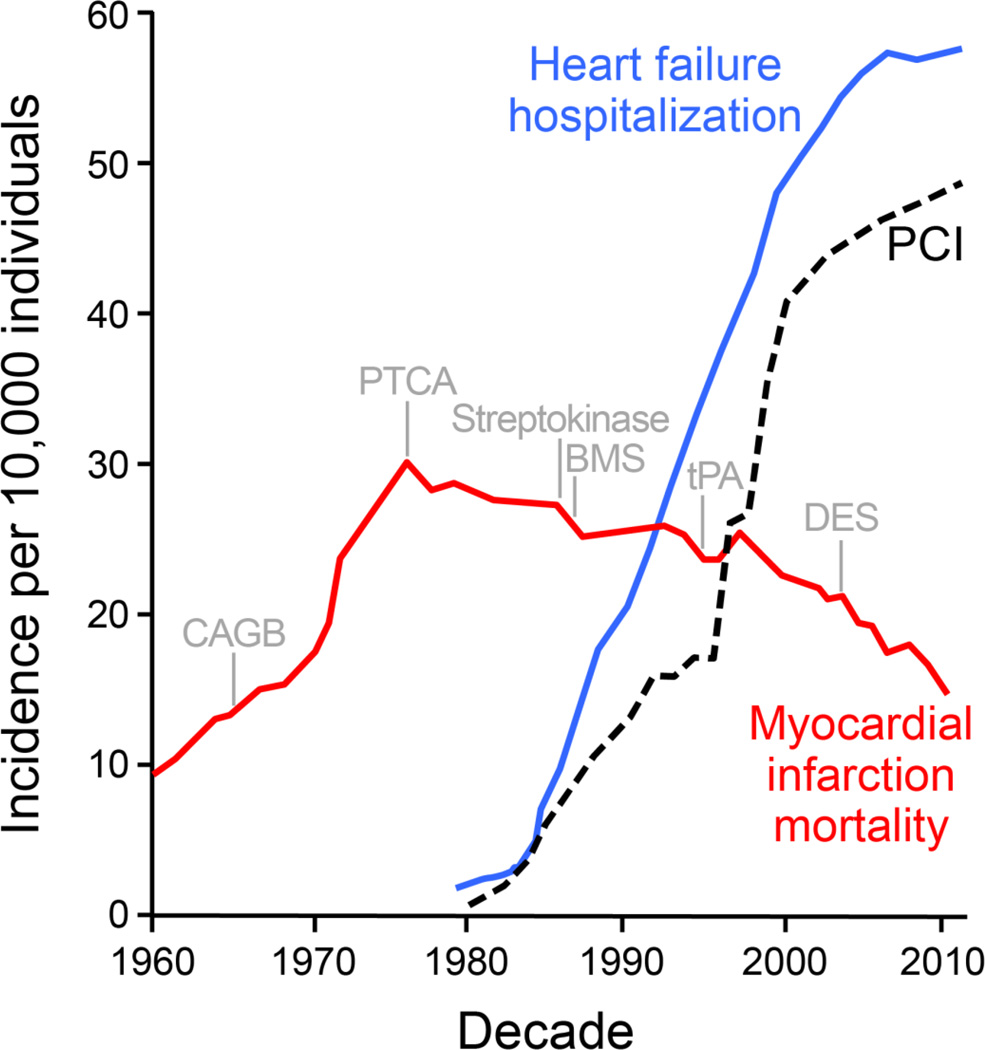
Scientific advances impact profoundly evidence-based systems of cardiovascular care [1]. In acute myocardial infarction, coronary reperfusion along with adjuvant pharmacotherapy has helped ensure a ≥96% in-hospital survival offering a contemporary exemplar of improved outcomes [2]. Despite reduced early mortality, 12% of patients die within 6 months post-infarction and 25% of infarction survivors progressively develop organ failure [3]. The emerging heart failure epidemic is hence regarded as a paradox of medical success (Fig. 1) [4].
Fig. 1.
The chronic heart failure epidemic in the context of advances in acute coronary care. Fifty year-long trends highlighting the impact of acute revascularization on myocardial infarction (MI) mortality (red line) and concomitant increase in heart failure hospitalizations (blue line). Data represented as incidence per 10,000 persons and normalized to US Census population figures. Vertical markers: Institution of coronary artery bypass grafting (CABG); percutaneous transluminal coronary angioplasty (PTCA); thrombolytics (streptokinase and tPA); bare metal stent (BMS); and drug eluting stent (DES). Dashed line: percutaneous coronary intervention (PCI).
Chronic heart failure affects up to 30 million people worldwide, and highlights the growing burden of degenerative diseases at a global scale [5]. About 1–2% of adults in developed countries suffer from heart failure, with prevalence rising to ≥10% in persons 70 years of age or older. Liable for >2 million yearly hospitalizations in the United States and Europe, heart failure is a primary indication for repeated in-hospital care across geographies [6]. Survival does not exceed 1 in 3 patients at 5-year follow-up [7]. These staggering trends underscore pressing unmet needs of a vulnerable aging population in spite of a generalized decline in cardiovascular mortality rates.
Coronary artery disease underpins two-thirds of all systolic heart failure – the best known form of disease associated with reduced ejection fraction. In patients that overcome acute ischemic insult, initial survival is offset by progressive organ failure requiring therapy escalation. As focus of therapy shifts from mortality to consequences of survival, the quest for treatments that reduce myocardial injury/limit adverse remodeling and restore parenchymal integrity/preserve ventricular function is paramount [8].
Disease reversal goals
Heart failure therapy entails syndrome relief, prevention of hospital admission, and mortality reduction [9]. To impact quality of life and survival, disease management relies largely on optimal titration of pharmacotherapy (i.e., beta-blockers, angiotensin-converting-enzyme inhibitors, aldosterone antagonists and neprilysin inhibition), and use of cardiac resynchronization as appropriate. Infarct size however remains the main determinant of adverse post-infarction aftermath, including a particularly poor clinical outcome in worsening heart failure [10]. Current approaches fail to address the fundamental issue of myocyte loss that underlies incipient cardiomyopathy. In end-stage disease, mechanical circulatory support and organ transplantation are extraordinary life-extending measures limited by cost and access. To enhance standard of care, innovative treatments aim to fundamentally alter the course of disease, and avert end-stage deterioration and need for transplantation [11].
The U.S. Department of Health and Human Services perspective, “2020: A New Vision”, singles out regenerative medicine at the core of healthcare innovation [12]. Exemplified by curative therapies offered in transfusion medicine and in defined hematological malignancies, regenerative technologies incorporate transplant of healthy tissues, induction of a healing response in diseased tissues, and/or implement tissue engineering to manufacture new tissue [13,14]. Regenerative innovations are introduced across medical and surgical specialties aiming at normative organ restitution integrated in whole-person care. With the prospect of functional and structural repair, regenerative solutions strive to achieve disease reversal goals reducing medical and societal imperatives of life-long disease management [15].
Regenerative equation
The notion of the heart as an organ permissive of regeneration is central in the roll-out of regenerative paradigms applied to cardiovascular medicine. Traditionally referred as a post-mitotic, terminally differentiated organ, newer evidence supports a dynamic view of the human heart. Cell death versus renewal incorporates vital components governing cardiac homeostasis, aging, and disease [16]. During a person’s life, revitalizing mechanisms – particularly operational at a younger age – contribute to ongoing renewal of the heart mass, securing physiological tissue safeguard [17]. Regenerative reserve reflects the ability to maintain homeostasis through self-reparative mechanisms [18]. In disease, this innate propensity becomes inadequate to cope with cardiomyocyte loss and ultimately fails to restore organ performance. In particular with aging, the rejuvenative reserve is compromised as decline in tissue health is compounded with accrual of senescent cells [19]. Clearance of senescent cell pools improves tissue function, yet falls short at restoring pre-aging status [20]. In a permissive myocardial environment, regenerative therapy is thus conceived as a boost to the innate repair capacity aiming to restore regenerative fitness.
Within a diverse and evolving regenerative toolkit, that includes standalone or combination techniques relaying on cells/tissues/biomaterials and/or molecules, stem cells and derivatives are the most commonly tested active ingredient [21–23]. Use of stem cells to buttress the regenerative fortitude of ailing hearts leverages a presumed capacity to recreate tissue and/or promote repair, and represents 25% of all clinical development efforts in cell-based therapies [24]. Stem cells, envisioned to fulfill a building-block role to rebuild compromised heart muscle, are increasingly thought to actually stimulate a multifaceted regenerative response leading to the overhaul of the disease substrate within the host myocardium. Indeed, a science that was initially highly cell-centric has undergone a fundamental reexamination, moving away from the premise of a direct exogenous stem cell-mediated regeneration towards the currently prevailing hypothesis that therapeutic activity reflects primarily an indirect, paracrine effect of delivered cells interacting with the diseased myocardium to trigger an endogenous regenerative cascade. Multimodal repair mechanisms, implicating both exogenous and endogenous progenitors, have in this regard been proposed [25].
Post-infarction, cell-based interventions aim at regenerative prophylaxis of fragile injured hearts, i.e., to limit early damage by altering the myocardial response to injury, averting adverse remodeling, and avoiding or delaying organ failure [26]. Beyond acute/subacute cardioprotection, in advanced heart failure associated with protracted systolic decompensation, the goal becomes cardiorestorative aimed at reversal of contractile dysfunction, structural restoration, and scar reduction [27]. Proposed strategies are supported by wide-ranging preclinical proof-of-concept studies that serve as a launch pad for testing in humans [28].
As a result, over the last decade, translation of stem cell technology in clinical trials has been increasingly realized. Across the cardiovascular disease spectrum, numerous phase I and a growing number of phase II clinical trials have been completed, testing various cell types and delivery protocols. Accumulating data from early phase clinical experience documents safety and feasibility of delivering autologous or allogeneic therapies in a range of cardiovascular conditions, and importantly provides a foundation to define parameters of clinical efficacy that justify further investigation in larger clinical trials [29]. Clinical progress in developing convincing and successful therapies, although steady, has been modest; in part attributed to rather small, underpowered trials using surrogate endpoints and open-label treatment approaches carrying the risk of bias [30]. A recent meta-analysis focused on heart failure reflects on the state-of-the-art [31]. This comprehensive study analyzed systematically 31 clinical trials including over 1500 total participants (882 cell-treated and 639 control patients). Collectively, these trials encompass an assortment of tested cell products ranging from bone marrow-derived mononuclear cells, including granulocyte-colony stimulating factor (G-CSF) mobilized subpopulations, and bone marrow-derived mesenchymal stem cells to cardiac stem cells, skeletal myoblast and adipose tissue-derived cells [31]. Supporting the safety record of cell-based therapies, this meta-analysis underscores overall safety with minimal major intervention-related adverse effects and no increase in the incidence of arrhythmias [31]. Moreover, reduction in mortality and re-hospitalization caused by worsening heart failure during long-term follow up, along with moderate improvement of left ventricular ejection fraction and improved heart failure symptoms including exercise capacity were documented. However, performance/selection bias was deemed considerable as only half of the analyzed trials reported blinding of participants/clinicians, and roughly half failed to report methods of allocation concealment [31]. In fact, when only double-blind studies were selected, the meta-analysis did not reveal statistical difference between cell-treated versus control groups [30,31]. Thus, encouraging feasibility and safety profiles observed repeatedly in clinical testing have yet to materialize into broadly validated clinical benefit, dictating the need for vigilant assessment of cell therapy practices [32]. In this regard, it should be noted that the presumed biological activity of a cellular product may greatly differ depending on the cell source, cell preparation, and/or cell administration. Moreover, among a number of variables, the state of the target cardiac microenvironment dictates the efficacy of mechanisms contributing to ultimate functional regeneration [30]. New emphasis is thus placed on establishing quality control procedures through development of standard operating practices for the harvesting, isolation, and expansion of cell populations. Insights into the composition of stem cell sources have for example paved the way towards approaches that would eliminate non-regenerative cells to expand cell populations that display multipotent traits possibly predicting regenerative potency before intervention [32].
Problem statement
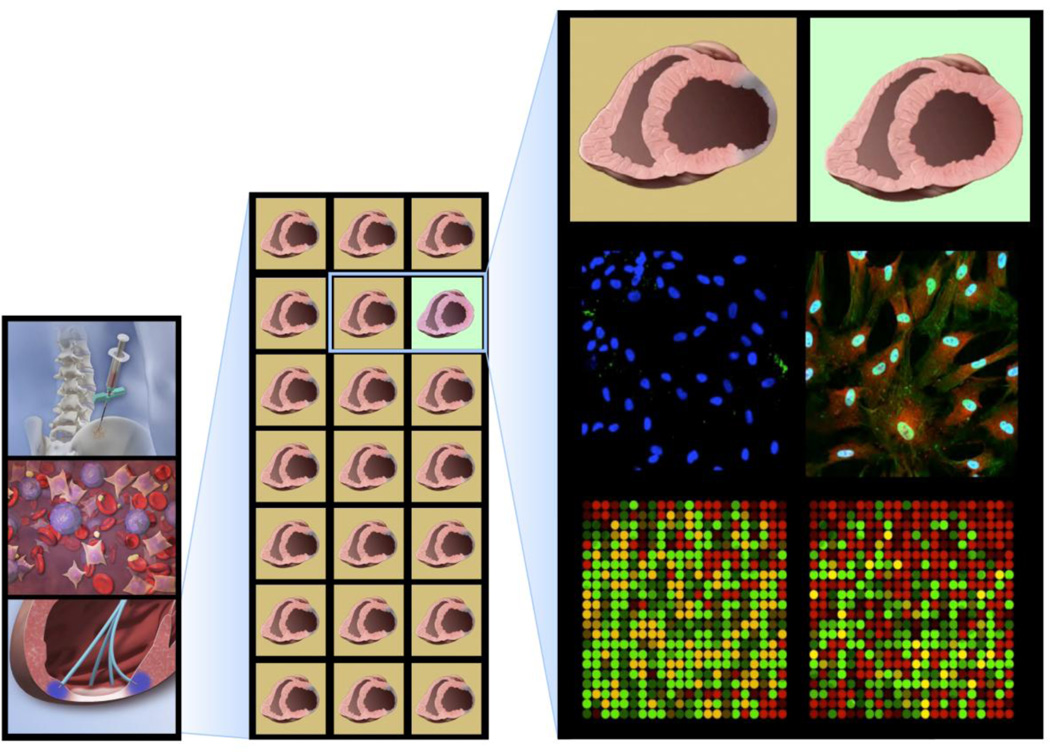
Regenerative science must achieve “validity” (potential effectiveness) and “utility” (likelihood of improved outcome) in clinical settings to extend current care models, and provide a value-added benefit for patients and society at large [33]. Build-out of regenerative service lines is predicated on effective clinical grade biotherapies suitable for scale-up and standardized production and application. A viable supply chain requires quality-controlled manufacturing and delivery of products that fulfill patient specifications [33]. At present, an essential point of vulnerability that constrains translational readiness and practice adoption is the inherent idiosyncrasy and aleatory bioactivity of stem cell populations (Fig. 2) [34].
Fig. 2.
Heterogenous regenerative proficiency. Left column: Harvest of unselected stem cell populations, which when delivered as a singular intervention produce mixed results. Middle column: Only 5% of patients with heart failure harbor stem cells associated with clinically demonstrable benefit. Right column: Reparative cell populations are distinguished from non-reparative counterparts by a distinct molecular signature reflecting functional plasticity.
Patient modifiers – such as age, sex, morbidities and concomitant therapies – impact regenerative fitness. Cell performance is also subject to influences during procurement, production, and/or delivery [35]. In fact, not all individuals harbor stem cells with a uniform reparative capacity. Systematic analysis of national trial experience reveals that, in patient cohorts, the incidence of reparative stem cells with a clinically measurable cardio-regenerative aptitude is quite rare – in the order of 5% [36]. The inconsistency in stem cell effectiveness mandates means that would ensure consistent efficacy of treatment, including quantitative surrogates to reliably predict the intended biological activity [37].
Informing biotherapy evolution
Clinical trial experience provides an irreplaceable avenue to inform the evolution of cardioregenerative stem cell therapies [30]. “First generation” therapies are typically comprised of mixed cell populations that generated largely mixed results [38,39]. Heterogeneous clinical outcomes offer however a unique opportunity to delineate molecular underpinnings of true responders from non-responders (Fig. 2). Surface markers alone may provide insufficient resolution to forecast cellular repair aptitude. Rather, regenerative from non-regenerative cytotypes are segregated based on distinctive molecular pathways that are starting to be elucidated through high-throughput clinomics approaches leveraging clinical trial specimens cross-referenced with individual patient outcomes [36,40]. Non-regenerative cells remain confined to a state of perpetual stemness [39]. In contrast, rare regenerative counterparts are milieu-responsive, plastic, with a definitive inclination for differentiation – traits of regenerative proficiency [40,41].
Accordingly, “next generation” therapies are designed to ensure that therapeutic stem cells will reliably function in the target organ [42]. This requirement can, in principle, be achieved through multiple strategies, including habituation of the myocardial environment to improve on stem cell homing upon delivery [43], anatomic matching of cell source with target organ relying on resident stem cell pools [44], or combined cell therapy (e.g., mesenchymal stem cells along with c-kit(+) cells) for synergistic effects that leverage cooperative cell-to-cell communication according to organ needs [45,46]. We here zoom-in on an alternative prototype platform – cardiopoiesis – developed to mitigate variability inherent to cell products/patients and integrate a quality system that certifies regenerative proficiency of a biotherapy candidate.
Cardiopoiesis fundamentals
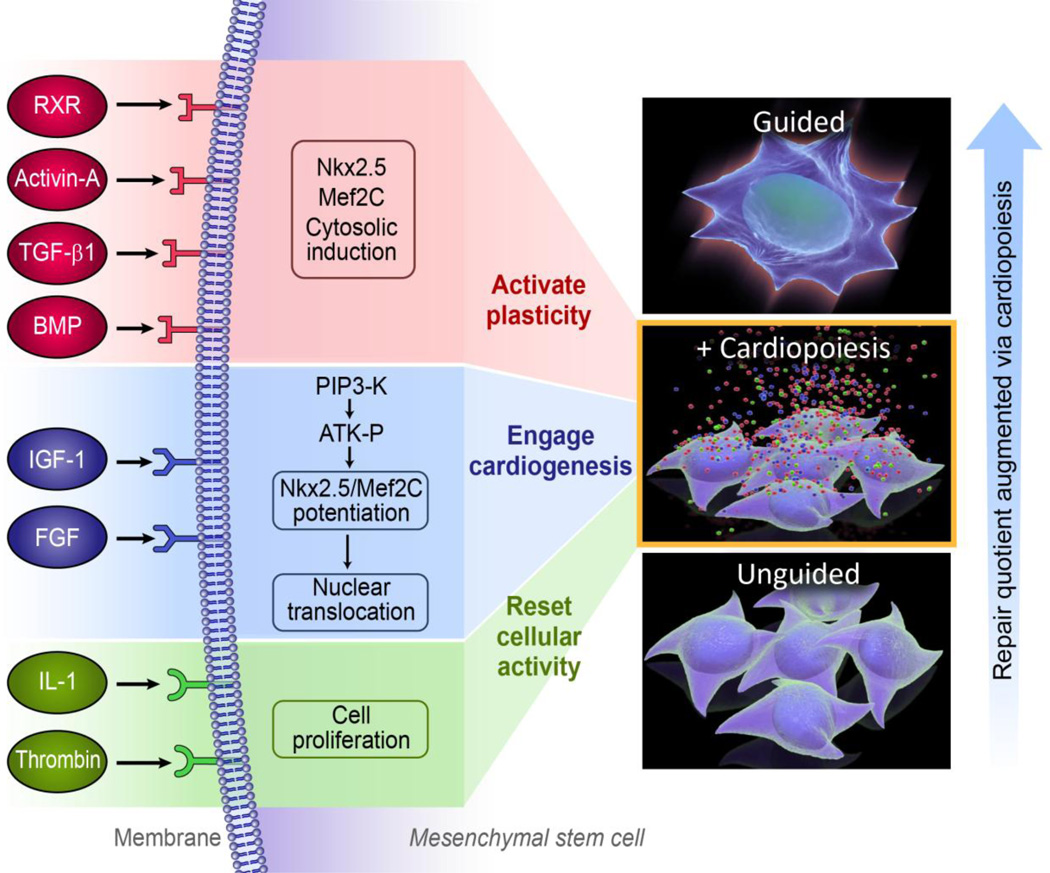
Cardiopoiesis imposes a lineage-specifying program on stem cells to reinvigorate function and promote cardioreparative proclivity [40,41]. Cardiopoiesis guides stem cells to (re)activate cellular plasticity, (re)engage into cardiovasculogenesis, and (re)set an active aptitude for repair (Fig. 3). This conditioning paradigm draws from embryonic signals that instruct pre-cardiac mesoderm to commit into the cardiomyogenic fate [47]. Cues germane to the ventral endoderm of a developing embryo guide the anterolateral mesoderm ensuring definitive cardiac program engagement, and avoidance of alternative fates or uncontrolled growth [48]. Narrow windows defining developmental stages dictate the delicate nature in which cardiogenic cues need to be introduced to promote cardiogenesis from an embryonic stem cell source, exemplified in the complex dynamics of TGF-β superfamily signaling guiding pluripotent stem cell fate choices [49,50]. A systems biology-resolved cardiopoietic atlas revealed an integrated and tractable molecular network fundamental to lineage specification [51]. Using endodermal cell lines, the cardio-inductive aptitudes of secreted cytokines and growth factors have been screened – a process facilitated by the stress cytokine TNFα that spikes the cardiogenicity of the endodermal secretome [52]. Resolving the unprimed endodermal secretome vis-a-vis that of the TNFα-enhanced endoderm enabled dissection of molecules that coax stem cells into cardiac fate. Through this approach, a cocktail of critical factors was formulated to recapitulate required cardiogenic cues [53]. An initial version included TGF-β1, BMP-2/4, FGF-2/4, IL-6, IGF-1/2, VEGF-A, EGF and Activin-A, where staged factor combinations created a synergistic environment that promotes the up-regulation and nuclear translocation of cardiac transcription factors, including homeobox transcription factor Nxk2.5, zing finger-containing transcription factor GATA-4, and myocyte enhancer factor MEF2C. Directed differentiation allows lineage mapping of embryonic stem cells as they transition from pluripotency to a cardiogenically-oriented multipotent fate. The distinguishing feature of the derived intermediate cell phenotype, termed cardiopoietic stem cell, is the capacity to uniquely yield cardiovascular lineages [40,48,53]. Cardiopoietic stem cells are defined by nuclear translocation of cardiac transcription factors (low in unguided stem cells) and absence of sarcomerogenesis (typical of mature cardiomyocytes). In density gradients, sarcomere-poor cardiopoietic stem cells are readily separated from cardiomyocytes. A low density cardiopoietic stem cell culture (1,500 cells/cm2) placed in the cardiogenic cocktail yields a 10, 30, and 65% population of cardiomyocytes by 3, 6, and 10 days, respectively [41]. In this way, cardiopoiesis enables targeted generation of lineage-specified stem cells [54].
Fig. 3.
Targeted (re)activation of latent plasticity in adult stem cells augments the repair quotient. Left: Cardiopoiesis, via cardiogenic cues, guides patient-derived stem cells into a state of active cellular plasticity and cardiovasculogenesis to augment repair aptitude. Right: Increase of cardiac repair propensity in stem cells following cardiopoietic guidance.
Translating cardiopoiesis
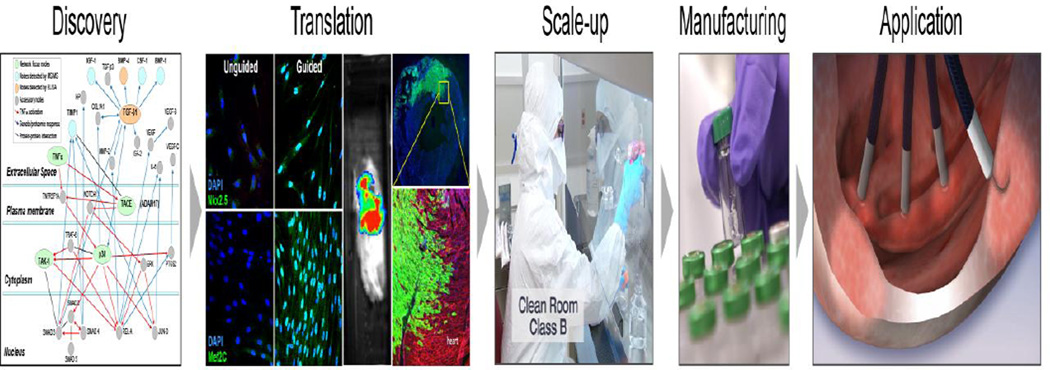
Principles discovered in embryonic platforms are translatable into clinically-apt practices (Fig. 4). A cardiogenic cocktail-rich milieu can guide patient-derived adult stem cells to acquire a repair potential associated with cardiac transcription factor expression [40,54]. Adult stem cells suffering from sequestered plasticity are resuscitated by priming with recombinant factors TGF-β, BMP-4, Activin-A, IGF-1, IL-6, FGF-2, thrombin, and retinoic acid that mimic signals and pathways activated in natural cardiogenesis. Of note, however, the biological outcome of cardiopoiesis applied to an adult stem cell population should be distinguished from that of pluripotent counterparts as it intends to achieve a regenerative paracrine function in the heart rather than to recapitulate embryonic cardiomyogenesis [40,48]. The first clinically tested example of such an approach is lineage specification through conditioning of bone marrow derived mesenchymal stem cells from patients with ischemic heart failure to yield the cardiopoietic stem cell phenotype [40,41,54]. In mesenchymal stem cells, simultaneous activation with TGF-β, BMP-4 and Activin-A along with retinoic acid induces cytosolic expression of cardiac transcription factors, while IGF-1 and IL-6 prompt their nuclear translocation (Fig. 3). Such co-stimulation typically results in cell cycle arrest of primed mesenchymal stem cells precluding cell propagation to achieve a therapeutic dose needed in man. To this end, FGF-2 and thrombin are utilized to maintain cell cycle activity (Fig. 3). Compared to lineage-unspecified mesenchymal stem cells, delivery of derived cardiopoietic stem cells into an infarcted failing heart demonstrates improved therapeutic impact on follow-up [40]. Limited cell grafting detectable long-term contrasts the maintained functional benefit, implicating indirect mode of action that harnesses endogenous repair pathways [40,55]. Although rare, head-to-head studies of different transplanted cell types indicate functional superiority of those whose phenotype is close to that of the target tissue, i.e., cells committed towards a cardiac lineage [55]. Pre-emptive cardiopoietic conditioning could thus serve to expand the number of patients potentially benefiting from stem cell therapy by converting the naïve, typically nonreparative, source into a reparative cytotype [56,57].
Fig. 4.
Cardiopoiesis platform: Translating discovery into application. Deconvoluted molecular events underlying cardiogenesis guided translation and scale-up of lineage-specified stem cells manufactured for clinical application.
A biomarker-based measure to anticipate therapeutic efficacy of adult stem cells prior to transplantation was in accordance developed. The “cardiopoietic index” employs a gene-expression profiling as a means to assess the regenerative quotient of patient derived cells [58]. The index reflects an integrated readout, based on the messenger RNA expression of cardiogenic transcription factors Nkx2.5, MEF2c, Gata4, Gata6, Fog-1, MESP1, and Tbx5. Application of this quality control standard allows pre-assessment of repair potential at time of cell harvest predicting individuals harboring stem cells with an innate capacity for repair versus those with non-reparative cells where switch-on of pro-regenerative signaling is needed. The cardiopoietic index is a gauge of functional benefit (measured as ejection fraction change) with a reported sensitivity and specificity of 91 and 95%, respectively [58].
Ensuring a robust cardiopoietic yield would be valuable, in particular in conditioning stem cells derived from elderly patients. An example of strategy currently investigated to maintain youthful status is the titration of nucleostemin functionality. This nucleolar stress sensor works by stabilizing stemness gene programs through pro-survival pathways with nucleostemin overexpression reducing senescent traits in support of tissue youth [59], thus providing a means to adjust regenerative potential on a need-be basis [19].
Cardiopoiesis in the clinic
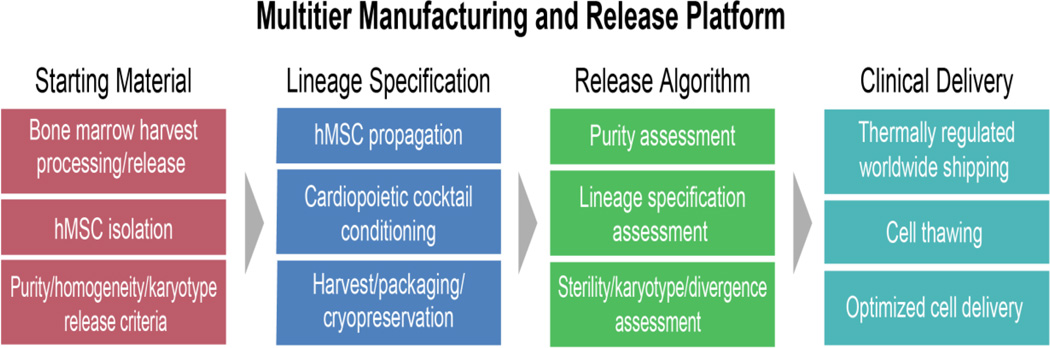
To achieve clinical application of a stem cell-based technology, scalable standard operating procedures are utilized. Proper dose ramp up, in tandem with suitable bio-distribution, are some of the basic requirements for safety and efficacy to reflect preclinical data [60,61]. The stringency of Good Manufacturing Practice is employed to ensure clinical-grade manufacturing of derived cellular products that must meet purity, potency, and sterility metrics. Manufactured cardiopoietic stem cells require a multitier release schedule which first establishes homogeneity of the mesenchymal stem cell source through cell surface marker profiling. This is followed by establishment of purity through gene profiling to ensure that the therapeutic formulation is devoid of divergent, non-cardioregenerative contaminants. Finally, documented nuclear translocation of a select cardiopoietic index marker ensures potency (Fig. 5).
Fig. 5.
Multitier release criteria offer a quality control system to ensure optimal regenerative proficiency. A quality system infrastructure conforming to Good Manufacturing Practice standards is needed for procurement, manufacture, and release of lineage-specified cellular product. A logistics-supervised distribution insures delivery of stable product for clinical use.
The impact of cardiopoietic stem cells on patients with established ischemic heart failure was investigated in the C-CURE trial (Cardiopoietic stem Cell therapy in heart failURE; ClinicalTrials.gov Identifier: NCT00810238; Fig. 6). This Phase II, randomized and prospective multicenter study evaluated the feasibility and safety of the cardiopoiesis-based technology in patients with chronic heart failure of ischemic origin while monitoring for efficacy signals [62]. Cardiopoietic stem cells were implanted, using direct endomyocardial delivery [63], on average 1,500 days after myocardial infarction. Patients were randomized to receive cardiopoietic stem cells plus standard of care, in the therapy arm, versus standard-of-care alone in the control arm. Following the cardiopoiesis algorithm, the C-CURE trial pre-emptively treated patient-derived mesenchymal stem cells with the cardiogenic cocktail to achieve guidance toward a lineage specified state [62]. There was no evidence of cardiac or systemic toxicity induced by cardiopoietic cell therapy. In addition, left ventricular ejection fraction was improved in the cardiopoietic stem cells therapy arm compared to standard-of-care alone, and associated with reduction in left ventricular end-systolic volume. A favorable impact on global parameters such as 6-min walk distance was also noted, along with benefit in a composite clinical score encompassing cardiac as well as general wellness parameters [62].
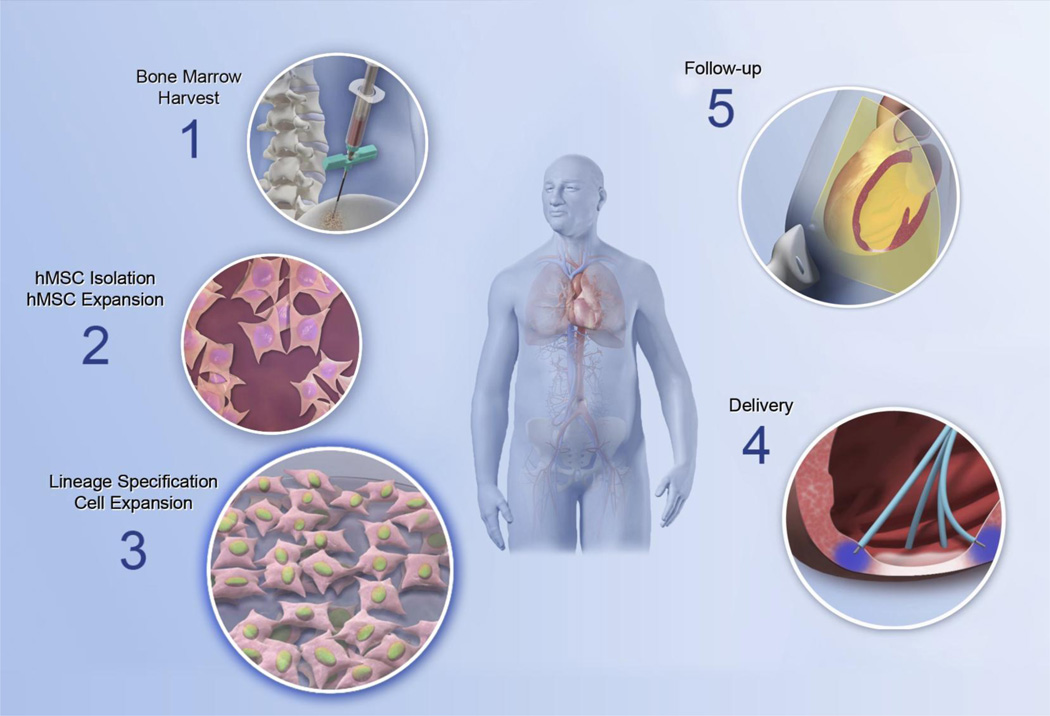
Fig. 6.
Clinical implementation of the lineage-guidance paradigm in cell therapy. The C-CURE (Cardiopoietic stem Cell therapy in heart failURE) trial was conducted in patients with ischemic heart failure. Bone marrow was harvested (step 1) and isolated mesenchymal stem cells (step 2) lineage-specified by cardiogenic cocktail priming (step 3). Cardiopoietic stem cells meeting release criteria were delivered by endomyocardial injections (step 4). On follow-up, signs of efficacy were documented (step 5).
These results serve to support further investigation [64], including a multinational phase III clinical trial, named CHART-1 (Congestive Heart Failure CArdiopoietic Regenerative Therapy), currently in the follow-up phase [65]. Patients with chronic heart failure secondary to ischemic heart disease, reduced left ventricular ejection fraction (<35%), and at high risk for recurrent heart failure-related events, were randomized in CHART-1 to receive 600×106 bone marrow-derived and lineage-directed autologous cardiopoietic stem cells (administered via a retention-enhanced intramyocardial injection catheter [66]) or a sham procedure (ClinicalTrials.gov Identifier: NCT01768702). The primary efficacy endpoint of the CHART-1 study is a hierarchical composite of mortality, worsening heart failure, Minnesota Living with Heart Failure Questionnaire score, 6-min walk test, left ventricular end-systolic volume, and left ventricular ejection fraction at 9 months [65]. The secondary efficacy endpoint is the time to cardiovascular death or worsening heart failure at 12 months. Safety endpoints include mortality, readmissions, aborted sudden deaths, and serious adverse events at 12 and 24 months. The CHART-1 clinical trial is powered to examine the therapeutic impact of lineage-directed stem cells as a strategy to achieve cardiac regeneration in heart failure populations [65]. On completion, the CHART-1 trial is designed to offer a definitive evaluation of the efficacy and safety of cardiopoietic stem cells in the treatment of chronic ischemic heart failure [67].
Outlook
Standard of care in heart failure aims to reverse disease course and reduce adverse outcomes. Countering post-infarction parenchymal loss, patients display different trajectories of disease progression [68] compounded by age-mediated cardiac vulnerability [69]. Introduction of regenerative regimens in management algorithms is conceived to complement, and potentially transform the available armamentarium. Early experience in clinical cardiac regeneration supports the compatibility of stem cell-based therapies as adjuvants to established practice [70]. However, lack of therapeutic consistency inherent to patient-derived stem cell populations remains a central hurdle limiting adoption.
The regenerative capacity of stem cells is influenced by multiple factors dictating the proclivity for tissue health restoration [71]. Importantly, therapeutic inconsistency in clinical trials provides a kaleidoscope of biological systems activity across the range of observed regenerative benefit (Fig. 7). Leveraging clinomics-based interrogation, biological deconvolution informs the development of new high-fidelity protocols endowed with a resolution needed to ensure cell repair potency prior to application. A prototype approach is cardiopoiesis that inculcates lineage-specification, conditioning stem cells with recombinant cardiogenic cues to endow therapeutic proficiency in heart failure. Accordingly, a multitier quality system to verify homogeneity, purity, and potency-associated markers for release of manufactured clinical grade cardiopoietic stem cells has been rolled-out. The cardiopoietic stem cell phenotype is currently tested in advanced clinical trials for chronic heart failure exemplifying a next-generation biotherapy optimized for regenerative proficiency.
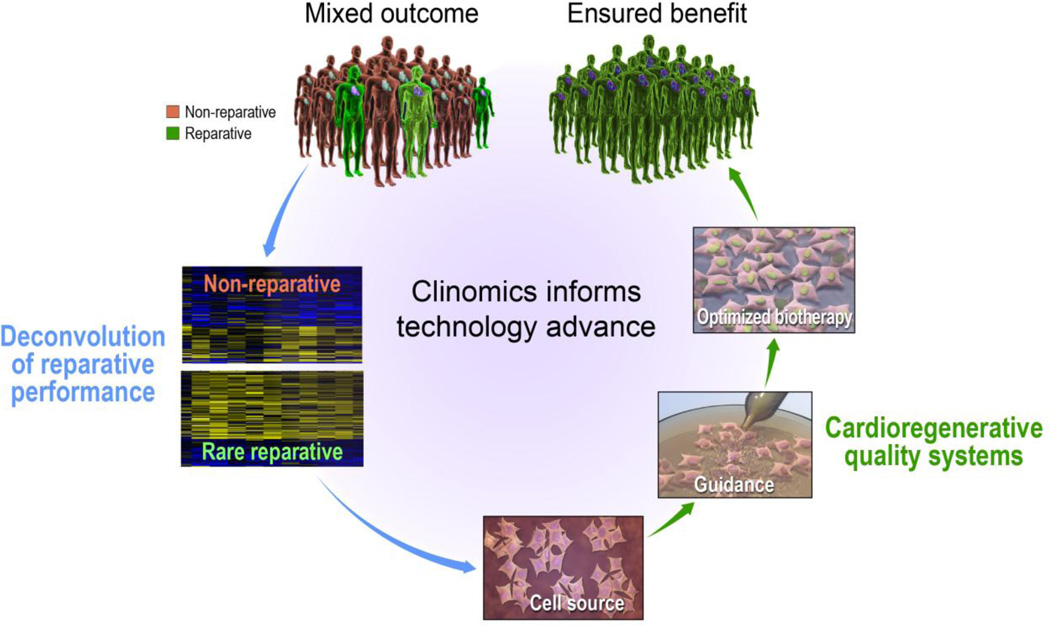
Fig. 7.
Clinomics-based optimization algorithm informs next generation regenerative biotherapies. Mixed outcomes documented in cardiovascular clinical trials underscore a limitation of first generation stem cell regimens. High-throughput clinomics strategies provide the opportunity to delineate the molecular underpinnings of responders versus non-responders informing next generation strategies. Use of a priming platform to guide patient-derived stem cells into a pro-reparative phenotype exemplifies such an optimizing approach aimed to ensure benefit in heart failure patient populations.
Beyond validity, the utility of newest regenerative options will inform adoption reflecting real-world experience with emerging treatments poised to address unmet needs of broader populations [72,73]. Modern clinical development algorithms of candidate technology incorporate multi-disciplinary assessment by healthcare providers, developers, regulators, and payers [74] and seek active patient engagement [75]. This evolving landscape heralds an evolution in the medical product development and authorization lifecycle of novel therapies, from a paradigm focused on the therapeutics to a holistic evaluation that integrates the patient within a healthcare regimen.
Acknowledgments
Drs. Terzic and Behfar report research grants, administered by Mayo Clinic, from Marriott Foundation, Michael S. and Mary Sue Shannon Family, Russ and Kathy VanCleve Foundation, Leducq Fondation, Florida Heart Research Institute, Celyad, and National Institutes of Health in support of work in the laboratory. Drs. Terzic and Behfar are listed as co-inventors on patents US 20080019944 and US 20120100533.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Braunwald E. The ten advances that have defined modern cardiology. Trends Cardiovasc Med. 2014;24:179–183. doi: 10.1016/j.tcm.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS. Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. doi: 10.1056/NEJMoa1208200. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2016 update. Circulation. 2016;132 [Google Scholar]
- 4.Jessup M. The heart failure paradox: an epidemic of scientific success. Circulation. 2014;129:2717–2722. doi: 10.1161/CIR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 5.Terzic A, Waldman S. Chronic diseases: the emerging pandemic. Clin Transl Sci. 2011;4:225–226. doi: 10.1111/j.1752-8062.2011.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–892. doi: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 7.Joffe SW, Webster K, McManus DD, Kiernan MS, Lessard D, Yarzebski J, et al. Improved survival after heart failure: a community-based perspective. J Am Heart Assoc. 2013;2:e000053. doi: 10.1161/JAHA.113.000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 10.Butler J, Gheorghiade M, Kelkar A, Fonarow GC, Anker S, Greene SJ, et al. In-hospital worsening heart failure. Eur J Heart Fail. 2015;17:1104–1113. doi: 10.1002/ejhf.333. [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E. The war against heart failure. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. 2020: A new vision – a future for regenerative medicine. [Accessed January 10, 2016]; http://medicine.osu.edu/regenerativemedicine/documents/2020vision.pdf.
- 13.Terzic A, Nelson TJ. Regenerative medicine primer. Mayo Clin Proc. 2013;88:766–775. doi: 10.1016/j.mayocp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Atala A, Murphy S. Regenerative medicine. JAMA. 2015;313:1413–1414. doi: 10.1001/jama.2015.1492. [DOI] [PubMed] [Google Scholar]
- 15.Terzic A, Harper CM, Jr, Gores GJ, Pfenning MA. Regenerative medicine blueprint. Stem Cells Dev. 2013;22(Suppl 1):20–24. doi: 10.1089/scd.2013.0448. [DOI] [PubMed] [Google Scholar]
- 16.Anversa P, Leri A. Innate regeneration in the aging heart: healing from within. Mayo Clin Proc. 2013;88:871–883. doi: 10.1016/j.mayocp.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Behfar A, Gersh B, Terzic A. Repetition rescues regenerative reserve. Eur Heart J. 2016;00:000–000. doi: 10.1093/eurheartj/ehv596. [DOI] [PubMed] [Google Scholar]
- 19.Behfar A, Terzic A. Stem cells versus senescence: the yin and yang of cardiac health. J Am Coll Cardiol. 2015;65:148–150. doi: 10.1016/j.jacc.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 20.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menasché P. Stem cells in the management of advanced heart failure. Curr Opin Cardiol. 2015;30:179–185. doi: 10.1097/HCO.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 22.Emmert MY, Hitchcock RW, Hoerstrup SP. Cell therapy, 3D culture systems and tissue engineering for cardiac regeneration. Adv Drug Deliv Rev. 2014;69–70:254–269. doi: 10.1016/j.addr.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Jackman CP, Shadrin IY, Carlson AL, Bursac N. Human cardiac tissue engineering: From pluripotent stem cells to heart repair. Curr Opin Chem Eng. 2015;7:57–64. doi: 10.1016/j.coche.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey AM, Mendicino M, Au P. An FDA perspective on preclinical development of cell-based regenerative medicine products. Nat Biotechnol. 2014;32:721–723. doi: 10.1038/nbt.2971. [DOI] [PubMed] [Google Scholar]
- 25.Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssens S. Stem cells in the treatment of heart disease. Annu Rev Med. 2010;61:287–300. doi: 10.1146/annurev.med.051508.215152. [DOI] [PubMed] [Google Scholar]
- 27.Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart. 2010;96:792–800. doi: 10.1136/hrt.2007.139394. [DOI] [PubMed] [Google Scholar]
- 28.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–814. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assmus B, Dimmeler S, Zeiher AM. Cardiac cell therapy: lost in meta-analyses. Circ Res. 2015;116:1291–1292. doi: 10.1161/CIRCRESAHA.115.306330. [DOI] [PubMed] [Google Scholar]
- 31.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 32.Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol. 2014;11:232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 33.Terzic A, Pfenning MA, Gores GJ, Harper CM., Jr Regenerative medicine build-out. Stem Cells Transl Med. 2015;4:1373–1379. doi: 10.5966/sctm.2015-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimmeler S, Ding S, Rando TA, Trounson A. Translational strategies and challenges in regenerative medicine. Nat Med. 2014;20:814–821. doi: 10.1038/nm.3627. [DOI] [PubMed] [Google Scholar]
- 35.Trounson A, McDonald C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Cogle CR, Wise E, Meacham AM, Zierold C, Traverse JH, Henry TD, et al. Cardiovascular Cell Therapy Research Network (CCTRN). Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014;115:867–874. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schüssler-Lenz M, Beuneu C, Menezes-Ferreira M, Jekerle V, Bartunek J, Chamuleau S, et al. Cell-based therapies for cardiac repair – a meeting report on scientific observations and European regulatory viewpoints. Eur J Heart Fail. 2016:18,000–18,000. doi: 10.1002/ejhf.422. [DOI] [PubMed] [Google Scholar]
- 38.Rosenzweig A. Cardiac cell therapy--mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 39.Marbán E, Malliaras K. Mixed results for bone marrow-derived cell therapy for ischemic heart disease. JAMA. 2012;308:2405–2406. doi: 10.1001/jama.2012.64751. [DOI] [PubMed] [Google Scholar]
- 40.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behfar A, Terzic A. Cardiopoietic stem cells for heart failure therapy. In: Perin EC, Miller LW, Taylor DA, Willerson JT, editors. Stem Cell and Gene Therapy for Cardiovascular Disease. Waltham: Academic Press; 2016. pp. 235–241. [Google Scholar]
- 42.Terzic A, Behfar A. Regenerative heart failure therapy headed for optimization. Eur Heart J. 2014;35:1231–1234. doi: 10.1093/eurheartj/ehu117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, et al. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309:1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 44.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS Trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, et al. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. J Am Coll Cardiol. 2015;66:1990–1999. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leri A, Anversa P. Stem cells and myocardial Regeneration: Cooperation wins over competition. Circulation. 2013;127:165–168. doi: 10.1161/CIRCULATIONAHA.112.153973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behfar A, Faustino RS, Arrell DK, Dzeja PP, Perez-Terzic C, Terzic A. Guided stem cell cardiopoiesis: discovery and translation. J Mol Cell Cardiol. 2008;45:523–529. doi: 10.1016/j.yjmcc.2008.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: lessons from development. Genes Dev. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai W, Guzzo RM, Wei K, Willems E, Davidovics H, Mercola M. A Nodal-to-TGFβ cascade exerts biphasic control over cardiopoiesis. Circ Res. 2012;111:876–881. doi: 10.1161/CIRCRESAHA.112.270272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faustino RS, Behfar A, Perez-Terzic C, Terzic A. Genomic chart guiding embryonic stem cell cardiopoiesis. Genome Biol. 2008;9:R6. doi: 10.1186/gb-2008-9-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arrell DK, Niederländer NJ, Faustino RS, Behfar A, Terzic A. Cardioinductive network guiding stem cell differentiation revealed by proteomic cartography of tumor necrosis factor alpha-primed endodermal secretome. Stem Cells. 2008;26:387–400. doi: 10.1634/stemcells.2007-0599. [DOI] [PubMed] [Google Scholar]
- 53.Behfar A, Terzic A. Derivation of a cardiopoietic population from human mesenchymal stem cells yields cardiac progeny. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S78–S82. doi: 10.1038/ncpcardio0429. [DOI] [PubMed] [Google Scholar]
- 54.Behfar A, Terzic A. Mesenchymal stem cells: engineering regeneration. Clin Transl Sci. 2008;1:34–35. doi: 10.1111/j.1752-8062.2008.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menasché P. Stem cells for the treatment of heart failure. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140373. doi: 10.1098/rstb.2014.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behfar A, Terzic A. Stem cell in the rough: repair quotient mined out of a bone marrow niche. Circ Res. 2014;115:814–816. doi: 10.1161/CIRCRESAHA.114.305075. [DOI] [PubMed] [Google Scholar]
- 57.Marban E, Malliaras K. Boot camp for mesenchymal stem cells. J Am Coll Cardiol. 2010;56:735–737. doi: 10.1016/j.jacc.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crespo-Diaz R, Yamada S, Bartunek J, Perez-Terzic C, de Waele P, Mauën S, et al. Cardiopoietic index predicts heart repair fitness of patient-derived stem cells. Biomark Med. 2015;9:639–649. doi: 10.2217/bmm.15.31. [DOI] [PubMed] [Google Scholar]
- 59.Hariharan N, Quijada P, Mohsin S, Joyo A, Samse K, Monsanto M, et al. Nucleostemin rejuvenates cardiac progenitor cells and antagonizes myocardial aging. J Am Coll Cardiol. 2015;65:133–147. doi: 10.1016/j.jacc.2014.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson TJ, Behfar A, Terzic A. Stem cells: biologics for regeneration. Clin Pharmacol Ther. 2008;84:620–623. doi: 10.1038/clpt.2008.146. [DOI] [PubMed] [Google Scholar]
- 61.O'Brien T, Creane M, Windebank AJ, Terzic A, Dietz AB. Translating stem cell research to the clinic: a primer on translational considerations for your first stem cell protocol. Stem Cell Res Ther. 2015;6:146. doi: 10.1186/s13287-015-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, et al. Cardiopoietic stem cell therapy in heart failure: The C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 63.Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clin Pharmacol Ther. 2008;85:548–552. doi: 10.1038/clpt.2008.295. [DOI] [PubMed] [Google Scholar]
- 64.Murry CE, Palpant NJ, MacLellan WR. Cardiopoietry in motion: primed mesenchymal stem cells for ischemic cardiomyopathy. J Am Coll Cardiol. 2013;61:2339–2340. doi: 10.1016/j.jacc.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 65.Bartunek J, Davison B, Sherman W, Povsic T, Henry TD, Gersh B, et al. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. Eur J Heart Fail. 2016;18:000–000. doi: 10.1002/ejhf.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Behfar A, Latere JP, Bartunek J, Homsy C, Daro D, Crespo-Diaz RJ, et al. Optimized delivery system achieves enhanced endomyocardial stem cell retention. Circ Cardiovasc Interv. 2013;6:710–718. doi: 10.1161/CIRCINTERVENTIONS.112.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheridan C. Cardiac stem cell therapies inch toward clinical litmus test. Nat Biotechnol. 2013;31:5–6. doi: 10.1038/nbt0113-5. [DOI] [PubMed] [Google Scholar]
- 68.Wilcox JE, Fonarow GC, Ardehali H, Bonow RO, Butler J, Sauer AJ, et al. "Targeting the Heart" in heart failure: Myocardial recovery in heart failure with reduced ejection fraction. JACC Heart Fail. 2015;3:661–669. doi: 10.1016/j.jchf.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 69.Jahangir A, Sagar S, Terzic A. Aging and cardioprotection. J Appl Physiol. 2007;103:2120–2128. doi: 10.1152/japplphysiol.00647.2007. [DOI] [PubMed] [Google Scholar]
- 70.Núñez García A, Sanz-Ruiz R, Fernández Santos ME, Fernández-Avilés F. “Second-generation” stem cells for cardiac repair. World J Stem Cells. 2015;7:352–367. doi: 10.4252/wjsc.v7.i2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 72.Behfar A, Bartunek J, Terzic A. Stem cell therapy for ischemic heart disease. In: Bartunek J, Vanderheyden M, editors. Translational Approach to Heart Failure. New York: Springer; 2013. pp. 449–466. [Google Scholar]
- 73.Filippatos G, Khan SS, Ambrosy AP, Cleland JGF, Collins SP, Lam CSP, et al. International REgistry to assess medical Practice with lOngitudinal obseRvation for Treatment of Heart Failure (REPORT-HF): rationale for and design of a global registry. Eur J Heart Fail. 2015;17:527–533. doi: 10.1002/ejhf.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terzic A, Behfar A, Filippatos G. Clinical development plan for regenerative therapy in heart failure. Eur J Heart Fail. 2016;00:00–00. doi: 10.1002/ejhf.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunter NL, O'Callaghan KM, Califf RM. Engaging patients across the spectrum of medical product development: View from the US Food and Drug Administration. JAMA. 2015;314:2499–2500. doi: 10.1001/jama.2015.15818. [DOI] [PubMed] [Google Scholar]