Abstract
The goal of the present research was to assess the degree to which a pattern completion process operates in cue-induced relapse to cocaine-seeking behavior. Using a novel cue-preference version of the place preference task, rats were administered cocaine or saline, which resulted in a preference for the cocaine-paired cues. After 21 days of abstinence and prior to the preference test, for one group, PBS or naloxone was injected into the CA3 subregion of the hippocampus and for a second group, saline or naloxone was injected systemically. The results indicated that infusions of naloxone into CA3 or systemic injections produced a marked disruption for one and two cues, but had minimal disruptive effect for three or four cues, suggesting that naloxone injections disrupt CA3 function and trigger a deficit in a pattern completion process. Thus, it appears that cue-based activation of the dorsal CA3 might be a critical trigger via a pattern completion process. Based on additional analyses it appears that there is a disruption primarily for object touches for one cue naloxone injections into the CA3 or systemic injections, but no effect on time (spatial context).
Keywords: CA3, hippocampus, cocaine-seeking, relapse, naloxone
Introduction
It is generally accepted that drug-associated environmental cues (e.g., associated drug paraphernalia or locations where a drug was previously consumed) can elicit drug craving and consequently motivate drug-seeking and drug-taking behaviors. Often, one specific salient cue or context can be sufficient to induce relapse to drug seeking and drug taking. One mechanism that could support the ability of a single cue to reinstate drug seeking is known as pattern completion, a process wherein presentation of an incomplete stimulus complex leads to recall of the complete, previously learned pattern. It has been proposed that during retrieval of information, the CA3 recurrent collaterals of the hippocampus play a major role in retrieving complete, previously learned, patterns in the face of an incomplete stimulus complex input to the hippocampus (pattern completion, Marr, 1971; Rolls and Kesner, 2006). For example, rats with CA3 lesions have impaired accuracy compared to controls on a cue-pattern completion task when only a subset of cues are available, suggesting impaired cue-pattern completion (Gold and Kesner, 2005). These data are consistent with proposed computational models suggesting that an auto-associative CA3 network is responsible for the completion of patterns based on incomplete input (Rolls, 1996; Rolls and Kesner, 2006).
In order to determine whether naloxone injections into the CA3 subregion of the hippocampus could mediate a cue-based pattern separation process for relapse mediation of drug seeking, visual objects were selected using a novel revised version of the place preference paradigm. It is well known that the lateral entorhinal cortex input into CA3 is opioid dependent and mediates object, but not spatial contextual information, whereas the medial entorhinal cortex is glutamate dependent and mediates spatial contextual, but not object, information (Hargreaves et al, 2005). Because it is easier to manipulate the number of objects to determine whether the CA3 plays a role during relapse a revised the place preference box was used by placing a door in the middle of the box and placing four different objects using the same spatial location pattern of the objects on each on the walls for each side of the box. It should be noted that the spatial aspect of the box itself did not differ between the two sides. It is also known that LTP produced by stimulation of the lateral entorhinal cortex perforant path is disrupted following injections of naloxone into the CA3, but LTP produced by stimulation of the medial entorhinal perforant path is not disrupted by naloxone injections into the CA3 (Do et al., 2002). Furthermore, naloxone injections into the CA3 disrupt a CA3 based pattern completion function for visual stimuli, but not for the spatial context (Kesner & Warthen, 2010). It should be noted that naltrexone, another opioid receptor antagonist, also disrupts reinstatement of alcohol-, as well as cocaine-, seeking behaviors by drug-associated stimuli (Burrattini et al, 2008; Ciccocioppo et al, 2002); Schmitz et al, 2001) and inhibits c-Fos expression in the CA3 (Dayas et al, 2007). Further, naltrexone has been shown to attenuate relapse in patients addicted to alcohol (Jaffe et al, 1996). Therefore, the possibility exists that the process of pattern completion mediated by opioid signaling in the CA3 region of the hippocampus may play a role in the ability of drug-associated cues to drive drug-seeking and -taking behaviors.
In the drug-reinstatement literature, there are a few studies examining the role of the hippocampus. For example, Fuchs et al, 2005). have shown that temporary inactivation of the dorsal hippocampus impairs context-induced reinstatement of cocaine seeking, but not reinstatement induced by discrete cocaine-paired cues or a cocaine priming injection. In a subsequent study, Lasseter et al, 2010) reported that temporary inactivation of the ventral hippocampus (CA1 and CA3), but not the ventral DG or posterior dorsal hippocampus (CA1 and CA3), produced a disruption in context-induced cocaine seeking. Rogers and See (2007) also have reported that selective inhibition at the junction between dorsal and ventral hippocampus attenuates relapse to cocaine-seeking following presentation of drug-paired cues or a priming injection of cocaine itself. However, to date, neither the specific role of the CA3 subregion nor the process of pattern completion has been examined in relation to the influence of drug-paired cues on drug-seeking and -taking behaviors. The focus of the present study, therefore, was to assess the degree to which a pattern completion process operates in cue-induced relapse to cocaine-seeking behavior. Furthermore, since naloxone can disrupt a CA3-mediated pattern-completion process, we examined whether the drug prevents cue-induced relapse to cocaine seeking.
Materials and Methods
Subjects
Rats were male, Long-Evans hooded rats weighing 300–350g. There were 16 rats in Experiment 1 and 22 rats in Experiment 2. An additional 8 rats were used to test whether naloxone induced a conditioned aversion. All rats were individually housed in plastic cages on a 12:12 light:dark cycle (8:00 AM: 8:00 PM) and had free access to food and water. All testing was performed during the light portion of the cycle.
Training apparatus
The training apparatus was a red Plexiglas box (91×29×29cm) that was constructed with a removable partition dividing the space in half, with each of the sides treated as a separate context. Four small children’s toys were located on the wall of each half of the box and served as environmental cues. These toys were attached with Velcro at the center of their respective wall, 15cm from the bottom and 15 cm apart (see Figure 1).
Figure 1.

Picture of the cue-based preference box.
Surgical procedures
Prior to training and testing in Experiment 1, naive rats underwent implantation of cannulae directed to the CA3. Subjects were anesthetized with isoflurane (1%–4% [vol/vol] at 1–2 L/min) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) on an isothermal pad. The coordinates for targeting the CA3 were 3.6 mm posterior, 3.6 mm lateral, and 3.6 mm ventral as measured from dura. Burr holes were drilled to allow for lowering of each cannula, as well as two holes for jeweler’s screws that were used as anchors for the dental cement. Cannulae implanted were 22 gauge (GA), through which 26-GA injection cannulae were subsequently inserted to project 1.0 mm from the bottom of the guide cannulae. Dorsoventral measurements were made from the tip of an injection cannula projecting through the guide cannulae. Cannulae supplies and screws were purchased from Plastics One, Inc. (Roanoke, VA). Once the screws and the guide cannulae were in place, dental cement (Orthojet Powder and Liquid, Lang Dental, Wheeling, IL) was mixed and placed on the skull to anchor the cannulae. Once the cement was dry and the cannulae were secure, the rat was removed from the earbars. As the rat recovered from the anesthesia, injection cannulae were removed from the guides, and dust caps with dummy cannulae were put in their place. After surgery, rats were allowed to recover in their home cage and given Carprofen (4 mg/kg) for seven days. All protocols were performed in concordance with the NIH Guide for the Care and Use of Laboratory Animals (2011) and were approved by the Institutional Animal Care and Use Committee at the University of Utah.
Behavioral methods
Acclimation Phase
Initially, rats were subjected to a 7–14 day acclimation period, during which time rats were handled daily for 10–15 minutes. Behavioral testing began once the rat displayed acclimation to the handling procedure.
Dependent Measures
Dependent measures were the number of touches of each object by the rat and the amount of time spent on each side of the box. A direct touch by the rat's body to any location on a cue was recorded. Touches were also recorded when the rat appeared to be whisker brushing the object.
Initial Preference Test – Day 1
Rats were placed in the center of the apparatus and allowed to explore freely for 15 minutes. During the trial, the amount of time spent on each side of the box was recorded, as was the number of touches for each cue. At the end of the 15 min, the rat was removed from the apparatus and placed back in the home cage. The touches for each cue on a side were summed, and the side with the highest number of touches was designated as the preferred context.
Conditioning Phase – Days 2–9
Rats were administered one intraperitoneal (i.p.) injection of cocaine•HCl (15 mg/mL, calculated as the salt; 1mL/kg) or saline (1 mL/kg) per day. Rats were kept in their home cages for 10–15 minutes following the injection. Following injection of cocaine, rats were placed in the non-preferred side as determined by the initial preference test. Following injections of saline, rats were placed in the preferred side. Rats received exposure to one trial/day pairing cocaine or saline with the designated side of the chamber, with the injected substance alternating daily. The order of injections was counterbalanced across rats.
Post-conditioning Preference Test – Day 10
Parameters of the post-conditioning test were identical to the preference test conducted on day 1. This test was used to evaluate whether a cocaine-induced cue preference was formed, as evidenced by more touches of cues on the side with which cocaine was paired. The order of cue preference for each object was also measured to establish a preference scale. Video recordings were made during the post-conditioning day-10 preference test for further analysis.
Abstinence phase – Days 11–31
Rats were then given a 21-day abstinence phase following the post-conditioning preference test. During abstinence, they were kept in their home-cages and handled daily.
Relapse phase – Days 32 – 44
Starting on day 32, the rats entered a relapse-testing phase conducted across 13 days. Each rat was run on a unique relapse-testing condition every 4th day (days 32, 36, 40, and 44), with the order of treatment and number of available cues on each side counterbalanced across rats. During this test the partition was removed from the apparatus, allowing for determination of preference for cues on the two sides of the chamber. In both Experiment 1 and Experiment 2, different cohorts of rats were used to test the role of different numbers of cues on each side, in order to decrease the number of relapse tests each rat experienced. In each Experiment, one cohort of rats was exposed to 1-and 4-cue conditions, whereas the other cohort was exposed to 2- and 3-cue conditions. Cues with the highest number of touches during the day-10 preference test were used during the relapse trials. Video recordings were made during the critical relapse trials for further analysis.
Drug treatments
In Experiment 1, rats were given infusions of naloxone (30mM, 0.4 µL, 1 µL/min) or PBS via the infusion cannulae and were then returned to their home cages for 15 min. The rats were then placed into the apparatus and allowed to explore for 15 minutes. During this time the number of touches of cues on each side was recorded. In Experiment 2, rats were injected with naloxone (2 mg/kg, 1 mL/kg, i.p.) or 0.9% saline (1 mL/kg, i.p.) and then tested as described for Experiment 1.
Histology
Following the completion of Experiment 1, rats were injected with a lethal dose of sodium pentobarbital (70 mg/mL; i.p.). The rats were then perfused transcardially with PBS followed by 10% (wt/vol) formaldehyde in 0.1 M phosphate buffer (formalin solution; Sigma-Aldrich, St. Louis, Missouri). The brains were removed and stored in 30% (wt/vol) sucrose-formalin for 1 week. Each brain was frozen and coronal sections (24 µm) were cut with a cryostat through the hippocampus and stained with cresyl violet. All sections were analyzed under a light microscope for cannulae placements.
Results
Experiment 1
Effects of naloxone injections into the CA3 subregion of the hippocampus on cued-based relapse to cocaine seeking.
The number of times each rat touched the different objects on the two sides was recorded for the day-10 preference test. At this point, the data from the two cohorts were combined for the cocaine-induced conditioned cue preference test and the time spent on the cocaine vs. saline sides. Cocaine-induced conditioned cue preference was assessed by subtracting the number of object touches on the saline-paired side from the number of object touches on the cocaine-paired side divided by the sum of the touches on both the cocaine- and saline-paired sides. The results indicated that the rats preferred the four cues on the cocaine-paired side compared to the four cues on the saline side, as reflected in a significant positive ratio of 0.32 which is significantly different from zero (t(1,14)=4.61, P< .01). We also assessed the mean time that the rats spent on the cocaine-paired side (8.67 min) compared to the mean time spent on the saline side (6.33 min; Figure 2). A one-way ANOVA revealed that rats spent more time on the cocaine-paired than the saline-paired side (F(1,17)=7.97, P=.0117). We also scaled the frequency of touches for each object to determine which objects each rat preferred the most, and these frequencies aided in the selection of cues for subsequent relapse trials. It should be noted that in all tests the objects in the back for the saline as well as the cocaine side received more exploration. Also across rats no specific object was explored more than others and there were many differences in exploration across rats. The selection for the 1 cue condition was based on the highest number of touches, i.e. the greatest preference; for the 2 cue condition the next frequently touched cue was added to the 1 cue; for the 3 cue condition the next frequently touched cue was added to the 1 and 2 cues and for the 4 cue condition all cues were used.
Figure 2.
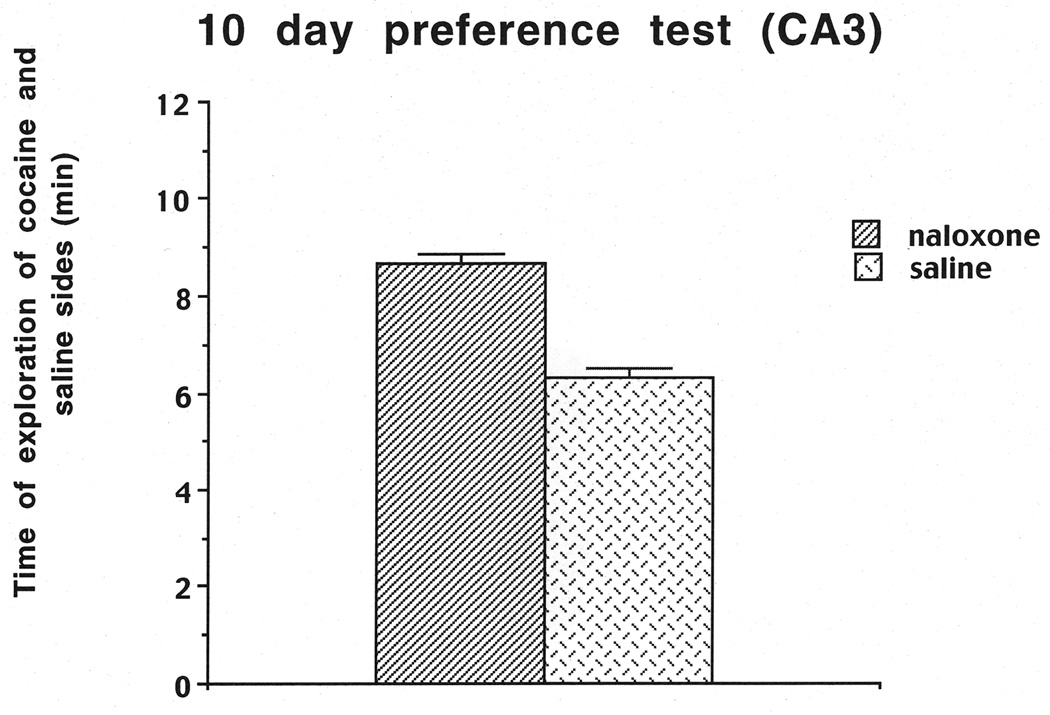
Duration of exploration of the cocaine vs. saline sides on the Day-10 preference test for the CA3 cohorts used in Experiment 1.
For the relapse test the same ratio described for the 10 day test was used. Results are shown in Figure 3.When rats received injections of saline into the CA3 bilaterally, they showed cue-induced relapse to cocaine-seeking, as evidenced by a positive ratio for touches of the cocaine-paired cues. Conversely, injections of naloxone into the CA3 led to a disruption of relapse when one or two cues were present, but no effect when three or four cues were present. A two-way ANOVA (group (naloxone, saline) × cue number (1 cue vs. 4 cues)) on the data from the first cohort of rats revealed a significant main effect of group (F (1,14)=20.47,P=.0005), as well as a significant interaction between group and cue number (F(1,14)=4.58,P=.05). A Newman-Keuls test based on the interaction revealed a significant decrease in cocaine seeking (relapse) for naloxone 1 cue vs. saline 1 cue (P< .01), but no significant decrease in cocaine seeking (relapse) for naloxone 4 cues vs. saline 4 cues. Likewise, the two-way ANOVA on the second cohort of animals revealed a significant main effect of group (F (1,16)=7.87,P=.013), as well as a significant interaction between group and cue number (F(1,16)=5.23,P=.036). A Newman-Keuls test based on the interaction revealed a significant decrease in cocaine seeking (relapse) for naloxone 2 cues vs. saline 2 cues (P< .05), but no significant decrease in seeking for naloxone 3 cues vs. saline 3 cues.
Figure 3.
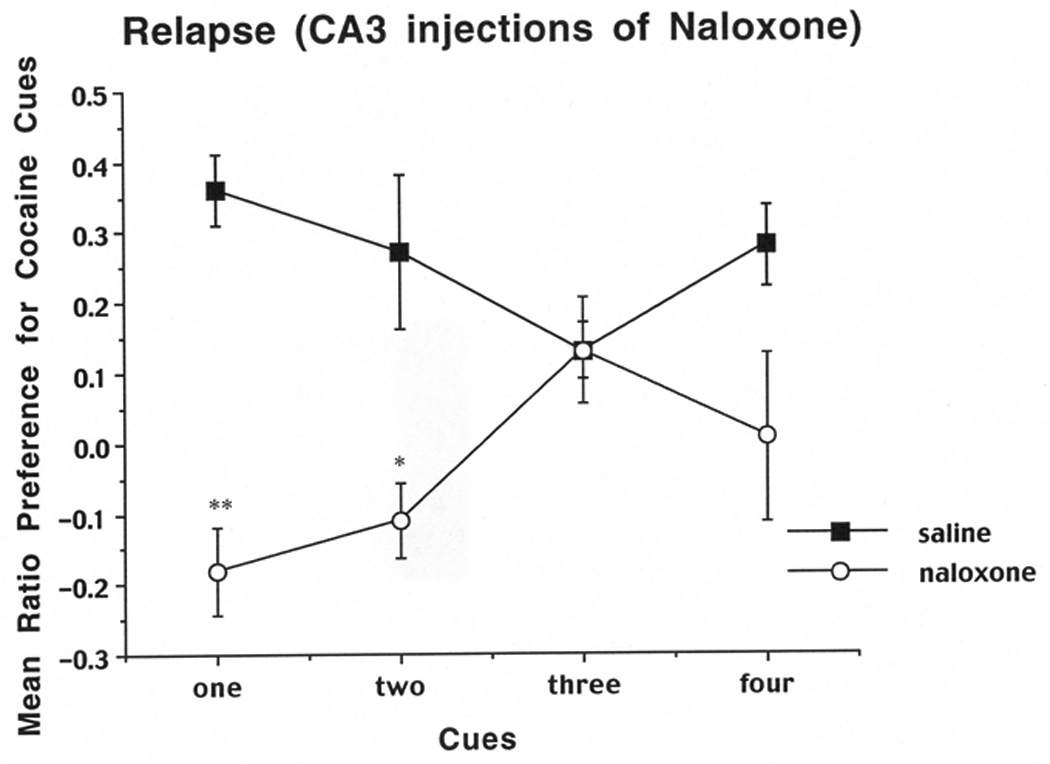
Mean preference for the cocaine side after local injections of naloxone or PBS into the CA3 as a function of the number of available cues on the preference tests on days 32–44. The cocaine-side preference was calculated as the total number of cue touches on the cocaine-paired side minus the number of touches on the saline-paired side divided by the total number of touches on both sides. Data shown are means (±SEM, n=16). **, significantly different from saline, one-cue group, P<0.01. *, significantly different from saline, two-cue group, p<0.05.
Histology
Based on cresyl violet staining of the dorsal CA3 region, the location of the cannulae placements are shown as stars in Figure 4 on a plate adapted from Paxinos and Watson (2009). An illustration of bilateral cannulae in the dorsal CA3 is shown in Figure 5.
Figure 4.
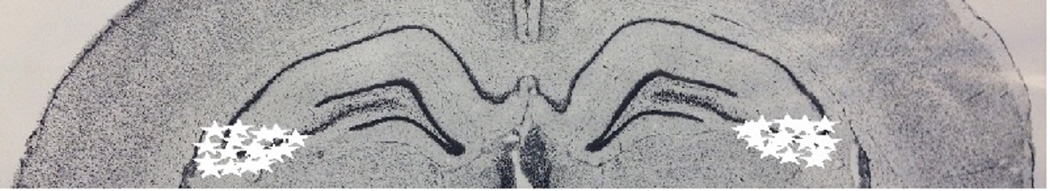
Schematic diagrams (adapted from Paxinos and Watson, 2009) showing placements of bilateral cannulae in the CA3 region of the hippocampus from rats used in Experiment 1. Cannula placements are indicated by stars.
Figure 5.
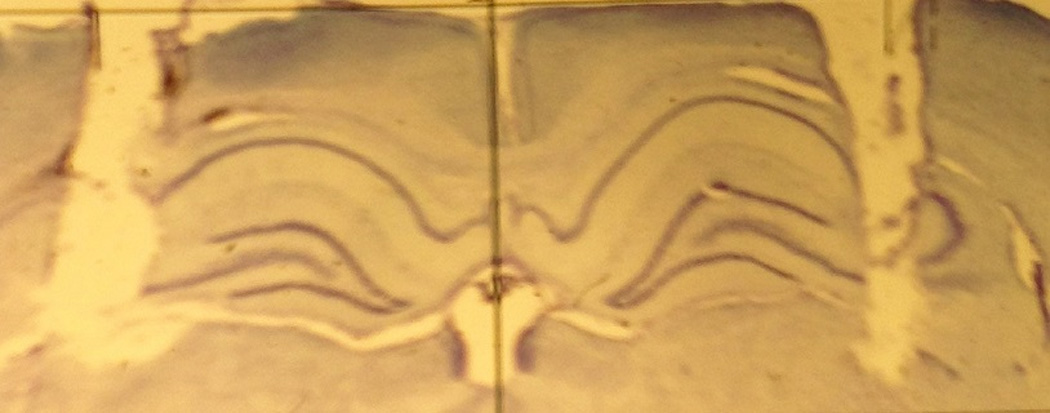
An illustration of bilateral cannulae in the dorsal CA3.
Experiment 2
Effects of systemic naloxone injections on cued-based relapse to cocaine seeking
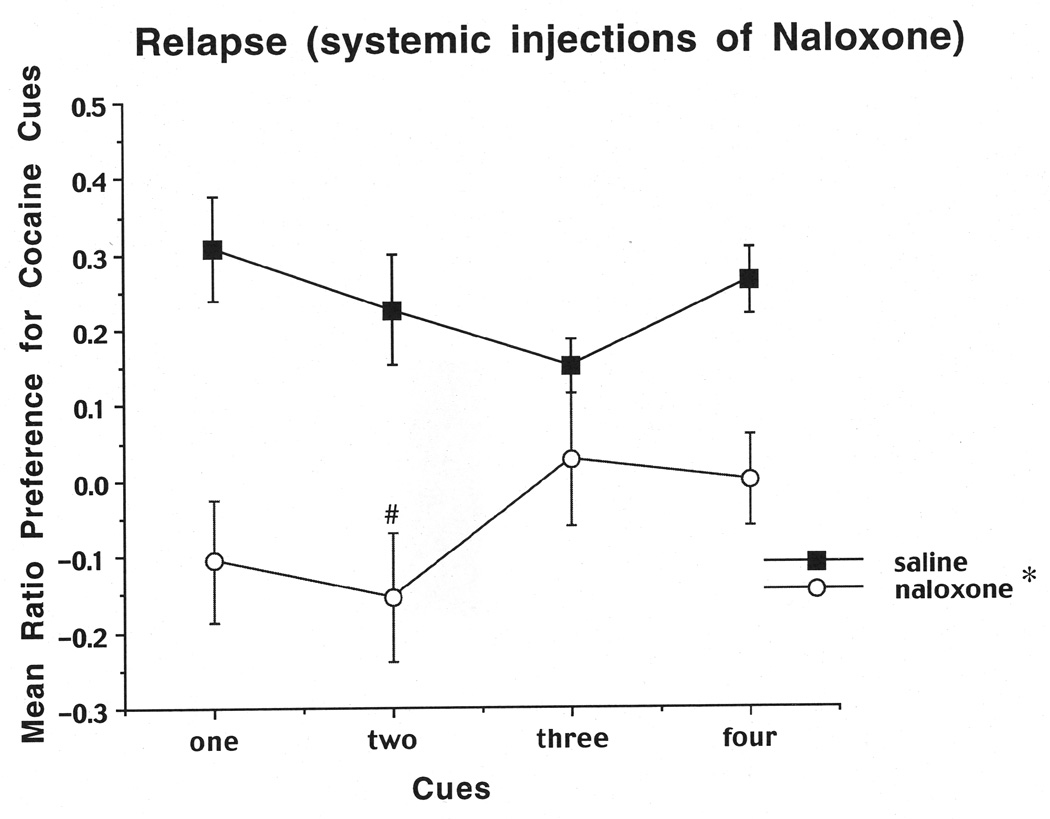
The results on the Day-10 preference test indicated that the rats preferred the four cues on the cocaine-paired side compared to the four cues on the saline side, as reflected in a significant positive ratio of .30, which is significantly different from zero (t (1,20)=5.16, P< .01). We also calculated the time that the rats spent on the cocaine-paired side (9.63 min) compared to the time spent on the saline side (5.47 min; see Figure 6). A one-way ANOVA revealed that rats spent more time on the cocaine side compared to the saline side (F (1,20)=30.05, P=.0001). We also scaled the frequency of touches for each object to determine which objects each rat preferred the most, and these frequencies aided in the selection of cues for subsequent relapse trials. For the relapse tests as shown in Figure 7, saline-pretreated rats showed cue-induced relapse to cocaine seeking, as evidenced by a positive ratio for touches of the cocaine-paired cues. Conversely, naloxone-pretreated rats showed a disruption of relapse when only one or two cues were present, but not when three or four cues were present. A two-way ANOVA (group (naloxone, saline) × cue (1 cue vs. 4 cues) revealed a significant main effect of group (F (1, 20) =18.97,P=.0003), but no significant interaction between cue and group number. The two-way ANOVA on the second cohort of animals (group (naloxone, saline) × cues (2 cues vs. 3 cues) also revealed a significant main effect of group (F (1, 20)=8.92,P=.0073), as well as a significant interaction between group and cues (F (1, 20)=4.65,P=.045). A Newman-Keuls test based on the interaction revealed a significant decrease in cocaine seeking (relapse) for naloxone 2 cues vs. saline 2 cues (P< .01), but no significant decrease for naloxone 3 cues vs. saline 3 cues (P=.10).
Figure 6.
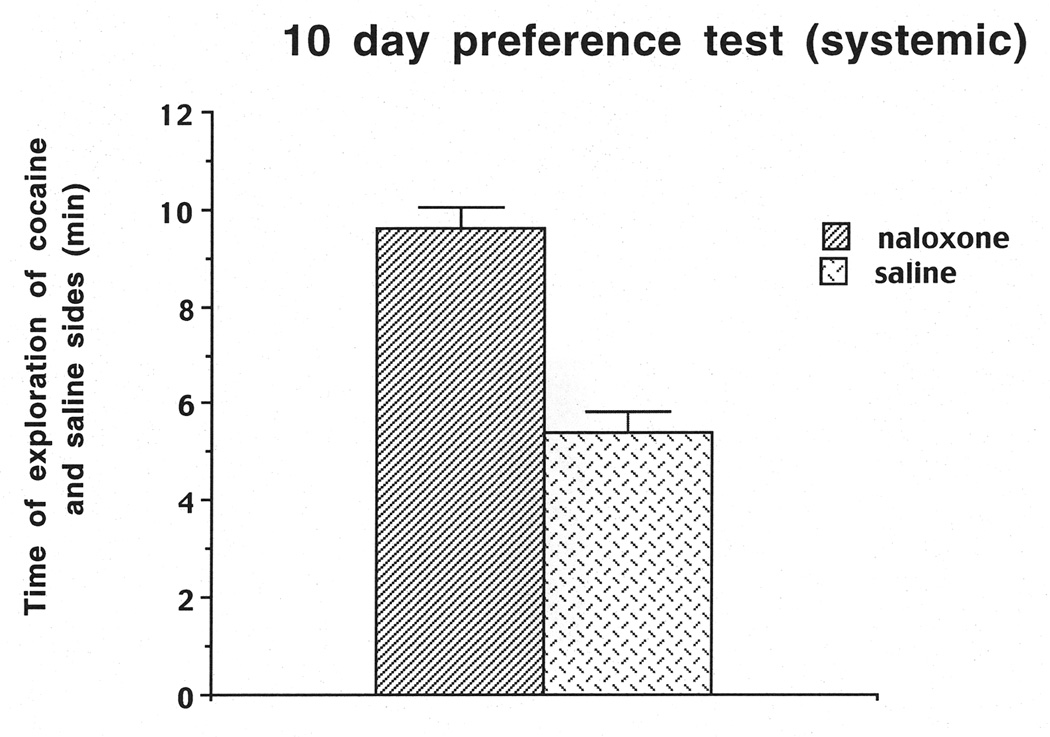
Duration (min) of exploration of cocaine vs. saline side on the Day-10 preference test for the system cohorts of rats used in Experiment 2.
Figure 7.
Mean preference for the cocaine side after systemic injections of naloxone or saline as a function of the number of available cues during the preference tests on days 32–44. The cocaine-side preference was calculated as described in the legend to Figure 3 and in the text. Data shown are means (±SEM, n=22). *, Significant main effect of group in 1–4 and 2–3 cue cohorts, p≤0.01. #, Significantly different from 2-cue saline group, p≤0.01.
Given the hypothesis that naloxone disrupts primarily object but not spatial context information, and that naloxone disrupts pattern completion especially for the presence of only one cue object, the following analysis was conducted to test the above-mentioned hypothesis. The number of times each rat touched the single cue object on the two sides for the CA3 injections of saline or naloxone was recorded for the one cue preference test. Based on CA3 injections of naloxone or saline into CA3, a ratio score was calculated. Subtracting the number of object touches on the cocaine-paired side from the number of object touches on the saline-paired side divided by the sum of the touches on both the cocaine and saline-paired sides. The results are shown in Table 1. A within group ANOVA for one object cue following naloxone or saline injection into CA3 revealed a significant reduction in the number of object touches for the naloxone side compared to number of object touches for saline side (F(1,7)=7.5, P=.03). Based on injections of naloxone or saline into CA3, a different ratio score was assessed by subtracting the amount of time (spatial context) spent on the cocaine-paired side from amount of time (spatial context) spent on the saline-paired side divided by the sum of time (spatial context) spent on both the cocaine and saline-paired sides. A within group ANOVA for one object cue following naloxone vs. saline injection into CA3 revealed a non-significant effect in time (spatial context) spent for naloxone side time, compared to time (spatial context) spent for saline side time(F(1,7)=.101, P= .76). The same ratio analysis was conducted for a comparison of object touches for cocaine side vs. saline side following systemic injection of naloxone or saline. The results are shown in Table 2. A within group ANOVA for one object cue following systemic injections of naloxone or saline revealed a significant reduction in the number of object touches for naloxone side compared to number of object touches for saline side (F(1,10)=5.14, P= .047). A within group ANOVA for one object cue following systemic injections of naloxone vs. saline revealed a non-significant effect in time (spatial context) spent for the naloxone side compared to time (spatial context) spent for saline side (F(1,10)=1.77, P= .21). The results suggest that there is a disruption primarily for object touches for one cue naloxone injections into the CA3 or systemic injections, but no effect on time (spatial context).
Table 1.
Based on one cue mean ratio preference for saline or naloxone injections into CA3
| CA3 Cue object touches | CA3 time (spatial context) | |||
|---|---|---|---|---|
| Saline | Naloxone | Saline | Naloxone | |
| Mean | .304 | .064* | .07 | .126 |
| S.E. | .076 | .057 | .122 | .190 |
Significant P=.03
Table 2.
Based on one cue mean ratio preference for systemic injections of saline or naloxone
| Systemic cue touches | Systemic time (spatial context) | |||
|---|---|---|---|---|
| Saline | Naloxone | Saline | Naloxone | |
| Mean | .259 | .017* | .097 | −.161 |
| S.E. | .097 | .065 | .123 | .149 |
Significant P=.047
To determine whether the dose of 2mg/kg naloxone produced possible aversive effects, 8 new rats were injected with 2mg/kg naloxone on one side of the cue-box and 0.9% saline on the other side of the box. In this case, on the day-10 preference test based on four cues on each side, there was a non-significant mean preference ratio of .08 for the number of touches on the saline side compared to the naloxone side, suggesting that 2mg/kg naloxone alone did not produce any rewarding or aversive effects.
Discussion
Previous research has shown that temporary inactivation of the dorsal hippocampus using tetrodotoxin (a sodium channel blocker) impairs contextual reinstatement of cocaine-seeking behavior, but not reinstatement induced by discrete cocaine-paired cues or a cocaine priming injection (Fuchs et al, 2005). Likewise, Lasseter et al, 2010) reported that temporary inactivation of the ventral hippocampus (CA1 and CA3), but not the ventral DG or posterior dorsal hippocampus (CA1 and CA3), using muscimol and baclofen (GABA receptor agonists) produced a disruption in cocaine seeking induced by exposure to the cocaine-paired context. Rogers and See (2007) also reported that injection of muscimol and bacoflen at the junction between the dorsal and ventral hippocampus attenuates relapse to cocaine-seeking following presentation of drug-paired cues or a priming injection of cocaine. In the above-mentioned studies, the CA3 region likely was impacted by the inactivation treatments; however, no previous studies have specifically examined the role of the CA3 subregion of the dorsal hippocampus in cue- induced reinstatement. Further, no prior work has examined hippocampal processes, particularly pattern completion, underlying cue- induced reinstatement of cocaine seeking. To address this lack of knowledge, the present work used a behavioral paradigm that can measure cue-based pattern completion associated with relapse to cocaine seeking.
In the present experiments, during a relapse test, infusions of naloxone into CA3 produced a marked disruption of cue-evoked cocaine seeking, particularly when only one or two cues were presented, suggesting that naloxone injections disrupt CA3 function and trigger a deficit in a pattern completion process. These results are consistent with previous research showing that rats with CA3 lesions display impaired accuracy compared to controls on a visual cue-based pattern-completion task when only a subset of cues are available, suggesting impaired cue pattern completion (Rolls and Kesner, 2006). Infusion of naloxone into CA3, as done herein, also disrupted visual cue-based pattern completion and mimicked the effects of a CA3 lesion (Kesner and Warthen, 2010). These data are consistent with proposed computational models and suggest that an auto-associative CA3 network is responsible for the completion of patterns based on incomplete input (Rolls, 1996; Rolls and Kesner, 2006). The present data, taken together with these prior results, implicates for the first time a pattern completion process mediated by the CA3 region of the hippocampus in cue-context-induced cocaine seeking.
Systemic injection of naloxone disrupted cue-evoked cocaine seeking in a manner similar to that observed for naloxone injections into the CA3 subregion of the hippocampus, suggesting that systemic injections of naloxone likely affect the CA3 subregion of the hippocampus as well, implicating an opioid modulation of the pattern completion process. Furthermore only the CA3, but not the dentate gyrus or CA1 support pattern completion for object based pattern completion. Based on additional analyses it appears that there is a disruption primarily for object touches for one cue naloxone injections into the CA3 or systemic injections, but no effect on time (spatial context).
It is possible that the disruption of the cue-evoked cocaine-seeking reflected an aversive effect of the naloxone. One argument against this interpretation is the observation that the effect of naloxone was sensitive to the number of cues presented, being greatest when one or two cues were presented. This significant interaction between naloxone administration and cue number favors the conclusion that naloxone was not simply aversive/impairing behavior, but rather affecting a pattern completion process, as previously described (Gold and Kesner, 2005; Kesner and Warthen, 2010). Further, when systemic administration of naloxone was presented on one side of the chamber and 0.9% saline on the other over 8 days, rats did not show an aversion to the naloxone-paired side, suggesting that the 2 mg/kg dose of naloxone did not produce aversive effects. Thus, it appears that cue-based activation of the dorsal CA3 might be the critical trigger to subsequently activate other brain regions, such as the amygdala, prefrontal cortex and nucleus accumbens (Fuchs et al, 2008; Kolb and Volkow (2010; Pelloux et al, 2013), that could be involved in context-induced relapse to drug seeking. Targeting CA3 function, particularly through the opioid system, may therefore be effective in managing relapse in addiction, particularly that being driven by cue-based contextual cues.
Acknowledgments
We would like to thank Isha Gupta for their help in the collection of data.
Funding
This work was supported by NIH/NIDA # 1R21DA031408 Kesner/Keefe (PIs).
Footnotes
Disclosure
The authors declare no conflict of interest.
Contributor Information
Raymond P. Kesner, Department of Psychology, 380S 1530 E Rm 502. Tel: 801-581-7430 Fax: 801-581 5841, ray.kesner@psych.utah.edu
Ryan A. Kirk, Department of Psychology, University of Utah, Utah 84112, USA, synapsekirk@gmail.com
Jascha K. Clark, Department of Psychology, University of Utah, Utah 84112, USA, jashaclark@gmail.com
Angela Moore, Department of Psychology, University of Utah, Utah 84112, USA, Angela Moorebiochem@gmail.com.
Kristen Keefe, Department of Pharmacology and Toxicology, University of Utah. Utah 84112, USA, k.keefe@utah.edu.
References
- Burattini C, Burbassi S, Aicardi G, Cervo L. Effects of naltrexone on cocaine-and sucrose-seeking behaviour in response to associated stimuli. Int J Neuropharmacol. 2008;11:103–109. doi: 10.1017/S1461145707007705. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of μ1 or б opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharm. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: Effects of naltrexone. Biol Psychiatry. 2007;6:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do VH, Martinez CO, Martinez JL, Jr, Derrick BE. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J. Neurophysiol. 2002;87:669–678. doi: 10.1152/jn.00938.2000. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharm. 2005;30:296–308. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models. 2008;5:251–258. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O’Malley SS. Naltrexone, relapse prevention, and supportive therapy with alcoholics; An analysis of patient treatment matching. J Consult Clin Psychol. 1996;64:1044–1053. doi: 10.1037//0022-006x.64.5.1044. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–557. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. Reviews. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine seeking behavior in rats. Neuroscience. 2010;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York, NY: Elsevier; 2009. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Europ. J. Neurosci. 2013;38:3018–3026. doi: 10.1111/ejn.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Stotts AL, Rhoades HM, Moeller FG, Grabowski J. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26:167–180. doi: 10.1016/s0306-4603(00)00098-8. [DOI] [PubMed] [Google Scholar]