Abstract
Background & Aims
The association between breastfeeding and thyroid cancer risk is not consistent from epidemiological studies. To better clarify the association including assessing a potential dose-response relationship, we conducted a comprehensive meta-analysis.
Methods
We searched PubMed (MEDLINE) up to November 2015 for prospective studies or case-control studies that evaluated the association between breastfeeding and risk of thyroid cancer. Effect estimates were pooled using a fixed-effects model.
Results
Nine reports (2 prospective studies, 6 case-control studies and 1 pooled analysis of 14 case-control studies) involving 2423 cases and 350081 non-cases were identified. After pooling relevant studies, there was a significant inverse association between ever breastfeeding and risk of thyroid cancer (RR=0.91, 95% CI 0.83–0.99), with minor heterogeneity (I2=10.1%). The dose-response analysis revealed a significant linear relationship between the duration of breastfeeding and risk of thyroid cancer. The summary RR for an increment of 1 month of breastfeeding with risk of thyroid cancer was 0.983 (95% CI 0.98–0.99). When focusing on cohort studies, a more prominent linear dose-response relationship was detected, with the combined RR for every increment of 1 month of breastfeeding to be 0.965 (95% CI 0.96–0.97).
Conclusions
This meta-analysis suggests that breastfeeding is potentially inversely associated with thyroid cancer risk. Also longer duration of breastfeeding may further decreases thyroid cancer risk. If validated in large-scale prospective studies, our findings may have implications for impacting women’s decision in breastfeeding.
Keywords: breastfeeding, epidemiological studies, meta-analysis, risk, thyroid cancer
Introduction
Among the various endocrine malignancies, thyroid cancer is the most common one and represents a huge public health burden, especially in females [1] [2]. It is estimated that there will be approximately 62,450 new incident cases in the US in 2015, including 47,230 females [3]. In the US alone, about 1,950 subjects will die from thyroid cancer in 2015 [3]. The incidence of thyroid cancer has kept increasing for the last decades [4]. To better understand the etiology is crucial for the disease prevention, risk stratification, and disease treatment. Supported by a significant incidence difference between males and females, reproductive factors were demonstrated to potentially affect thyroid cancer risk [2] [5]. This is further corroborated by the fact that during pregnancy, thyroid activity is considerably increased [6]. Furthermore, research shows that estrogens are able to significantly influence malignant thyroid cells [7–9].
As one of the most relevant reproductive factors which have potential practical implication, breastfeeding is a plausible factor that may affect risk of thyroid cancer. Several epidemiological studies have demonstrated evidence for supporting such a relationship. For example, ever breastfeeding was inversely associated with thyroid cancer risk in females based on a case-control study [10]. Besides, the highest category vs. the lowest category of duration of breastfeeding was suggested to be significantly associated with thyroid cancer risk in several cohort studies and case-control studies [10, 11]. On the other hand, some other epidemiological studies did not suggest a clear relationship between breastfeeding and thyroid cancer risk [12–19]. Considering that for each individual study the detected effect size may be limited by the relevant sample size, a summary of available evidence will be needed to clarify whether breastfeeding is significantly associated with thyroid cancer risk. To better characterize the research question of interest including understanding the dose-response relationship, we conducted a comprehensive systematic review and meta-analysis.
Materials and Methods
Data Sources and Search Strategies
This meta-analysis was performed according to the recommendations of the Meta-analysis of Observational Studies in Epidemiology group [20]. We conducted a literature search of PubMed (MEDLINE) from the inception to January 2015 for humans’ studies. No language or population restrictions were applied. We used the following search keywords and Medical Subject Heading terms: (breastfeeding OR breast feed OR lactation OR infant nutrition OR breast milk OR milk human) and thyroid and (cancer or neoplasm or carcinoma or tumor). References of related review articles were also screened to search for additional potential studies. The literature search was updated at November 12, 2015.
Study Selection
The study eligibility criteria were: (i) case–control studies or prospective studies; (ii) evaluated the association between breastfeeding and thyroid cancer risk; (iii) presented relative risk (RR), hazard ratio (HR), or odds ratio (OR) estimates with 95% confidence intervals (CI) or sufficient data to determine the risk estimates. No studies were excluded based on publication status, sample size or length of follow-up. In situations when multiple publications involving same individuals were detected, we used only the study with the largest number of patients, like previous studies [21–24].
Data Extraction and Quality Assessment
Data extraction was performed independently by two authors using a pre-designed standardized data extraction form. The form was pre-tested in a pilot scenario to ensure the validity. Data extracted from each study included: the first author’s last name, year of publication, study region, study design, characteristics of study population (sample size, participants’ age, length of follow-up, categories of breastfeeding, and effect sizes of the association). When there was more than 1 estimate of the association, we used the estimate from the model that maximally adjusted for relevant covariates, like previous studies [25, 26]. If only unadjusted estimate was provided, we used the crude estimate in the analysis.
For the quality assessment of relevant studies, we used the Newcastle-Ottawa Quality Assessment Scale [27]. This assessment scale involved evaluating methods of sampling, descriptions of exposure and outcome, and data matching and statistical adjustments. For each study we assigned a maximum of 9 points. If studies had scores of 7 or above, they were categorized as studies with high quality, otherwise they were categorized as studies with low quality.
Statistical Methods
The RR and 95% CI from each included study were used to measure the association. Due to the relative rarity of thyroid cancer in the general population, ORs and HRs were thought to be equivalent to RRs and we used RRs to represent measures. We used the I2 to assess the heterogeneity across studies, where I2>50% suggests high heterogeneity [28]. By assuming that a common effect size is shared by all included studies, we pooled the log transformed RRs using the fixed-effects model [29]. Subgroup analyses based on study design (prospective vs. case-control studies), study quality (high vs. low), and study location (America, Europe, Asia, Oceania or International) were also performed. We also conducted sensitivity analyses excluding 1 study at a time to evaluate whether any specific study significantly influenced the overall pooled results.
Additionally, we explored potential linear and non-linear dose-response relationship between the duration of breastfeeding and thyroid cancer risk [30]. When the categories of breastfeeding duration were reported in ranges, we determined the midpoints of categories by averaging the lower and upper bounds. In situations when the upper bound was not specified for the highest category, the width of the open ended interval was assumed to be as same as that of the second highest category. For determining the dose-response relationship, the numbers of cases and overall subjects or person-years, as well as the risk estimates for at least 3 categories of breastfeeding duration are required. We assessed a potential nonlinear dose-response relationship between the duration of breastfeeding and thyroid cancer risk with fractional polynomial models, using restricted cubic splines with 3 knots at fixed percentiles (10%, 50% and 90%) of the distribution [31, 32]. We then conducted a likelihood ratio test for evaluating the difference between the linear and nonlinear models, to determine whether linear or nonlinear model was more appropriate.
Publication bias was evaluated through Egger’s test [33] and Begg’s test [34]. A P-value threshold of 0.05 was used to determine whether there was significant publication bias. All statistical analyses were performed with Stata (version 13; StataCorp, College Station, TX).
Results
Literature Search and Study Characteristics
The detailed procedures of the article screening were demonstrated in Figure 1. Overall, 9 reports were included in the current meta-analysis [10–13, 15–19]. The detailed characteristics of these studies were demonstrated in Table 1. In total, 2 prospective studies (1 cohort study and 1 case cohort study), 6 case-control studies and 1 pooled analysis of 14 case-control studies were included. Several eligible studies were already incorporated in the large pooled analysis by Negri et al and were thus not individually included in the current meta-analysis to avoid duplication. Overall, 3 studies were conducted in Europe, 2 in America, 2 in Asia, 1 in Oceania and 1 in multiple continents. The studies enrolled 2423 patients and 350081 non-thyroid cancer individuals. For all studies, the risk estimates of breastfeeding focused on parous women. The lowest category of breastfeeding duration ranged from never to less than 1 month. And the highest category of breastfeeding duration varied from ≥4 months to >54 months.
Figure 1.
Flow chart for selection of eligible studies
Table 1.
Characteristics of Studies of Breastfeeding and Thyroid Cancer Risk
| First author, publication year (reference), Country, Study design | Cases/controls (age) | Breastfeeding categories (exposure/case assessment) | RR (95% CI) | Matched/Adjusted factors |
|---|---|---|---|---|
| Case-control studies | ||||
| Xhaard, 2014, France, PC-CS | 246/235(10–40y) | Never Ever <4 months ≥4 months (Trained interviewer/Cancer registry) |
1.0 (ref) 0.84 (0.70–0.995) 0.9 (0.4–2.0) 0.3 (0.1–0.7) |
Ethnic group, level of education, height, BMI, smoking status, number of children |
| Mack, 1999, USA, PC-CS | 88/90(15–54y) | Never Ever 1–6 months >6 months (Trained interviewer/medical record) |
1.0 (ref) 0.6 (0.3–1.2) 0.91 (0.63–1.31) 0.83 (0.55–1.24) |
prior thyroid disease, number of births |
| Rossing, 2000, Washington, USA, PC-CS | 183/243(18–64y) | Never or <1 month Ever 1–5 months 6–11 months 12–23 months ≥24 months (Trained interviewer/Cancer registry) |
1.0 (ref) 1.2 (0.9–1.6) 1.1 (0.6–2.0) 1.0 (0.5–1.8) 1.5 (0.8–2.7) 1.7 (0.9–3.3) |
Age, county of residence, smoking |
| Negri, 1999, International, pooled analysis of case-control studies | 767/1194 (NA) | Never Ever |
1.0 (ref) 1.0 (0.7–1.3) |
Study, age, history of radiation, parity, use of hormones for lactation suppression |
| Brindel, 2008, French Polynesia, PC-CS | 201/324 (NA) | Never Ever 1–6 months 7–18 months 19–30 months 31–54 months >54 months (Trained interviewer/cancer registry and histology review) |
1.0 (ref) 1.2 (0.7–2.1) 1.1 (0.6–2.2) 1.3 (0.7–2.7) 2.7 (1.0–7.5) 1.0 (0.4–2.3) 1.2 (0.6–2.6) |
Ethnic group, educational level, height BMI, interviewer, number of livebirths |
| Lee, 2010, Korea, HC-CS | 257/260 (NA) | Never Ever (self-administered questionnaire/unclear) |
1.0 (ref) 0.94 (0.77–1.15) |
Age |
| Przybylik-Mazurek, 2012, Poland, HC-CS | 150/32 (mean 41/37) | Never Ever (questionnaire/unclear) |
1.0 (ref) 0.84 (0.63–1.11) |
None |
| First author, publication year (reference), Country, Study design | Cases/subject (age), duration of follow up | Breastfeeding categories (exposure/case assessment) | RR (95% CI) | Matched/Adjusted factors |
|---|---|---|---|---|
| Prospective studies | ||||
| Zamora-Ros, 2014, Europe, CS | 406/345,157(mean 51y),11y | Never Ever <1 month 1–6 months 6–12 months 12–24 months >24 months (Self-questionnaire/Cancer registry) |
1.0 (ref) 0.86 (0.67–1.10) 0.87 (0.65–1.17) 0.85 (0.66–1.11) 0.65 (0.48–0.88) 0.51 (0.36–0.73) 0.25 (0.15–0.44) |
Age, study center, age at recruitment |
| Wong, 2006, China, Case cohort study | 125/3077(30–69), 10y | Never Ever <6 months 7–12 months 13–24 months 25–36 months 37–48 months 49+ months (Trained interviewer/Cancer registry) |
1.0(ref) 0.70 (0.44–1.11) 1.18 (0.69–2.01) 0.69 (0.42–1.15) 0.42 (0.23–0.76) 0.38 (0.19–0.73) 0.27 (0.12–0.62) 0.21 (0.10–0.45) |
age |
BMI: body mass index; CI: confidence interval; CS: cohort study; HC-CS: hospital-based case-control study; N/A: not available; OR: odds ratio; PC-CS: population-based case-control study; Ref: reference; RR: relative risk.
The detailed quality ratings of each study were shown in Table 2. Overall, the methodological quality of included studies was fair. Among the 2 prospective studies and 6 case-control studies, 5 were with high quality and 3 were with low quality. The pooled analysis by Negri et al was not assigned a quality score because it involved a lot of individual studies with potentially mixing quality and not all of them had sufficient information for the quality assessment.
Table 2.
Quality Assessment of Reviewed Case-Control and Prospective Studies
| Case-Control Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Case defined with independent validation | Representativeness of the cases | Selection of controls from community | Statement that controls have no history of outcome | Cases and controls matched and/or adjusted by factors | Ascertain exposure by blinded structured interview | Same method of ascertainment for cases and controls | Same response rate for both groups | Overall Score |
| Rossing,2000 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Xhaard,2014 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Brindel, 2008 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Lee, 2010 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 5 |
| Przybylik-Mazurek, 2012 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
| Mack, 1999 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 6 |
| Prospective Studies | |||||||||
| Study | Exposed cohort represents average in community | Selection of the non-exposed cohort from same community | Ascertain exposure through records or structured interviews | Demonstrate that outcome not present at study start | Exposed and non-exposed matched and/or adjusted by factors | Ascertain outcome via independent blind assessment or record linkage | Follow-up long enough for outcome to occur | Loss to follow-up<20% | Overall Score |
| Zamora-Ros, 2014 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 |
| Wong, 2006 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
1 means study adequately fulfilled a quality criterion (2 for case-control or exposed-non exposed fully matched and adjusted), 0 means it did not. Quality scale does not imply that items are of equal relevant importance
Ever vs. never breastfeeding
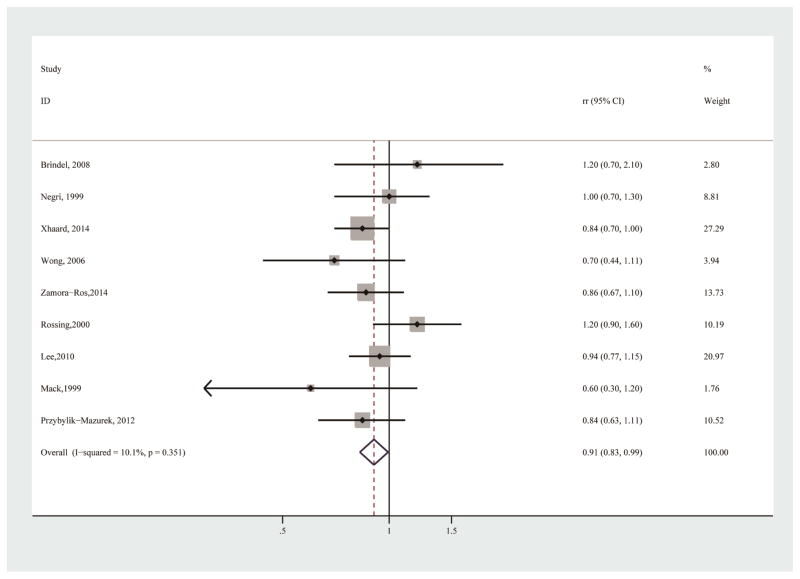
All 9 included studies provided sufficient information for the association between ever vs. never breastfeeding and thyroid cancer risk [10–13, 15–19]. The pooled analysis revealed a significant inverse association (RR=0.91, 95% CI 0.83–0.99), with little heterogeneity (I2=10.1%) (Table 3; Figure 2). No publication bias was detected by Egger’s test (p for bias: 0.926) or Begg’s test (p for bias: 0.917). In the sensitivity analysis, the 9 study-specific RRs of ever breastfeeding ranged from as low as 0.88 (95% CI 0.80–0.97; I2=0.0%) after omission of Rossing et al [13] to as high as 0.93 (95% CI 0.84–1.04; I2=11.7%) after omission of Xhaard et al [10] (Figure 3). The subgroup analyses revealed that there was also a significant inverse association in studies conducted in Europe (Table 3). Although statistical significance was not detected for analyses of other subgroups, the trend of decreased risk was observed in the majority of subgroups (Table 3). Analyses in these subgroups might be limited by the available numbers of studies and sample sizes.
Table 3.
Summary Risk Estimates of the Association between Breastfeeding and Thyroid Cancer Risk (Ever versus Never)
| No of reports | RR (95% CI) | I2 | P for heterogeneity | |
|---|---|---|---|---|
| Overall | 9 | 0.91 (0.83–0.99) | 10.1% | 0.351 |
| Subgroup analysis | ||||
| Study design | ||||
| Prospective | 2 | 0.82 (0.66–1.02) | 0.0% | 0.442 |
| Case-control | 7 | 0.93 (0.84–1.02) | 18.6% | 0.288 |
| Study quality | ||||
| High | 5 | 0.90 (0.80–1.02) | 40.7% | 0.150 |
| Low | 3 | 0.89 (0.76–1.04) | 0.0% | 0.431 |
| Location | ||||
| Europe | 3 | 0.85 (0.74–0.96) | 0.0% | 0.987 |
| America | 2 | 1.08 (0.83–1.41) | 69.5% | 0.070 |
| Asia | 2 | 0.90 (0.75–1.08) | 23.8% | 0.252 |
| Oceania | 1 | 1.20 (0.70–2.10) | - | - |
| International | 1 | 1.00 (0.70–1.30) | - | - |
| Confounder adjustment | ||||
| Yes | 8 | 0.91 (0.83–1.01) | 18.5% | 0.283 |
| No | 1 | 0.84 (0.63–1.11) | - | - |
| Adjustment of other reproductive or hormone factors | ||||
| Yes | 4 | 0.88 (0.76–1.02) | 9.7% | 0.345 |
| No | 5 | 0.92 (0.82–1.04) | 25.1% | 0.254 |
Figure 2.
Forest plot (fixed effects model) of breastfeeding (ever vs. never) and thyroid cancer risk
Figure 3.
Sensitivity analyses of the association between of breastfeeding (ever vs. never) and thyroid cancer risk by excluding one study at a time
Dose-response relationship assessment
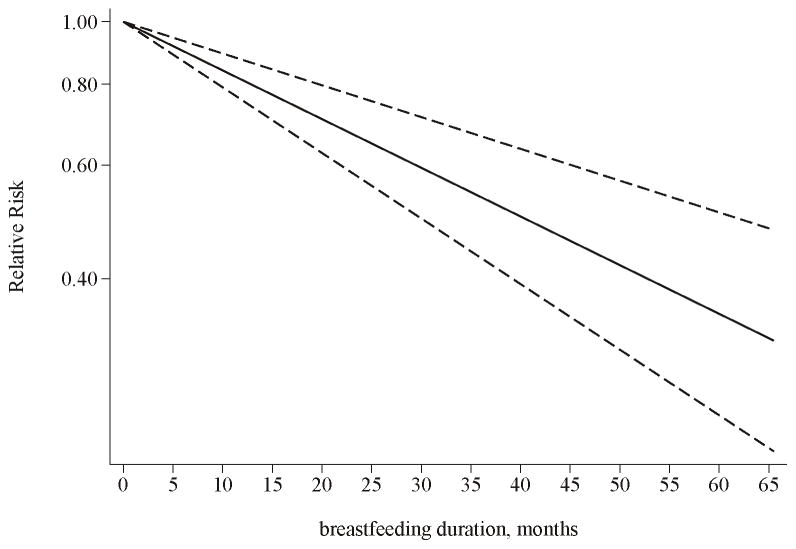
Two prospective studies and 4 case-control studies provided sufficient information and were thus included in the analyses for evaluating the dose-response relationship [10–13, 15, 18]. Based on the analyses, a significant linear relationship between the duration of breastfeeding and risk of thyroid cancer was detected (P<0.0001 for the linear trend). The overall RR for every increment of 1 month of breastfeeding was 0.983 (95% CI 0.98–0.99) with thyroid cancer risk, with high heterogeneity (P for heterogeneity: <0.0001) (Figure 4). After estimating the dose-response relationship with a nonlinear model, the likelihood ratio test suggested that there was no sufficient evidence to reject the linear relationship model (p=0.09). Because of the significant heterogeneity detected in this analysis, we further conducted dose-response analyses in subgroups according to study design (prospective or case-control studies). Although there was no significant linear or non-linear relationship based on the analysis of case-control studies (P=0.39 and 0.12 for the linear trend and non-linear trend respectively), analysis of cohort studies demonstrated a more prominent linear relationship (P<0.0001 for the linear trend), with no considerable heterogeneity (p=0.36). Based on the dose-response analysis in cohort studies, the combined RR for every increment of 1 month of breastfeeding was 0.965 (95% CI 0.96–0.97). The likelihood ratio test suggested that the linear relationship might be more appropriate for this analysis (p=0.41).
Figure 4.
Dose-response relationship for the association between the duration of breastfeeding and thyroid cancer risk. The solid line represents the estimated relationship. The dashed line represents the 95 % confidence interval of the estimated relationship
Discussion
To the best of our knowledge, this is the most comprehensive quantitative dose-response meta-analysis up to date to assess the relationship between breastfeeding and thyroid cancer risk. Since the conduction of the current study, several meta-analyses focusing on similar research questions have been published [35, 36]. In the study by Cao et al [35], duration of breastfeeding was significantly associated with decreased thyroid cancer risk in cohort studies. The association of breastfeeding duration in overall studies and the associations of breastfeeding were not statistically significant. However, the upper bounds of the associations were relatively close to 1, suggesting that a moderate association may be able to be detected when there is sufficient power. In the study by Wang et al [36], no significant association was detected focusing on papillary thyroid cancer. However, in these analyses several relevant original studies were not included for the evidence synthesization. Besides, the dose-response relationship of the association between breastfeeding and thyroid cancer was not assessed in these studies. In our study, after summarizing available evidence from epidemiological studies, ever breastfeeding was associated with a decreased risk of developing thyroid cancer for females. We did not detect considerable heterogeneity across studies and evidence of publication bias. Furthermore, we identified a significant linear dose-response relationship for the duration of breastfeeding with risk of thyroid cancer. Due to that the highest category of breastfeeding in individual studies varied considerably from ≥4 months to >54 months, we did not perform the analysis of comparing the highest versus lowest category of breastfeeding with disease risk. All together, this meta-analysis suggested a potential inverse association between breastfeeding and thyroid cancer risk, revealing potential important roles of reproductive factors in thyroid cancer risk in females, which is consistent with another meta-analysis we performed suggesting an association between oral contraceptive use and thyroid cancer risk [37].
Although it remains not fully understood for the exact biological mechanism underlying the inverse association between breastfeeding and risk of thyroid cancer, plausible explanations have been suggested. It is demonstrated that estrogens could affect the proliferation of malignant thyroid cells; besides, the adhesion, migration and invasiveness of malignant thyroid cells could be enhanced [7–9]. Estrogens can also alter apoptotic pathways through interacting with estrogen receptors, which is known to be linked with cancer development [38–40]. Breastfeeding is demonstrated to be able to inhibit ovulation, and can thus decrease the exposure to endogenous estrogens, which may eventually decrease thyroid cancer risk. Further studies are warranted to clarify the exact mechanism.
The associations between breastfeeding and risks of human diseases have been extensively evaluated. With regards to cancer, meta-analyses or pooled studies have revealed that breastfeeding is associated with reduced risks of ovarian cancer [41], breast cancer [42], oesophageal and gastric junction adenocarcinoma [43], childhood acute lymphoblastic leukemia and acute myeloblastic leukemia [44, 45], Hodgkin’s lymphoma [45] and neuroblastoma [45]. With regards to other diseases, breastfeeding is shown to be potentially related to decreased risks of type 2 diabetes [46, 47], type 1 diabetes [48], childhood obesity [49], and pneumonia morbidity and mortality [50]. Consistent with the beneficial effect identified in the current meta-analysis, it may be warranted to promote breastfeeding for decreasing risks of chronic diseases for both mothers and infants.
Our study has several strengths. This is the most comprehensive quantitative meta-analysis for evaluating the association between breastfeeding and risk of thyroid cancer in females, including assessing the dose-response relationship. Besides conducting numerous subgroup analyses and sensitivity analyses to assess the association, we performed dose-response analyses to further clarify the relationship. Our analyses summarized evidence from epidemiological studies and demonstrated that breastfeeding could potentially decrease thyroid cancer risk in women. Our study provides new knowledge beyond other meta-analyses and systematic reviews [35, 36].
Several potential limitations should be considered for the interpretation of our findings. First, evidence from observational studies is known to prone to bias and should be interpreted with caution. Second, we could not conduct analyses with individualized data, in which case there was possibility that the used risk estimates from individual studies might not be fully adjusted for. We noticed that at least 5 of included studies did not adjust for other relevant reproductive or hormone factors, which may confound the findings. Aligning with the fact that the detected association for ever breastfeeding was very close to 1 for the upper 95% CI bound, we need to be cautious that the detected association may be due to residual confounding, although the detected dose-response relationship strengths the validity of the association. Further well-designed prospective studies adjusting for all relevant confounders are needed to validate our findings.
Conclusion
In conclusion, based on a quantitative summary of evidence from relevant epidemiological studies, breastfeeding, especially with a longer duration, was potentially associated with a decreased risk of thyroid cancer. If validated by future well-designed prospective studies, our findings may have implications for impacting women’s decisions in breastfeeding for decreasing risks of diseases.
Acknowledgments
Funding sources
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Xiao Zhu was supported by National Natural Science Foundation of China (81541153), Guangdong Provincial Research Project of Science and Technology (2015A050502048, 2014A020212295 and 2014A020212653) and Science and Technology Research Project in Dongguan City (2013508152011 and 2013508152002). The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations used
- OR
Odds ratio
- RR
relative risk
- HR
hazard ratio
- CI
confidence intervals
Footnotes
Statement of Authorship
Lang Wu designed the study, performed the study, analyzed and interpreted the data, and drafted the manuscript; Xingyang Yi performed the study, interpreted the data and significantly revised the manuscript; Jingjing Zhu performed the study, analyzed and interpreted the data, and significantly revised the manuscript; Guang Jian Liu performed the study, interpreted the data, and significantly revised the manuscript; Xiao Zhu participated in the study and revised the manuscript; all authors approved the final version of manuscript.
Conflict of Interest Statement
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wartofsky L. Increasing world incidence of thyroid cancer: increased detection or higher radiation exposure? Hormones (Athens) 2010;9:103–8. doi: 10.14310/horm.2002.1260. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari SM, Politti U, Spisni R, Materazzi G, Baldini E, Ulisse S, et al. Sorafenib in the treatment of thyroid cancer. Expert Rev Anticancer Ther. 2015;15:863–74. doi: 10.1586/14737140.2015.1064770. [DOI] [PubMed] [Google Scholar]
- 5.Sakoda LC, Horn-Ross PL. Reproductive and menstrual history and papillary thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:51–7. [PubMed] [Google Scholar]
- 6.Sack J. Thyroid function in pregnancy - maternal-fetal relationship in health and disease. Pediatr Endocrinol Rev. 2003;1(Suppl 2):170–6. discussion 6. [PubMed] [Google Scholar]
- 7.Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab. 2001;86:1072–7. doi: 10.1210/jcem.86.3.7283. [DOI] [PubMed] [Google Scholar]
- 8.Glinoer D, de Nayer P, Bourdoux P, Lemone M, Robyn C, van Steirteghem A, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276–87. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 9.Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20:33–41. doi: 10.1089/thy.2009.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xhaard C, Rubino C, Clero E, Maillard S, Ren Y, Borson-Chazot F, et al. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in young women in france: a population-based case-control study. Am J Epidemiol. 2014;180:1007–17. doi: 10.1093/aje/kwu220. [DOI] [PubMed] [Google Scholar]
- 11.Wong EY, Ray R, Gao DL, Wernli KJ, Li W, Fitzgibbons ED, et al. Reproductive history, occupational exposures, and thyroid cancer risk among women textile workers in Shanghai, China. Int Arch Occup Environ Health. 2006;79:251–8. doi: 10.1007/s00420-005-0036-9. [DOI] [PubMed] [Google Scholar]
- 12.Zamora-Ros R, Rinaldi S, Biessy C, Tjonneland A, Halkjaer J, Fournier A, et al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: The EPIC study. Int J Cancer. 2014 doi: 10.1002/ijc.29067. [DOI] [PubMed] [Google Scholar]
- 13.Rossing MA, Voigt LF, Wicklund KG, Daling JR. Reproductive factors and risk of papillary thyroid cancer in women. Am J Epidemiol. 2000;151:765–72. doi: 10.1093/oxfordjournals.aje.a010276. [DOI] [PubMed] [Google Scholar]
- 14.Kabat GC, Kim MY, Wactawski-Wende J, Lane D, Wassertheil-Smoller S, Rohan TE. Menstrual and reproductive factors, exogenous hormone use, and risk of thyroid carcinoma in postmenopausal women. Cancer Causes Control. 2012;23:2031–40. doi: 10.1007/s10552-012-0084-x. [DOI] [PubMed] [Google Scholar]
- 15.Mack WJ, Preston-Martin S, Bernstein L, Qian D, Xiang M. Reproductive and hormonal risk factors for thyroid cancer in Los Angeles County females. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1999;8:991–7. [PubMed] [Google Scholar]
- 16.Lee SM, Kwak KH. Risk factors and a predictive model for thyroid cancer in Korean women. Cancer Nurs. 2010;33:310–9. doi: 10.1097/NCC.0b013e3181cd2844. [DOI] [PubMed] [Google Scholar]
- 17.Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, et al. A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control. 1999;10:143–55. doi: 10.1023/a:1008880429862. [DOI] [PubMed] [Google Scholar]
- 18.Brindel P, Doyon F, Rachedi F, Boissin JL, Sebbag J, Shan L, et al. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in native women in French Polynesia: a population-based case-control study. Am J Epidemiol. 2008;167:219–29. doi: 10.1093/aje/kwm288. [DOI] [PubMed] [Google Scholar]
- 19.Przybylik-Mazurek E, Hubalewska-Dydejczyk A, Fedorowicz A, Pach D. Factors connected with the female sex seem to play an important role in differentiated thyroid cancer. Gynecol Endocrinol. 2012;28:150–5. doi: 10.3109/09513590.2011.563909. [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Wang Z, Zhu J, Murad A, Prokop L, Murad M. Nut consumption and risk of cancer and type 2 diabetes: A systematic review and meta-analysis. Nutr Rev. 2015;73:409–25. doi: 10.1093/nutrit/nuv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L, Zhu J, Prokop L, Murad M. Pharmacologic therapy of diabetes and overall cancer risk and mortality: A meta-analysis of 265 studies. Sci Rep. 2015;5:10147. doi: 10.1038/srep10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu QJ, Wu L, Zheng LQ, Xu X, Ji C, Gong TT. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev. 2015 doi: 10.1097/CEJ.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 24.Wang YZ, Wu QJ, Zhu J, Wu L. Fish consumption and risk of myeloma: a meta-analysis of epidemiological studies. Cancer Causes Control. 2015;26:1307–14. doi: 10.1007/s10552-015-0625-1. [DOI] [PubMed] [Google Scholar]
- 25.Wu QJ, Li YY, Tu C, Zhu J, Qian KQ, Feng TB, et al. Parity and endometrial cancer risk: a meta-analysis of epidemiological studies. Sci Rep. 2015;5:14243. doi: 10.1038/srep14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu QJ, Tu C, Li YY, Zhu J, Qian KQ, Li WJ, et al. Statin use and breast cancer survival and risk: a systematic review and meta-analysis. Oncotarget. 2015 doi: 10.18632/oncotarget.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells GABS, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Cook NR, Bergstrom A, Hsieh CC. A two-stage hierarchical regression model for meta-analysis of epidemiologic nonlinear dose–response data. Computational Statistics and Data Analysis. 2009;53:4157–67. [Google Scholar]
- 32.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 35.Cao YWZ, Gu J, Hu F, Qi Y, Yin Q, et al. Reproductive factors but not hormonal factors associated with thyroid cancer risk: a systematic review and meta-analysis. BioMed Research International. 2015 doi: 10.1155/2015/103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Lv L, Qi F, Qiu F. Increased risk of papillary thyroid cancer related to hormonal factors in women. Tumour Biol. 2015 doi: 10.1007/s13277-015-3165-0. [DOI] [PubMed] [Google Scholar]
- 37.Wu L, Zhu J. Linear reduction in thyroid cancer risk by oral contraceptive use: a dose-response meta-analysis of prospective cohort studies. Hum Reprod. 2015;30:2234–40. doi: 10.1093/humrep/dev160. [DOI] [PubMed] [Google Scholar]
- 38.Lee ML, Chen GG, Vlantis AC, Tse GM, Leung BC, van Hasselt CA. Induction of thyroid papillary carcinoma cell proliferation by estrogen is associated with an altered expression of Bcl-xL. Cancer J. 2005;11:113–21. doi: 10.1097/00130404-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–23. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 40.Chen GG, Vlantis AC, Zeng Q, van Hasselt CA. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets. 2008;8:367–77. doi: 10.2174/156800908785133150. [DOI] [PubMed] [Google Scholar]
- 41.Luan NN, Wu QJ, Gong TT, Vogtmann E, Wang YL, Lin B. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020–31. doi: 10.3945/ajcn.113.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan H, He Z, Ling L, Ding Q, Chen L, Zha X, et al. Reproductive factors and breast cancer risk among BRCA1 or BRCA2 mutation carriers: results from ten studies. Cancer Epidemiol. 2014;38:1–8. doi: 10.1016/j.canep.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Cronin-Fenton DP, Murray LJ, Whiteman DC, Cardwell C, Webb PM, Jordan SJ, et al. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: a pooled analysis. Eur J Cancer. 2010;46:2067–76. doi: 10.1016/j.ejca.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwan ML, Buffler PA, Abrams B, Kiley VA. Breastfeeding and the risk of childhood leukemia: a meta-analysis. Public Health Rep. 2004;119:521–35. doi: 10.1016/j.phr.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin RM, Gunnell D, Owen CG, Smith GD. Breast-feeding and childhood cancer: A systematic review with metaanalysis. Int J Cancer. 2005;117:1020–31. doi: 10.1002/ijc.21274. [DOI] [PubMed] [Google Scholar]
- 46.Jager S, Jacobs S, Kroger J, Fritsche A, Schienkiewitz A, Rubin D, et al. Breast-feeding and maternal risk of type 2 diabetes: a prospective study and meta-analysis. Diabetologia. 2014;57:1355–65. doi: 10.1007/s00125-014-3247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aune D, Norat T, Romundstad P, Vatten LJ. Breastfeeding and the maternal risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Nutr Metab Cardiovasc Dis. 2014;24:107–15. doi: 10.1016/j.numecd.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 48.Patelarou E, Girvalaki C, Brokalaki H, Patelarou A, Androulaki Z, Vardavas C. Current evidence on the associations of breastfeeding, infant formula, and cow’s milk introduction with type 1 diabetes mellitus: a systematic review. Nutr Rev. 2012;70:509–19. doi: 10.1111/j.1753-4887.2012.00513.x. [DOI] [PubMed] [Google Scholar]
- 49.Yan J, Liu L, Zhu Y, Huang G, Wang PP. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267. doi: 10.1186/1471-2458-14-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamberti LM, Zakarija-Grkovic I, Fischer Walker CL, Theodoratou E, Nair H, Campbell H, et al. Breastfeeding for reducing the risk of pneumonia morbidity and mortality in children under two: a systematic literature review and meta-analysis. BMC Public Health. 2013;13(Suppl 3):S18. doi: 10.1186/1471-2458-13-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]