Abstract
The Veterans Aging Cohort Study (VACS) Index was developed as a risk index for health outcomes in HIV, and it has been consistently associated with mortality. It shows a significant, yet relatively weak, association with neurocognitive impairment, and little is known about its utility among ethnic/racial minority groups. We examined whether the association between the VACS Index and neurocognition differed by ethnic/racial group. Participants included 674 HIV-infected individuals (369 non-Hispanic whites, 111 non-Hispanic blacks, and 194 Hispanics). Neurocognitive function was assessed via a comprehensive battery. Scaled scores for each neurocognitive test were averaged to calculate domain and global neurocognitive scores. Models adjusting for demographics and HIV disease characteristics not included in the VACS Index showed that higher VACS Index scores (indicating poorer health) were significantly associated with worse global neurocognition among non-Hispanic whites. This association was comparable in non-Hispanic blacks, but nonsignificant among Hispanics (with similar results for English and Spanish speaking). We obtained comparable findings in analyses adjusting for other covariates (psychiatric and medical comorbidities and lifestyle factors). Analyses of individual neurocognitive domains showed similar results in learning and delayed recall. For other domains, there was an effect of the VACS Index and no significant interactions with race/ethnicity. Different components of the VACS Index were associated with global neurocognition by race/ethnicity. In conclusion, the association between the VACS Index and neurocognitive function differs by ethnic/racial group. Identifying key indicators of HIV-associated neurocognitive impairment by ethnic/racial group might play an important role in furthering our understanding of the biomarkers of neuroAIDS.
Keywords: HIV, Cognition, Comorbidity, Latino, African American
Introduction
Hispanics and non-Hispanic (NH) blacks/African-Americans represent the largest minority groups in the USA. While there is considerable heterogeneity within ethnic/racial groups, persons from these underserved segments of the population tend to be disproportionally affected by HIV/AIDS. As a group, Hispanics and NH blacks have higher rates of infection than NH whites in the USA (Centers for Disease Control and Prevention 2013) and tend to be tested for HIV and engage in care later in the course of the disease (Chen et al. 2012; Dennis et al. 2011; Shapiro et al. 1999; Turner et al. 2000). Thus, not surprisingly, they tend to present with worse HIV disease characteristics as compared to NH whites (Swindells et al. 2002).
HIV-infected Hispanics and NH blacks also appear to be at increased risk for worse neurocognitive outcomes than HIV-infected NH whites (Durvasula et al. 2001; Heaton et al. 2014; Manly et al. 1998; Mindt et al. 2008; Ryan et al. 2005; Wojna et al. 2006). Despite the availability of more effective combination antiretroviral treatment (cART), neurocognitive impairment (NCI) continues to be common and impactful in HIV. It is observed in approximately half of HIV-infected persons overall (Heaton et al. 2011), with elevated rates of impairment in minority groups, and is associated with worse everyday functioning, such as medication nonadherence and driving problems (Heaton et al. 2004; Marcotte et al. 1999). The reasons for ethnic/racial differences in neurocognitive performance are not fully understood. There are likely multiple variables associated with race/ethnicity, both psychosocial and biomedical, which impact the expression of NCI in HIV-infected persons of diverse backgrounds. Most biomedical factors underlying NCI are likely to occur across ethnic groups, yet they might be differentially prevalent and impactful. Bidirectional associations and complex interactions among the various risk factors may lead to them having a differential impact among ethnic/racial groups with varying vulnerabilities.
Both biomarkers of HIV disease burden and common co-morbidities have been related to HIV-associated NCI (Cysique et al. 2010; Heaton et al. 2014; McCutchan et al. 2012). Furthermore, examination of a large diverse cohort of HIV-infected persons showed that non-white ethnic/racial groups had higher rates of comorbid conditions that contribute to NCI in HIV (Heaton et al. 2010). Thus, an index of disease status that incorporates both traditional HIV disease characteristics and biomarkers of common comorbidities, such as the Veterans Aging Cohort Study (VACS) Index, might prove to be a particularly good marker of neurocognitive status among minority groups. The VACS Index was developed as a tool to monitor the impact of dysfunction involving multiple organs commonly affected in HIV, using objective measures that are collected routinely in HIV clinics (Justice et al. 2010, 2012, 2013; Tate et al. 2013). It includes age, traditional HIV biomarkers (HIV-1 plasma RNA and current CD4 count), and non-HIV biomarkers (indicators of renal and liver function, anemia, and hepatitis C coinfection). The VACS Index has been consistently validated for estimating risk for mortality among HIV-infected persons, including in diverse cohorts (Justice et al. 2010, 2012, 2013; Tate et al. 2013). There is one published study showing a significant, yet weak, association with NCI (Marquine et al. 2014), suggesting that other factors not considered in the VACS Index may play important roles. Race/ethnicity might prove to be a key one. As described above, racial/ethnic identity is associated with multiple biomedical (e.g., genetics) and psychosocial factors (e.g., educational exposure and healthcare access), which interact with one another resulting in their impact being greater than the sum of its parts, and potentially leading to varying influences on VACS Index scores by ethnic/racial group.
The main purpose of the present study was to examine whether the association between the VACS Index and neurocognitive function differed among ethnic/racial groups. In order to do so, we examined this association in large and well-characterized cohorts of HIV-infected NH whites, NH blacks, and Hispanics living in the US-Mexico border region. We hypothesized that, by incorporating both “traditional” HIV markers and biomarkers of common comorbidities, the VACS Index might better capture the complexity of factors leading to NCI in HIV-infected persons from minority groups, and thus be more strongly associated with neurocognitive function in these groups than in NH whites. We were also interested in exploring whether the components of the VACS Index might be differentially associated with neurocognitive function by ethnic/racial group.
Methods
Participants
Six hundred and seventy-four HIV-infected individuals (369 NH whites, 111 NH blacks, and 194 Hispanics) enrolled in NIH-funded observational studies at the University of California San Diego HIV Neurobehavioral Research Program from May 1, 1999, to June 1, 2012, participated in the current study. Details on these studies have been published previously (Heaton et al. 1995; Rippeth et al. 2004; Woods et al. 2004). Participants were excluded if they had a history of head injury with loss of consciousness greater than 30 min, a neurologic or psychiatric illness that may affect cognitive functioning (e.g., stroke and schizophrenia), or significant sensory or physical problems that would interfere with neurocognitive testing. Inclusion criteria for the present study were as follows: (1) presence of HIV infection as determined by enzyme-linked immunosorbent assays (ELISA) with Western blot confirmatory test; (2) having laboratory data available to calculate the VACS Index; (3) completing neurocognitive testing within 2 months of laboratory data collection; (4) self-identification as Hispanic/Latino, NH white, or NH black/African American; and (5) proficiency in English or Spanish.
Materials and procedures
Study procedures were in accordance with the institutional and national ethical standards of the responsible committee on human experimentation. After providing written informed consent, participants completed comprehensive neuromedical, neurocognitive, psychiatric, and substance use assessments, consistent with our previous research on the VACS Index and NCI (Marquine et al. 2014). Evaluations were conducted in English or Spanish. For participants who reported some degree of bilingualism, language of evaluation was determined via participant’s report of preferred language and performance-based fluency in English and Spanish (Cherner et al. 2007).
Neuromedical evaluation
Neuromedical evaluations included a medical history, structured neurological and medical examination, and collection of blood and urine samples. Laboratory measurement included routine clinical chemistry panels, complete blood counts, rapid plasma reagin, HCV antibody, and CD4+ T cells (flow cytometry) performed at a Clinical Laboratory Improvement Amendments (CLIA)-certified, or CLIA equivalent, laboratory. HIV-RNA levels in plasma were measured by reverse transcriptase polymerase chain reaction (Roche Amplicor, v. 1.5; lower limit of quantitation, 50 copies per milliliter). We calculated the VACS Index as previously described (Justice et al. 2012). We assessed via self-report whether participants had a history of hypertension, hyperlipidemia, and diabetes.
Neurocognitive evaluation
Neurocognitive scores from the neuropsychological testing date closest to blood draw were used for the present analysis (interval number of days: mean = 1.31, SD = 4.79, range = 0–49). Participants completed a standardized neurocognitive test battery covering seven domains: learning, recall, verbal fluency, speed of information processing, executive function, working memory, and fine motor skills (see Cysique and colleagues for a list of specific tests (Cysique et al. 2011)). For participants who had previously completed neurocognitive testing during their participation in studies at our center, raw test scores were transformed into practice-adjusted scaled scores (SS), using published norms for change (Cysique et al. 2011). SS for tests comprising each domain were averaged to obtain domain scores. Global neurocognitive function represented the average of SS for all tests. We also computed rates of overall NCI (including scores from all tests) based on previously published standard regression-based models. In addition to adjusting for the effects of repeated testing when appropriate, overall NCI rates adjusted for the effects of age, education, gender, and race/ethnicity (Cysique et al. 2011).
The development of the Spanish language neurocognitive battery has been described elsewhere (Artiola i Fortuny et al. 1999; Cherner et al. 2007). Briefly, it included already existing translated test versions where appropriate and adaptation of other tests into Spanish. This adaptation was done through back translation and group consensus in order to obtain a neutral Spanish language and culturally relevant version of the neurocognitive tests, while preserving their comparability to the English version.
Psychiatric and substance use characteristics
We obtained past and current history of substance use and major depressive disorder in a portion of our sample (n = 493) via structured interviews with available English and Spanish versions (First et al. 2002; Hasin et al. 1996; World Health Organization 1997) and which follow Diagnostic and Statistical Manual-Fourth Edition criteria (American Psychiatric Association 1994). A substance use disorder was determined to be present if participants met criteria for either abuse or dependence for the following substances: alcohol, cannabis, opioids, methamphetamine, cocaine, sedatives, and/or hallucinogens. Current mood was assessed with the English and Spanish versions of the Beck Depression Inventory (BDI) versions one and two (Beck et al. 1961, 1996a, b; Beck and Steer 1993), using cutpoints appropriate for the version administered.
Lifestyle factors
Participants underwent urine drug screen (UDS) for the following drugs: marijuana, cocaine, opiates, barbiturate, benzodiazepines, phencyclidine (PCP), methamphetamine, and other amphetamines. We assessed history of smoking (lifetime and current) via self-report. We also evaluated participants’ adherence to their ART regimen by asking them to indicate the percent ART doses that they took as prescribed over the past 4 weeks. We considered a self-report of greater than 95 % of ART doses taken as prescribed as “adherent.”
Statistical analyses
Ethnic/racial group differences on demographic factors, HIV disease characteristics, psychiatric and medical comorbidities, lifestyle factors, global and domain neurocognitive scores, overall NCI, and VACS Index (overall score and individual components) were assessed via analyses of variance (ANOVA) and follow-up pairwise comparisons with Tukey’s correction (or nonparametric equivalent) and chi-squared tests. To examine the association between the VACS Index and neurocognitive function, we ran separate multivariable linear regression models in the overall sample on global and domain SS with NH whites as the reference group. These models adjusted for demographic characteristics (age, education, gender) and HIV disease characteristics that differed across groups, and included terms for the interaction between the VACS Index and race/ethnicity. For models with a significant interaction, we then ran separate analyses by ethnic/racial group adjusting for the same variables and examined the impact of language use within our Hispanic sample. To explore which VACS Index components might be more strongly associated with neurocognitive function by ethnic/racial group, we ran separate linear regression models on global SS with the VACS Index components as predictors. We used standard specifications and weightings of these components (Justice et al. 2012). We adjusted for education and gender in these models given the known effect of demographics on neurocognition. In initial analyses, we did not adjust for age, however, because age (as a categorical variable) is a component of the VACS Index, and we were interested in evaluating its effect as a component of the index.
All analyses on global SS were performed on rates of NCI using logistic regression methods. The global NCI score adjusts for demographics (age, education, gender, race/ethnicity) as well as a correction for repeated exposures to the individual neuropsychological tests (Cysique et al. 2011). We chose to make the primary focus of the manuscript on analyses using the SS approach given that results based on these scores are easier to interpret in the context of investigating the impact of race/ethnicity on the association between the VACS Index and neurocognitive functioning. In order to facilitate comparisons with prior relevant findings (e.g., Marquine et al. 2014), we also report results on overall NCI. For all models including the VACS Index overall score, estimates are provided per 10-unit change in this index.
Results
Demographic factors, HIV disease characteristics, and neurocognitive function by ethnic/racial group
NH whites were older, better educated, had a lower proportion of women, and had longer estimated duration of infection than NH blacks and Hispanics. They also were less likely to be on cART than NH blacks. The ethnic/racial groups differed on various aspects of neurocognitive function in analyses unadjusted for demographics (Table 1). Analyses on NCI (this type of score includes adjustments for demographic factors including race/ethnicity) indicated overall ethnic/racial group differences in NCI (p = 0.03), such that rates of NCI were higher in Hispanics (46 %) than blacks (31 %; p < 0.01), with no significant differences between NH whites (40 %) and NH blacks (p = 0.08) or NH whites and Hispanics (p = 0.17). Among Hispanic participants, 44 % reported being born in Mexico, 30 % in the USA, 2 % in Central America, and 1 % in South America (23 % data missing on this variable), and 60 % were primarily Spanish speaking.
Table 1.
Demographic factors, HIV disease characteristics, neurocognitive function, and VACS Index by study group
| Term | NH whites (W), n = 369 | NH blacks (B), n = 111 | Hispanics (H), n = 194 | Group comparisons
|
|
|---|---|---|---|---|---|
| p valuea | Pairwiseb | ||||
| Demographic factors | |||||
| Age, M (SD); range | 43.08 (9.64); 20–76 | 39.71 (8.52); 18–63 | 37.84 (9.51); 20–66 | <0.001 | W > B, W > H |
| Education, M (SD); range | 13.85 (2.52); 6–20 | 12.66 (2.00); 7–20 | 11.36 (3.36); 4–20 | <0.001 | W > B > H |
| Male gender | 91 % | 79 % | 79 % | <0.001 | W > B, W > H |
| HIV disease characteristics | |||||
| Nadir CD4 | 140 (26, 300) | 159 (10, 300) | 88 (18, 264) | 0.39 | |
| Current CD4 count (cells/mm3) | 388 (184, 585) | 349 (163, 536) | 339 (161, 547) | 0.39 | |
| ART prescribed | 60 % | 72 % | 68 % | 0.02 | W < B |
| Detectable plasma RNA | 59 % | 59 % | 56 % | 0.68 | |
| AIDS | 64 % | 61 % | 64 % | 0.87 | |
| EDI (years) | 10 (4, 14)c | 7 (2, 13)d | 6 (2, 11)e | <0.001 | W > B, W > H |
| Neurocognitive performance (SS), M (SD) | |||||
| Global | 9.36 (2.14) | 8.04 (2.13) | 8.81 (2.04) | <0.001 | W > H > B |
| Learning | 8.49 (2.71) | 7.10 (2.58) | 8.53 (2.65) | <0.001 | W > B, H > B |
| Recall | 8.53 (3.13) | 7.01 (2.84) | 8.69 (2.92) | <0.001 | W > B, H > B |
| Verbal fluency | 10.28 (2.53) | 9.64 (2.53) | 9.60 (2.55) | <0.01 | W > H |
| Speed of information | 9.96 (2.56) | 8.43 (2.61) | 9.02 (2.69) | <0.001 | W > B, W > H |
| Executive function | 9.79 (2.72) | 8.40 (2.71) | 8.56 (2.64) | <0.001 | W > B, W > H |
| Working memory | 9.75 (2.69) | 7.95 (2.76) | 8.06 (2.55) | <0.001 | W > B, W > H |
| Fine motor skills | 8.64 (2.75) | 7.84 (2.94) | 9.31 (2.96) | <0.001 | H > W > B |
| VACS Index | 18 (10, 33) | 23 (10, 45) | 17 (7, 34) | 0.02 | B > W, B > H |
Values represent median (IQR) unless otherwise noted
ART antiretroviral therapy, EDI estimated duration of infection, NH non-Hispanic, VACS Veterans Aging Cohort Study
Results from analyses of variance (ANOVAs) and chi-squared tests
Indicates significant group differences (p < 0.05) based on results from follow-up pairwise comparisons with Tukey’s correction and chi-squared tests
Forty-two missing
Thirteen missing
Twenty-seven missing
VACS Index scores and its components by ethnic/racial group
VACS Index scores were higher in NH blacks than NH whites (p = 0.03) and Hispanics (p = 0.04), with no significant differences between NH whites and Hispanics (Table 1). Follow-up analyses adjusting for age and gender demonstrated comparable findings.
Among the VACS Index components (Table 2), ethnic/ racial groups were comparable on traditional HIV biomarkers and HCV coinfection and differed significantly on indicators of anemia (hemoglobin), liver function (fibrosis index-4 (FIB-4)), and renal function (estimated glomerular filtration rate (eGFR)).
Table 2.
Components of the Veterans Aging Cohort Study (VACS) Index by group
| VACS component | Ptsa | NH whites (n = 369), n (%) | NH blacks (n = 111), n (%) | Hispanics (n = 194), n (%) | Group comparisonb
|
|
|---|---|---|---|---|---|---|
| p value | Pairwise | |||||
| Age | ||||||
| <50 | 0 | 287 (78 %) | 100 (90 %) | 173 (89 %) | <0.01 | W > B, W > H |
| 50–64 | 12 | 71 (19 %) | 11 (10 %) | 18 (9 %) | ||
| ≥65 | 27 | 11 (3 %) | 0 (0 %) | 3 (2 %) | ||
| CD4 count (cells/mm3) | ||||||
| ≥500 | 0 | 125 (34 %) | 37 (33 %) | 60 (31 %) | 0.24 | |
| 350–499 | 6 | 73 (20 %) | 18 (16 %) | 33 (17 %) | ||
| 200–349 | 6 | 71 (19 %) | 22 (20 %) | 33 (17 %) | ||
| 100–199 | 10 | 54 (15 %) | 14 (13 %) | 35 (18 %) | ||
| 50–99 | 28 | 14 (4 %) | 3 (3 %) | 16 (8 %) | ||
| <50 | 29 | 32 (9 %) | 17 (15 %) | 17 (9 %) | ||
| HIV-1 RNA (copies/mL) | ||||||
| <500 | 0 | 187 (51 %) | 60 (54 %) | 110 (57 %) | 0.48 | – |
| 500–1 × 105 | 7 | 135 (37 %) | 36 (32 %) | 67 (35 %) | ||
| ≥1 × 105 | 14 | 47 (13 %) | 15 (14 %) | 17 (9 %) | ||
| Hemoglobin (g/dL) | ||||||
| ≥14 | 0 | 224 (61 %) | 38 (34 %) | 109 (56 %) | <0.001 | W > H > B |
| 12–13.9 | 10 | 121 (33 %) | 41 (37 %) | 53 (27 %) | ||
| 10–11.9 | 22 | 21 (6 %) | 28 (25 %) | 28 (14 %) | ||
| <10 | 38 | 3 (1 %) | 4 (4 %) | 4 (2 %) | ||
| FIB-4 | ||||||
| <1.45 | 0 | 265 (72 %) | 85 (77 %) | 169 (87 %) | <0.001 | H < W |
| 1.45–3.25 | 6 | 92 (25 %) | 20 (18 %) | 20 (10 %) | ||
| >3.25 | 25 | 12 (3 %) | 6 (5 %) | 5 (3 %) | ||
| eGFR | ||||||
| ≥60 | 0 | 346 (94 %) | 106 (95 %) | 187 (96 %) | <0.01 | W > B, H > B |
| 45–59.9 | 6 | 20 (6 %) | 0 (0 %) | 6 (3 %) | ||
| 30–44.9 | 8 | 2 (1 %) | 2 (2 %) | 1 (1 %) | ||
| <30 | 26 | 1 (0 %) | 3 (3 %) | 0 (0 %) | ||
| HCV infection | 5 | 27 (7 %) | 12 (11 %) | 19 (10 %) | 0.35 | – |
Fibrosis index (FIB-4) = (years of age × AST) / (platelets in 109/L × square root of ALT); estimated glomerular filtration rate (eGFR) = 186.3 × (serum creatinine – 1.154) × (age – 0.203) × (0.742 for women) × (1.21 if black)
ALT alanine transaminase, AST aspartate transaminase
Points assigned as indicated by Justice et al. (2012)
Group comparisons based on chi-squared or Fisher’s exact tests
Association of the VACS Index to neurocognitive function by ethnic/racial group
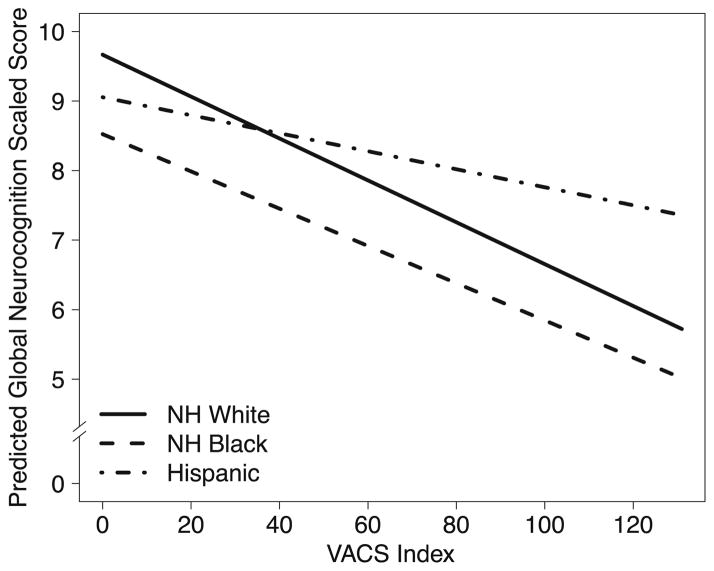
Multivariable analyses predicting global neurocognition with NH whites as the reference group showed a significant main effect of the VACS Index (p < 0.001), indicating a significant association in NH whites (Fig. 1). There was also a significant VACS Index × Hispanic interaction (p = 0.04) but no significant interaction for NH blacks (p = 0.74), indicating that the strength of the association between the VACS Index and neurocognition was significantly weaker in Hispanics than NH whites, but comparable between NH blacks and whites. Follow-up analyses stratified by race/ethnicity showed that higher VACS Index scores (worse health) were significantly associated with worse global neurocognitive function in NH whites (estimate = −0.32, SE = 0.06, p < 0.001, semi-partial R2 = 0.07) and NH blacks (estimate = −0.24, SE = 0.08, p < 0.01, semi-partial R2 = 0.06), but not in Hispanics (estimate = −0.09, SE = 0.07, p = 0.21, semi-partial R2 = 0.01). We obtained similar results in comparable analyses on NCI, including a significant VACS Index × Hispanic interaction (p = 0.02), but no significant VACS Index × NH black interaction (p = 0.16). In stratified analyses, higher VACS Index scores were associated with increased NCI in NH whites (OR = 1.29, CI = 1.15–1.47, p = 0.02) and NH blacks (OR = 1.41, CI = 1.15–1.75, p = 0.03), but not in Hispanics (OR = 1.08, CI = 0.94–1.24, p = 0.29).
Fig. 1.
Results from multivariable models on global neurocognitive function adjusting for covariates (age, education, gender, ART status) and predictors being VACS Index and terms for the interaction of this index with race/ethnicity
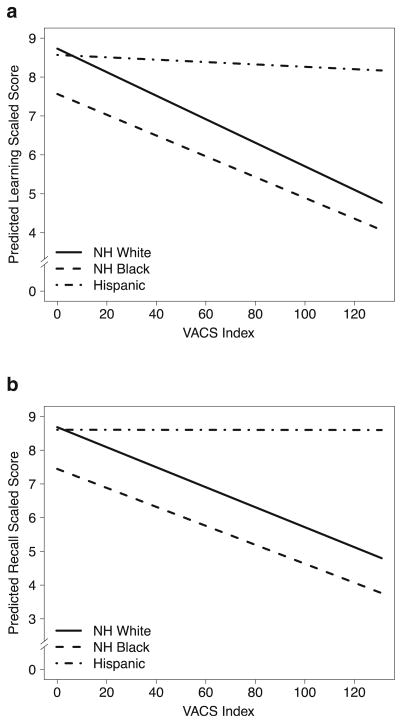
Analyses of the overall sample on the seven neurocognitive domains with NH whites as the reference group showed a main effect of the VACS Index for all domains (all ps< 0.01). There were no significant VACS Index × NH black interactions for any of the domains (ps > 0.25). The VACS Index × Hispanics terms were significant for learning (p = 0.01; Fig. 2a) and recall (p = 0.02; Fig. 2b), and there was a nonsignificant trend for verbal fluency (p = 0.08), with this interaction term being non-significant for other domains (ps > 0.26). Follow-up analyses showed that higher VACS Index scores were associated with worse learning and recall in NH whites (p < 0.001 for both domains) and NH blacks (p = 0.03 and p = 0.02, respectively), but not in Hispanics (p = 0.94 and 0.84, respectively). Separate analyses on verbal and visual learning and memory tests showed comparable findings.
Fig. 2.
Results from multivariable models on learning (a) and recall (b) neurocognitive domains adjusting for covariates (age, education, gender, ART status) and predictors being VACS Index and terms for the interaction of this index with race/ethnicity
Analyses examining the association between the VACS Index components and global neurocognitive function (Table 3), showed that age (as weighted in the VACS Index) was a significant predictor in all ethnic/racial groups, while the other components varied by group. FIB-4 and hemoglobin were significant predictors for NH whites, CD4 cell counts for NH blacks, and HCV status for Hispanics. We obtained similar results in comparable analyses on NCI.
Table 3.
Association between the components of the VACS Index and global neurocognitive function by ethnic/racial group
| NH white
|
NH black
|
Hispanic
|
||||
|---|---|---|---|---|---|---|
| p | Estimatea | p | Estimatea | p | Estimatea | |
| Age | <0.001 | – | <0.001 | – | 0.008 | – |
| <50 | Reference | Reference | Reference | |||
| 50–64 | −1.25 | −2.27 | −0.96 | |||
| ≥65 | −2.81 | −5.10 | −2.16 | |||
| CD4 count | 0.17 | – | 0.003 | – | 0.61 | – |
| HIV-1 RNA | 0.59 | – | 0.33 | – | 0.17 | – |
| Hemoglobin | 0.004 | – | 0.52 | – | 0.21 | – |
| ≥14 | Reference | – | – | |||
| 12–13.9 | −0.47 | – | – | |||
| 10–11.9 | −1.03 | – | – | |||
| <10 | −1.79 | – | – | |||
| FIB-4 | 0.004 | – | 0.27 | – | 0.73 | – |
| <1.45 | Reference | – | – | |||
| 1.45–3.25 | −0.37 | – | – | |||
| >3.25 | −1.53 | – | – | |||
| eGFR | 0.44 | – | 0.99 | – | 0.62 | – |
| HCV | 0.68 | – | 0.21 | – | 0.03 | −0.77 |
Represents results from three separate multivariable linear regression models on global neurocognitive function (scaled scores) by ethnic/racial group with predictors being the components of the VACS Index and adjusting for years of education and gender
Estimates are given for levels of significant predictors only
The impact of psychiatric and medical comorbidities and lifestyle factors
Considering that VACS Index scores may be impacted by lifestyle factors and psychiatric and other medical comorbidities, we evaluated the potential contribution of these factors to the different associations found between the VACS Index and neurocognition among ethnic/racial groups. The groups did not differ significantly on most psychiatric and substance use comorbidities, except Hispanics were less likely to have a positive UDS. Hispanics were also less likely to report a history of smoking (lifetime and current) than both NH groups, and NH blacks reported reduced ART adherence compared to NH whites and Hispanics. The ethnic/racial groups also reported varying rates of medical comorbidities (Table 4).
Table 4.
Psychiatric and medical comorbidities and lifestyle factors among the three ethnic/racial groups
| Term | NH whites (W), n = 369 | NH blacks (B), n = 111 | Hispanics (H), n = 194 | Group comparisons
|
|
|---|---|---|---|---|---|
| pa | Pairwiseb | ||||
| Psychiatric comorbidities | |||||
| Current major depressive disorder | 21 % c | 17 %d | 17 %e | 0.58 | |
| Lifetime major depressive disorder | 54 %c | 51 %d | 48 %e | 0.60 | |
| Current substance use disorder | 16 %c | 13 %d | 11 %e | 0.32 | |
| Lifetime substance use disorder | 69 %c | 66 %d | 61 %e | 0.26 | |
| Current depressed mood (BDI) | 56 % | 56 % | 51 % | 0.53 | |
| Medical comorbidities | |||||
| Hypertension | 22 % | 17 % | 10 % | 0.001 | W > H |
| Hyperlipidemia | 18 % | 6 % | 12 % | <0.001 | W > B, W > H |
| Diabetes | 7 % | 2 % | 5 % | 0.10 | |
| Lifestyle factors | |||||
| UDS+ (all drugs except marijuana) | 17 % | 22 % | 9 % | <0.01 | W > H, B > H |
| UDS+ for marijuana | 21 % | 25 % | 13 % | 0.02 | W > H, B > H |
| Current smoking (%) | 24 % | 29 % | 12 % | <0.001 | W > H, B > H |
| Ever smoked | 27 % | 31 % | 14 % | <0.001 | W > H, B > H |
| ART adherence | 77 % | 57 % | 80 % | 0.003 | W > H, H > B |
ART antiretroviral therapy, BDI Beck Depression Inventory, NH non-Hispanic, UDS+ positive urine drug screen
Results from chi-squared tests
Indicates significant group differences (p < 0.05) based on results from follow-up pairwise chi-squared tests
Ninety-five missing
Twenty-nine missing
Eighty-two missing
Of the comorbidities and lifestyle factors that differed across ethnic/racial groups, positive UDS (all tested substances except marijuana), hypertension, and medication adherence were significantly associated with global SS (ps < 0.05). We ran a linear regression model on global SS similar to the one in the original analyses (adjusting for demographics and ART status, and including terms for the VACS Index, race/ethnicity, and the interaction of these two latter factors) and also including terms for UDS status and hypertension. Results from this model showed similar results to those reported above, including a main effect of the VACS Index (p < 0.001), a nonsignificant VACS × NH black interaction (p = 0.52), and a significant VACS Index × Hispanic interaction (p = 0.04). We obtained comparable findings on NCI.
Within our group of patients on ART, we ran a comparable linear regression adjusting for demographics and medication adherence (NH whites: n = 203; NH blacks, n = 66; and Hispanics, n = 111). Results from this analysis showed a main effect of the VACS Index, but no significant VACS Index × race/ethnicity interactions. Because our smaller sample might have precluded us from finding a significant interaction, we ran separate models by ethnic/racial groups. Results from these analyses showed a pattern consistent with our larger sample size analyses, where the VACS Index was significantly associated with global neurocognition in NH whites (estimate = −0.31, SE = 0.09, p < 0.001) and NH blacks (estimate = −0.30, SE = 0.11, p < 0.01), but not in Hispanics (estimate = −0.16, SE = 0.09, p = 0.08). We obtained similar findings in NCI (NH whites: OR = 1.31, CI = 1.08–1.61, p = 0.005; NH blacks: OR = 1.48, CI = 1.15–1.98, p = 0.002; Hispanics: OR = 1.05, CI = 0.87–1.30, p = 0.63).
Follow-up subgroup analyses
Age- and gender-matched samples of NH whites and Hispanics
Given significant differences in demographic factors among ethnic groups, we selected (blinded to all nondemographic data) a subset of NH white participants (n = 194), comparable in age (M = 38.04; SD = 8.37) and gender (85 % male) to our total group of Hispanics (n = 194; see Table 1 for descriptive characteristics). We also made efforts to have years of education be equivalent, but there continued to be significant ethnic differences (NH whites education: M = 12.63, SD = 2.10; p < 0.001). We excluded NH blacks from these analyses given the smaller size of this group. Hispanics and the selected subgroup of NH whites did not differ significantly on the VACS Index (NH whites: median = 18, IQR = 10, 33), but NH whites had higher nadir CD4 (median = 194, IQR = 39, 333; p < 0.001), were less likely to be currently on ART (53 %; p < 0.01) and were more likely to have detectable plasma RNA (67 %; p = 0.02). The groups were comparable on other HIV disease characteristics.
Consistent with findings of analyses with the overall sample, multivariable analyses with this subset of participants on global SS adjusting for demographics and HIV disease characteristics that differed significantly between groups (education, nadir CD4, ART status, detectable plasma RNA) and including the VACS Index and its interaction with ethnicity (with NH whites as the reference group) showed a main effect of the VACS Index (p<0.0001) and a significant VACS Index × Hispanic interaction (p = 0.02). Within our subset of NH whites, the VACS Index was significantly associated with global neurocognition (estimate = −0.43, SE = 0.08, p < 0.001, semi-partial R2 = 0.10). We obtained similar findings in analyses adjusting for comorbidities and lifestyle factors that differed between groups (i.e., hypertension, smoking, marijuana-positive UDS; NH whites: estimate =−0.38, SE= 0.09, p <0.001), and when we used NCI as the outcome (NH whites: OR = 1.23, CI = 1.01–1.51, p = 0.04). Comparable analyses on neurocognitive domains yielded largely similar results to those in the larger sample including all NH whites. Analyses investigating the VACS Index components most predictive of global neurocognitive function in the subset of NH whites also yielded similar results to those of the larger NH white sample.
The effect of language use among Hispanics
Table 5 presents characteristics of Hispanics by language use. To explore whether language modified the association between the VACS Index and neurocognition, we ran a multivariable model on global SS within our Hispanic sample, adjusting for age, gender, education, and including a VACS Index and language (English/Spanish) interaction term. Results showed there was not a significant main effect of the VACS Index (p = 0.82) or VACS Index × language interaction (p = 0.50). Comparable analyses also adjusting for current and lifetime substance use disorder yielded similar findings. We also obtained similar results on rates of NCI.
Table 5.
Characteristics of Hispanics (n = 194) by language background
| Term | Spanish speakers, n = 116 | English speakers, n = 78 | p valuea |
|---|---|---|---|
| Demographic factors | |||
| Age, M (SD); range | 38.30 (9.74) | 37.14 (9.19) | 0.40 |
| Education, M (SD); range | 10.49 (3.60) | 12.64 (2.49) | <0.001 |
| Male gender | 74 % | 87 % | 0.02 |
| HIV disease characteristics | |||
| Nadir CD4 | 77 (17, 230) | 111 (19, 501) | 0.33 |
| Current CD4 count (cells/mm3) | 329 (152, 534) | 386 (161, 563) | 0.37 |
| ART prescribed | 30 % | 36 % | 0.32 |
| Detectable plasma RNA | 54 % | 58 % | 0.64 |
| AIDS | 67 % | 61 % | 0.43 |
| EDI (years) | 6 (2, 10)b | 5 (1, 12)c | 0.84 |
| Psychiatric comorbidities | |||
| Current major depression | 15 %d | 19 %e | 0.56 |
| Lifetime major depression | 46 %d | 50 %e | 0.66 |
| Current substance use disorder | 2 %d | 17 %e | <0.01 |
| Lifetime substance use disorder | 31 %d | 83 %e | <0.001 |
| Current depressed mood (BDI) | 52 % | 50 % | 0.80 |
| Medical comorbidities | |||
| Hypertension | 8 % | 14 % | 0.16 |
| Hyperlipidemia | 14 % | 8 % | 0.18 |
| Diabetes | 6 % | 3 % | 0.31 |
| Lifestyle factors | |||
| UDS+ (all drugs except marijuana) | 8 % | 9 % | 0.76 |
| UDS+ for marijuana | 11 % | 16 % | 0.29 |
| Current smoking | 8 % | 16 % | 0.10 |
| Ever smoked | 11 % | 18 % | 0.13 |
| ART adherence | 80 % | 79 % | 0.83 |
| Global SS, M (SD) | 8.84 (2.13) | 8.77 (1.91) | 0.81 |
| VACS Index | 17 (10, 35) | 17 (6, 27) | 0.43 |
Values represent median (IQR) unless otherwise noted
ART antiretroviral therapy, BDI Beck Depression Inventory, EDI estimated duration of infection, NH non-Hispanic, UDS+ positive urine drug screen, VACS Veterans Aging Cohort Study
Results from independent sample t tests (or Wilcoxon rank-sum test) and chi-squared tests
Fifteen missing
Twelve missing
Sixty-eight missing
Fourteen missing
Discussion
Previous findings showed a significant, yet relatively weak, association between higher VACS Index scores and increased concurrent risk for NCI (Marquine et al. 2014). Results from the present study extend these prior findings by showing that the strength of the association between the VACS Index and global neurocognition differed by ethnic/ racial groups. In contrast to our primary hypothesis, however, higher VACS Index scores were significantly associated with worse global neurocognition among NH whites and blacks, but this association was notably weaker and nonsignificant among Hispanics.
The reasons for these ethnic/racial differences are likely to be varied. Results from our study suggest that they cannot be fully explained by group differences in demographic factors (age, gender, education, language background), HIV disease characteristics that are not part of the VACS Index, psychiatric and medical comorbidities, or certain lifestyle factors (smoking, ART adherence). These differences in global neurocognitive function seem to be primarily driven by differential associations of the VACS Index to learning and recall domains across ethnic/racial groups. These neurocognitive domains are among the most affected in HIV infection in the cART era (Heaton et al. 2011) and are key predictors of functional outcomes in HIV (Heaton et al. 2004), highlighting their relevance among HIV-infected persons. Learning and memory tend to be mediated by medial temporal lobe structures and underlying subcortical pathways, indicating that disease processes primarily impacting these brain regions among HIV-infected Hispanics might not be fully captured in the VACS Index. While there are a number of diseases that lead to problems in learning and memory and our cohort was fairly young, studies in older non-HIV infected samples have shown an increased risk for mild cognitive impairment and dementia, including Alzheimer’s disease, among Hispanics (Manly et al. 2008; Tang et al. 2001), and an earlier onset of dementia symptoms in Hispanics (Clark et al. 2005). The identification of optimal biomarkers of these disease processes in older age is underway, and there is likely much to be gained by incorporating such biomarkers in the development of indexes for persons aging with HIV.
Components of the VACS Index were differentially associated with global neurocognition among ethnic/racial groups. Among NH whites biomarkers not specific to HIV disease (hemoglobin and FIB-4) were more strongly associated with neurocognition, whereas markers of infectious diseases were more relevant in minority groups; detectable plasma RNA for NH blacks and HCV coinfection for Hispanics. Poor or late treatment for infectious diseases among minority groups might be one of the factors explaining these findings. The biomarkers that were most important for a given ethnic/ racial group were not necessarily more severe or problematic in that group. This is in line with the notion that other factors not included in the index, such as biomarkers of inflammation or host genetic influences, may be particularly important for neurocognition, especially among HIV-infected Hispanics. Interactions between these factors and those that are part of the VACS Index might also result in the various biomarkers having a differential effect on neurocognitive function across ethnic/racial groups. It might also be that the components of the index need to be weighted differently across ethnic groups, at least when considering its association with neurocognition. Age, as a component in the VACS Index, was an important predictor of neurocognition in all groups. This is not surprising given the known effect of age on neurocognitive function in the general population. Age as a component of the VACS Index might also be capturing the effect of other factors associated with neurocognitive function in aging with HIV that are not included in the index (Marquine et al. 2014).
It is also possible that there are “protective” factors among Hispanics that were not assessed in the current study that attenuate the association between poor health, as measured in the VACS Index, and neurocognitive function. We assessed a limited number of comorbidity and lifetime factors that were more favorable among Hispanics in the present study (positive UDS, hypertension, hyperlipidemia, smoking, medication adherence). None of these factors seemed to explain the lack of a significant association between the VACS Index and neurocognition among Hispanics. Yet, our assessment of these factors was fairly limited (e.g., medication adherence was assessed by participant-report on a single assessment question, medical comorbidities were assessed via self-report), There are many other culturally relevant factors that warrant examination (e.g., increased social support, genetic factors), and could explain, at least in part, the current findings suggesting a weaker association between the VACS Index and neurocognition among Hispanics.
While we did not find an association between the VACS Index and neurocognitive function in Hispanics, work from the North American AIDS Cohort Collaboration Study (NA-ACCORD) showed that the VACS Index was predictive of mortality in a diverse cohort, including Hispanics, NH whites, and NH blacks (Justice et al. 2013). In addition to the difference in outcomes between this past study and ours, the NA-ACCORD study did not investigate interactions between race/ ethnicity and the VACS Index. Furthermore, the present sample of Hispanics (residing in the US-Mexico border region) likely differed in various ways from that of the NA-ACCORD study (which included sites across the USA and Canada). There is great heterogeneity within all ethnic/racial groups, particularly Hispanics, and regional differences may influence adherence to specific cultural norms. Thus, caution is warranted in generalizing present findings to other subgroups not included in our sample. Hispanics are highly diverse, comprising multiple national origins, racial groups, patterns of immigration, educational backgrounds, and language use, which have implications for health care use and outcomes (Guarnaccia et al. 2007). Nonetheless, there are also notable cultural aspects that are shared by many Hispanics (Anez et al. 2005; Guarnaccia and Rodriguez 1996). In the present study, we focus on Hispanics living in the US-Mexico border, which are primarily of Mexican origin/ descent. Importantly, Mexican Americans represent the majority (63 %) of the total US Hispanic population (9 % Puerto Ricans; 4 % Cubans, 3 % Dominican, 8 % Central American, 6 % South American, and 7 % other).
One limitation of the present study is that our cohort, particularly the NH black and Hispanic groups, were relatively young, and rates of severe comorbidities were fairly low across all groups (partly due to our exclusion criteria). Future studies including older HIV-infected persons, particularly members of minority groups, are a vital next step. Importantly, though, present findings indicate that the differential association between the VACS Index and neurocognitive function across ethnic/racial groups, cannot be explained by group differences in age, or severity of the index or its individual components. Other than language spoken and country of birth, we had limited data regarding culturally relevant characteristics of our group of Hispanics (e.g., years in the USA, ancestry). Future studies might investigate potential differences among subgroups of Hispanics, particularly given prior findings on the association between the VACS Index and mortality (Justice et al. 2013). The cross-sectional nature of the present study precludes ascribing directionality to our findings and highlights the need for future longitudinal studies in this area. The main strengths of our study include having a relatively large, diverse, and well-characterized group of HIV-infected adults, and the use of a comprehensive neurocognitive test battery that is sensitive to HIV-associated NCI and is used in both national and international HIV studies (Heaton et al. 2008, 2010).
Overall, results from the present study indicate that the association between the VACS Index and neurocognitive function differs by ethnic/racial group. In its current form the index does not appear to be an optimal indicator of risk for NCI, particularly among Hispanics. Thus, caution is warranted when utilizing the index as an indicator of NCI in this group. More broadly, present findings highlight the importance of examining the role of race/ethnicity when studying the association of HIV and non-HIV biomarkers to neurocognitive function. Identification of the key factors leading to NCI among different ethnic/racial groups of HIV-infected persons might prove to be an important step in developing tailored interventional approaches and furthering our understanding of biomarkers of neuroAIDS.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers P30 MH062512, U24 MH100928, U01 MH083506, R24 MH59745, P01 DA12065, T32MH019934, R25MH080663, K23 MH105297 and 1 K99 AG048762-01]. The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes the following: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobe-havioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
Footnotes
This work was previously presented at the 4th International Workshop on HIV & Aging meeting, October 30–31, 2013, Baltimore, MD, USA.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: 1994. [Google Scholar]
- Anez LM, Paris M, Jr, Bedregal LE, Davidson L, Grilo CM. Application of cultural constructs in the care of first generation Latino clients in a community mental health setting. J Psychiatr Pract. 2005;11(4):221–230. doi: 10.1097/00131746-200507000-00002. [DOI] [PubMed] [Google Scholar]
- Artiola i Fortuny L, Hermosillo Romo D, Heaton RK, Pardee RE. Manual de Normas y Procedimientos para la Batería Neuropsicológica en Español. m Press; Tucson: 1999. [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory Spanish Translation. The Psychological Corporation; San Antonio: 1993. [Google Scholar]
- Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996a;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory –Second Edition Manual. The Psychological Corporation; San Antonio: 1996b. [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed 09 Jun 2015];HIV Surveillance Report, 2011. 2013 23 Avaialable via http://www.cdc.gov/hiv/pdf/statistics_2011_HIV_Surveillance_Report_vol_23.pdf. [Google Scholar]
- Chen NE, Gallant JE, Page KR. A systematic review of HIV/AIDS survival and delayed diagnosis among Hispanics in the United States. J Immigr Minor Health. 2012;14(1):65–81. doi: 10.1007/s10903-011-9497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Suarez P, Lazzaretto D, Fortuny LA, Mindt MR, Dawes S, Marcotte T, Grant I, Heaton R. Demographically corrected norms for the Brief Visuospatial Memory Test-revised and Hopkins Verbal Learning Test-revised in monolingual Spanish speakers from the U.S.-Mexico border region. Arch Clin Neuropsychol. 2007;22(3):343–353. doi: 10.1016/j.acn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CM, DeCarli C, Mungas D, Chui HI, Higdon R, Nunez J, Fernandez H, Negron M, Manly J, Ferris S, Perez A, Torres M, Ewbank D, Glosser G, van Belle G. Earlier onset of Alzheimer disease symptoms in latino individuals compared with anglo individuals. Arch Neurol. 2005;62(5):774–778. doi: 10.1001/archneur.62.5.774. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Murray JM, Dunbar M, Jeyakumar V, Brew BJ. A screening algorithm for HIV-associated neurocognitive disorders. HIV Med. 2010;11(10):642–649. doi: 10.1111/j.1468-1293.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Franklin D, Abramson I, Ellis RJ, Letendre S, Collier A, Clifford D, Gelman B, McArthur J, Morgello S, Simpson D, McCutchan JA, Grant I, Heaton RK. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33(5):505–522. doi: 10.1080/13803395.2010.535504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis AM, Napravnik S, Sena AC, Eron JJ. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clin Infect Dis. 2011;53(5):480–487. doi: 10.1093/cid/cir434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol. 2001;23(2):149–163. doi: 10.1076/jcen.23.2.149.1211. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research. New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Guarnaccia PJ, Rodriguez O. Concepts of culture and their role in the development of culturally competent mental health services. Hisp J Behav Sci. 1996;8(4):419–443. [Google Scholar]
- Guarnaccia PJ, Martinez Pincay I, Alegria M, Shrout PE, Lewis-Fernandez R, Canino G. Assessing diversity among Latinos: results from the NLAAS. Hisp J Behav Sci. 2007;29(4):510–534. doi: 10.1177/0739986307308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric research interview for substance and mental disorders (PRISM): Reliability for substance abusers. Am J Psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, Wolfson T, Velin R, Marcotte TD, Hesselink JR, Jernigan TL, Chandler J, Wallace M, Abramson I the HNRC Group. The HNRC 500–neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I the HNRC Group. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, Franklin DR, Ake C, Vigil O, Atkinson JH, Marcotte TD, Grant I, Wu ZY. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2008;14(6):536–549. doi: 10.1080/13550280802378880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I The CHARTER & HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I. Neurocognitive Change in the Era of HIV Combination Antiretroviral Therapy: The Longitudinal CHARTER Study. Clin Infect Dis. 2014;60(3):473–480. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, Rimland D, Rodriguez-Barradas MC, Oursler KK, Brown ST, Braithwaite RS, May M, Covinsky KE, Roberts MS, Fultz SL, Bryant KJ VP Team. Towards a combined prognostic index for survival in HIVinfection: the role of ‘non-HIV’ biomarkers. HIV Med. 2010;11(2):143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, Fiellin DA, Vanasse GJ, Butt AA, Rodriguez-Barradas MC, Gibert C, Oursler KA, Deeks SG, Bryant K VP Team. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging With HIV? Clin Infect Dis. 2012;54(7):984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, Kitahata MM, Horberg MA, Brooks JT, Buchacz K, Rourke SB, Rachlis A, Napravnik S, Eron J, Willig JH, Moore R, Kirk GD, Bosch R, Rodriguez B, Hogg RS, Thorne J, Goedert JJ, Klein M, Gill J, Deeks S, Sterling TR, Anastos K, Gange SJ. Predictive accuracy of the Veterans Aging Cohort Study Index for mortality with HIV infection: A North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–163. doi: 10.1097/QAI.0b013e31827df36c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly JJ, Miller SW, Heaton RK, Byrd D, Reilly J, Velasquez RJ, Saccuzzo DP, Grant I. The effect of African-American acculturation on neuropsychological test performance in normal and HIV-positive individuals. The HIV Neurobehavioral Research Center (HNRC) Group. J Int Neuropsychol Soc. 1998;4(3):291–302. [PubMed] [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JPG, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhassoon O, Arfaa K, Grant I. The impact of HIV-related neuropsychological dysfunction on driving behavior. J Int Neuropsychol Soc. 1999;5(7):579–592. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- Marquine MJ, Umlauf A, Rooney AS, Fazeli PL, Gouaux BD, Paul Woods S, Letendre SL, Ellis RJ, Grant I, Moore DJ. The Veterans Aging Cohort Study Index is associated with concurrent risk for neurocognitive impairment. J Acquir Immune Defic Syndr. 2014;65(2):190–197. doi: 10.1097/QAI.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan JA, Marquie-Beck JA, FitzSimons CA, Letendre SL, Ellis RJ, Heaton RK, Wolfson T, Rosario D, Alexander TJ, Marra C, Ances BM, Grant I, Grp C. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology. 2012;78(7):485–492. doi: 10.1212/WNL.0b013e3182478d64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt MR, Byrd D, Ryan EL, Robbins R, Monzones J, Arentoft A, Germano KK, Morgello S, Henniger D. Characterization and sociocultural predictors of neuropsychological test performance in HIV+ Hispanic individuals. Cultur Divers Ethnic Minor. 2008;14(4):315–325. doi: 10.1037/a0012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I the HNRC Group. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Ryan EL, Baird R, Mindt MR, Byrd D, Monzones J, Bank SM HIVBB Manhattan. Neuropsychological impairment in racial/ethnic minorities with HIV infection and low literacy levels: effects of education and reading level in participant characterization. J Int Neuropsychol Soc. 2005;11(7):889–898. doi: 10.1017/S1355617705051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, Athey LA, Keesey JW, Goldman DP, Berry SH, Bozzette SA Consortium H. Variations in the care of HIV-infected adults in the United States—results from the HIV Cost and Services Utilization Study. J Am Med Assoc. 1999;281(24):2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- Swindells S, Cobos DG, Lee N, Lien EA, Fitzgerald AP, Pauls JS, Anderson JR. Racial/ethnic differences in CD4 T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS. 2002;16(13):1832–1834. doi: 10.1097/00002030-200209060-00020. [DOI] [PubMed] [Google Scholar]
- Tang MX, Cross P, Andrews H, Jacobs DM, Small S, Bell K, Merchant C, Lantigua R, Costa R, Stern Y, Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, Nattermann J, Lampe FC, Bucher HC, Sterling TR, Crane HM, Kitahata MM, May M, Sterne JA. An internationally gener-alizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27(4):563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Cunningham WE, Duan N, Andersen RM, Shapiro MF, Bozzette SA, Nakazono T, Morton S, Crystal S, St Clair P, Stein M, Zierler S. Delayed medical care after diagnosis in a US national probability sample of persons infected with human immunodeficiency virus. Arch Intern Med. 2000;160(17):2614–2622. doi: 10.1001/archinte.160.17.2614. [DOI] [PubMed] [Google Scholar]
- Wojna V, Skolasky RL, Hechavarria R, Mayo R, Selnes O, McArthur JC, Melendez LM, Maldonado E, Zorrilla CD, Garcia H, Kraiselburd E, Nath A. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12(5):356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview. Geneva: 1997. [Google Scholar]