Abstract
Background
Ectopic pregnancy (EP) is the commonest cause of maternal mortality-related death in the first trimester. Methotrexate (MTX) remains the first-line treatment in optimally selected patients.
Objective
To evaluate the success rate and predictors of success of a single-dose MTX treatment in EP.
Subjects and Method
We studied retrospectively 109 patients with unruptured EP who were treated with Intramuscular MTX administered in a dose of 50 mg/m2 on days 0 and in additional doses on day 7 if β-hCG levels did not decrease by 15 % during the follow-up period. The study was conducted at the Maternity and Children Hospital Buraidah, Saudi Arabia from June 2013 to December 2013. Pretreatment β-β-hCG, EP mass diameter, peritoneal fluid, and fetal cardiac activity were evaluated. The main outcome measures were success rate, the predictors of success without surgical treatment.
Result
Under this regime, the overall success rate was 60.6 % of patients. Of the failure group, only 4.7 % of patients experienced rupture of EP. No side effects were reported. The main predictors of failure were initial β-hCG value ≥ 3.500 mIU/mL OR 4.11 (1.646–12.248, 0.043) and EP diameter 3.73 (1.646–12.10, p = 0.003).
Conclusion
The success rate of MTX in this study was 60.6 %, and the initial β-hCG concentration and EP diameter were the best predictors of successful treatment with MTX. Furthermore, MTX should be offered only to those patients with β-hCG <2,000 mIU/mL and EP mass size <3.5 cm.
Keywords: Ectopic pregnancy, Methotrexate, Success rate, Predictors of failure complications
Introduction
Ectopic pregnancy (EP) is major health problem accounting for 13 % of all pregnancy-related deaths [1]. EP complicated 1–2 % of all pregnancies; recent studies have shown that the incidence of EP is on a rising trend as a result of assisted reproductive techniques [2].
MTX was proved to be safe and effective alternative drug with a similar outcome to surgical intervention in properly selected cases [3].
There are many regimes used for MTX in ectopic pregnancy, but the most popular is a single-dose regime in which a single intramuscular dose of MTX is given at day 0; additional subsequent doses is given when necessary as determined by serial β-hCG concentration [4].
In optimally selected candidate, MTX therapy has an overall success rate of nearly 90 % [5]. MTX should be used in hemodynamically stable patients with human chorionic gonadotropin beta-subunit (β-hCG) with concentration ≤ 5,000 mIU/mL [6], gestational sac size ≤ 3.5 cm, and negative cardiac action [7]. The patient should be willing and able to comply with post-treatment follow-up, and there is a contraindication to MTX [8, 9], while other studies reported a comparatively lower success for MTX [10]. However, rates of success of MTX in each of these studies depend on the number of prognostics factors used and the cut-off points for both β-hCG and EP mass diameter. However, there is no consensus on many of these prognostics factors used in MTX therapy.
The current study was conducted to determine the success rate and predictors of MTX after single-dose treatment.
Materials and Methods
This cross-sectional retrospective study was conducted on 109 patients with EP treated with single-dose MTX 50 mg/m2 at Maternity and Children Hospital, Buraidah, Saudi Arabia. The study was conducted between June 2013 and December 2013 after receiving approval from the Ethics and research committee of the concerned institute. All records of patients with EP were reviewed electronically and then manually from the patient’s notecase.
Patients included in the study were those who received a single dose of MTX and met the following inclusion criteria: (1) hemodynamically stable, (2) intra-abdominal fluid limited to pelvic cavity with no evident of intra-abdominal bleeding based on clinical and sonographic findings, (3) negative fetal cardiac action, (4) initial beta β-hCG concentration ≤ 10,000 β-hCG mIU/dL [11], and (5) ectopic mass diameter ≤ 4 cm.
Patients excluded were those who (1) were hemodynamically unstable, (2) had signs of acute abdomen, and/or (3) showed abnormal hematologic, renal, or hepatic laboratory studies, and those who did not receive MTX.
Patients were instructed before starting the protocol to report for regular follow-up for the following reasons: a second dose of MTX may be required and β-hCG level assessment, and to report immediately if they developed abdominal pain.
The variables included in the study were maternal height and weight. Maternal height (cm) and maternal weight (kg) were measured and expressed as BMI body mass index [BMI—weight (kg) height (m)]. Other variables we included were the number of previous miscarriages, past surgical history, history of pelvic inflammatory diseases, and history of IVF. Clinical and laboratory variables included were initial β-hCG and sonographic findings such as EP mass diameter and fluid in the peritoneal cavity. The variables used in the analysis of predictors of treatment failure which were continuous were categorized to determine the cut-off points that influence MTX the treatment failure.
Diagnosis of EP was determined by a combination of serial β-hCG concentration and trans-vaginal scanning without surgical intervention [12].
Patients were classified into either successful to MTX treatment defined as the resolution of the β-hCG concentration to <5 mIU/mL or treatment failure defined as those required surgical intervention following MTX administration.
Statistical Analysis
The Statistical Package for the Social Sciences for Windows, version 15.0 (SPSS Inc., Chicago, Illinois) was used for analyzing data. Means and proportions were compared between successfully treated group and failure group by student’s t test and X2, respectively. Univariate and multivariate analysis was performed where the outcome (success or failure) is the dependent variable and age, height, weight, and fetal gestational age, Past surgical history, PID, IVF, Fluid in Cul de sac, β-hCG ≥ 3,500 mIU/mL, mass ≥ 3.5 cm maternal weight, maternal height and body mass index were the independent variables. Adjusted odds ratios (OR) and the corresponding 95 % confidence interval (CI) were estimated. A p value of less than 0.05 was considered statistically significant.
Results
Complete resolution of EP after the first dose of MTX was 63.63 % (42/66) and after the second dose of MTX was 36.36 % (24/66). The remaining cases 43 (38.3 %) required surgical intervention after the first dose in 97.7 % (42/43) of cases and 2.3 % (1/43) after the second dose owing to persistent abdominal pain. Thus, the overall success rate of MTX in this study was 60.6 %.The majority of cases that required surgical intervention occurred in the first week of treatment 97.7 % (42/43). The overall frequency of ruptured ectopic pregnancy which required surgical intervention was 4.7 % (2/43).
The commonest presentations of studied women were vaginal bleeding in 88.1 %, abdominal pain in 5.5 %, and combined presentation was in 6.4 % of cases. There were no significant differences between those treated successfully and failure group in their clinical presentations p = 0.3864, p = 1.0000, and p = 0.7043, respectively.
The basic characteristics of successful and failure group with EP are shown in Table 1, and there were no significant differences between both the groups in term of obstetrical history such as gravidity, parity, and number of previous miscarriages (p = 0.734, p = 0.720, and p = 0.0867, respectively). Trans-vaginal ultrasound revealed free fluid in the peritoneal cavity in 65.2 % of the success group and 88.4 % in the failed group (p = 0.09). Similarly, both groups showed no significant differences in age, height, weight, BMI, and gestational age at admission (p > 0.05). Both groups had a similar history of pelvic inflammatory disease (4.6 % vs. 7 %, p = 0.068). Patients in the failure group meaningfully had a higher incidence of PCOs than those in the success group (48.8 % vs. 21.2 %, p = 0.003). Also, they tend to have a higher percentage of irregular menses, but the difference was not statistically significant (p = 0.06).
Table 1.
Demographic characteristics related to the efficacy of MTX treatment in women with EP
| Variable | Success | Failed | p value |
|---|---|---|---|
| Age | 29.5 ± 5.23 | 29.84 ± 5.75 | 0.813 |
| Weight | 73.6 ± 13.49 | 73.5 ± 10.52 | 0.974 |
| Height | 157.8 ± 5.15 | 158.8 ± 3.47 | 0.244 |
| Gravidity | 3.8 ± 2.72 | 3.7 ± 2.11 | 0.734 |
| Parity | 1.9 ± 2.07 | 1.8 ± 1.58 | 0.720 |
| Abortion | 0.86 ± 1.52 | 0.81 ± 1.5 | 0.0867 |
| β-hCG | 1380.7 ± 1341.9 | 3199.2 ± 2445.6 | <0.001 |
| Gestational age | 6.5 ± 1.02 | 6.6 ± 0.92 | 0.671 |
| Body mass index | 29.5 ± 4.77 | 29.1 ± 4.05 | 0.691 |
| Free fluid | 49(65.2) | 38(88.4) | 0.09 |
| PID | 3(4.6) | 3(7) | 0.68 |
| Ruptured ectopic | – | 2(4.7) | 0.1622 |
| PCO | 14(21.2) | 21(48.8) | 0.003 |
Data are presented as mean ± SD. p value was significant at less than 0.05
PID pelvic inflammatory diseases; PCOs polycystic ovarian syndrome; β-hCG human chorionic gonadotrophin
The failure group had a significantly higher initial human chorionic gonadotrophins concentrations compared to successful group (3199.2 ± 2445.6 vs. 1380.7 ± 1341.9, and p = <0.0001, respectively), ranged (107–8753). Moreover, the success rate of MTX therapy decreased as the β-hCG level increased. Women with initial β-hCG < 2000 mI/mL showed 78.8 % success rate (p = 0.0199) in comparison with a β-hCG value greater than 4,000 mL/mL (p = 0.0525) as shown in Table 2.
Table 2.
The success rates of MTX in 109 women with ectopic pregnancies as a function of their initial β-hCG concentrations
| β-hCG concentrations | Success 66 | Failed 43 | p value |
|---|---|---|---|
| 0–1,999 | 52 (78.8) | 14 (32.6) | 0.0199 |
| 2,000–2,999 | 9 (13.6) | 11 (25.6) | 0.2941 |
| 3,000–3,999 | – | 7 (16.3) | 0.0061 |
| 4,000 and more | 5 (7.6) | 11 (25.6) | 0.0525 |
Data are presented as frequency (percentage); p value was significant at less than 0.05
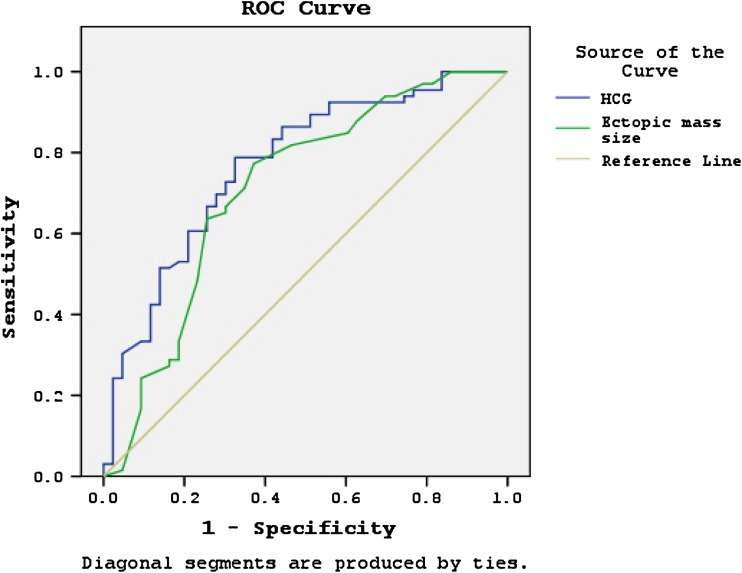
The logistic regression analysis of a cut-off point of ≥3,500 mIU/mL of β-hCG showed increased risk of treatment failure of MTX with an OR of 4.11(95 % CI 1.646–12.248, p = 0.043). Similarly, logistic regression showed that increased failure of medical treatment was associated with an increase of EP mass diameter of greater than 3.5 cm with an OR of 3.73 (CL 1.646–12.109, p = 0.003), as shown in Table 3. The ROC curve analysis indicated that the sensitivity and specificity of the successful group were 80 and 70 %, respectively, at β-hCG cut-off point of 2000 mIU/mL (area under the curve, 0.77, 95 % CI 0.679–0.861, p < 0.0001). In addition, the sensitivity and specificity of mass size of 3.55 cm were 78 and 64 %, respectively (area under the curve, 0.711, 95 % CI 0.606–0.816, p < 0.0001), term of identifying the patients who were successfully responded to a single-dose MTX in EP (Fig. 1).
Table 3.
Predictor factors of response to a single dose of MTX in 109 ectopic pregnant women
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95 % CI | p value | OR | 95 % CI | p value | |
| Maternal age | 1.009 | 0.939–1.083 | 0.811 | 1.002 | 0.916–1.097 | 0.960 |
| No. abortion | 0.97 | 0.831–1.138 | 0.866 | 1.004 | 0.730–1.380 | 0.982 |
| Gestational age | 1.09 | 0.736–1.614 | 0.668 | 1.28 | 0.814–2.011 | 0.286 |
| Past surgical history | 1.04 | 0.486–2.25 | 0.906 | 1.29 | 0.471–3.580 | 0.614 |
| PID | 1.575 | 0.303–8.190 | 0.589 | 1.441 | 0.182–11.38 | 0.729 |
| IVF | 0.762 | 0.067–8.670 | 0.827 | 5.956 | 0.374–94.736 | 0.206 |
| Fluid in cul de sac | 2.63 | 0.89–7.7 | 0.079 | 1.28 | 0.373–4.397 | 0.695 |
| β-hCG ≥ 3500 mIU/mL | 1.001 | 1.000–1.001 | 0.000 | 4.11 | 1.646–12.248 | 0.043 |
| Mass | 4.07 | 1.8–9.23 | 0.001 | 3.73 | 1.646–12.109 | 0.003 |
| Maternal weight | 0.99 | 0.96–1.03 | 0.974 | 0.982 | 0.941–1.026 | 0.422 |
| Maternal height | 1.05 | 0.96–1.1 | 0.245 | 1.127 | 0.916–1.097 | 0.038 |
| Body mass index | 0.98 | 0.90–1.07 | 0.688 | 0.971 | 0.874–1.078 | 0.580 |
p value was set significantly at less than 0.05
PID pelvic inflammatory diseases; IVF in viro fertilization; β-hCG human chorionic gonadotrophins, OR odd ratio; CI confident interval
Fig. 1.
β-hCG, area under the curve (AUC) = 0.770, (95 % CI 0.679–0.861, p < 0.001). Mass diameter, area under the curve (AUC) = 0 0.711, (95 % CI 0.606–0.816, p < 0.001)
Discussion
The putative effect of MTX is via multiple of actions including reduction of cell proliferation, increase of endogenous adenosine release, increase of apoptosis of T cells, alteration of expression of cellular adhesion molecules, and influence on production of cytokines [13, 14]. These effects are lethal to the growing human embryo even in a single dose of 50 mg/m2 particularly between 6 and 8 weeks of gestation [15].
The main findings in the present study were success and rupture rates of 60.6 and 4.7 %, respectively. The main predictors of this success of MTX were initial quantitative β-hCG and EP diameter.
There is a wide disparity in the literature on the success rate of MTX in treating ectopic pregnancy. Watters et al. [16] have reported a lower success rate of 29.6 %, and the rate of 35 % was reported with β-hCG concentrations of ≥4,000 mIU/mL [17]. Later authors commented when the cut-off point of β-hCG was taken as ≤4,000 mIU/mL and the success rate of MTX increased to 92.5 %. On the other hand, success rate as high as 98 % with a β-hCG concentration of ≤1,000 mIU/mL was reported [18].
Our success rate is comparable to a 65 % rate reported by Hansa Dhar et al. [19] from the same region. The available literature showed a global success rate ranging from 67 to 100 % [7]. Higher success rates were obtained with multiple and double-dose regimes [7, 20]. These results were confirmed by a meta-analysis study [5].
Most of studies that reported higher success rates have limited number of recruited cases [21, 22] compared to our sample size. Sample size, population, population heterogeneity, section criteria, patients’ compliance, the type of MTX protocol, and most importantly the lack of specific criteria for failed treatment that require surgical intervention could explain these differences between various studies.
The unsuccessfully treated group in the present study had tubal rupture rate of 4.7 % which is higher compared with other reports [7, 20]. This could be explained by high frequency of patients (65.2 %) with intra-pelvic fluid collection. Patients with hemoperitoneum were at a higher risk of treatment failure [10]. Moreover, the presence of hemoperitoneum even if confined to pelvic cavity does exclude the possibility of ongoing intra-abdominal hemorrhage.
While many studies proved the efficacy of systemic MTX in the treatment of EP with a similar outcome to laparoscopic surgery [23], clinical trials failed to demonstrate any value of this treatment over expectant management [24]. Authors concluded that single-dose MTX does not have any significant effect compared with expectant management in women with an EP or a pregnancy of unknown location with low and plateauing serum β-hCG level. Authors in their clinical trials used highly selective criteria “Dutch national guideline Tubal Ectopic Pregnancy, Diagnosis and Treatment” for patients’ eligibility in which the cut-off point for Β-HCG is <2,000 mIU/mL that is very low compared to other studies which used higher concentrations of β-hCG [24].
The receiver operator curve in the present study showed that a β-hCG concentration ≤ 2000 mIU/mL has a good sensitivity and specificity in identifying patients that could be successfully treated with a single dose of MTX. Moreover, patients with β-hCG concentration <2000 mIU/dL responded significantly to treatment (0.0199) compared to those with β-hCG greater than 2,000 mIU/mL. A similar result was obtained by Sagiv et al. [25] in a study determining the optimal cut-off serum level of β-hCG for efficacy of MTX treatment in women with EP, and they found that β-hCG value of >2,000 mIU/mL increased the odds of failure by about 4.5 times. In the current study, logistic regression indicated when β-hCG concentration exceeded 3,500 mIU/mL MTX failure increased by 4.11-folds. (95 % CI 1.646–12.248, p = 0.043) which is consistent with results obtained by Sagiv et al. [25]; in our study, we included patients with initial β-β-hCG ≤ 10,000 mIU/mL. A systematic review and meta-analysis have shown that a rate of failure of MTX is primarily dependant on initial β-hCG concentrations [6].
The present study showed that EP mass diameter of ≥3.5 cm is associated with 3.73-folds increased in MTX failure. The cut-off point for ectopic diameter is diverging among experts. Admissions of treatment according to mass size vary some limited treatments to mass diameter of ≤3.5 cm [26], while others use a higher cut-off point larger than 3.5 cm [27]. Cobellis et al. [28] reported a success rate of 91 % in patients with a mass size of up to 5 cm. These diverging results among reports can explain by the difficulty of obtaining the exact size of the gestational sac from a surrounding hematoma or tissues.
The shortcomings of this report are its retrospective nature, relatively small sample size, and a single-center study rather than multicenter which is more informative.
Conclusion
An initial β-hCG concentration and EP diameter were the only predictors of successful treatment with MTX. These two factors may act as one cause and effect string, and with larger EP mass size, there is a possibility of high β-hCG production due and consequently higher failure rate. This study indicated that β-hCG <2,000 mIU/mL and EP mass size <3.5 cm are ideal cut-off points for successful treatment with a single dose of MTX. Moreover, ROC indicated that MTX should be offered to patients with β-hCG <2,000 mIU/mL and EP mass size <3.5 cm. Therefore, further studies are needed to clarify these points.
Acknowledgments
Compliance with Ethical Requirements and Conflict of Interest
The study was approved by the institutional Ethics committees of Qassim University and Saudi medical specialization board. We declare no conflict of interest.
Mohamed Alkhatim Alsammani
is currently working as an associate professor, Department of Obstetrics and Gynecology, College of Medicine Qassim University, KSA, and Bahri University, Sudan. After obtaining his MBBS from the University of Gezira and MD from the University of Khartoum, he persuaded an academic career in teaching and assessment of medical students and was trained in scientific writing in joint courses conducted by University of Khartoum and the WHO in 2002. He is credited with many publications in reputable journals. He also served as reviewer at the International Journal of Health Sciences, the World Journal of Gastroenterology, and Dove Medical Press Ltd., a science domain and premier publisher group.
References
- 1.Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin. 2000;27:653–657. doi: 10.1016/S0889-8545(05)70162-5. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JL, Diamandis EP, Horne AW, et al. Ectopic pregnancy. Clin Chem. 2012;58:1278–1285. doi: 10.1373/clinchem.2012.184168. [DOI] [PubMed] [Google Scholar]
- 3.Pekhlivanov B, Milchev N. Conservative treatment of EP with MTX. Akush Ginekol (Sofiia). 2002;41:53–56. [PubMed] [Google Scholar]
- 4.ACOG practice bulletin. Medical management of tubal pregnancy. Number 3, December 1998. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynecol Obstet 1999;65:97–103. [PubMed]
- 5.Barnhart K, Hummel AC, Sammel MD, et al. Use of “2-dose” regimen of MTX to treat ectopic pregnancy. Fertil Steril. 2007;87:250–256. doi: 10.1016/j.fertnstert.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 6.Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide MTX treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87:481–484. doi: 10.1016/j.fertnstert.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Barnhart KT, Gosman G, Ashby R, et al. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol. 2003;101:778. doi: 10.1016/s0029-7844(02)03158-7. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar CM. Ectopic pregnancy. Lancet. 2005;366:583–591. doi: 10.1016/S0140-6736(05)67103-6. [DOI] [PubMed] [Google Scholar]
- 9.Morlock RJ, Lafata JE, Eisenstein D. Cost-effectiveness of single-dose MTX compared with laparoscopic treatment of ectopic pregnancy. Obstet Gynecol. 2000;95:407. doi: 10.1016/s0029-7844(99)00548-7. [DOI] [PubMed] [Google Scholar]
- 10.Gnisci A, Stefani L, Bottin P, et al. Predictive value of hemoperitoneum for outcome of MTX treatment in ectopic pregnancy: an observational comparative study. Ultrasound Obstet Gynecol. 2014;43:698–701. doi: 10.1002/uog.13255. [DOI] [PubMed] [Google Scholar]
- 11.Mukul LV, Teal SB. Current management of ectopic pregnancy. Obstet Gynecol Clin North Am. 2007;34:403–419. doi: 10.1016/j.ogc.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Sivalingam VN, Duncan WC, Kirk E, et al. Diagnosis and management of ectopic pregnancy. J Fam Plan Reprod Health Care. 2011;37:231–240. doi: 10.1136/jfprhc-2011-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of MTX in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47:249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 14.Kremer JM. MTX treatment of rheumatic diseases: can we do better? Arthritis Rheum. 2008;58:3279–3282. doi: 10.1002/art.24032. [DOI] [PubMed] [Google Scholar]
- 15.Feldkamp M, Carey JC. Clinical teratology counseling and consultation case report: low dose MTX exposure in the early weeks of pregnancy. Teratology. 1993;47:533–539. doi: 10.1002/tera.1420470605. [DOI] [PubMed] [Google Scholar]
- 16.Watters AN, Barber EL, Roston A, et al. MTX: an appropriate treatment for EP in an inner-city population? Obstet Gynecol. 2014;123(Suppl 1):130S–131S. doi: 10.1097/01.AOG.0000447097.15673.ac. [DOI] [Google Scholar]
- 17.Tawfiq A, Agameya AF, Claman P. Predictors of treatment failure for EP treated with single-dose MTX. Fertil Steril. 2000;74:877–880. doi: 10.1016/S0015-0282(00)01547-8. [DOI] [PubMed] [Google Scholar]
- 18.Lipscomb GH, McCord ML, Stovall TG, et al. Predictors of success of MTX treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999;341:1974–1978. doi: 10.1056/NEJM199912233412604. [DOI] [PubMed] [Google Scholar]
- 19.Dhar H, Hamdi I, Rathi B. MTX treatment of ectopic pregnancy: experience at Nizwa hospital with literature review. Oman Med J. 2011;26:94–98. doi: 10.5001/omj.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamed HO, Ahmed SR, Alghasham AA. Comparison of double- and single-dose MTX protocols for treatment of ectopic pregnancy. Int J Gynaecol Obstet. 2012;116:67–71. doi: 10.1016/j.ijgo.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Srivichai K, Uttavichai C, Tongsong T. Medical treatment of ectopic pregnancy: a ten-year review of 106 cases at Maharaj Nakorn Chiang Mai Hospital. J Med Assoc Thai. 2006;89:1567–1571. [PubMed] [Google Scholar]
- 22.Soliman KB, Saleh NM, Omran AA. Safety and efficacy of systemic MTX in the treatment of unruptured tubal pregnancy. Saudi Med J. 2006;27:1005–1010. [PubMed] [Google Scholar]
- 23.Mol F, Mol BW, Ankum WM, et al. Current evidence on surgery, systemic MTX and expectant management in the treatment of tubal ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod Update. 2008;14:309–319. doi: 10.1093/humupd/dmn012. [DOI] [PubMed] [Google Scholar]
- 24.van Mello NM, Mol F, Verhoeve HR, et al. MTX or expectant management in women with an EP or pregnancy of unknown location and low serum β-hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60–67. doi: 10.1093/humrep/des373. [DOI] [PubMed] [Google Scholar]
- 25.Sagiv R, Debby A, Feit H, et al. The optimal cutoff serum level of human chorionic gonadotropin for efficacy of MTX treatment in women with extrauterine pregnancy. Int J Gynaecol Obstet. 2012;116:101–104. doi: 10.1016/j.ijgo.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 26.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: medical management of ectopic pregnancy. Obstet Gynecol. 2008;111:1479–85. [DOI] [PubMed]
- 27.Practice Committee of American Society for Reproductive Medicine Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100:638–644. doi: 10.1016/j.fertnstert.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Cobellis G, Pierno G, Pecori E, et al. MTX treatment for tubal pregnancy. Criteria for medical approach. Minerva Ginecol. 2003;55:531–535. [PubMed] [Google Scholar]