Abstract
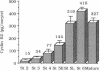
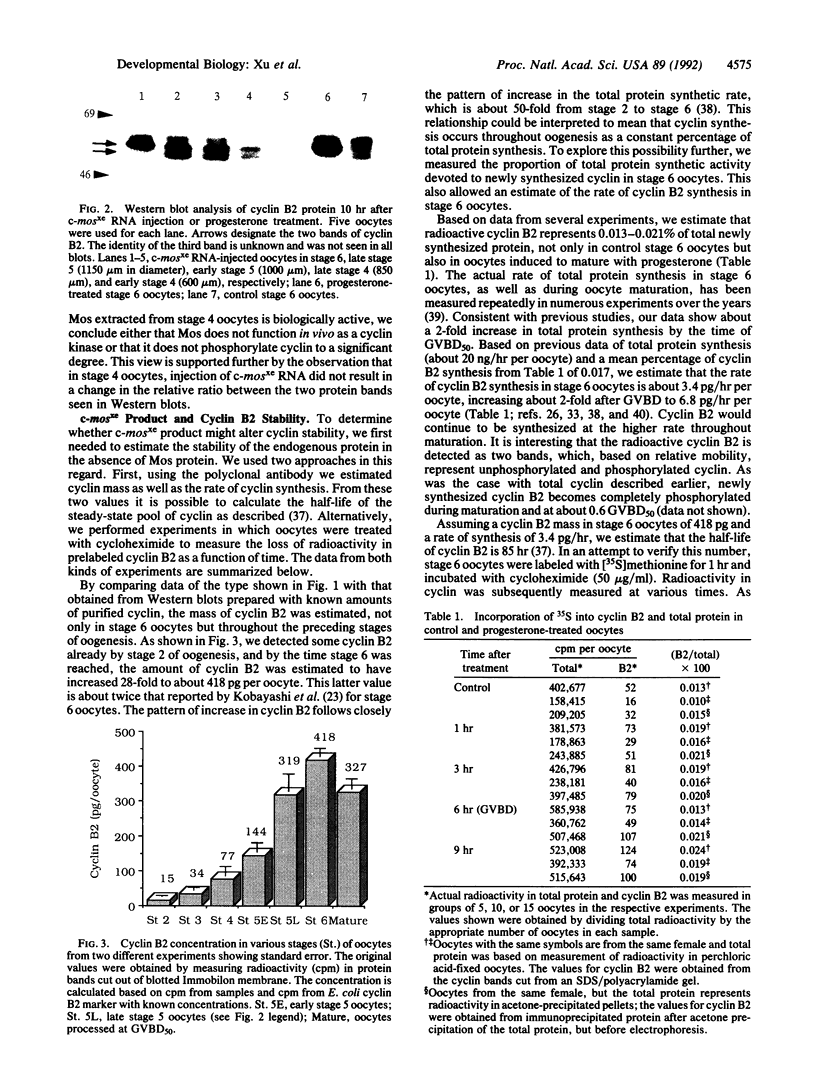
Using affinity-purified antiserum we have examined cyclin B2 levels in Xenopus oocytes at various stages of oogenesis. We found that cyclin B2 is detected from stage 2 to stage 6 as two bands, one of which is phosphorylated, and that cyclin B2 mass increases about 28-fold between stage 2 and stage 6. To examine the effect of Mos protein on cyclin phosphorylation, we microinjected synthetic Xenopus c-mos (c-mosxe) RNA into stages 4, 5, and 6 Xenopus oocytes. In stage 6 oocytes, maturation was induced by c-mosxe RNA, and, as is the case with progesterone treatment, all cyclin B2 was shifted to the phosphorylated form. However, c-mosxe RNA injected into stage 4 or 5 oocytes did not induce maturation or cause a shift in the relative proportion of the two cyclin B2 bands. These data suggest that Mos does not act directly to phosphorylate cyclin B2, causing the band shift during maturation. Cyclin B2 synthesis increases about 2-fold during maturation, in concert with total protein synthesis. Data from experiments on cyclin B2 stability indicate that the half-life of cyclin B2 is about 85 hr in stage 6. This suggests that if Mos protein has a direct effect on cyclin stability, it does so only at a later stage in oocyte maturation, but not at the onset.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cicirelli M. F., Pelech S. L., Krebs E. G. Activation of multiple protein kinases during the burst in protein phosphorylation that precedes the first meiotic cell division in Xenopus oocytes. J Biol Chem. 1988 Feb 5;263(4):2009–2019. [PubMed] [Google Scholar]
- Cyert M. S., Kirschner M. W. Regulation of MPF activity in vitro. Cell. 1988 Apr 22;53(2):185–195. doi: 10.1016/0092-8674(88)90380-7. [DOI] [PubMed] [Google Scholar]
- Daar I., Paules R. S., Vande Woude G. F. A characterization of cytostatic factor activity from Xenopus eggs and c-mos-transformed cells. J Cell Biol. 1991 Jul;114(2):329–335. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Freeman R. S., Ballantyne S. M., Donoghue D. J. Meiotic induction by Xenopus cyclin B is accelerated by coexpression with mosXe. Mol Cell Biol. 1991 Mar;11(3):1713–1717. doi: 10.1128/mcb.11.3.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. S., Pickham K. M., Kanki J. P., Lee B. A., Pena S. V., Donoghue D. J. Xenopus homolog of the mos protooncogene transforms mammalian fibroblasts and induces maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5805–5809. doi: 10.1073/pnas.86.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Maller J. L. Cyclin B in Xenopus oocytes: implications for the mechanism of pre-MPF activation. EMBO J. 1991 Jan;10(1):177–182. doi: 10.1002/j.1460-2075.1991.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Maller J. L. Phosphorylation of Xenopus cyclins B1 and B2 is not required for cell cycle transitions. Mol Cell Biol. 1991 Aug;11(8):3860–3867. doi: 10.1128/mcb.11.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Smith L. D. In vivo regulation of MPF in Xenopus oocytes. Development. 1990 May;109(1):149–156. doi: 10.1242/dev.109.1.149. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Minshull J., Ford C., Golsteyn R., Poon R., Hunt T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol. 1991 Aug;114(4):755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Hayes M. K., Maller J. L. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci U S A. 1988 May;85(9):3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L. Xenopus oocytes and the biochemistry of cell division. Biochemistry. 1990 Apr 3;29(13):3157–3166. doi: 10.1021/bi00465a001. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Minshull J., Murray A., Colman A., Hunt T. Xenopus oocyte maturation does not require new cyclin synthesis. J Cell Biol. 1991 Aug;114(4):767–772. doi: 10.1083/jcb.114.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Reynhout J. K., Smith L. D. Studies on the appearance and nature of a maturation-inducing factor in the cytoplasm of amphibian oocytes exposed to progesterone. Dev Biol. 1974 Jun;38(2):394–400. doi: 10.1016/0012-1606(74)90016-5. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Wasserman W. J., Smith L. D. The mechanism for increased protein synthesis during Xenopus oocyte maturation. Dev Biol. 1982 Jan;89(1):159–167. doi: 10.1016/0012-1606(82)90304-9. [DOI] [PubMed] [Google Scholar]
- Roy L. M., Singh B., Gautier J., Arlinghaus R. B., Nordeen S. K., Maller J. L. The cyclin B2 component of MPF is a substrate for the c-mos(xe) proto-oncogene product. Cell. 1990 Jun 1;61(5):825–831. doi: 10.1016/0092-8674(90)90192-h. [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar I., Oskarsson M., Showalter S. D., Vande Woude G. F. The product of the mos proto-oncogene as a candidate "initiator" for oocyte maturation. Science. 1989 Aug 11;245(4918):643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Cyclic AMP-mediated control of meiosis: effects of progesterone, cholera toxin, and membrane-active drugs in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):850–854. doi: 10.1073/pnas.79.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. D. The induction of oocyte maturation: transmembrane signaling events and regulation of the cell cycle. Development. 1989 Dec;107(4):685–699. doi: 10.1242/dev.107.4.685. [DOI] [PubMed] [Google Scholar]
- Taylor M. A., Smith L. D. Induction of maturation in small Xenopus laevis oocytes. Dev Biol. 1987 May;121(1):111–118. doi: 10.1016/0012-1606(87)90144-8. [DOI] [PubMed] [Google Scholar]
- Taylor M. A., Smith L. D. Quantitative changes in protein synthesis during oogenesis in Xenopus laevis. Dev Biol. 1985 Jul;110(1):230–237. doi: 10.1016/0012-1606(85)90079-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Masui Y. Effects of cyclohexamide on a cytoplasmic factor initiating meiotic naturation in Xenopus oocytes. Exp Cell Res. 1975 Mar 15;91(2):381–388. doi: 10.1016/0014-4827(75)90118-4. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Richter J. D., Smith L. D. Protein synthesis during maturation promoting factor- and progesterone-induced maturation in Xenopus oocytes. Dev Biol. 1982 Jan;89(1):152–158. doi: 10.1016/0012-1606(82)90303-7. [DOI] [PubMed] [Google Scholar]
- Webb A. C., LaMarca M. J., Smith L. D. Synthesis of mitochondrial RNA by full-grown and maturing oocytes of Rana pipiens and Xenopus laevis. Dev Biol. 1975 Jul;45(1):44–55. doi: 10.1016/0012-1606(75)90239-0. [DOI] [PubMed] [Google Scholar]