Abstract
Aflatoxins contamination of maize exhibits a serious threat to human and animal health over the past few decades. To protect the safety of food commodities, regular monitoring for afltoxins in food is necessary. In the proposed study, we have followed a rapid and sensitive biosensor approach as well as thin layer chromatography method for quantification of aflatoxins. Our data demonstrate that all the samples tested were beyond the safety level of aflatoxins as determined by Food and Drug Administration and European Union. Results of fungal mycoflora evidenced the massive presence of Aspergillus species (75 %) followed by Fusarium (11 %), Penicillium (8 %) and Trichoderma (6 %) as characterized by biochemical and sporulation properties. Use of internationally developed biosensor for detection of fungal toxin in this work is the first approach that was utilized in the developing country like Ethiopia. In the end, we conclude that fungal contaminant and there metabolites are potential threat to the agricultural industry and require urgent intervention.
Keywords: Mycotoxins, Biosensor, Cancer, Aspergillus, Thin layer chromatography
Background
Mycotoxins i.e. aflatoxins represents the class of fungal polyketide secondary metabolites which are mainly produced by two fungi viz. Aspergillus flavus and Aspergillus parasiticus (Bennett and Klich 2003). Both the fungi are reported to produce four principle kinds of aflatoxins i.e. aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2). Among these four classes of aflatoxins, AFB1 is predominant in nature and functionally carcinogenic in animal models if the toxicity exceeds threshold level (CAST 2003; Bennett and Klich 2003). The agricultural commodities that are prone to aflatoxins toxicity are corn and corn products, peanuts, cottonseed, milo, animal feed and majority of tree nuts (Beatriz et al. 2005; Binder et al. 2007). Aflatoxins toxicity has always remained a topic of debate in terms of international market as well as economic development of country which are part of trade market. To overcome these challenges many countries have set maximum acceptable levels of aflatoxins in food and food products and animal feed (Diener et al. 1987; European Commission 2006).
Previous studies proposed that the occurrence of aflatoxins in food products mainly influenced by favorable conditions such as high moisture content and temperature (Wu et al. 2011). The extent of contamination by aflatoxins also varies with different geographic location, agricultural and agronomic practices, storage condition of crops and more importantly processing of food materials under favorable temperature and humidity conditions (Chauhan et al. 2008). In many developing countries of Africa continent, aflatoxins toxicity of food have been companion with increase risk of hepatocellular carcinoma in the presence of hepatitis B virus infection (Henry et al. 1999) and esophageal cancer respectively (Wild and Turner 2002). Intensive exposures of AFB1 at a concentration in excess of 2 ppm are reported to cause non-specific liver problems and death within few days. Whereas, chronic effect of AFB1 leads to immunosuppression and nutritional deficiency (Peraica et al. 1999).
Maize as an agricultural commodity is considered as one of the best substrate for the fungi to grow and produce toxicogenesis. Many surveys across the globe showed that this crop can be highly contaminated with aflatoxins (Munkvold 2003). Ethiopia crop agriculture is very complicated, involving substantial alteration in crops cultivated around the different parts of country (Chilot et al. 1998). The maize crop is third most important crop in Ethiopia after wheat and teff and accounts for largest share in total crop production (Befekadu and Berhanu 2000). Production of maize sharply expanded from 2.5 million tons in 2003–2004 to 8 million tons in 2012–2013 (Bonger et al. 2004). Maximum quantities of maize produced are stored under poor and unsatisfactory storage conditions for considerable period of time. Traditional storage of maize in Ethiopia is like made up of mud, bamboo strips, and pits. In comparison of these storage conditions, recent technology involves storage of maize in polyethylene bags and gunny bags (Anjum et al. 2012). Previous reports proposed that extended storage of maize under unacceptable storage conditions enhances fungal growth which promotes the production of respective mycotoxins (Chauhan et al. 2008).
Despite the fact that maize is a crucial food to Ethiopian population and is vulnerable to mycotoxins risk due to different geographical and climatic conditions and poor handling of crop and storage, limited surveys have been reported on the relation of fungal mycotoxins in the crop and ways to protect the food from contamination in Ethiopia (Alemu 2008). Therefore the aim of the proposed work is to determine the fungal load of maize sample from Dilla region of Ethiopia and quantify the concentration of aflatoxins by using rapid and sensitive technique. In the present study, we used an immunochromatographic assay and thin layer chromatography assay for quantification of aflatoxin in maize samples. Thus, use of internationally developed biosensor for detection of aflatoxins in this work is the first approach that was developed in the developing countries like Ethiopia and results are discussed below.
Methods
Materials
Reveal Q+ aflatoxin test kit (Lot No. 203322, Neogen Corporation, USA) was used for quantitative analysis of aflatoxins in maize. Mycotoxin biosensor was purchased from Mobile Assay Inc., 150 Murray Street, PO Box 96, Nowot with Wireless Nexus 7 inch Tablet inbuilt with Android 4.0 operating system, GPS tracking and mReader Software for measuring the intensity of band developed on Reveal Q+ Aflatoxin Test Strips (Neogen Corporation, USA). The assay is based on single-step lateral flow immunocharomatographic principle with competitive immunoassay format (Mobile Assay Inc; Neogen Corporation, USA).
Analytical standard chemicals
Different standards of aflatoxins were obtained from Hi-Media Laboratories Ltd. Mumbai, India. Preparation of standard solution was done by referring to the Manual of Official Methods of Analysis of Association of Official Analytical Chemists (AOAC 1995). From the stock solutions of each toxin as determined by UV-Spectrophotometer (UV-1800, Shimadzu, Japan), a working standard of 25, 50, 75, 100, 125, 150, and 200 ppb for AFB1, AFB2, AFB3 and AFB4 was prepared in benzene: acetonitrile (98:2 v/v) solution. All the media components and chemicals were purchased from Hi-Media Laboratories Ltd. Mumbai, India.
Study site
The study was carried out in Dilla town of Gedeo zone located in South Nations Nationalities and Peoples Region (SNNPR) of south Ethiopia. The place is located at 86 km from regional capital Hawassa and 359 km from nation capital Addis Ababa. Five different Gedeo zones namely Dilla Zuria, Yirgachaffe, Kochere, Qisha and Wonago were visited for collection of maize samples.
Sampling
A total number of 150 different maize samples were collected from different Gedeo zones as stated above. All the samples were randomly selected from local markets, store house, flour mills, grain retailers and street corn fruit seller. Commodities samples included dry maize flour, freshly harvested corn fruits and dry maize kernels (Table 1).
Table 1.
Distribution of maize samples on the basis of location and types
| Sample matrix | Sample location | Sample code | Number of samples | Percentage of samples from total samples |
|---|---|---|---|---|
| Dry maize flour | Dilla Zuria | MS1-MS26 | 26 | 39 |
| Yirgachaffe | MS56-MS70 | 15 | 23 | |
| Kochere | MS86-MS98 | 13 | 20 | |
| Qisha | MS107-MS113 | 7 | 11 | |
| Wonago | MS136-MS139 | 4 | 6 | |
| Freshly harvested corn fruit | Dilla Zuria | MS27-MS47 | 21 | 32 |
| Yirgachaffe | MS71-MS79 | 9 | 14 | |
| Kochere | MS99-MS104 | 6 | 9 | |
| Qisha | MS114-MS124 | 11 | 17 | |
| Wonago | MS140-MS146 | 7 | 11 | |
| Dry maize kernels | Dilla Zuria | MS48-MS55 | 8 | 12 |
| Yirgachaffe | MS80-MS85 | 6 | 9 | |
| Kochere | MS105-MS106 | 2 | 3 | |
| Qisha | MS125-MS135 | 11 | 17 | |
| Wonago | MS147-MS150 | 4 | 6 |
All the samples were randomly selected from local markets, store house, flour mills, grain retailers and street corn fruit seller from different location as shown in table
Sample preparation and aflatoxin quantification
Aflatoxin quantification by using biosensor based immunochromatographic assay
The aflatoxins were extracted as per manufactures protocol (Mobile Assay Inc; Neogen Corporation, USA). Briefly, different samples were bring to laboratory and grind or paste so at least 75 % of material passes through a 20 mesh sieve, about the particle size of fine instant coffee. Aflatoxins were extracted by mixing 1 part of sample to 5 parts of 65 % ethanol (HPLC grade, HI-Media Laboratories Ltd. Mumbai, India) and were vigorously vortex for 3 min. The samples were allowed to settled and then filter with syringe filter and finally utilized for quantification of aflatoxins by using Reveal Q+ aflatoxin test strip (Neogen Corporation, USA).
Quantification of aflatoxin by using thin layer chromatography
Quantification of aflatoxin was done according to the methodology described previously (Soares and Rodriguez-Amaya 1989) by using thin layer chromatography. Briefly, 50 grams of sample were homogenized in a blender containing a solution mixture of 270 ml of methanol and 30 ml of potassium chloride for 5 min. The mixture was filtered using Whatmann filter paper. 150 ml of filtrate was transferred to a glass containing a solution mixture of 150 ml of 30 % ammonium sulfate and 50 ml of Celite. Again the mixture was filter using Whatmann filter paper. 150 ml of filtrate was transferred to a separating funnel and was filled with 150 ml of water and twice partitioned with 10 ml of chloroform. 5 ml of solution from both the chloroform partition were combined. The mixture was evaporated in a water bath at 80 °C. The extract was spotted along with working standards with the use of Autospotter on TLC plate (Silica Gel 60G, Merck). The plate was developed in an unsaturated tank containing toluene–ethyl acetate–chloroform–formic acid (70:50:50:20, v/v). The aflatoxins were visualized by the incidence of UV light. For quantification of afltoxin, known volume of samples and standards were applied to TLC plate. The plates were developed as described above in the respective solvent. All calculations were done according to the manual of to the Manual of Official Methods of Analysis of AOAC (AOAC 1995). The identity of aflatoxins was also confirmed by reaction with its derivatives i.e. trifluoroacetic acid according to Przybykski (1975).
Determination of fungal species and population
To detect the presence of fungi in maize samples fungal bioassay was done. Briefly, twenty gram of each sample was dissolved in 180 ml of sterile saline solution. One ml of above solution was aseptically spread on Potato Dextrose Agar (Hi-Media Laboratories Ltd. Mumbai, India) and plates were incubated at 30 °C for 7 days and After incubation they were identified to genus and species level according to taxonomic keys and guides available for the kingdom fungi (Pitt and Hocking 2009).
Statistical analysis
The differences in aflatoxins concentration in maize between the Gedeo zones, Ethiopia were compared by ANOVA in PAST 3.11 software (Hammer et al. 2001). P < 0.05 was considered statistically significant.
Results
Aflatoxins contamination of maize samples
All the maize samples intended for human consumption tested by us shown aflatoxins toxicity higher than those recommended by Food and Drug Administration (FDA) and European Union (EU) regulatory levels as determined by immunochromatographic assay and thin layer chromatography. Results of immunochromatographic assay reveal that mean aflatoxins concentration for all samples was observed as 53 ppb. Out of total 150 numbers of samples, 53 % (80 samples) possesses more than 50 ppb concentration of aflatoxins while, 38 % (57 samples) have the aflatoxins level of 40–50 ppb. In the remaining 9 % (13 samples), aflatoxins concentration was found to be in the range of 20–40 ppb (Table 2). Whereas, results of thin layer chromatography demonstrated 52.1 ppb as a mean aflatoxin concentration for all 150 samples tested. Among 150 samples tested, 56 % (84 samples) possesses aflatoxin concentration more than 50 ppb. While, 28 % (42 samples) showed aflatoxin concentration in the range of 40–50 ppb and 16 % (24 samples) has aflatoxin concentration in the range of 20–40 ppb (Table 3). There was no significant differences were observed in the different maize commodities as well as no correlation with different locality devoid of the two different methodologies used for quantification of aflatoxin in this study (P = 0.567). Average aflatoxins concentration for dry maize flour, corn fruit and dry maize seeds resulted in 53.89, 52.47 and 49.79 ppb respectively as determined by immunochroatographic assay (Table 2). While mean concentrations of aflatoxins for dry maize flour, corn fruit and dry maize seeds were found to be 54.86, 50.87 and 48.29 ppb as determined by thin layer chromatography (Table 3).
Table 2.
Concentrations of aflatoxins contaminated maize samples as determined by immunochromatographic assay
| Sample no. | Concentration of aflatoxins (ppb) | Sample no. | Concentration of aflatoxins (ppb) | Sample no. | Concentration of aflatoxins (ppb) |
|---|---|---|---|---|---|
| MS1 | 40.2 | MS26 | 46.8 | MS51 | 34.23 |
| MS2 | 38.6 | MS27 | 50.67 | MS52 | 44.08 |
| MS3 | 40.71 | MS28 | 33.49 | MS53 | 43.28 |
| MS4 | 43.21 | MS29 | 47.07 | MS54 | 43.59 |
| MS5 | 43.73 | MS30 | 51.78 | MS55 | 64.7 |
| MS6 | 39.94 | MS31 | 55.26 | MS56 | 50.29 |
| MS7 | 57.7 | MS32 | 60.29 | MS57 | 41.86 |
| MS8 | 56.9 | MS33 | 49.53 | MS58 | 51.62 |
| MS9 | 43.38 | MS34 | 48.13 | MS59 | 80.98 |
| MS10 | 47.35 | MS35 | 54.45 | MS60 | 79.26 |
| MS11 | 50.23 | MS36 | 63 | MS61 | 77.67 |
| MS12 | 43.48 | MS37 | 43.9 | MS62 | 88.47 |
| MS13 | 45.38 | MS38 | 43.77 | MS63 | 66.5 |
| MS14 | 32.34 | MS39 | 50.48 | MS64 | 83.23 |
| MS15 | 74.09 | MS40 | 43.04 | MS65 | 53.77 |
| MS16 | 47.11 | MS41 | 38.45 | MS66 | 66.55 |
| MS17 | 52.25 | MS42 | 41.8 | MS67 | 49.19 |
| MS18 | 53.12 | MS43 | 61.24 | MS68 | 44.14 |
| MS19 | 47.4 | MS44 | 38.14 | MS69 | 45.4 |
| MS20 | 54.84 | MS45 | 45.61 | MS70 | 51.74 |
| MS21 | 44.96 | MS46 | 43.29 | MS71 | 53.76 |
| MS22 | 50.14 | MS47 | 43.44 | MS72 | 73.49 |
| MS23 | 60.25 | MS48 | 42.6 | MS73 | 83.7 |
| MS24 | 45.48 | MS49 | 46.09 | MS74 | 43.1 |
| MS25 | 52.52 | MS50 | 53.53 | MS75 | 42.49 |
| MS76 | 45.65 | MS101 | 53.26 | MS126 | 45.12 |
| MS77 | 53.04 | MS102 | 50.35 | MS127 | 43.88 |
| MS78 | 54.64 | MS103 | 60.89 | MS128 | 45.02 |
| MS79 | 67.87 | MS104 | 59.96 | MS129 | 46.9 |
| MS80 | 64.1 | MS105 | 46.72 | MS130 | 54.6 |
| MS81 | 59.19 | MS106 | 47.93 | MS131 | 43.77 |
| MS82 | 45.33 | MS107 | 41.7 | MS132 | 91.04 |
| MS83 | 44.66 | MS108 | 61.74 | MS133 | 59.07 |
| MS84 | 43.46 | MS109 | 90.4 | MS134 | 44.67 |
| MS85 | 56.5 | MS110 | 57.47 | MS135 | 59.7 |
| MS86 | 50.94 | MS111 | 62.52 | MS136 | 81.31 |
| MS87 | 31.61 | MS112 | 53.82 | MS137 | 70.2 |
| MS88 | 43.42 | MS113 | 53.78 | MS138 | 64.51 |
| MS89 | 38.4 | MS114 | 51.79 | MS139 | 86.28 |
| MS90 | 67.22 | MS115 | 59.49 | MS140 | 91.4 |
| MS91 | 44.04 | MS116 | 53.08 | MS141 | 59.05 |
| MS92 | 91.4 | MS117 | 50.75 | MS142 | 49.35 |
| MS93 | 51.08 | MS118 | 52.38 | MS143 | 38.11 |
| MS94 | 60.9 | MS119 | 49.4 | MS144 | 33.07 |
| MS95 | 33.68 | MS120 | 59.1 | MS145 | 57.17 |
| MS96 | 43.47 | MS121 | 53.55 | MS146 | 41.21 |
| MS97 | 55.28 | MS122 | 47.91 | MS147 | 56.87 |
| MS98 | 50.42 | MS123 | 54.7 | MS148 | 28.24 |
| MS99 | 68.38 | MS124 | 47.81 | MS149 | 51.69 |
| MS100 | 66.57 | MS125 | 45.41 | MS150 | 47.49 |
Aflatoxins concentrations were quantified by using mReader Software by measuring the intensity of band developed on Reveal Q+ aflatoxin test strips. Detection limit for aflatoxins was 2–150 ppb
Table 3.
Concentrations of aflatoxins contaminated maize samples as determined by thin layer chromatography
| Sample no. | Concentration of aflatoxins (ppb) | Sample no. | Concentration of aflatoxins (ppb) | Sample no. | Concentration of aflatoxins (ppb) |
|---|---|---|---|---|---|
| MS1 | 43 | MS26 | 56 | MS51 | 35 |
| MS2 | 36 | MS27 | 51 | MS52 | 44 |
| MS3 | 51 | MS28 | 34 | MS53 | 36 |
| MS4 | 44 | MS29 | 37 | MS54 | 44 |
| MS5 | 39 | MS30 | 52 | MS55 | 65 |
| MS6 | 45 | MS31 | 51 | MS56 | 52 |
| MS7 | 57 | MS32 | 62 | MS57 | 49 |
| MS8 | 57 | MS33 | 36 | MS58 | 52 |
| MS9 | 35 | MS34 | 47 | MS59 | 78 |
| MS10 | 48 | MS35 | 48 | MS60 | 76 |
| MS11 | 52 | MS36 | 61 | MS61 | 81 |
| MS12 | 48 | MS37 | 44 | MS62 | 85 |
| MS13 | 55 | MS38 | 31 | MS63 | 62 |
| MS14 | 36 | MS39 | 51 | MS64 | 83 |
| MS15 | 75 | MS40 | 46 | MS65 | 54 |
| MS16 | 48 | MS41 | 42 | MS66 | 65 |
| MS17 | 59 | MS42 | 43 | MS67 | 43 |
| MS18 | 57 | MS43 | 65 | MS68 | 31 |
| MS19 | 37 | MS44 | 39 | MS69 | 35 |
| MS20 | 55 | MS45 | 51 | MS70 | 52 |
| MS21 | 45 | MS46 | 44 | MS71 | 54 |
| MS22 | 52 | MS47 | 35 | MS72 | 74 |
| MS23 | 63 | MS48 | 43 | MS73 | 81 |
| MS24 | 46 | MS49 | 47 | MS74 | 43 |
| MS25 | 55 | MS50 | 51 | MS75 | 31 |
| MS76 | 45 | MS101 | 51 | MS126 | 46 |
| MS77 | 53 | MS102 | 52 | MS127 | 44 |
| MS78 | 51 | MS103 | 61 | MS128 | 45 |
| MS79 | 68 | MS104 | 48 | MS129 | 48 |
| MS80 | 64 | MS105 | 45 | MS130 | 51 |
| MS81 | 59 | MS106 | 42 | MS131 | 44 |
| MS82 | 56 | MS107 | 42 | MS132 | 81 |
| MS83 | 48 | MS108 | 62 | MS133 | 52 |
| MS84 | 37 | MS109 | 91 | MS134 | 41 |
| MS85 | 51 | MS110 | 58 | MS135 | 60 |
| MS86 | 51 | MS111 | 57 | MS136 | 79 |
| MS87 | 30 | MS112 | 59 | MS137 | 75 |
| MS88 | 41 | MS113 | 56 | MS138 | 61 |
| MS89 | 32 | MS114 | 52 | MS139 | 87 |
| MS90 | 64 | MS115 | 60 | MS140 | 82 |
| MS91 | 47 | MS116 | 54 | MS141 | 60 |
| MS92 | 86 | MS117 | 51 | MS142 | 51 |
| MS93 | 54 | MS118 | 53 | MS143 | 35 |
| MS94 | 62 | MS119 | 47 | MS144 | 32 |
| MS95 | 34 | MS120 | 60 | MS145 | 51 |
| MS96 | 38 | MS121 | 57 | MS146 | 42 |
| MS97 | 56 | MS122 | 48 | MS147 | 51 |
| MS98 | 52 | MS123 | 54 | MS148 | 20 |
| MS99 | 65 | MS124 | 42 | MS149 | 58 |
| MS100 | 69 | MS125 | 41 | MS150 | 48 |
Aflatoxins concentrations were quantified by comparing with the standards developed on thin layer chromatography. Detection limit for aflatoxins was 2–200 ppb
Fungal mycoflora of different maize samples
The different load for fungal mycoflora of maize samples from Dilla region is highlighted in Fig. 1. Identification of fungal strain by standard protocol revealed that Aspergillus genus was predominant among maize samples which accounts for 75 % (113 samples). Among Aspergillus species, A. flavus accounts for 64 % (96 samples) followed by A. parasiticus with a frequency of 11 % (17 samples). Apart from Aspergillus fungi, Fusarium spp, Penicillium spp and Trichoderma spp were also isolated among various maize samples studied. Fusarium spp contamination contributed 11 % (17 samples) while, Penicillium spp and Trichoderma spp shares 8 % (12 samples) and 6 % (8 samples) respectively (Fig. 1).
Fig. 1.
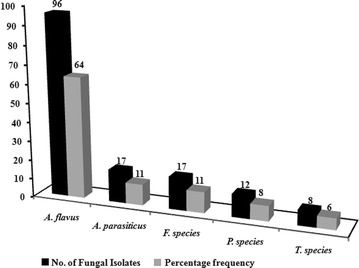
Distribution of fungal mycoflora among maize samples from Dilla region, Ethiopia. One ml of sample solution was aseptically spread on Potato Dextrose Agar and plates were incubated at 30 °C for 3–5 days. After incubation fungus were identified to genus and species level by referring standard protocol
Discussion
Aflatoxins contamination of crops possesses a serious threat to human and animal health as well as consider as danger in trade market (Bennett and Klich 2003). Among various mycotoxins produced by fungus, aflatoxins has distinct relation with maize requires serious concerns in decontamination of toxicity in many agricultural commodities (Trung et al. 2008). Even though maize in one of the most important crop than wheat and teff in Ethiopia, maize are not well studied for the toxicity generated by aflatoxins. Aflatoxins are reported to be prevalent through the west and east Africa. Some of the previous studies reported that 90 % of east African maize samples showed the evidence of high level of aflatoxins, and some parts of West Africa the exposure of aflatoxins is as high as 99 % (Doko et al. 1995; Shephard 2004; Rodrigues et al. 2011). In comparison to east and West Africa, Ethiopia has a serious problem with aflatoxins though the exact levels of exposures are uncertain due to lack of data or testing (Bernard et al. 2008). In the proposed study, all the samples come from the regions within the temperature ranges from 20 to 31 °C (Alene et al. 2000). Earlier studies demonstrated that higher temperature supports the growth of Aspergillus species (Chauhan et al. 2008). In addition to the above factor, farmers are not aware of handling of crops and storage in this part of country. They did not follow the standards for the processing of maize samples. Therefore possibilities of contamination of food commodities employed for human consumption in this region cannot be ruled out. The results of our study confirmed that all the samples utilized in this study are at a risk of contamination of aflatoxins. As shown in Tables 2 and 3, more than 50 % of samples possess aflatoxin concentration more than 50 ppb. In addition to this, the average mean concentration of aflatoxin was resulted as 53 and 52.1 ppb as determined by immunochromatograhpic assay and thin layer chromatography respectively.
Aflatoxins not only support severe health risk but also favours significant economic lost to farmers whether their crops must be rejected or accepted for buyers. For example in Kenya, two World Food Program of the United Nation purchased maize samples were confiscated and destroyed because of the lack of acceptable levels of aflatoxins in the purchased crops (Hassan et al. 1998). This is of particular concerns to smallholder farmers as aflatoxins toxicity primarily emerge out where there is high moisture content and high temperatures which is supported by inadequate storage structures. The place visited in this study fulfils all of these criteria and was confirmed by our study that aflatoxins contamination is serious challenge to smallholder farmers especially in this part of country.
Previous studies from neighbouring countries of Ethiopia like Kenya, Somalia, Uganda and Sudan demonstrate that A. flavus and A. parasiticus can invade maize seed in the field before harvest, during post harvest, drying and curing as well as during storage and transportation. Since, spores of both the species can survive for a long period of time in air and can get disseminated over a long period of distance from one place to another (Bhat et al. 1997; Gao et al. 2007). Since, Dilla town is located on the Addis Ababa-Nairobi international highway, there is potential of dissemination of spores from Kenya to Ethiopian commercial outlets as well as in maize fields. Our data confirmed the presence of Aspergillus as dominant fungal mycoflora among all which accounts for 75 % of samples followed by Fusarium (11 %), Penicillium (8 %) and Trichoderma (6 %) (Fig. 1).
The prevalence of contamination of maize sample in this study by aflatoxins is consistent with previous reports from this country (Abera and Admssu 1988; Habtamu and Kelbessa 2001) and in other countries with same climatic conditions (Shephard 2004). However within Ethiopia, a national standard has yet to be set the regulatory acceptable levels of aflatoxins. Therefore it is difficult to say that really the maize samples are acceptable or rejectable for human consumption base on our study. But in comparison to regulatory levels of aflatoxins with other countries the concentration of aflatoxins found in the samples of this study are quite higher when compared with their respective setting limits. Based on that we can recommend that maize samples analyzed in these findings correspond to heavier toxicity of aflatoxins and requires setting of safety levels for mycotoxins by respective bodies of the countries immediately.
Humans are exposed to aflatoxins mostly by consuming contaminated foods containing fungal metabolites at threshold levels. Most of the developing countries in Africa, risk of aflatoxins contamination have been companion with increase risk of hepatocellular carcinoma and esophageal cancer respectively (CAST 2003; Murugavel et al. 2007). Although there is no direct evidence still available that demonstrate that aflatoxins affected food consumption leads to cancer in Ethiopia. Therefore findings of these reports emphasize that the presence of aflatoxins at high concentration in maize samples may related to serious public health concerns and assured that fungal toxicity is a major problem in this country. Since no agricultural commodities are not directly prone to mycotoxins contamination, results of this work will guide the identification of various factors responsible for contamination and the areas where control measures requires serious intervention. Implementation of national prevention and control strategies like proper pre-harvest and pro-harvest treatment of infected maize and standard storage facilities are required to reduce the risk of aflatoxin contamination by fungi. In addition to this more studies are required from different parts of Ethiopia to generate data for Ethiopian government to work on policy making decision strategy. More importantly there is a need to find out whether aflatoxins are dominant among mycotoxins in maize or chances of contamination of other mycotoxins other than aflatoxins are prevalent. Since in our study, 25 % of mycoflora was not Aspergillus but governed by other fungal species like Fusarium, Penicillium, Trichoderma that are known to produce different kinds of mycotoxins.
Conclusions
The maize samples collected from Gedeo zone, Ethiopia were contaminated with aflatoxins. Due to the levels of aflatoxins observed in this work posses a potential threat to the agricultural industry and require urgent intervention. It is important to undertake control strategies and to distinguish the maize samples whether suitable for human consumptions and animal feed or not. These results emphasize the need for future research to reduce the occurrence of aflatoxins contamination in Ethiopian maize.
Authors’ contributions
NMC carried out all experimental work, data acquisition and analysis, literature search. He was also responsible for study concept, designing and coordinating the research and supervising the work. APW contributed to writing and manuscript preparation. TN carried our preliminary analysis and contributed to experiments. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to Dilla University Research, Dissemination and Community Services Office for supporting the financed by a Grant for the proposed work.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Nitin M. Chauhan, Phone: +251-960908760, Email: nitinchauhan25@gmail.com
Alemayehu P. Washe, Email: alemayehu.washe@gmail.com
Tesfaye Minota, Email: taltamo82@yahoo.com.
References
- Abera G, Admssu M. A survey of aflatoxin in maize, sorghum and teff samples. Ethiop J Health Dev. 1988;2:59–70. [Google Scholar]
- Alemu D. Grain markets in Ethiopia: a literature review. Addis Ababa: United Nation Wold Food Program; 2008. [Google Scholar]
- Alene AD, Poonyth D, Hassan RM. Determinants of the adoption and intensity of use of improved maize varieties in the central highlands of Ethiopia: a tobit analysis. Agrekon. 2000;39:633–643. doi: 10.1080/03031853.2000.9523679. [DOI] [Google Scholar]
- Anjum MA, Khan SH, Sahota AW, Sardar R. Assessment of aflatoxin B1 in commercial poultry feed and feed ingredients. J Anim Plant Sci. 2012;22:268–272. [Google Scholar]
- AOAC (1995) Official methods of analysis of the association of official analytical chemists, 16th edn. Method 971.22; 985.17. The Association, Arlington, Washington
- Beatriz LS, Alessandra BR, Paulo AM, Miguel MJ. Aflatoxins, ochratoxin A and zearalenone in maize-based food products. Braz J Microbiol. 2005;36:289–294. doi: 10.1590/S1517-83822005000300016. [DOI] [Google Scholar]
- Befekadu D, Berhanu N. Annual report on the Ethiopian economy. Addis Ababa: The Ethiopian Economic Association; 2000. [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard T, Alemayehu S, Gabre-Madhin E. Impact of co-operatives on smallholders’ commercialization behaviour: evidence from Ethiopia. Agric Econ. 2008;39:147–161. doi: 10.1111/j.1574-0862.2008.00324.x. [DOI] [Google Scholar]
- Bhat RV, Sherry PH, Amruth RP, Sudershan RV. A food borne disease outbreak due to the consumption of mouldy sorghum and maize containing fumonisis mycotoxins. J Toxicol. 1997;35:249–255. doi: 10.3109/15563659709001208. [DOI] [PubMed] [Google Scholar]
- Binder EM, Tan LM, Chin LJ, Hadle J, Richard J. Worldwide occurrence of mycotoxins in commodities feeds and feed ingredients. Anim Feed Sci Technol. 2007;137:265–282. doi: 10.1016/j.anifeedsci.2007.06.005. [DOI] [Google Scholar]
- Bonger T, Ayele G, Kumsa T. Agricultural extension, adoption and diffusion in Ethiopia. Research report no. 1. Addis Ababa: Ethiopian Development Research Institute; 2004. [Google Scholar]
- Chauhan Y, Wright G, Rachaputi N. Modelling climatic risks of aflatoxin contamination in maize. Aust J Exp Agric. 2008;48:358–366. doi: 10.1071/EA06101. [DOI] [Google Scholar]
- Chilot Y, Shapiro BI, Mulat D. Factors influencing adoption of new wheat technologies in Walmara and Addis Alem areas of Ethiopia. Ethiop J Agric Econ. 1998;1:63–84. [Google Scholar]
- Council for Agricultural Science and Technology (CAST) (2003) Mycotoxins: risks in plant, animal and human systems. Task Force Report No. 139, Ames, IA, USA
- Diener U, Cole R, Sanders T, Payne G, Lee L, Klich M. Epidemiology of aflatoxin formation by Aspergillus flavus. Ann Rev Phytopathol. 1987;25:249–270. doi: 10.1146/annurev.py.25.090187.001341. [DOI] [Google Scholar]
- Doko MB, Rapior S, Visconti A, Schjoth JE. Incidence and levels of fumonisin contamination in maize genotypes grown in Europe and Africa. J Agric Food Chem. 1995;43:429–434. doi: 10.1021/jf00050a032. [DOI] [Google Scholar]
- European Commission (EC) Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union. 2006;L364:5–24. [Google Scholar]
- Gao J, Liu Z, Yu J. Identification of Aspergillus section flavi in maize in northeastern China. Mycopathologia. 2007;11:91–95. doi: 10.1007/s11046-007-9029-4. [DOI] [PubMed] [Google Scholar]
- Habtamu F, Kelbessa U. Survey of aflatoxin contamination in Ethiopia. Ethiop J Health Sci. 2001;11:17–25. [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:1–9. [Google Scholar]
- Hassan RM, Onyango R, Rutto JK. Relevance of maize research in Kenya to maize production problems perceived by farmers. In: Hassan RM, editor. Maize technology development and transfer: a GIS approach to research planning in Kenya. London: CAB International; 1998. [Google Scholar]
- Henry SH, Bosch FX, Troxell TC, Bolger PM. Reducing liver cancer—global control of aflatoxin. Science. 1999;286:2453–2454. doi: 10.1126/science.286.5449.2453. [DOI] [PubMed] [Google Scholar]
- Mobile Assay Inc, Neogen Corporation, Lansing, USA. http://www.mobileassay.com
- Munkvold GP. Cultural and genetic approaches to managing mycotoxins in maize. Ann Rev Phytopathol. 2003;41:99–116. doi: 10.1146/annurev.phyto.41.052002.095510. [DOI] [PubMed] [Google Scholar]
- Murugavel KG, Naranatt PP, Shankar EM, Mathews S, Raghuram K, Rajasambandam P, Jayanthi V, Surendra R, Murali A, Srinivas U, Palaniswamy KR, Srikumari D, Thyagarajan SP. Prevalence of aflatoxin B1 in liver biopsies of proven hepatocellular carcinoma in India determined by an in-house immunoperoxidase test. J Med Microbiol. 2007;56:1455–1459. doi: 10.1099/jmm.0.47151-0. [DOI] [PubMed] [Google Scholar]
- Neogen Corporation. Mycotoxin handbook. 3rd edn. http://www.neogen.com/FoodSafety/pdf/MycotoxinHandbook_12.pdf
- Peraica M, Radic B, Lucic A, Pavlovic M. Toxic effects of mycotoxins in human health. Bull WHO. 1999;77:754–766. [PMC free article] [PubMed] [Google Scholar]
- Pitt JI, Hocking AD. Fungi and food spoilage. 3. New York: Springer; 2009. [Google Scholar]
- Przybykski W. Formation of aflatoxin derivatives on thin layer chromatographic plates. J Assoc Anal Chem. 1975;58:163–164. [PubMed] [Google Scholar]
- Rodrigues I, Handl J, Binder EM. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Add Cont Part B Surveill. 2011;4:168–179. doi: 10.1080/19393210.2011.589034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard GS. Mycotoxins worldwide: current issues in Africa. In: Barug D, van Egmond H, Lopez-Garcia R, van Ossenbruggen T, Visconti A, editors. Meeting the Mycotoxin Menace. Wageningen: Wageningen Academic Publishers; 2004. pp. 81–88. [Google Scholar]
- Soares LMV, Rodriguez-Amaya DB. Survey of aflatoxins, ochratoxin A, zearalenone, and sterigmatocystin in some Brazilian foods by using multi-toxin thin-layer chromatographic method. J Assoc Off Anal Chem. 1989;72:22–26. [PubMed] [Google Scholar]
- Trung TS, Tabuc C, Bailly S, Querin A, Guerre P, Bailly JD. Fungal mycoflora and contamination of maize from Vietnam with aflatoxin B1 and fumonisin B1. World Mycotox J. 2008;1:87–94. doi: 10.3920/WMJ2008.x010. [DOI] [Google Scholar]
- Wild CP, Turner PC. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis. 2002;17:471–481. doi: 10.1093/mutage/17.6.471. [DOI] [PubMed] [Google Scholar]
- Wu F, Bhatnagar D, Bui-Klimke T, Carbone I, Hellmich R, Munkvold G, Paul P, Payne G, Takle E. Climate change impacts on mycotoxin risks in US maize. World Mycot J. 2011;4:79–93. doi: 10.3920/WMJ2010.1246. [DOI] [Google Scholar]


