Abstract
The aim of this study was to assess the antiglycation and antioxidant properties of aqueous extract of Anethum graveolens (dill). In the in vivo and in vitro experiments, antioxidant properties, blood glucose, and AGEs formation were determined. Dill extract was given orally to healthy and diabetic rats. Our results illustrated that different concentrations of dill extract (0.125, 0.25, 0.5, and 1 mg/ml) have potential antiradical and antioxidant activity. Aqueous extract of dill significantly reduced AGEs formation and fructosamine levels, protein carbonyl and also thiol group’s oxidation, amyloid cross-β and fragmentation. After 2 months, blood glucose levels (P=0.006) and AGEs formation (P=0.003) significantly reduced in dill treated group compared with untreated diabetic animals. In conclusion, dill can be recommended as herbal medicine for the control and prevention of diabetic complications.
Keywords: Anethum graveolens, Antioxidants, Herbal medicine, Hypoglycemic agents
What’s Known
Administration of dill, whose antioxidant properties have been reported, reduces blood glucose and improves lipid profile in patients with diabetes and dyslipidemia and also in animal models of these disorders.
The exact hypoglycemic and hypolipidemic mechanisms of dill are yet to be fully elucidated.
What’s New
Dill extract inhibited AGE and fructosamine formation and also reduced protein carbonyl content and prevented thiol group oxidation in vitro and in vivo.
Different concentrations of the dill extract showed potential antiradical and antioxidant properties.
Introduction
Researchers have confirmed that foods rich in natural antioxidants play a significant role in the prevention and treatment of many disorders, particularly diabetes, cancers, and cardiovascular disease.1 In traditional medicine, Anethum graveolens has been used as an anticancer, antimicrobial, antidiabetic, anti-gastric irritation, anti-inflammatory, and antioxidant.2 Administration of dill in diabetic animals, has significantly reduced blood glucose and cholesterol levels.2 Inhibitory properties of dill extract on glycation of protein have not been reported yet. Some studies reported that aqueous or hydroalcoholic extracts of dill reduce blood glucose and shows antioxidant activity more than other fractions.1,3 The exact hypoglycemic and hypolipidemic mechanisms of dill are not clearly understood so far. The reported studies did not survey all of the antioxidant and antiradical tests for Anethum graveolens. On the other hand, differences in cultivation area of plant and the method of extraction lead to different antioxidant ability. Therefore, we were mainly interested in determining the effects of aqueous extract of cultivated dill in Hamadan, west of Iran, on protein glycation and antioxidant parameters.
Materials and Methods
Extraction of Plant Materials
Anethum graveolens leaves (dill) were purchased from Hamadan and identified by our colleague in the Department of Biology (Faculty of Sciences, Bu-Ali Sina University, Hamadan, Iran). Aqueous extract of dill was prepared according to the previously published method.4
Antioxidant and Antiradical Activity and Phytochemical Evaluation
The phytochemical evaluation, antioxidant and antiradical activity of dill extracts (0.125, 0.25, 0.5 and 1 mg/ml), butylated hydroxytoluene (BHT) and ascorbic acid as standards were determined according to the Salmanian et al. method.5
Glycation of BSA
Glycated bovine serum albumin (BSA) was prepared according to the Aramsri et al.5 method using two concentrations of fructose (200 or 500 mM) for 1, 2, 3, and 4 weeks. The fluorescence intensity of the samples was determined using Jasco FP-6200 spectrofluorometer at 340 nm and 440 nm for the excitation and emission wavelengths, respectively. Fructosamine protein carbonyl content and thiol group were determined according to the Jariyapamornkoon et al. method.6 The fragmentation of protein in the presence of dill extract and aminoguanidine was tested by SDS-PAGE.6
In Vivo Studies
Male rats with an average weight of 220-250 g were purchased from Hamadan University of Medical Sciences. Animals maintained at 12:12 h light-dark cycles at the temperature of 22±1 °C. Following one week of acclimatization in an animal house, rats were randomly divided into three groups (n=6-8). Groups 1 included normal rats, group 2 diabetic rats, and group 3 diabetic rats receiving 300 mg/kg aqueous dill extract orally. Diabetes in rats was induced according to the previously published method, using Streptozotocin.1 After 2 months, animals were anesthetized using diethyl ether, sacrificed and blood samples were collected from their hearts. This study was approved by the Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran.
Blood Glucose and AGEs Assay
Fasting blood glucose (FBS) was measured enzymatically using a commercial kit (Pars Azmoon Co., Tehran, Iran). For the determination of AGEs, the serum was diluted 1:50 with PBS. Fluorescence intensity was expressed as arbitrary units (AU).6
Statistical Analysis
The results of this study are presented as means±SEM of three replicate measurements. Statistical analyses were carried out using the SPSS statistical software package, version 16 (SPSS Inc., USA). The one-way analysis of variance (ANOVA) followed by Tukey and Dunnett’s tests was used for data analysis. P<0.05 were considered statistically significant.
Results
Antiradical and Antioxidant Activity
Dill showed strong DPPH radical scavenging activity (table 1). Dill extract also had FRAP value of 0.78-2.39 mM equivalent to FeSO4.7H2O. As demonstrated in table 1, dill extract in the concentration of 1 mg/mL showed H2O2 and O2- scavenging ability similar to those of BHT and ascorbic acid. Ferrous chelating activities, metal chelating capacity, and reducing power of dill extract and BHT are shown in table 2. Dill extract showed nitric oxide scavenging activity in a dose-dependent manner. Total phenolic, flavonols, and flavonoid compounds in dill were 163±3.8, 136±4.2, and 112±3.5 mg/g, respectively. The total alkaloids, anthocyanin, total tannin and saponin of dill were 76±2.5 mg/g, 23 mg/g and 59±4.0, 45 mg/g, respectively.
Table 1.
Feric Reducing Antioxidant power (FRAP), DPPH-, superoxide- and H2O2- scavenging activity of different concentrations of dill, BHT, and Vitamin C (in vitro)
| Concentration mg/ml | DPPH radical scavenging activity (%) | FRAP (mM) equivalent to FeSO4 7H2O | Superoxide scavenging activity (%) | H2O2 scavenging (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dill | Vit C | BHT | Dill | Vit C | Dill | Vit C | BHT | Dill | Vit C | BHT | |
| 0.125 | 64 | 79 | 81 | 1.26 | 1.57 | 58 | 69 | 65 | 59 | 70 | 63 |
| 0.250 | 67 | 81 | 78 | 1.59 | 1.75 | 64 | 71 | 69 | 68 | 81 | 66 |
| 0.500 | 70 | 82 | 85 | 1.80 | 1.90 | 83 | 87 | 83 | 84 | 84 | 80 |
| 1.0 | 86 | 94 | 93 | 2.39 | 2.65 | 96 | 95 | 92 | 96 | 95 | 95 |
BHT: butylated hydroxytoluene; Dill: Anethum graveolens
Table 2.
Metal chelating capacity, reducing power and NO scavenging activity of different concentrations of dill, BHT, and vitamin C (in vitro)
| Concentration mg/ml | Metal chelating capacity (%) | Reducing power (absorbance at 700 nm) | No scavenging (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Dill | BHT | Dill | Vit C | BHT | Dill | Vit C | BHT | |
| 0.125 | 48 | 62 | 1.09 | 1.50 | 1.35 | 56.87 | 74.16 | 72.92 |
| 0.250 | 58 | 69 | 1.17 | 1.89 | 1.68 | 69.92 | 86.24 | 83.95 |
| 0.500 | 62 | 69 | 1.52 | 2.05 | 1.86 | 74.69 | 93.91 | 94.26 |
| 1.0 | 74 | 80 | 2.00 | 2.31 | 2.08 | 96.64 | 97.53 | 96.64 |
Antiglycation Properties
After 4 weeks incubation of BSA with 200 and 500 mM fructose and dill, AGEs formation significantly reduced and fructosamine levels considerably declined (figure 1).
Figure 1.
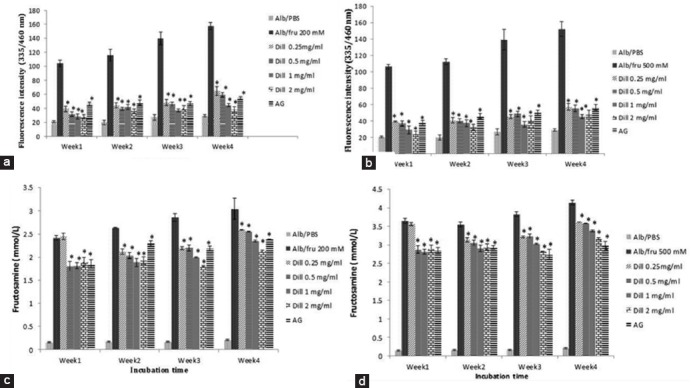
The glycation of fructose treated BSA according to AGE formation (fluorescent intensity) and fructosamine level in the presence and absence of dill extract. A: AGE in BSA/200 mM fructose; B: AGE in BSA/500 mM fructose; C: Fructosamine in BSA/200 mM fructose; D: Fructosamine in BSA/500 mM fructose; Data are expressed as the mean±SEM (n=3); *P<0.01 when compared to BSA/fructose at the same incubation time; AG: Aminoguanidine.
Protein carbonyl content and thiol group oxidation significantly inhibited by aminoguanidine (AG) and dill (figure 2). As shown in figure 3, dill extracts and AG significantly inhibited formation of amyloid cross-β structure. The fragmentation of protein was lower when samples were incubated with aminoguanidine or dill extract (figure 4).
Figure 2.
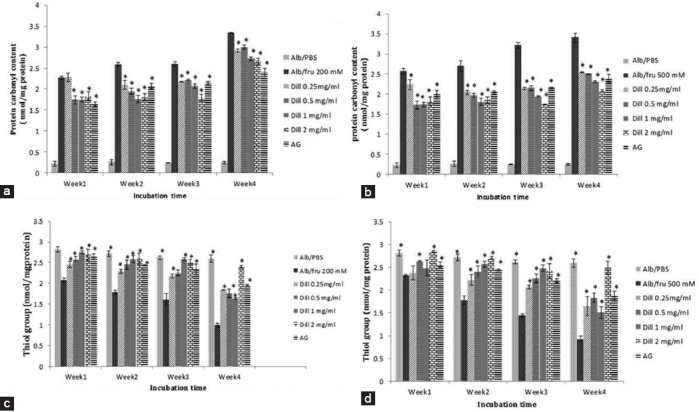
Dill extract effects on protein carbonyl content and thiol groups in fructose treated BSA. A: Carbonyl contents in BSA/200 mM fructose; B: Carbonyl contents in BSA/500 mM fructose; C: Thiol groups in BSA/200 mM fructose; D: Thiol groups in BSA/500 mM fructose; Data are expressed as the mean±SEM (n=3); *P<0.01 when compared to BSA/fructose at the same incubation time; AG: Aminoguanidine.
Figure 3.
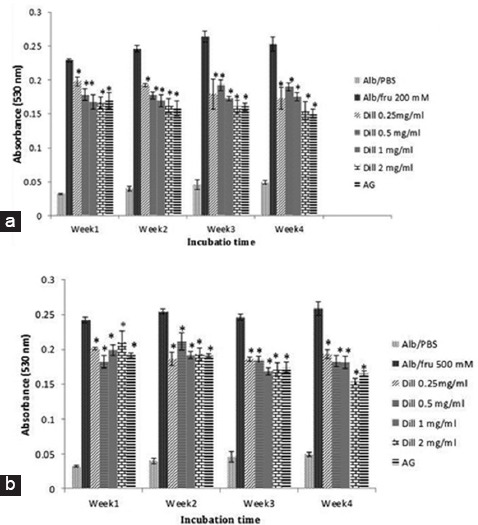
Dill extract effects on the formation of amyloid cross-β structure in BSA incubated with 200 mM (A) and 500 mM fructose (B). Data are expressed as the mean±SEM (n=3); *P<0.01 when compared to BSA/fructose at the same incubation time; AG: Aminoguanidine.
Figure 4.
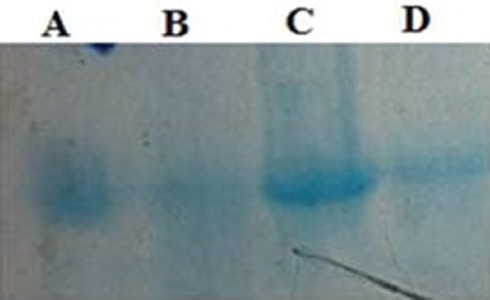
Electrophertic pattern (SDS-PAGE) shows the dill extract and aminoguanidine effects on protein fragmentation of BSA incubated with 200 mM fructose in presence of Cu+2 ion for one week. Protein fragmentation inhibited by aminoguanidine (lane C) and dill extract (lane D) compared with BSA/fructose. A-lane: 10 mg/ml BSA; B-lane: 10 mg/ml BSA+200 mM fructose; C-lane: 10 mg/ml BSA+200 mM fructose+aminoguanidine; D-lane: 10 mg/ml BSA+200 mM fructose+dill extract.
In Vivo Study
Fasting blood glucose levels markedly increased in diabetic animals compared with normal group (340±7.10 vs. 97±6.10 mg/ml, P=0.001), while significantly reduced in dill-treated group compared with untreated diabetic animals (115.33±6.38 vs. 340±7.10 mg/ml, P=0.006). After 2 months, AGEs formation significantly increased in diabetic animals compared with normal group (6.87±0.53 vs. 3.49±0.36 AGEs unit, P=0.003). Treatment with dill extract considerably declined AGEs formation compared with diabetic animals (5.37±0.37 vs. 6.87±0.53 AGEs unit, P=0.04).
Discussion
Antioxidant and Antiradical Activity
In this study, dill showed potential DPPH radical scavenging activity and had the highest FRAP value. Our findings are in agreement with Bahramikia et al.3 who reported that dill displayed radical scavenging activity. In our study, FRAP value was detected to be greater than that observed in a study by Shyu et al., which used different fractions of dill.7 Ferrous ions chelating capacity may give defense against oxidative damage by the reduction of free radical production and consequently lipid peroxidation.5 The reducing power could supply a significant antioxidant activity and consequently certain natural antioxidants are famous for their reducing activity. Donating hydrogen by dill may be responsible for its reducing power.5 Nitric oxide shows poisonous activity following reaction with oxygen and superoxide radicals.1 In a study by Orhan et al.,8 the ethanol extract of dill (leaf) had 78.49% of nitric oxide scavenging activity.
Antiglycation Properties
In the intracellular condition, the glycation rate by fructose is more rapid than glucose. Consequently, fructose and its metabolites are recognized to be significant precursors for AGEs formation. Therefore, we used fructose for BSA glycation in the in vitro experiment. In this study, AGEs formation was significantly reduced by dill extract in both in vivo and in vitro experiments. Fructosamine in clinic is used for short time monitoring of hyperglycemia in diabetic patients.9 In this experiment, dill extracts significantly reduced fructosamine levels in a dose dependent manner. The results of this study demonstrated a significant reduction in free thiol groups when albumin was incubated with fructose and this reduction was inhibited in the presence of aminoguanidine and dill extract. The ability of dill to inhibit oxidation of glycated BSA might be contributed to its anti-oxidant activity. Ramadan et al. reported that aqueous extract of Anethum graveolens had β-carotene-linoleate and DPPH free radicals scavenging activity and also normalized total antioxidant status in rat.10 Some properties such as metal chelating activity is known as one of the chief actions mediated anti-glycation property.9 In this study, dill extract markedly showed iron chelating activity. According to the results reported by Adisakwattana et al.,11 polyphenolic compounds in plant extracts may play a major role in the inhibition of AGEs formation.
In this experiment, dill extract significantly reduced protein aggregation. Increase of amyloid cross β-structures in some tissues, especially in pancreatic islets may lead to progression of amyloidosis, which directly devastates β-cell and impaired secretion of insulin.12
Sakai et al.13 reported that incubation of BSA with 200 mM fructose and Cu2+ significantly increased protein fragmentation while AG inhibited this process.
In this study, dill extract significantly reduced blood glucose. Mishra et al. reported that aqueous extract of Anethum graveolens significantly reduced blood glucose levels in diabetic mice.1 Whereas, the effect of ethanol extract showed trivial antidiabetic properties compared with aqueous extract. We showed that Anethum graveolens markedly reduced blood glucose in diabetic rat. Previous studies also showed that some natural products have useful properties such as antihypertensive and oxidative stress attenuation in diabetic rats.14-16
Incubation of glycated BSA with Cu2+ is accompanied by the decline of protein-bound glucose, showing that fragmentation happened at the expense of BSA-bound glucose. Ahmad et al. showed that aged garlic extract and S-allyl cysteine significantly inhibited formation of AGEs, fructoseamine, and also protein fragmentation.17
Since another group of AGE products is non-florescent compounds that have not been measured in this study, it can be considered as a limitation of this study.
Conclusion
According to the result of this study, dill showed potential antiglycation and antioxidant activity; therefore, it can be recommended for the prevention of diabetic complications.
Acknowledgment
The authors would like to thank Hamadan University of Medical Sciences for financial support. This article is based on the results of a PhD thesis by E. Abassi.
Conflict of Interest: None declared.
References
- 1.Mishra N. Haematological and hypoglycemic potential Anethum graveolens seeds extract in normal and diabetic Swiss albino mice. Vet World. 2013;6:502–7. doi: 10.5455/vetworld.2013.502-507. [DOI] [Google Scholar]
- 2.Jana S, Shekhawat GS. Anethum graveolens:An Indian traditional medicinal herb and spice. Pharmacogn Rev. 2010;4:179–84. doi: 10.4103/0973-7847.70915. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahramikia S, Yazdanparast R. Antioxidant and free radical scavenging activities of different fractions of Anethum graveolens leaves using in vitro models. Pharmacology online. 2008;2:233–19. [Google Scholar]
- 4.Mohammadi A, Bazrafshani MR, Oshaghi EA. Effect of garlic extract on some serum biochemical parameters and expression of npc1l1, abca1, abcg5 and abcg8 genes in the intestine of hypercholesterolemic mice. Indian J Biochem Biophys. 2013;50:500–4. [PubMed] [Google Scholar]
- 5.Salmanian S, Sadeghi Mahoonak A, Alami M, Ghorbani M. Phenolic Content, Antiradical, Antioxidant, and Antibacterial Properties of Hawthorn (Crataegus elbursensis) Seed and Pulp Extract. J Agric Sci Technol. 2014;16:343–54. [Google Scholar]
- 6.Jariyapamornkoon N, Yibchok-anun S, Adisakwattana S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med. 2013;13:171. doi: 10.1186/1472-6882-13-171. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyu YS, Lin JT, Chang YT, Chiang CJ, Yang DJ. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chemistry. 2009;115:515–21. doi: 10.1016/j.foodchem.2008.12.039. [DOI] [Google Scholar]
- 8.Orhan IE, Senol FS, Ozturk N, Celik SA, Pulur A, Kan Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem Toxicol. 2013;59:96–103. doi: 10.1016/j.fct.2013.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications:a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279–88. doi: 10.1016/S2213-8587(13)70199-2. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramadan MM, Abd-Algader NN, El-kamali HH, Ghanem KZ, Farrag ARH. Volatile compounds and antioxidant activity of the aromatic herb Anethum graveolens. JASMR. 2013;8:79. doi: 10.4103/1687-4293.123791. [DOI] [Google Scholar]
- 11.Adisakwattana S, Thilavech T, Chusak C. Mesona Chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complement Altern Med. 2014;14:130. doi: 10.1186/1472-6882-14-130. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzban L, Park K, Verchere CB. Islet amyloid polypeptide and type 2 diabetes. Exp Gerontol. 2003;38:347–51. doi: 10.1016/S0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 13.Sakai M, Oimomi M, Kasuga M. Experimental studies on the role of fructose in the development of diabetic complications. Kobe J Med Sci. 2002;48:125–36. [PubMed] [Google Scholar]
- 14.Nekooeian AA, Eftekhari MH, Adibi S, Rajaeifard A. Effects of pomegranate seed oil on insulin release in rats with type 2 diabetes. Iran J Med Sci. 2014;39:130–5. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 15.Mozafari M, Nekooeian AA, Panjeshahin MR, Zare HR. The effects of resveratrol in rats with simultaneous type 2 diabetes and renal hypertension:a study of antihypertensive mechanisms. Iran J Med Sci. 2015;40:152–60. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajizadeh MR, Eftekhar E, Zal F, Jafarian A, Mostafavi-Pour Z. Mulberry leaf extract attenuates oxidative stress-mediated testosterone depletion in streptozotocin-induced diabetic rats. Iran J Med Sci. 2014;39:123–9. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad MS, Pischetsrieder M, Ahmed N. Aged garlic extract and S-allyl cysteine prevent formation of advanced glycation endproducts. Eur J Pharmacol. 2007;561:32–8. doi: 10.1016/j.ejphar.2007.01.041. [DOI] [PubMed] [Google Scholar]


