Abstract
The left atrium is the most common location of myxomas, which are benign tumors. Only a few cases of myxomas in pregnancies have been reported. Our thorough medical literature search showed only 17 reported cases in the course of pregnancy. Myxomas during pregnancy and in the preterm period constitute a serious phenomenon that can mimic an early sign of a life-threatening pathology like severe mitral stenosis. We describe a 33-year-old woman, who presented with acute dyspnea to a gynecology center and was referred to our hospital for further evaluation of pulmonary embolism. Transthoracic echocardiography showed a huge left atrial myxoma, and computed tomography scan illustrated paradoxical pulmonary embolism in the left upper lung lobe via a large patent foramen ovale. The tumor required urgent cardiac surgery. In this article, we review causes of dyspnea in pregnancy and the cardiovascular effects of myxomas in pregnancy. We also describe the pathophysiological effects of cardiopulmonary bypass on the mother, fetus, and the feto-placental system during open-heart surgery. We performed a successful surgical resection of a myxoma in a pregnant woman. Given the rarity of such cases, individual multidisciplinary assessment and management strategies are essential.
Keywords: Cardiopulmonary bypass, Myxoma, Pregnancy complications
What’s Known
Cardiac myxomas are rare in pregnancy.
Clinical characteristics of this entity have been insufficiently elucidated.
What’s New
We reported a large left atrial cardiac myxoma and paradoxical pulmonary emboli to the upper lobe of the left lung from a patent large foramen ovale subsequently.
Introduction
Cardiac myxomas are considered an uncommon cause of peripartum dyspnea, with only 17 cases having been reported during pregnancy during the last 40 years.1 The important causes of dyspnea in pregnant women with left atrial myxomas include functional mitral stenosis, functional mitral regurgitation, peripheral thromboembolism from peripheral veins, and paradoxical embolism from a large foramen ovale. Functional mitral regurgitation and stenosis caused by left atrial myxomas is a frequent occurrence preoperatively, and damage caused by these tumors to the mitral valvular apparatus requiring mitral valve repair or replacement is a rare occurrence during pregnancy.2-4 Other possible causes of mitral regurgitation in a patient with a left atrial myxoma are tumor prolapse causing volume overload and leading to left atrial and left ventricular dilatation and annular dilatation and possible mechanical trauma to the mitral valve leaflets. The striking feature of dyspnea in our patient was the paradoxical embolization of the myxoma from a patent foramen ovale (PFO) to the pulmonary circulation. However, the myxoma caused distortion in the mitral valve (posterior annular distortion), giving rise to pulmonary hypertension and dyspnea. Myxoma-induced dyspnea in a pregnant woman with any causes (paradoxical embolism, peripheral embolism, function mitral stenosis or functional mitral regurgitation, pulmonary edema, or fluid overload) causes hypoxia, which is fatal to the fetus. Preoperative hypoxia combined with the effect of cardiopulmonary bypass (CPB) on the cardiorespiratory system during myxoma surgery is known to increase respiratory dysfunction.3 Aside from myxoma-induced dyspnea, venous thromboembolism complications, pulmonary tumor embolism (PTE), and deep venous thrombosis also occur in 1 in every 1,000 pregnancies. Nonetheless, venous thromboembolism is deemed one of the serious complications of pregnancy. The presence of Virchow’s triad in pregnancy, comprising thrombophilia, stasis, and vascular injury, may be associated with venous thromboembolism. Venous thromboembolism is regarded as an important cause of maternal death during peripartum. In contrast to our patient, all patients with venous thromboembolism have peripheral thrombophlebitis and edema and erythema. A prolonged postoperative stay in the intensive care unit (ICU) caused by the complications of CPB may be associated with stasis in peripheral veins; nevertheless, not only did our patient have no signs and symptoms of thrombophlebitis but also she had a very short ICU stay.4 The differential diagnoses of amniotic fluid embolism include pulmonary embolism; air embolism; acute myocardial infarction; sepsis; dilated or restricted cardiomyopathy; anaphylactic shock; anesthesia approach via the upper spinal cord or upper epidural block; anaphylactic reaction to local, inhaled, or intravenous anesthetic drugs or opioids; aspiration; bleeding due to placental abnormality or uterine rupture; eclampsia; seizures in pre-eclampsia; and postpartum bleeding. In the case of our patient, we ruled out all these differential diagnoses through clinical, laboratory, and imaging studies. The only important disease in the differential diagnosis of pulmonary emboli is thrombophlebitis, which was ruled out in our patient by the absence of clinical thrombophlebitis; however, both clot emboli and myxoma had the same radiographic view (i.e., cut-off in the pulmonary artery branches). Amniotic fluid embolism has different radiological signs and its symptoms typically start within 30 minutes of Cesarean section; it, therefore, has a stormy clinical course. Another extremely significant adverse effect of CPB in pregnancy is hypothermia-induced acute respiratory distress syndrome (ARDS). Hypothermia affects both respiratory function and uterine muscle function during CPB. Deep hypothermia may be associated with a greater risk of premature and sustained uterine contraction, amniotic fluid embolism, and fetal death. CPB in pregnancy causes severe placental vasoconstriction, which leads to fetal death from hypoxemia and respiratory acidosis. This response can be minimized by the administration of some drugs, high flow bypass, and normothermia. However, the development of fatal metabolic acidosis in the fetus is a serious phenomenon.5 The initial response of the fetus to CPB is marked by a decreased fetal heart rate caused by uterine contractions, placental arterial spasm, and subsequent hypoperfusion. If placental hypoperfusion continues, it may lead to severe bradycardia and dysrhythmia, which are reversible by increasing the CPB perfusion rate. Fetal heart rate monitoring is essential to allow a safe conduction of CPB with minimum damage to the fetus. The most important factor in determining the ideal prognosis of the fetus in a pregnant woman managed by CPB is related to the time of surgery. The time of surgery is one of the most challenging issues in decision-making and in the care of pregnant patients with cardiac surgery and needs to be done on the basis of presenting clinical variables. Although early cardiac surgery may be associated with reduced maternal risk, it may contribute to fetal mortality. Alternatively, delaying cardiac surgery until after delivery may result in maternal death. There are 2 different approaches in dealing with a pregnant woman who needs open-heart surgery. Normal delivery if there is enough fetal gestational age to tolerate the delivery and preoperative delivery if we predict a complicated maternal surgery with a prolonged CPB time, which may be associated with coagulopathy. If the gestational age of the fetus is <24 weeks and the risk of embryopathy is also low, the live fetus may be persevered by maintaining appropriate CPB oxygen saturation and normal maternal blood glucose levels, which are important for avoiding fetal bradycardia and arrhythmia and abortion. Other variables contributing to an increased chance of a normal delivery following cardiac surgery include controlling the fetal heart rate during CPB, adjusting the CPB flow rate, preserving appropriate mean arterial pressure, and maternal normothermia. These factors are crucial in maintaining fetal heart rates within a range of 110 and 160 bpm. If maternal condition permits, cardiac surgery should be delayed to allow the woman to have a normal delivery and minimize the risks of prematurity and fetal demise.6
Case Report
A 33-year-old woman, who was 8 months’ pregnant, was referred to our center from a gynecological center because of severe dyspnea. On her arrival at our center, she had dyspnea and pleuritic chest pain. According to obstetric sonography, the fetus was at a size consistent with an intrauterine pregnancy of 32 weeks and appeared to be normal. Transthoracic echocardiography (TTE) showed a large mass in the left atrium, causing a blockage in the mitral valve and severe stenosis and moderate regurgitation (figures 1 and 2). The PFO was not reported on TTE. Median sternotomy was performed, and CPB was established under full (3 mg/kg) systemic heparinization via the ascending aorta and bicaval cannulation. The ascending aorta was cross-clamped, and diastolic cardiac arrest was induced with antegrade cardioplegia. A nasopharyngeal temperature >34 °C and a perfusion pressure >80 mm Hg were maintained during CPB. Additional strategies were used to minimize fetal risks; the strategies included minimizing intraoperative blood loss by careful hemostasis, maintaining uterine displacement to avoid aortocaval compression by putting a roll under the left hemithorax, using normothermic CPB, minimizing CPB times, maintaining a high flow rate (>2.6 L/min/m2), and maintaining mean arterial pressure >80 mm Hg. Continuous fetal heart rate and uterine monitoring was performed using cardiotocography throughout the procedure. The large mass was removed with its adhered bases to the atrial septal defect, and the septal defect was repaired with fresh pericardium. The patient’s surgical procedures were unremarkable, and the estimated postoperative blood loss was 450mL. She was scheduled for fast tract extubation and was extubated within 2 hours after ICU arrival. After extubation, pregnancy was complicated by the premature rupture of the membranes at the 7th postoperative hour, requiring normal vaginal delivery. She developed preterm contractions at 32 weeks’ gestation, prompting us to deliver a 1,800 g baby, whose condition was stable, with Apgar scores of 5 at 1 minute and 7 at 5 minutes. The neonate survived the complications of preterm labor. Immediately after extubation, the patient experienced moderate dyspnea with pleuritic left chest pain, which resembled the preoperative signs and symptoms. Her vital sign abnormalities progressed to a more concerning state of respiratory distress as her heart rate increased to >115bpm and her respiratory rate to >26/min. A diagnosis of preoperative pulmonary embolism of the myxoma was considered, and a spiral computed tomography (CT) scan was ordered. The preliminary read of the CT scan demonstrated left-sided small pleural effusions without parenchymal airspace disease or areas of patchy consolidation in the parenchyma of both lungs, which ruled out underlying infection, hemorrhage, evolving ARDS, or progressive pulmonary edema. However, evidence of PTE was noted on the preliminary read in terms of a filling defect in the left upper lobe pulmonary artery branches. Symptom-related acute therapy based on clinical experience of PTE had good supporting evidence. The highest priority in this case of suspected PTE is to safeguard the airways using endotracheal intubation and early, sufficient oxygenation using an optimized Fi02: PEEP (positive end-expiratory pressure) ratio. Reliable prevention against aspiration was performed. Because of hypotension, the early use of vasopressors (adrenaline and dobutamine) was started in addition to crystalloid-based volume replacement. Blood was taken immediately for laboratory tests, including coagulation tests, cross-matching, and blood gas analysis. Following successful treatment, the neonate benefited from immediate delivery, and its care was optimized by the involvement of a neonatology team. Postoperatively, the neonate was in good condition, although it was preterm. Post partially, uterotonics were administered forthwith to prevent uterine atony. Over the next 6 hours, the severity of the patient’s tachycardia and tachypnea decreased (heart rate=100bpm and respiratory rate =21/min). She underwent treatment with heparin and lasix. The dyspnea was further decreased on the next day. Over the next 5 days, she was dyspneic but had a clinically stable condition. Her condition completely improved, and she was discharged with her neonate to home on the 15th day of hospitalization. The tumor was completely resected and sent for pathological examination. Microscopic analysis revealed a benign atrial myxoma.
Figure 1.
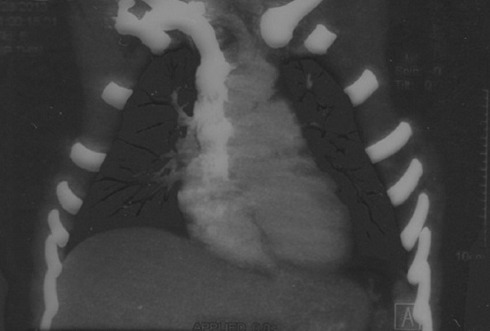
Computed tomography scan with contrast reveals the cut-off of the left upper lobe of the bronchial artery.
Figure 2.
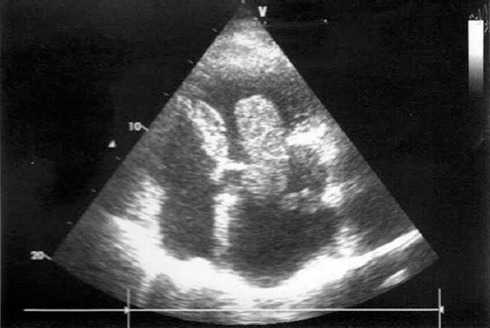
Echocardiography in the 4-chamber view shows a huge left atrial myxoma.
Discussion
It has been shown that 3% of women in the reproductive age group suffer from a variety of acquired or congenital heart diseases such as myxomas, which exhibit themselves as dyspnea. Dyspnea is a common phenomenon during pregnancy and is a result of such physiological changes in pregnancy as edema, weight gain, dilutional anemia, and sex hormones-induced hyperventilation. Differential diagnosis between pathological and physiological dyspnea in pregnancy is difficult; however, dyspnea is not as serious a problem as is hypoxia.7 The cardiac causes of hypoxia in the peripartum period include congestive heart failure from pre-existing cardiac conditions (e.g., cardiomyopathy, valvular disease, and congenital heart disease) and congestive heart failure attributable to pregnancy-induced cardiomyopathy or acute ischemic events. Pre- and postoperative TTE excludes the above-mentioned cardiac pathologies. Pulmonary causes such as pulmonary thromboembolism and amniotic fluid embolism, previously mentioned in the Introduction, also need to be considered.8 Pregnancy is associated with voluminous plasma expansion, related to reduced systemic vascular resistance and consequent renin angiotensin system stimulation. The absorption of water and sodium from renal tubules is associated with dilutional anemia. This plasma expansion with a large hemodynamic load on the cardiovascular system may not be tolerated with the additional burden of a new pathological process in pregnancy such as the presence of a myxoma. At delivery, this plasma expansion is increased further by the return of the uteroplacental blood to the maternal circulation, increased systemic vascular resistance, pain, and exclusion of the low vascular resistance of the placental system. In the presence of a left atrial myxoma, the hemodynamic changes caused by the mitral stenosis-like effect of the myxoma concomitant with the circulatory burden of pregnancy may result in cardiac decompensation and congestive heart failure and lead to the death of the mother or fetus.9 In large left atrial myxomas, both systolic and diastolic murmurs may be heard in the precordial area; this may be considered a normal murmur. In late pregnancy, the compression of the gravid uterus on the inferior vena cava in the supine decubitus position decreases venous return, reduces cardiac output, and causes dyspnea. In myxomas, sometimes mitral valve function is disturbed by the obstruction of the valve, which mimics mitral stenosis, reduces cardiac output, and exaggerates mitral stenosis. In some cases, geographical changes in the mitral valve cause regurgitation.10 Dyspnea induced by left atrial myxomas is aggravated by the fall in systemic and pulmonary vascular resistances in consequence of the blockage of the mitral valve, the effects of circulating prostaglandins and other hormones, and the low resistance of the placental circulation.11 All the adverse effects of CPB in pregnancy such as hemodilution, changes in coagulation, complement activation, release of vasoactive substances by leukocytes and particulates, air embolization, and hypotension further add to the deleterious effect of myxomas on the respiratory system and cause hypoxia and early uterine contraction.12-15
Conclusion
Myxomas and mitral stenosis have the same presentation in pregnancy. However, uncommon myxomas with pulmonary embolism should also be considered in the differential diagnosis of patients with dyspnea during pregnancy.
Acknowledgment
We extend our sincere appreciation to the staff of the departments of cardiovascular surgery in Imam Ali Hospital, without whose efforts we would not have been able to complete this project.
Ethical Approval: This work was approved by the Ethics Committee of Kermanshah University of Medical Sciences.
Conflict of Interest: None declared.
References
- 1.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–72. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock R, Evans R, Lonn E, Teoh K. Giant left atrial myxoma and associated mitral valve pathology. J Cardiothorac Vasc Anesth. 2007;21:103–5. doi: 10.1053/j.jvca.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Abbas AE, Lester SJ, Connolly H. Pregnancy and the cardiovascular system. Int J Cardiol. 2005;98:179–89. doi: 10.1016/j.ijcard.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Veldtman GR, Connolly HM, Grogan M, Ammash NM, Warnes CA. Outcomes of pregnancy in women with tetralogy of Fallot. J Am Coll Cardiol. 2004;44:174–80. doi: 10.1016/j.jacc.2003.11.067. [DOI] [PubMed] [Google Scholar]
- 5.Dodd JM, Reid K. Tocolysis for assisting delivery at caesarean section. Cochrane Database Syst Rev. 2006:CD004944. doi: 10.1002/14651858.CD004944.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Yaju Y, Nakayama T. Effectiveness and safety of ritodrine hydrochloride for the treatment of preterm labour:a systematic review. Pharmacoepidemiol Drug Saf. 2006;15:813–22. doi: 10.1002/pds.1317. [DOI] [PubMed] [Google Scholar]
- 7.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–21. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 8.Drenthen W, Pieper PG, Roos-Hesselink JW, van Lottum WA, Voors AA, Mulder BJ, et al. Outcome of pregnancy in women with congenital heart disease:a literature review. J Am Coll Cardiol. 2007;49:2303–11. doi: 10.1016/j.jacc.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113:517–24. doi: 10.1161/CIRCULATIONAHA.105.589655. [DOI] [PubMed] [Google Scholar]
- 10.John AS, Connolly HM, Schaff HV, Klarich K. Management of cardiac myxoma during pregnancy:a case series and review of the literature. Int J Cardiol. 2012;155:177–80. doi: 10.1016/j.ijcard.2011.05.069. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari E, Tozzi P, von Segesser LK. Spontaneous coronary artery dissection in a young woman:from emergency coronary artery bypass grafting to heart transplantation. Eur J Cardiothorac Surg. 2005;28:349–51. doi: 10.1016/j.ejcts.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Dob DP, Yentis SM. Practical management of the parturient with congenital heart disease. Int J Obstet Anesth. 2006;15:137–44. doi: 10.1016/j.ijoa.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita T, Huynh AT, Singh T, Hayes P, Armarego S, Seah PW. Mitral valve annular dilatation caused by left atrial myxoma. Heart Lung Circ. 2009;18:145–7. doi: 10.1016/j.hlc.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Formica F, Sangalli F, Paolini G. Unusually large left atrial myxoma causing mitral valve occlusion and hiding a severe mitral regurgitation:a case report. Heart Surg Forum. 2006;9:E849–50. doi: 10.1532/HSF98.20061077. [DOI] [PubMed] [Google Scholar]
- 15.Germing A, Lindstaedt M, Mugge A, Laczkovics A, Fritz M. Severity of mitral regurgitation may be underestimated in the presence of a left atrial myxoma. J Heart Valve Dis. 2006;15:830–2. [PubMed] [Google Scholar]


