Abstract
Three named cell types degrade and remove skeletal tissues during growth, repair, or disease: osteoclasts, chondroclasts, and septoclasts. A fourth type, unnamed and less understood, removes non-mineralized cartilage during development of secondary ossification centers. “Osteoclasts,” best known and studied, are polykaryons formed by fusion of monocyte precursors under the influence of CSF-1 (M-CSF) and RANKL. They resorb bone during growth, remodeling, repair, and disease. “Chondroclasts”, originally described as highly similar in cytological detail to osteoclasts, reside on and degrade mineralized cartilage. They may be identical to osteoclasts, since to date there are no distinguishing markers for them. Because osteoclasts also consume cartilage cores along with bone during growth, the term “chondroclast” might best be reserved for cells attached only to cartilage. “Septoclasts” are less studied and appreciated. They are mononuclear perivascular cells rich in cathepsin B. They extend a cytoplasmic projection with a ruffled membrane and degrade the last transverse septum of hypertrophic cartilage in the growth plate, permitting capillaries to bud into it. To do this, antiangiogenic signals in cartilage must give way to vascular trophic factors, mainly VEGF. The final cell type excavates cartilage canals for vascular invasion of articular cartilage during development of secondary ossification centers. The “clasts” are considered in the context of fracture repair and diseases such as arthritis and tumor metastasis. Many observations support an essential role for hypertrophic chondrocytes in recruiting septoclasts and osteoclasts/chondroclasts by supplying VEGF and RANKL. The intimate relationship between blood vessels and skeletal turnover and repair is also examined.
Keywords: growth plate, bone repair, arthritis, endochondral ossification, angiogenesis, pericyte
Introduction: Breaking rocks, the meaning of “clast”
The three known cell types that carry out degradation and removal of skeletal elements are osteoclasts, chondroclasts, and septoclasts. The combining form “-clast” in English, used in these names, comes from the Greek root klastos (κλαστός), referring to fragments of broken rock. In particular it refers to fragments derived from smashing iconic statues; hence also the English word “iconoclast.” “Osteoclast” denotes cells that resorb bone, “chondroclast,” cells that resorb cartilage, and “septoclast” denotes specialized perivascular cells, or pericytes, that remove the terminal transverse cartilage septum from growth plates. Finally, there are cells, as yet unnamed, which degrade non-mineralized articular cartilage to form secondary ossification centers in bones during development. In addition to these normal processes, catabolic cells also take part in pathological events such as inflammatory bone loss, osteoarthritis, periodontal disease and tumor metastasis. In this perspective, we consider identifying characteristics, factors that control differentiation, the intimate association of blood and bone, and how the “clasts” participate in bone repair and disease.
Endochondral ossification, converting cartilage to bone
To consider the “clasts” in their normal context, we briefly consider bone formation mechanisms. In vertebrates with mineralized skeletons, most of the initial skeleton must first be destroyed to allow it to grow. “Vertebrates,” it should be noted, are sometimes mistakenly thought to include only those classes of animals having a mineralized skeleton. The Vertebrata, however – animals with vertebrae – also include classes with cartilage skeletons, including the lampreys, sharks, and rays (e.g., see (1)). Among the vertebrate classes with mineralized skeletons – Osteicthyes, Amphibia, Reptilia, Aves, and Mammalia – the remarkable process of endochondral ossification creates most skeletal elements.
Endochondral ossification is the regulated, systematic vascular invasion of cartilage models of bones (anlagen) during development and growth, and their replacement by mineralized bone (2–4). This strategy enables bones – which, due to the rigidity of the calcified matrix cannot expand interstitially, nor bend significantly, nor swell – to bear weight during growth in length by protecting a disc of growth plate cartilage inside, where chondrocyte hypertrophy drives bone elongation. The majority of skeletal elements are built in this manner, including the limbs, digits, skull base, pelvis, and spine. The cranial vault, some facial bones, the anterior mandible, and the proximal clavicles form directly by intramembranous ossification without a cartilage intermediary, but resorption and remodeling also occur in those elements virtually from their earliest embryonic formation. Space must be created inside bones to accommodate enclosed organs and tissues, including brain, nerves, vessels, and marrow. Some of the same developmental mechanisms are also involved in the repair of fractures, and in progression of diseases such as osteoarthritis, periodontal disease, or tumor metastasis (reviewed in (4)).
Regulatory pathways which drive bone growth by controlling chondrocyte proliferation, growth, and terminal differentiation are complex and dynamic. They involve both positive and negative signaling by secreted factors including growth hormone, Wnt’s, fibroblast growth factors, parathyroid hormone-related peptide, and Indian hedgehog, along with TGF-β and members of its superfamily, the bone morphogenetic proteins (BMPs) (5–9). The interplay of these factors, plus other stimuli such as mechanical inputs and oxygen tension, coordinates the control of chondrocyte gene expression by transcription factors that include Sox9, GLI2 and 3, and Runx2 (10). The extracellular matrix, in particular heparan sulfate, has also been implicated in controlling growth cartilage regulation (11). For the purposes of this paper, the final steps of these processes, including release of vascular endothelial growth factor (VEGF) from the cartilage matrix, are the most critical for vessel incursion into cartilage at the chondroosseous junction (COJ).
From a histological viewpoint, endochondral ossification proceeds when chondrocytes in an anlage (Figure 1A) stop proliferating and switch from expressing type II collagen to type X. They then expand in height up to ten-fold in as little as 48 hours (12), and secrete matrix vesicles to mineralize the adjacent matrix (13) (Figure 1B, C). After mineralization of hypertrophic zone cartilage matrix in the center of a skeletal anlage, a bone collar forms on its surface (Figure 1C). Osteoblasts differentiate from perichondrium covering the central hypertrophic chondrocyte zone and deposit a layer of bone matrix on the surface of the hypertrophic cartilage, forming the bone collar (14). Once the bone collar forms, osteoclasts can resorb the matrix, and vessels are able to invade. In doing so, they bring all the cells and factors necessary for conversion to bone (Figure 1D). In contrast to events at later times during bone growth and development, the initial mineralization of hypertrophic cartilage does not depend on capillary invasion of the mineralized region of the anlage since it remains avascular until after the bone collar forms.
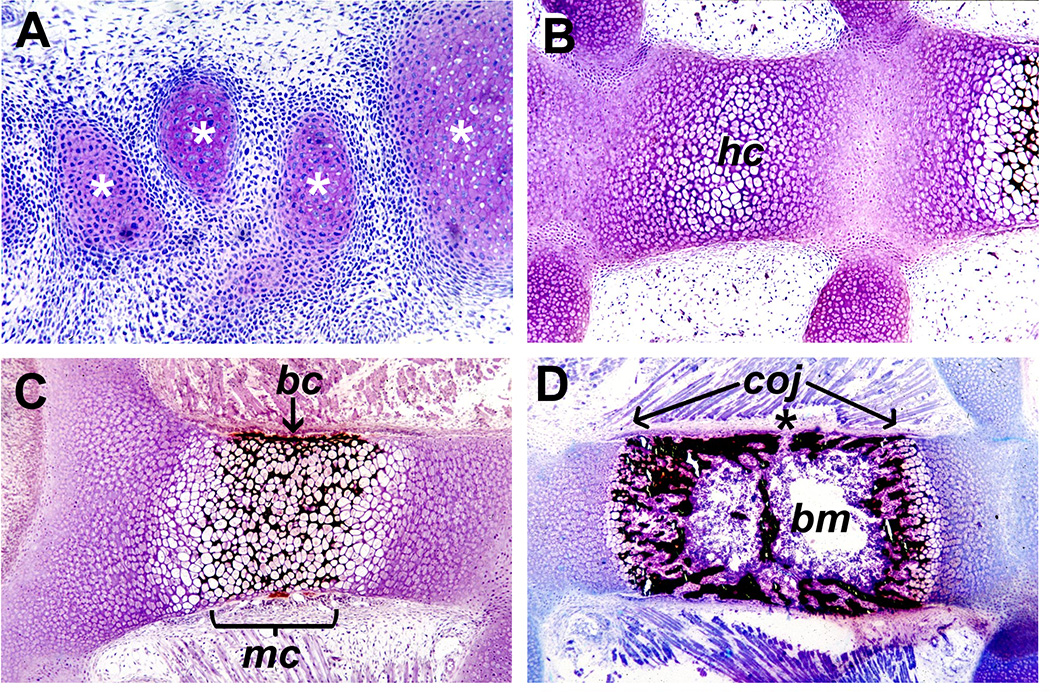
Figure 1. Steps in endochondral bone formation (non-demineralized methacrylate sections, toluidine blue and von Kossa stain, developing mouse).
Panel A shows condensations of chondrocytes (*) forming metatarsal anlagen in the paw, with pink-purple metachromasia indicating cartilage matrix being secreted. In panel B, showing sternebrae, chondrocytes in the central area have become hypertrophic (hc). In C, mineralized cartilage (mc, stained black) is seen due to the secretion of matrix vesicles containing mineral by the hypertrophic chondrocytes before blood vessel invasion. The bone collar (bc) is forming (arrow) by the action of osteoblasts differentiated from the perichondrium. In D, bone formation is taking place, with abundant, mineralized bone and a marrow cavity hosting active, hematopoietic bone marrow (bm). Through openings in the bone matrix such as the one indicated by the asterisk (*), vessels have invaded and brought with them stromal cells and mesenchymal stem cells. The position of the chondroosseous junction (coj) at either end of the growing bone is indicated by the arrows, where septoclasts are degrading matrix to permit capillaries to bud into the mineralized cartilage zone. As capillaries elongate, osteoclasts/chondroclasts follow and remove most of the mineralized cartilage. The distance between the COJs increases due to chondrocyte proliferation and hypertrophy at both ends.
Vascularization of growth and articular cartilage
The programmed cell death of hypertrophic chondrocytes at the COJ is dependent upon vascular invasion, without which they can be surprisingly persistent. To study this, Trueta and Amato (15) blocked communication between the cells of the metaphysis and the hypertrophic chondrocyte zone: they surgically placed polyethylene sheets at the COJ, between the lower limit of the growth plate and the metaphyseal bone/marrow space of rabbit proximal tibiae. They observed failure of vascularization, accompanied by a remarkable lengthening of the chondrocyte columns, with some 50 or 60 hypertrophic chondrocytes accumulating in each column over the course of the study. Mineralization of the hypertrophic cartilage ceased, but the biology of the growth plate remained intact, as shown by the restoration of normal histological appearance upon removal of the block. The failure of mineralization is interesting, implying that communication with vessels is necessary for matrix vesicle secretion by hypertrophic chondrocytes in growing bones. In some respects this is similar to rickets, in which vitamin D deficiency leads to elongation of hypomineralized growth plates. Mineralization and normal growth can be restored if vitamin D levels and mineralization are normalized soon enough to prevent deformation of the bones (16). This is in contrast to the mineralization of the hypertrophic chondrocytes in anlagen before formation of the bone collar, which occurs without vascular invasion (Figure 1C). This difference suggests that there may be a critical size limit for diffusion of necessary factors into the hypertrophic zone. It may be, for example, that oxygen tension in small anlagen is still high enough to meet a mineralization threshold, and that as bones grow, hypoxia induces a circulation-dependent state for mineralization. This could be mediated by Hif1α, for example, a transcription factor induced in the low oxygen conditions found deep within the growth plate (17). Alternatively, specific developmental signals could permit mineralization of anlagen to proceed even without vessels in intimate contact with the mineralization front.
Besides that fascinating study over 55 years ago (15), a more molecular approach to vascular invasion was undertaken in the late 1990’s. Gerber et al. (18) showed that VEGF is necessary and sufficient for vascular invasion of mouse growth plates. This was done using systemic injections of soluble VEGF receptor, mFlt(1–3)-IgG, into growing mice. The treatment blocked vascular invasion much as had the plastic sheets, and it led to a 3-to-6-fold thickening of the growth plate, a halving of limb bone growth in length, and failure to recruit TRAP-positive multinucleated cells to the mineralized cartilage trabeculae of the lower hypertrophic zone. Also strikingly similar to the 1960 study, cessation of treatment allowed recovery of vascular invasion and growth.
The production and availability of VEGF by chondrocytes is not limited to the COJ of established, growing bones. VEGF production and availability in cartilage has also been found at very early stages. Sox9, the key chondrocyte transcription factor, was found to control the production of VEGF by chondrocytes at the onset of patterning during limb bud formation. In the process of forming primitive anlagen, VEGF secretion by the differentiating chondrocytes recruited the formation of a vascular meshwork surrounding the condensation (19). Expression of VEGF (specifically, the VEGFA isoform) came in 2 waves, first to vascularize the perichondrium, then to vascularize the hypertrophic chondrocyte zone (20). Additionally, VEGF is secreted into the cartilage matrix by hypertrophic chondrocytes during bone growth and fracture repair, the latter being a process which locally recapitulates endochondral ossification. VEGF binds to heparan sulfate in the matrix, and is released by the action of osteo/chondroclast-derived heparanse (21). Whether the osteoclast itself is pro-angiogenic remains unresolved (22). Cackowski et al. (23) did show that angiogenesis in bone explants and in vivo was dependent on osteoclast number, being inhibited by blocking RANKL via OPG, and being promoted by RANKL-induced increases in osteoclast number. They also showed that the angiogenic effects were dependent on MMP9. They suggested that those effects were caused by enhanced osteoclast migration due to their expression of MMP9, although it is possible that the collagenase activity of MMP9 was required to release VEGF from the matrix.
Osteoclasts
Having considered the developmental and growth patterns affecting the “clasts,” we now look at their similarities and differences more closely. Osteoclasts, first described in 1873 ((24); see also (25)), have been investigated far more than either chondroclasts or septoclasts. They are multinucleated cells formed by the fusion of hematopoietic cells of the monocyte lineage (26–28). At this writing, a search of PubMed for articles with “osteoclast” in the title yielded some 3529 publications. As a general search term in PubMed, “osteoclast” yielded 22,327 hits. Many of the discoveries concerning their origin, differentiation, and mechanisms of bone removal have been decisively informed by studies of mutations that cause osteopetrosis in human patients and in animal models (29, 30). These and related investigations have revealed numerous steps required for osteoclast differentiation and bone resorptive activity. Some mutations in genes needed for osteoclast differentiation lead to so-called osteoclast-poor osteopetrosis, and mutations in genes needed for function lead to osteoclast-rich osteopetrosis. Such loss-of-function mutations, combined with molecular, cellular, and microscopic studies, have more recently led to greater understanding of the role of osteoclasts in providing the long-sought signals for anabolic feedback, or “coupling” (31), to osteoblastic cells for skeletal maintenance (reviewed in (32, 33)), but that is beyond the scope of this paper.
Structurally, the mature, multinucleated osteoclast is a large, highly polarized cell with an extremely convoluted plasma membrane region called the ruffled border. The ruffled border is closely apposed to the bone surface and is the site of secretion of HCl and proteases – predominantly cathepsin K and MMP9 – to degrade the mineralized matrix, as well as for endocytosis of the dissolved bone. A sealing zone surrounds the ruffled border and consists largely of a thick ring of filamentous actin. The vitronectin receptor, αvβ3 integrin, provides attachment to the bone substrate. Extremely active vesicle transport is essential for delivery of the acidification pumps and channels to the ruffled border, for exocytosis of proteases, and for endocytosis and subsequent transcytosis and secretion of solubilized bone matrix (34, 35).
Osteoclast differentiation from precursor cells is stimulated by locally high concentrations of first, colony stimulating factor 1 (CSF-1; M-CSF), signaling through its receptor c-fms (36–40). This stimulates expression of the TNF receptor superfamily member, receptor activator of NF-κB (RANK), which can then bind its ligand, the TNF superfamily member RANK ligand (RANKL) (41, 42). Normally, both CSF-1 and RANKL are provided in the bone microenvironment by osteoblasts, which also supply an antagonist to RANKL, osteoprotegerin (OPG). OPG acts as a soluble “decoy” receptor to lower the effective local level of RANKL (43, 44). The dynamic balance of RANKL and OPG is a critical regulator not only of bone development, but also of the timing of tooth eruption (45).
Besides these growth factors and their signaling pathways, there are essential transcription, signaling, and effector proteins necessary for osteoclast differentiation and activity which have been reviewed in some detail elsewhere (29, 30, 46, 47). Among the transcription factors are c-fos, PU.1, NFATc1, Mitf, and NF-κB. There are also many signaling proteins and effector proteins needed by osteoclasts for such tasks as vesicle trafficking, extracellular matrix breakdown, and acidification, including Snx10, c-Src, vATPase, TRAF6, ClC7, cathepsin K, carbonic anhydrase II, and plekhm1.
Chondroclasts
Despite common usage in discussions among bone biologists and its fairly frequent mention in the literature, the term “chondroclast” has not been consistently or rigorously defined. It is generally used in reference to cells that look like osteoclasts but which seem to be digesting cartilage, not bone. “Chondroclast” is not present in classic histology texts, including, for example, the Piersol 1930 English translation of the 4th German edition of the Sobotta atlas (48), nor 56 years later in the influential Bloom and Fawcett text (49). A more recent histology text does give chondroclast a single mention (50). Searching PubMed for articles with “chondroclast” in the title yielded only two. One examined the loss of multinucleated cells resorbing mineralized cartilage in growth plates of ovariectomized rats and their restoration by estrogen treatment (51). The other also looked at multinucleated cell formation in cartilaginous bone models, and it proposed that chondroitin sulfate in the extracellular matrix might have an inhibitory effect on their differentiation (52). As a general PubMed search term, “chondroclast” yielded 19 citations. The first occurrences of “chondroclast” in the literature that can be searched electronically are in papers by Savostin-Asling and Asling (53, 54). They showed light, transmission and scanning electron microscopic studies of calcified cartilage resorption. Specifically, they looked at the central region of Meckel’s cartilage in embryonic day 19 rat fetuses and describe their findings as follows:
“Chondroclasts (multinucleated cells identical with osteoclasts) dominate the erosion front.” (53)
“The many features which they showed in common with osteoclasts included abundant mitochondria, vacuolation, lysosomes, sparsity of rough-surfaced endoplasmic reticulum, and deep infoldings at loci of contact with calcified matrix. Crumbling of matrix (with mineral crystals penetrating between these foldings) and fragmentation of collagen fibrils were also seen.” (54)
Note that, in contrast to the termini of Meckel’s cartilage, which contribute to the mandible and auditory apparatus, the central portion under study in those papers is simply removed (55). Thus, from their earliest description, chondroclasts were seen as essentially identical to osteoclasts with the exception of their location on mineralized cartilage, as opposed to bone.
Underscoring the similarity, if not identity, of osteoclasts and chondroclasts are the observations that: 1) human osteoclasts differentiated in vitro from peripheral blood monocytes were able to resorb cartilage explants, an activity also seen in human osteo- and rheumatoid arthritis; and 2) there is very strong similarity in gene expression profiles of chondroclasts and osteoclasts (56). There have been reports of relatively minor differences between osteoclasts and chondroclasts. A comparative analysis of osteoclasts vs. chondroclasts along the COJ of growth plates showed poorly developed ruffled borders and clear zones (actin ring) in chondroclasts, along with higher intracellular TRAP and lower extracellular TRAP in chondroclasts (57).
The latter suggests that the substrate to which the cell attaches may affect its structure and perhaps function, but it is at odds with the original description of chondrocytes on calcified Meckel’s cartilage, where well-developed ruffled borders were present (54). Alternatively, it is possible, perhaps even likely, that the chondroclasts at the COJ are in quite an early stage of differentiation and activity, which could render the ruffled border, the actin ring, and TRAP exocytosis all immature. There is a report that chondroclasts in mouse embryos degrading hypertrophic Meckel’s cartilage express more MMP-13 than do osteoclasts on trabecular bone of the femur (58), but beyond this, there is little strong evidence for differences between osteoclasts and chondroclasts in: cellular origin; most cytological details; the factors required for their differentiation; or the repertoire of expressed genes needed for function. Mutations that eliminate osteoclasts, thus causing osteoclast-poor osteopetrosis, also lead to loss of chondroclasts at the chondroosseous junction (COJ) and to growth plate dysplasias (8). This provides some evidence that osteoclasts and chondroclasts may be identical, excepting only their substrate.
Septoclasts
“Septoclast” is the term coined by Lee and co-workers for a specialized cell that breaks down the last transverse cartilage septum in columns of growth plate chondrocytes (59). Note that, unlike the longitudinal septa, the transverse septa are generally not mineralized in hypertrophic cartilage. For septoclasts, as for chondroclasts, there were only two titular citations in PubMed; moreover, using septoclast as a general search term yielded only the same two (if one ignores the attempt by the PubMed search engine to substitute “septoplast”!). One is the original report describing the septoclast. The other is from our laboratory, in which we examined septoclasts at the chondroosseous junction of the proximal tibia of wild type and osteoclast-deficient osteopetrotic rats with and without osteoclastogenic treatments (60).
In the original report, Lee and co-workers examined active cathepsin B by immunostaining rat tibia COJ’s. They also looked at protein synthesis, and they carefully examined TEM images to characterize this unusual cell. The septoclast is a perivascular cell adjacent to the tip of budding capillaries (Figure 2). They are elongated, somewhat similar in appearance to fibroblasts, but are very rich in the lysosomal cysteine protease cathepsin B (Figure 3). They are polarized, with a basal nucleus and cytoplasm that extends toward the terminal transverse septum of the growth plate. A ruffled membrane protrudes from the cytoplasm and closely approaches that septum. Cathepsin B is present in septoclasts in multivesicular bodies and dense bodies which are tightly packed in the cytoplasm (59). Septoclasts were markedly more active in protein synthesis than their immediate neighbors. The cartilage of the adjacent septum in the growth plate appeared partially degraded in the area of the septoclast ruffled membrane. The authors proposed that the septoclast is responsible for removal of the terminal septum, thereby permitting capillary invasion of the growth plate, contact with the hypertrophic cell, and release of contents of the chondrocyte lacuna.
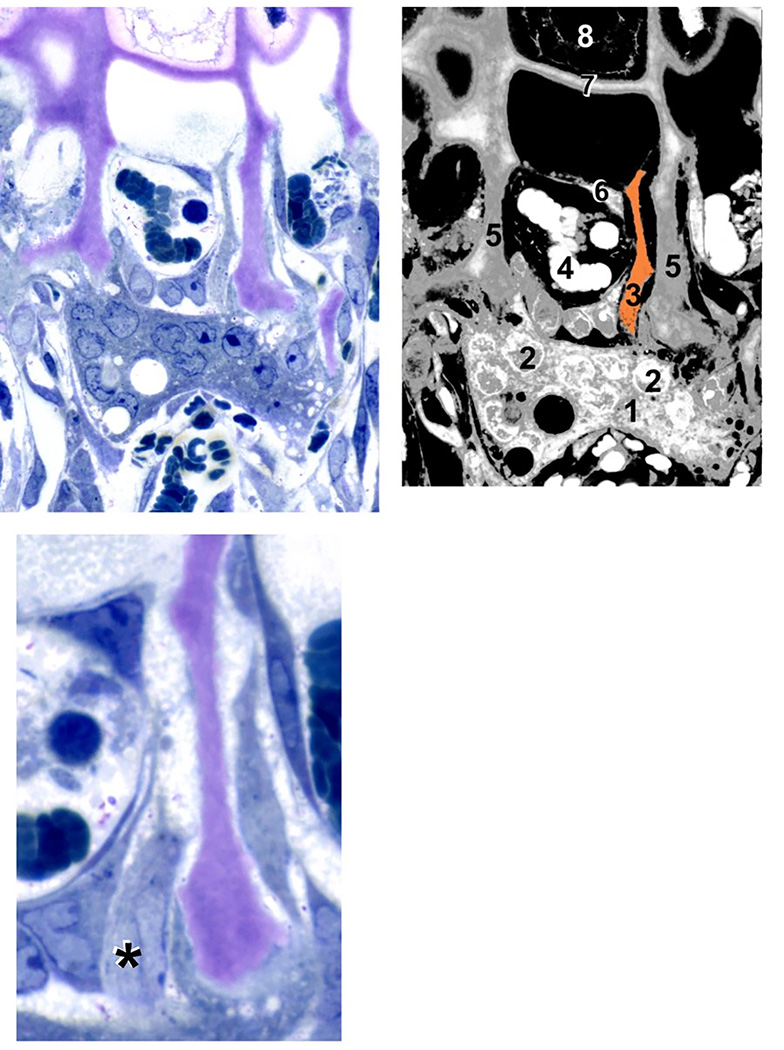
Figure 2. Septoclast in the context of the chondroosseous junction.
(A) Light micrograph of a 4-week-old wild-type rat proximal tibia, demineralized, stained with toluidine blue, 1 µm Epon section. (B) Diagram of (A) with various cellular and structural constituents numbered: 1, osteo/chondroclast; 2, some of the multiple nuclei in that cell; 3, septoclast cell body and cytoplasmic extension, pseudo-colored light orange; 4, red blood cells in budding capillary; 5, longitudinal septa of the hypertrophic cartilage zone (cartilage matrix is stained purple due to toluidine blue metachromasia); 6, endothelial cell of the capillary; 7, terminal growth plate transverse septum; 8, a hypertrophic chondrocyte in the terminal chondrocyte lacuna. (C) Serial section to (A) at higher magnification in which the septoclast cell body and nucleus are visible, but the cytoplasmic extension is out of the plane. Asterisk (*) is adjacent to the nucleus. Note that the septoclast and its capillary precede the front of osteoclasts/chondroclasts into the growth plate. Scale bar: 25 µm (A and B); 13 µm (C) (reprinted with permission from Gartland et al., 2009).
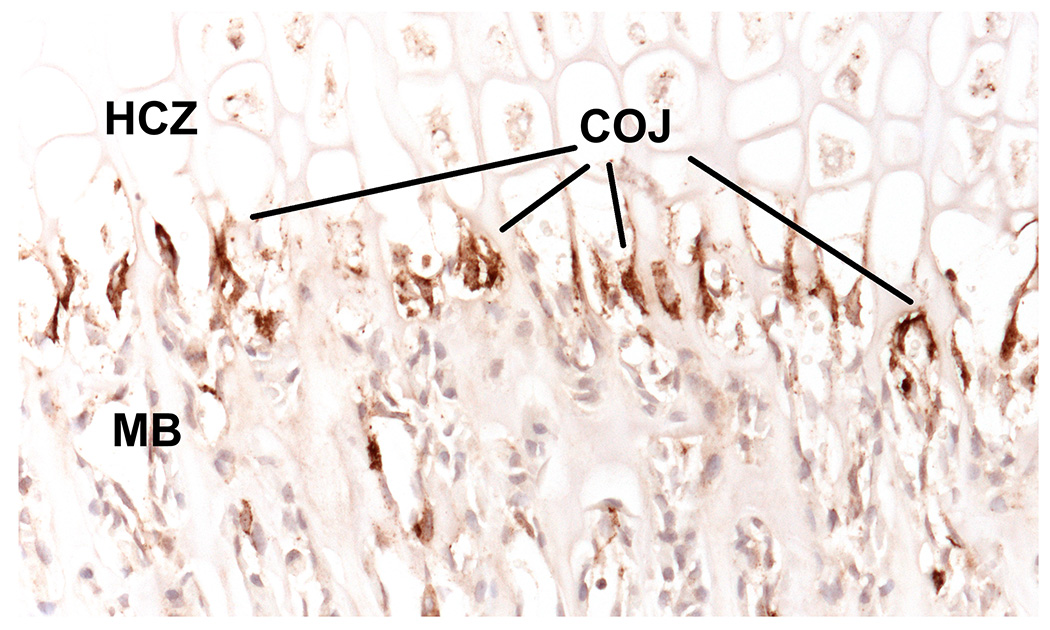
Figure 3. Septoclasts stain intensely for cathepsin B.
5 µm paraffin section of 2-week-old wild type rat proximal tibia immunostained for cathepsin B (brown) with light hematoxylin counterstain. Cathepsin B intensely labels the septoclasts, which are evident all along the chondroosseous junction (COJ), between the hypertrophic chondrocyte zone (HCZ) and the metaphyseal bone (MB). See (60) for methodological details.
Perivascular cells, or pericytes, are known to include a subset which are mesenchymal stem cells capable of differentiating into osteoblasts, chondrocytes, myocytes, and adipocytes (61, 62). In addition, other stem cells inhabit the perivascular space, including hematopoietic stem cells and neuro-progenitors (63). Overall, the septoclast, compared with other perivascular cells, remains understudied and poorly understood. No cellular origin of the septoclast among the known subsets of pericytes has yet been identified.
Growth plate dysplasias in osteoclast-poor models
The toothless (tl) rat mutation is a CSF-1 loss-of-function that causes severe osteoclast-poor osteopetrosis in homozygous mutants (36, 37, 64). Similar to other mutations in that class, growth is stunted, and growth plate cartilage is not removed, nor mineralized, nor invaded by vessels, and it accumulates over the post-natal period (8, 65–68). The putative role of septoclasts in vascularization and removal of growth plate cartilage led us to hypothesize that septoclast abnormalities would underlie that aspect of the observed histopathology at the COJ of tl/tl rats. Indeed, deficiency and misdirection of septoclasts was observed, suggesting a relationship between septoclasts, capillary invasion of growth cartilage, and osteoclast differentiation factors (60). Also of note, while systemic (intraperitoneal) injections of CSF-1 in tl/tl rats from birth restored osteoclast populations, bone resorption, removal of metaphyseal trabecular bone, and tooth eruption (69–71), even up to six weeks of daily injections from birth did not restore vascular invasion, mineralization, or greater order of chondrocytes in the growth plate (67). Septoclasts remained fewer in number and disorganized despite CSF-1 injections (60). Another rat model, osteopetrosis (op – n.b., not to be confused with the op mouse, which is CSF-1 deficient and osteoclast-poor) is a recessive allele at an unknown locus on chromosome 10 (72, 73) which causes bone disease very similar in severity to that seen in the tl rat (74); however the op/op rat has abundant, but non-functioning osteoclasts (osteoclast-rich osteopetrosis), and its growth plates are not abnormally thickened over time (8).
Similar pathology is seen in many mouse osteopetrotic mutations. Most osteoclast-rich osteopetrotic mice have normal-looking growth plates (e.g., cathepsin K, c-src, and ClC7), while osteoclast-poor mutants (e.g., CSF-1, c-fms, RANK, RANKL, or TRAF-6 mutations) show failure of capillary invasion of the COJ and resultant accumulation of growth plate cartilage (8, 60, 75, 76). Together, these observations imply that a trophic factor or factors required for capillary invasion, specifically for recruitment of active septoclasts to the site, depend on the same CSF-1/RANKL pathway needed for osteoclastogenesis. The thickened growth plates in those mutants are reminiscent of the growth plates isolated by polyethylene from capillaries (15) or by elimination of active VEGF from the area (18), as described above. Further, they imply that the chondrocytes themselves are the source of those signals. The most likely factor is of course VEGF. Since osteoclasts are present in the immediate vicinity of the growth plate in CSF-1 injected tl/tl rats and in RANKL knockout mice carrying a lymphocyte-specific rescue allele (67, 76), but there is no establishment of vascularization of the growth plate, clearly osteoclasts themselves are not sufficient to recruit vessels. It may be that CSF-1 and/or RANKL regulate VEGF expression by growth plate chondrocytes in an autocrine or paracrine manner. Indeed, RANKL expression has been detected in hypertrophic chondrocytes in normal and arthritic chondrocytes (77, 78). Although it has not been directly demonstrated that hypertrophic chondrocytes also supply CSF-1 at the COJ, it seems likely when one takes together the above-noted failure of systemic treatment or rescue, and the ability of hypertrophic cartilage implanted into renal capsules to support osteoclast differentiation (79). Osteoclasts might also play an auxiliary signaling role in that process, perhaps analogous to their role in stimulating bone formation, as has been shown by the increased rates of bone synthesis in osteoclast-rich osteopetrosis compared with the osteoclast poor types (reviewed in (80)). A mouse or rat with a chondrocyte-specific deletion of CSF-1, or rescue of tl rats or op mice (also CSF-1-deficient) with chondrocyte-specific expression are two means to test that idea.
Cartilage canals: no known “clasts”
In addition to septoclasts at the COJ, a further potential role for pericytes in cartilage is vascular invasion of non-mineralized articular cartilage. Secondary ossification centers form in epiphyses following the excavation of cartilage canals into the outer surface of epiphyseal cartilage. To accomplish this invasion, the physiological challenge is for capillaries to invade non-mineralized cartilage despite its active defenses against vascularization. In fact, due to its distinctive avascular nature, cartilage was correctly envisioned by Folkman and co-workers as a source of angiogenesis inhibitors to fight cancer progression. They had succeeded in demonstrating anti-angiogenic properties of cartilage explants by 1975 (81) and in isolating the first anti-angiogenic substance from cartilage by 1976 (82). Among the factors that repress vascularization of cartilage, TGF-β is the best-studied (reviewed in (6)). It is synthesized by chondrocytes and promotes their proliferation and the synthesis of cartilage matrix components, including aggrecan and type II collagen. TGF-β suppresses hypertrophy of chondrocytes and mineralization of the matrix. It has also been shown to stimulate production of protease inhibitors by chondrocytes (5, 9, 83). Mechanisms by which the vascularization of epiphyseal cartilage is regulated are not well understood, although they have potential significance in pathological cartilage loss, for example in osteoarthritis (84).
In human development, capillary invasion of non-mineralized joint cartilage occurs in the femur in two phases during mid-gestation, from about week 8 through weeks 15–16. Interestingly, the canals appear to be cut by small clusters of fibroblastoid mesenchymal cells without any accompanying vessel formation (85). The canals then remain devoid of cells for several weeks before vessels and perivascular tissues eventually form within them. Thus, this process is fundamentally different from what occurs on the diaphyseal side of the growth plate.
In rats, cartilage canal formation in the proximal tibia occurs through approximately the first 3 postnatal weeks, and it was studied in some detail by Lee and co-workers, in particular with respect to the degradative enzymes actively breaking down the cartilage (86, 87). They concluded that both matrix metalloproteinases and aggrecanases were active in degrading aggrecan, and that MMP9 and MMP13 (collagenase 3) then further broke down the partially digested “pre-resorptive layer.” No osteoclasts, chondroclasts, nor other specific cell types were noted to be responsible for this process, and, unlike in the human femur described above, vasculogenesis occurred concomitantly with canal excavation. Endothelial cells were observed in intimate contact with the degrading cartilage surface. In mice, the cells of the canals themselves appear to give rise to osteoblasts (88, 89), but whether this occurs in human cartilage canal/secondary ossification center formation remains to be determined. Osterix-driven excision of the IGF1 receptor resulted in mice with a slight delay and reduction in canal formation, suggesting a possible role for IGF1 from osteoblasts in the process (90). The cells causing canal formation appeared to originate as perichondrial cells, but are otherwise not characterized.
Repair and disease
We will now look briefly at the role of the “clasts” in removal of skeletal tissue in repair and disease. As in development and growth, these take place in close association with blood or marrow. Remodeling and repair of bone go on throughout life, with roughly 10% of the skeleton replaced annually in adult humans. It occurs both on bone surfaces and within bone in close proximity either to the (vascularized) extracellular milieu, to the marrow, or adjacent to vessels in Haversian canals. There is no cartilage intermediary in normal bone turnover. In remodeling of the mature skeleton, there are by definition no chondroclasts because the basic multicellular unit removes bone that does not possess cartilage cores. The recruitment of osteoclasts to remodeling sites is likely mediated by osteocyte-derived signals, giving rise to high local concentrations of the canonical CSF-1/RANKL growth factors (32). Due to the purely osseous matrix being degraded, the catabolic cells at these sites are unequivocally osteoclasts.
In fracture repair the cell type name is not so clear-cut, given the comparative disorganization of the callus, with mineralized cartilage and bone matrix often intimately intertwined. Fractures can be repaired by both endochondral and intramembranous mechanisms. The degree of endochondral ossification reflects the relative openness of the fracture site, in particular how tightly bone segments are re-connected and/or pinned (reviewed in (91)). At the site of injury, a hematoma forms and is soon followed by an inflammatory response. This in turn leads either to direct bone deposition in the form of intramembranous ossification, or to the recruitment of periosteal cells which migrate in, proliferate, and adopt a chondrocyte phenotype. The chondrocytes secrete a cartilaginous fracture callus that bridges the bone parts and also occupies a significant volume surrounding the injury site. Unlike in mature cartilage, the matrix fibers are disorganized. The chondrocytes undergo hypertrophy, switching from type II to type X collagen expression, and mineralize the callus. At various sites throughout the callus, vessels invade and the cartilage is replaced by bone. The bony callus generally exceeds the normal perimeter of the bone, most notably when the fracture is poorly reduced and/or not pinned. Over time, the excess bone is removed to restore the normal bone contours. The importance of the RANKL antagonist OPG, and the agonist, RANKL, in fracture repair were demonstrated in OPG −/− mice and derived cells (79). OPG −/− mice had faster fracture unions, the callus was resorbed faster, and greater numbers of osteo/chondroclasts were present at bone/cartilage interfaces than in wild type mice. Also, cultured OPG −/− chondrocytes were able to induce greater numbers of osteo/chondroclasts than wild type cells when transplanted under renal capsules. This confirms the ability of chondrocytes to recruit osteo/chondroclast differentiation directly, and not simply passively as an accompaniment to vessel recruitment.
A key role for angiogenesis in fracture repair was also recently shown. Cilostazol, a phosphodiesterase-3 inhibitor, is a promotor of angiogenesis. Mice given cilostazol had significant improvements in fracture healing as measured by histology, gene expression, and mechanical testing (92). Other observations showed that capillary tips that sprouted into fracture calluses lacked a basement membrane but did have an extracellular accumulation of laminin, and they were accompanied by perivascular cells. Those cells may be septoclasts or a related cell type (93); however, immunostained calluses in displaced fractures in mice showed no strong evidence of intense cathepsin B-positive staining of perivascular cells (94). This leaves unresolved the question of whether there are septoclasts or an equivalent cell type that mediate vascular invasion of mineralized cartilage during fracture repair.
In osteoarthritis, mechanical damage to joints can induce stable, articular cartilage to undergo hypertrophic differentiation and expression of terminal hypertrophic markers, including MMP 13. MMP 13 is a collagenase directly involved in initial cartilage degradation. In fact, overexpression of MMP13 increased osteoarthritis in mice, whereas chondrocyte-specific knockout was protective (4). Osteo/chondroclasts are seen at sites of bone loss in osteoarthritis, notably in subchondral bone, where bone loss can destabilize joints (reviewed in (95). Similarly, in rheumatoid arthritis, RANKL in the synovial fluid can drive osteoclast differentiation and resulting bone loss (96). We have not seen reports of differences in cytology, gene expression, or other features when comparing arthritic osteo/chondroclasts to those present in normal bone modeling or remodeling.
The recruitment and activation of osteoclasts to attack bone and provide a niche for tumor metastasis is an area of intense, long-standing, and ongoing study. Recent reviews have examined roles for micro-RNAs in this process (61, 97). Many therapeutic strategies for management of bone metastasis target osteoclasts with anti-resorptive drugs, including bisphosphonates and anti-RANKL antibody (denosumab), e.g., (98–102). There are some striking similarities between bone loss in arthritis and the invasion of bone by metastatic tumors. Analogous to its role in arthritic progression, MMP13 was shown to be expressed by breast cancer cells and to permit osteoclast differentiation (103), paving the way for osteoclasts to remove bone. The ability of chondrosarcoma to invade bone was recently shown to be dependent on osteoclasts, as osteoclast-poor tl/tl osteopetrotic rats were resistant to bone metastasis of chondrosarcoma (104), reminiscent of the resistance of tl/tl rat growth plates to vascular invasion and degradation. A role for epigenetic regulation of chromatin architecture in controlling osteosarcoma invasion of bone was recently demonstrated. Bromodomains are protein motifs that act as “readers” of chromatin architecture. Bromodomain-containing proteins bind to acetylated histones, and influence chromatin structure and gene expression (105–107). Using the bromodomain protein inhibitor JQ1, Lameroux et al. were able to block both the bone-forming sarcoma progression and the osteoclast-dependent local bone loss necessary for engraftment (108). In summary, metastatic invasion of bone by carcinomas and sarcomas appears to depend on osteoclasts, which are to all appearances normal.
Conclusion
An overview of the “clasts” at the COJ is depicted in Figure 4. Note that the chondroclasts and osteoclasts follow the capillary front, they do not lead it. The term osteoclast is well-understood, non-controversial, and those cells remain under active investigation in health and disease. The term chondroclast is less rigorously defined, and the cell it names may be identical with the osteoclast, save only for the substrate. The term septoclast is very specifically applied to an under-appreciated cell type that assists in the capillary invasion of growth cartilage and is strikingly rich in cathepsin B. All three are directly or indirectly dependent on the canonical CSF-1/RANKL pathway. All three are closely associated with blood vessels, and are VEGF-dependent in many anatomical locations. Since all three degrade the skeletal matrix, they remain important objects of study. The fourth cell type excavates cartilage canals in non-mineralized epiphyseal cartilage, and it may hold clues to some degenerative joint diseases. If and when such a link is found, those cells will undoubtedly receive a name. As to the others, our preference is that the term chondroclast, unless and until strong cellular distinctions are identified, be reserved for cells attached only to cartilage. You are what you eat.
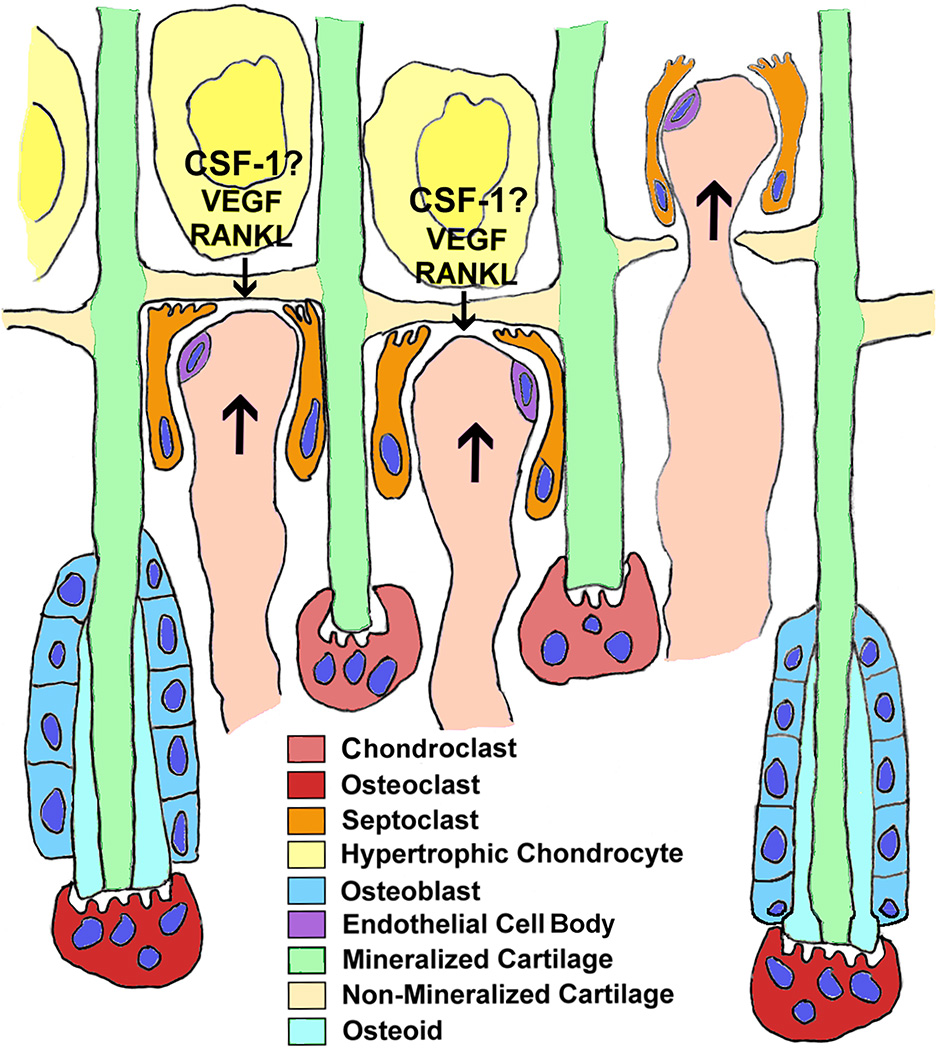
Figure 4. Catabolism at the chondroosseous junction.
The diagram represents events that take place during endochondral ossification, and, in a much less organized spatio-temporal fashion, during fracture repair. Hypertrophic chondrocytes produce VEGF and RANKL, and probably CSF-1 (see text) to recruit both vessels and osteoclasts. Capillaries bud into the growth plate by means of the septoclasts breaking down the non-mineralized transverse septa at the bottom of the growth plate. Immediately behind the capillary front, chondroclasts attach to and degrade 1/2 to 2/3 of the mineralized longitudinal septa. Immediately behind the chondroclast front, rows of osteoblasts actively secrete bone matrix onto the longitudinal septa that have escaped removal. Those trabeculae, consisting of mineralized cartilage covered by bone, are in turn degraded by osteoclasts in the metaphysis. The diet of both cartilage and bone at this location raises the question of whether the term “chondroclast” should be reserved for cells that remove only cartilage at anatomical sites not destined to become bone.
Acknowledgments
The authors thank Editor Dr. Gary Gibson for bringing the fascinating study by Trueta and Amato to our attention. We thank Drs. John Mort and Eunice Lee for anti-cathepsin B antibody. We thank Dr. Yasmin Carter for critical reading of the manuscript. This work was supported in part by grants RO1 DE-007444 and RO1 DE-13961 to PRO from the US National Institute of Dental and Craniofacial Research and grant RO1 AR061504 to PRO from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. The views expressed are those of the authors do not necessarily reflect those of the National Institutes of Health.
References
- 1.Margulis L, Schwartz KV. Five Kingdoms: an Illustrated Guide to the Phyla of Life on Earth. 3rd. New York: W. H. Freeman; 1998. p. 520. [Google Scholar]
- 2.Marks SC, Jr, Gartland A, Odgren PR. Skeletal Development. In: Martini L, editor. Encyclopedia of Endocrine Diseases. Vol. 4. San Diego: Elsevier; 2004. pp. 261–272. [Google Scholar]
- 3.Marks SC, Jr, Odgren PR. The structure and development of the skeleton. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2nd. Vol. 1. New York: Academic Press; 2002. pp. 3–15. [Google Scholar]
- 4.Staines KA, Pollard AS, McGonnell IM, Farquharson C, Pitsillides AA. Cartilage to bone transitions in health and disease. The Journal of endocrinology. 2013;219(1):R1–R12. doi: 10.1530/JOE-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez J, Horton J, Sohn P, Serra R. The perichondrium plays an important role in mediating the effects of TGF-beta1 on endochondral bone formation. Dev Dyn. 2001;221(3):311–321. doi: 10.1002/dvdy.1141. [DOI] [PubMed] [Google Scholar]
- 6.Finnson KW, Chi Y, Bou-Gharios G, Leask A, Philip A. TGF-b signaling in cartilage homeostasis and osteoarthritis. Frontiers in bioscience. 2012;4:251–268. doi: 10.2741/S266. [DOI] [PubMed] [Google Scholar]
- 7.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 8.Odgren PR, Philbrick WM, Gartland A. Perspective. Osteoclastogenesis and growth plate chondrocyte differentiation: emergence of convergence. Crit Rev Eukaryot Gene Expr. 2003;13(2–4):181–193. [PubMed] [Google Scholar]
- 9.Wang L, Almqvist KF, Veys EM, Verbruggen G. Control of extracellular matrix homeostasis of normal cartilage by a TGFbeta autocrine pathway. Validation of flow cytometry as a tool to study chondrocyte metabolism in vitro. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2002;10(3):188–198. doi: 10.1053/joca.2001.0492. [DOI] [PubMed] [Google Scholar]
- 10.Wuelling M, Vortkamp A. Chondrocyte proliferation and differentiation. Endocrine development. 2011;21:1–11. doi: 10.1159/000328081. [DOI] [PubMed] [Google Scholar]
- 11.Jochmann K, Bachvarova V, Vortkamp A. Heparan sulfate as a regulator of endochondral ossification and osteochondroma development. Matrix biology : journal of the International Society for Matrix Biology. 2014;34:55–63. doi: 10.1016/j.matbio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Breur GJ, VanEnkevort BA, Farnum CE, Wilsman NJ. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res. 1991;9(3):348–359. doi: 10.1002/jor.1100090306. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovitch AL, Anderson HC. Biogenesis of matrix vesicles in cartilage growth plates. Fed Proc. 1976;35(2):112–116. [PubMed] [Google Scholar]
- 14.Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Developmental biology. 2004;269(1):55–69. doi: 10.1016/j.ydbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Trueta J, Amato VP. The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. The Journal of bone and joint surgery British volume. 1960;42-B:571–587. doi: 10.1302/0301-620X.42B3.571. [DOI] [PubMed] [Google Scholar]
- 16.Lips P, van Schoor NM, Bravenboer N. Vitamin D-related disorders. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Eigth. Singapore: John Wiley and Sons; 2013. pp. 613–623. [Google Scholar]
- 17.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes & development. 2001;15(21):2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature medicine. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 19.Eshkar-Oren I, Viukov SV, Salameh S, Krief S, Oh CD, Akiyama H, et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009;136(8):1263–1272. doi: 10.1242/dev.034199. [DOI] [PubMed] [Google Scholar]
- 20.Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129(8):1893–1904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 21.Saijo M, Kitazawa R, Nakajima M, Kurosaka M, Maeda S, Kitazawa S. Heparanase mRNA expression during fracture repair in mice. Histochemistry and cell biology. 2003;120(6):493–503. doi: 10.1007/s00418-003-0589-1. [DOI] [PubMed] [Google Scholar]
- 22.Cackowski FC, Roodman GD. Perspective on the osteoclast: an angiogenic cell? Ann N Y Acad Sci. 2007;1117:12–25. doi: 10.1196/annals.1402.073. [DOI] [PubMed] [Google Scholar]
- 23.Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ, et al. Osteoclasts are important for bone angiogenesis. Blood. 2010;115(1):140–149. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kölliker A. Leipzig: Vogel; Die Normale resorption des Knochengewebes und Ihre Bedeutung für die Entstehung der typischen Knochenformen; p. 1873. [Google Scholar]
- 25.Nijweide PJ, Burger EH, Feyen JH. Cells of bone: proliferation, differentiation, and hormonal regulation. Physiol Rev. 1986;66(4):855–886. doi: 10.1152/physrev.1986.66.4.855. [DOI] [PubMed] [Google Scholar]
- 26.Marks SC, Jr, Walker DG. The hematogenous origin of osteoclasts: experimental evidence from osteopetrotic (microphthalmic) mice treated with spleen cells from beige mouse donors. Am J Anat. 1981;161(1):1–10. doi: 10.1002/aja.1001610102. [DOI] [PubMed] [Google Scholar]
- 27.Walker DG. Spleen cells transmit osteopetrosis in mice. Science. 1975;190(4216):785–787. doi: 10.1126/science.1198094. [DOI] [PubMed] [Google Scholar]
- 28.Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. 1975;190(4216):784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- 29.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 30.Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nature reviews Endocrinology. 2013;9(9):522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- 31.Hattner R, Epker BN, Frost HM. Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature. 1965;206(983):489–490. doi: 10.1038/206489a0. [DOI] [PubMed] [Google Scholar]
- 32.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy reports. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda K, Takeshita S. The role of osteoclast differentiation and function in skeletal homeostasis. J Biochem. 2015 doi: 10.1093/jb/mvv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenbeck G, Coxon FP. Role of vesicular trafficking in skeletal dynamics. Curr Opin Pharmacol. 2014;16:7–14. doi: 10.1016/j.coph.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Salo J, Lehenkari P, Mulari M, Metsikko K, Vaananen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997;276(5310):270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]
- 36.Dobbins DE, Sood R, Hashiramoto A, Hansen CT, Wilder RL, Remmers EF. Mutation of macrophage colony stimulating factor (Csf1) causes osteopetrosis in the tl rat. Biochem Biophys Res Commun. 2002;294(5):1114–1120. doi: 10.1016/S0006-291X(02)00598-3. [DOI] [PubMed] [Google Scholar]
- 37.Van Wesenbeeck L, Odgren PR, MacKay CA, D'Angelo M, Safadi FF, Popoff SN, et al. The osteopetrotic mutation toothless (tl) is a loss-of-function frameshift mutation in the rat Csf1 gene: Evidence of a crucial role for CSF-1 in osteoclastogenesis and endochondral ossification. Proc Natl Acad Sci U S A. 2002;99(22):14303–14308. doi: 10.1073/pnas.202332999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 39.Stanley ER, Berg KL, Einstein DB, Lee PS, Pixley FJ, Wang Y, et al. Biology and action of colony--stimulating factor-1. Mol Reprod Dev. 1997;46(1):4–10. doi: 10.1002/(SICI)1098-2795(199701)46:1<4::AID-MRD2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 40.Stanley ER, Chen DM, Lin HS. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978;274(5667):168–170. doi: 10.1038/274168a0. [DOI] [PubMed] [Google Scholar]
- 41.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 42.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF Family Member, Activates Akt/PKB through Signaling Complex Involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 43.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 44.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 45.Wise GE, Yao S, Odgren PR, Pan F. CSF-1 regulation of osteoclastogenesis for tooth eruption. J Dent Res. 2005;84(9):837–841. doi: 10.1177/154405910508400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aker M, Rouvinski A, Hashavia S, Ta-Shma A, Shaag A, Zenvirt S, et al. An SNX10 mutation causes malignant osteopetrosis of infancy. Journal of medical genetics. 2012;49(4):221–226. doi: 10.1136/jmedgenet-2011-100520. [DOI] [PubMed] [Google Scholar]
- 47.Ye L, Morse LR, Zhang L, Sasaki H, Mills JC, Odgren PR, et al. Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption. PLoS genetics. 2015;11(3):e1005057. doi: 10.1371/journal.pgen.1005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sobotta J. Atlas of Human Histology and Microscopic Anatomy. New York: G. E. Stechert; 1930. [Google Scholar]
- 49.Fawcett DW. A Textbook of Histology. Eleventh. Philadelphia: W. B. Saunders; 1986. Bloom and Fawcett; p. 1017. [Google Scholar]
- 50.Ross MH, Pawlina W. Histology: a Text and Atlas: with Correlated Cell and Molecular Biology. Baltimore: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 51.Turner RT, Evans GL, Wakley GK. Reduced chondroclast differentiation results in increased cancellous bone volume in estrogen-treated growing rats. Endocrinology. 1994;134(1):461–466. doi: 10.1210/endo.134.1.7506213. [DOI] [PubMed] [Google Scholar]
- 52.Smetana K, Jr, Vilim V. Biochemical properties of cartilage and multinucleate chondroclast formation. Funct Dev Morphol. 1992;2(3):163–165. [PubMed] [Google Scholar]
- 53.Savostin-Asling I, Asling CW. Resorption of calcified cartilage as seen in Meckel's cartilage of rats. The Anatomical record. 1973;176:345–360. doi: 10.1002/ar.1091760310. [DOI] [PubMed] [Google Scholar]
- 54.Savostin-Asling I, Asling CW. Transmission and scanning electron microscope studies of calcified cartilage resorption. The Anatomical record. 1975;183(3):373–391. doi: 10.1002/ar.1091830303. [DOI] [PubMed] [Google Scholar]
- 55.Amano O, Doi T, Yamamda T, Sasaki A, Sakiyama K, Kanegae H, et al. Meckel’s Cartilage : Discovery, Embryology and Evolution. J Oral Biosci. 2010;52(2):125–135. [Google Scholar]
- 56.Knowles HJ, Moskovsky L, Thompson MS, Grunhen J, Cheng X, Kashima TG, et al. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Arch. 2012;461(2):205–210. doi: 10.1007/s00428-012-1274-3. [DOI] [PubMed] [Google Scholar]
- 57.Nordahl J, Andersson G, Reinholt FP. Chondroclasts and osteoclasts in bones of young rats: comparison of ultrastructural and functional features. Calcif Tissue Int. 1998;63(5):401–408. doi: 10.1007/s002239900548. [DOI] [PubMed] [Google Scholar]
- 58.Sakakura Y, Hosokawa Y, Tsuruga E, Irie K, Nakamura M, Yajima T. Contributions of matrix metalloproteinases toward Meckel's cartilage resorption in mice: immunohistochemical studies, including comparisons with developing endochondral bones. Cell Tissue Res. 2007;328(1):137–151. doi: 10.1007/s00441-006-0329-7. [DOI] [PubMed] [Google Scholar]
- 59.Lee ER, Lamplugh L, Shepard NL, Mort JS. The septoclast, a cathepsin B-rich cell involved in the resorption of growth plate cartilage. J Histochem Cytochem. 1995;43(5):525–536. doi: 10.1177/43.5.7730591. [DOI] [PubMed] [Google Scholar]
- 60.Gartland A, Mason-Savas A, Yang M, MacKay CA, Birnbaum MJ, Odgren PR. Septoclast deficiency accompanies postnatal growth plate chondrodysplasia in the toothless (tl) osteopetrotic, colony-stimulating factor-1 (CSF-1)-deficient rat and is partially responsive to CSF-1 injections. The American journal of pathology. 2009;175(6):2668–2675. doi: 10.2353/ajpath.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3(3):229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Cotton WR, Gaines JF. Unerupted dentition secondary to congenital osteopetrosis in the Osborne-Mendel rat. Proc Soc Exp Biol Med. 1974;146(2):554–561. doi: 10.3181/00379727-146-38146. [DOI] [PubMed] [Google Scholar]
- 65.Seifert MF. Lack of evidence for rickets in the osteopetrotic rat mutation, toothless. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1994;9(11):1813–1821. doi: 10.1002/jbmr.5650091119. [DOI] [PubMed] [Google Scholar]
- 66.Seifert MF. Abnormalities in bone cell function and endochondral ossification in the osteopetrotic toothless rat. Bone. 1996;19(4):329–338. doi: 10.1016/s8756-3282(96)00220-7. [DOI] [PubMed] [Google Scholar]
- 67.Odgren PR, Popoff SN, Safadi FF, MacKay CA, Mason-Savas A, Seifert MF, et al. The toothless osteopetrotic rat has a normal vitamin D-binding protein-macrophage activating factor (DBP-MAF) cascade and chondrodysplasia resistant to treatments with colony stimulating factor-1 (CSF-1) and/or DBP-MAF. Bone. 1999;25(2):175–181. doi: 10.1016/s8756-3282(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 68.Devraj K, Bonassar LJ, MacKay CA, Mason-Savas A, Gartland A, Odgren PR. A new histomorphometric method to assess growth plate chondrodysplasia and its application to the toothless (tl, Csf1null) osteopetrotic rat. Connect Tissue Res. 2004;45:1–10. doi: 10.1080/03008200490278016. [DOI] [PubMed] [Google Scholar]
- 69.Iizuka T, Cielinski M, Aukerman SL, Marks SC., Jr The effects of colony-stimulating factor-1 on tooth eruption in the toothless (osteopetrotic) rat in relation to the critical periods for bone resorption during tooth eruption. Arch Oral Biol. 1992;37(8):629–636. doi: 10.1016/0003-9969(92)90125-r. [DOI] [PubMed] [Google Scholar]
- 70.Marks SC, Jr, Iizuka T, MacKay CA, Mason-Savas A, Cielinski MJ. The effects of colony-stimulating factor-1 on the number and ultrastructure of osteoclasts in toothless (tl) rats and osteopetrotic (op) mice. Tissue Cell. 1997;29(5):589–595. doi: 10.1016/s0040-8166(97)80059-6. [DOI] [PubMed] [Google Scholar]
- 71.Marks SC, Jr, Mackay CA, Jackson ME, Larson EK, Cielinski MJ, Stanley ER, et al. The skeletal effects of colony-stimulating factor-1 in toothless (osteopetrotic) rats: persistent metaphyseal sclerosis and the failure to restore subepiphyseal osteoclasts. Bone. 1993;14(4):675–680. doi: 10.1016/8756-3282(93)90091-n. [DOI] [PubMed] [Google Scholar]
- 72.Dobbins DE, Joe B, Hashiramoto A, Salstrom JL, Dracheva S, Ge L, et al. Localization of the mutation responsible for osteopetrosis in the op rat to a 1.5-cM genetic interval on rat chromosome 10: identification of positional candidate genes by radiation hybrid mapping. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17(10):1761–1767. doi: 10.1359/jbmr.2002.17.10.1761. [DOI] [PubMed] [Google Scholar]
- 73.Perdu B, Odgren PR, Van Wesenbeeck L, Jennes K, Mackay CC, Van Hul W. Refined Genomic Localization of the Genetic Lesion in the Osteopetrosis (op) Rat and Exclusion of Three Positional and Functional Candidate Genes, Clcn7, Atp6v0c, and Slc9a3r2. Calcif Tissue Int. 2009 doi: 10.1007/s00223-009-9229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seifert MF, Popoff SN, Marks SC., Jr Skeletal biology in the toothless (osteopetrotic) rat. Am J Anat. 1988;183(2):158–165. doi: 10.1002/aja.1001830206. [DOI] [PubMed] [Google Scholar]
- 75.Kim N, Odgren PR, Kim DK, Marks SC, Jr, Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A. 2000;97(20):10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Odgren PR, Kim N, MacKay CA, Mason-Savas A, Choi Y, Marks SC., Jr The role of RANKL (TRANCE/TNFSF11), a tumor necrosis factor family member, in skeletal development: effects of gene knockout and transgenic rescue. Connect Tissue Res. 2003;44(Suppl 1):264–271. [PubMed] [Google Scholar]
- 77.Martinez-Calatrava MJ, Prieto-Potin I, Roman-Blas JA, Tardio L, Largo R, Herrero-Beaumont G. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis research & therapy. 2012;14(3):R149. doi: 10.1186/ar3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakakura Y, Tsuruga E, Irie K, Hosokawa Y, Nakamura H, Yajima T. Immunolocalization of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG) in Meckel's cartilage compared with developing endochondral bones in mice. Journal of anatomy. 2005;207(4):325–337. doi: 10.1111/j.1469-7580.2005.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ota N, Takaishi H, Kosaki N, Takito J, Yoda M, Tohmonda T, et al. Accelerated cartilage resorption by chondroclasts during bone fracture healing in osteoprotegerin-deficient mice. Endocrinology. 2009;150(11):4823–4834. doi: 10.1210/en.2009-0452. [DOI] [PubMed] [Google Scholar]
- 80.Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res. 2007;22(4):487–494. doi: 10.1359/jbmr.070109. [DOI] [PubMed] [Google Scholar]
- 81.Brem H, Folkman J. Inhibition of tumor angiogenesis mediated by cartilage. The Journal of experimental medicine. 1975;141(2):427–439. doi: 10.1084/jem.141.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Langer R, Brem H, Falterman K, Klein M, Folkman J. Isolations of a cartilage factor that inhibits tumor neovascularization. Science. 1976;193(4247):70–72. doi: 10.1126/science.935859. [DOI] [PubMed] [Google Scholar]
- 83.Mukherjee A, Dong SS, Clemens T, Alvarez J, Serra R. Co-ordination of TGF-beta and FGF signaling pathways in bone organ cultures. Mechanisms of development. 2005;122(4):557–571. doi: 10.1016/j.mod.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44(1):7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 85.Burkus JK, Ganey TM, Ogden JA. Development of the cartilage canals and the secondary center of ossification in the distal chondroepiphysis of the prenatal human femur. The Yale journal of biology and medicine. 1993;66(3):193–202. [PMC free article] [PubMed] [Google Scholar]
- 86.Davoli MA, Lamplugh L, Beauchemin A, Chan K, Mordier S, Mort JS, et al. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats aged 0–21 days: II. Two proteinases, gelatinase B and collagenase-3, are implicated in the lysis of collagen fibrils. Dev Dyn. 2001;222(1):71–88. doi: 10.1002/dvdy.1160. [DOI] [PubMed] [Google Scholar]
- 87.Lee ER, Lamplugh L, Davoli MA, Beauchemin A, Chan K, Mort JS, et al. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats ages 0–21 days: I. Two groups of proteinases cleave the core protein of aggrecan. Dev Dyn. 2001;222(1):52–70. doi: 10.1002/dvdy.1168. [DOI] [PubMed] [Google Scholar]
- 88.Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat. 2008;190(4):305–315. doi: 10.1016/j.aanat.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Blumer MJ, Longato S, Schwarzer C, Fritsch H. Bone development in the femoral epiphysis of mice: the role of cartilage canals and the fate of resting chondrocytes. Dev Dyn. 2007;236(8):2077–2088. doi: 10.1002/dvdy.21228. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y, Menendez A, Fong C, ElAlieh HZ, Kubota T, Long R, et al. IGF-I Signaling in Osterix-Expressing Cells Regulates Secondary Ossification Center Formation, Growth Plate Maturation, and Metaphyseal Formation during Postnatal Bone Development. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015 doi: 10.1002/jbmr.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abou-Khalil R, Colnot C. Cellular and molecular bases of skeletal regeneration: what can we learn from genetic mouse models? Bone. 2014;64:211–221. doi: 10.1016/j.bone.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 92.Herath SC, Lion T, Klein M, Stenger D, Scheuer C, Holstein JH, et al. Stimulation of angiogenesis by cilostazol accelerates fracture healing in mice. J Orthop Res. 2015;33(12):1880–1887. doi: 10.1002/jor.22967. [DOI] [PubMed] [Google Scholar]
- 93.Mark H, Penington A, Nannmark U, Morrison W, Messina A. Microvascular invasion during endochondral ossification in experimental fractures in rats. Bone. 2004;35(2):535–542. doi: 10.1016/j.bone.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 94.Uusitalo H, Hiltunen A, Soderstrom M, Aro HT, Vuorio E. Expression of cathepsins B, H, K, L, and S and matrix metalloproteinases 9 and 13 during chondrocyte hypertrophy and endochondral ossification in mouse fracture callus. Calcif Tissue Int. 2000;67(5):382–390. doi: 10.1007/s002230001152. [DOI] [PubMed] [Google Scholar]
- 95.Karsdal MA, Bay-Jensen AC, Lories RJ, Abramson S, Spector T, Pastoureau P, et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments? Ann Rheum Dis. 2014;73(2):336–348. doi: 10.1136/annrheumdis-2013-204111. [DOI] [PubMed] [Google Scholar]
- 96.Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43(2):250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 97.Kagiya T. MicroRNAs and Osteolytic Bone Metastasis: The Roles of MicroRNAs in Tumor-Induced Osteoclast Differentiation. J Clin Med. 2015;4(9):1741–1752. doi: 10.3390/jcm4091741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, Rugo HS. Bisphosphonates and their impact on disseminated tumor cells in early stage breast cancer. Breast Dis. 2011;33(2):83–92. doi: 10.3233/BD-2010-0326. [DOI] [PubMed] [Google Scholar]
- 99.Brown JE, Coleman RE. Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat Rev Clin Oncol. 2012;9(2):110–118. doi: 10.1038/nrclinonc.2011.197. [DOI] [PubMed] [Google Scholar]
- 100.Gnant M, Dubsky P, Hadji P. Bisphosphonates: prevention of bone metastases in breast cancer. Recent Results Cancer Res. 2012;192:65–91. doi: 10.1007/978-3-642-21892-7_3. [DOI] [PubMed] [Google Scholar]
- 101.Kopper L. Denosumab--a powerful RANKL inhibitor to stop lytic metastases and other bone loss actions by osteoclasts. Pathol Oncol Res. 2012;18(4):743–747. doi: 10.1007/s12253-012-9538-4. [DOI] [PubMed] [Google Scholar]
- 102.Deng X, He G, Liu J, Luo F, Peng X, Tang S, et al. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat Rev. 2014;40(6):730–738. doi: 10.1016/j.ctrv.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pivetta E, Scapolan M, Pecolo M, Wassermann B, Abu-Rumeileh I, Balestreri L, et al. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011;13(5):R105. doi: 10.1186/bcr3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otero JE, Stevens JW, Malandra AE, Fredericks DC, Odgren PR, Buckwalter JA, et al. Osteoclast inhibition impairs chondrosarcoma growth and bone destruction. J Orthop Res. 2014 doi: 10.1002/jor.22714. [DOI] [PubMed] [Google Scholar]
- 105.Filippakopoulos P, Knapp S. The bromodomain interaction module. FEBS letters. 2012;586(17):2692–2704. doi: 10.1016/j.febslet.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 106.Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149(1):214–331. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knapp S, Weinmann H. Small-molecule modulators for epigenetics targets. ChemMedChem. 2013;8(11):1885–1891. doi: 10.1002/cmdc.201300344. [DOI] [PubMed] [Google Scholar]
- 108.Lamoureux F, Baud'huin M, Rodriguez Calleja L, Jacques C, Berreur M, Redini F, et al. Selective inhibition of BET bromodomain epigenetic signalling interferes with the bone-associated tumour vicious cycle. Nature communications. 2014;5:3511. doi: 10.1038/ncomms4511. [DOI] [PubMed] [Google Scholar]