Abstract
O-linked β-N-acetylglucosamine (O-GlcNAc) is a regulatory post-translational modification of intracellular proteins. The dynamic and inducible cycling of the modification is governed by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) in response to UDP-GlcNAc levels in the hexosamine biosynthetic pathway (HBP). Due to its reliance on glucose flux and substrate availability, a major focus in the field has been on how O-GlcNAc contributes to metabolic disease. For years this post-translational modification has been known to modify thousands of proteins implicated in various disorders, but direct functional connections have until recently remained elusive. New research is beginning to reveal the specific mechanisms through which O-GlcNAc influences cell dynamics and disease pathology including clear examples of O-GlcNAc modification at a specific site on a given protein altering its biological functions. The following review intends to focus primarily on studies in the last half decade linking O-GlcNAc modification of proteins with chromatin-directed gene regulation, developmental processes, and several metabolically related disorders including Alzheimer’s, heart disease and cancer. These studies illustrate the emerging importance of this post-translational modification in biological processes and multiple pathophysiologies.
Keywords: Alzheimer’s disease, cancer, cardiac disease, cellular differentiation, chromatin, O-GlcNAc, O-GlcNAcase, O-GlcNAc transferase
Introduction
Post-translational protein modifications (PTMs) are critical for imparting microheterogeneity and increasing protein functional diversity in biological systems. Several classes of PTMs have been identified, including: phosphorylation, ubiquitination, acetylation, SUMOylation, glycosylation, etc. Phosphorylation is the most established regulatory moiety, but interestingly, it took nearly 25 years after its discovery before groups began determining its functional roles (Fleckenstein et al., 1954; Krebs, 1993). A similar evolutionary timeframe is taking shape for O-GlcNAc. Initial studies investigating O-GlcNAc were aimed at determining its regulation and identifying processes it affected. Over the last several years, technological advancements have enabled the field to ask and begin to answer complex questions regarding O-GlcNAc’s mechanistic role in human disease.
O-GlcNAc: a post-translational protein modification
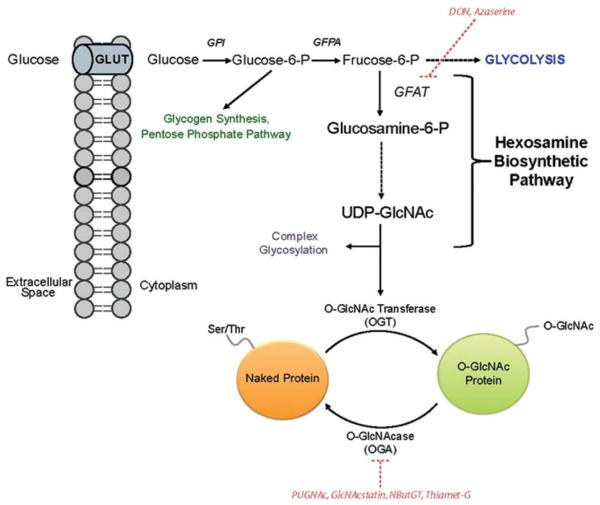
O-GlcNAc is a single monosaccharide regulatory modification occurring on nucleocytoplasmic proteins (Gao et al., 2001; Wells et al., 2001, 2003b). Approximately 2–5% of cellular glucose enters the nutrient sensing hexosamine biosynthetic pathway (HBP). The transaminase reaction of fructose-6-phosphate by glutamine fructose-6-phosphate amidotransferase (GFAT) to yield glucosamine-6-phosphate is the rate-limiting step of the pathway (Kornfeld et al., 1964; Marshall et al., 1991). The end product of the pathway is the nucleotide sugar donor UDP-GlcNAc that is used as the substrate for O-GlcNAc modification. UDP-GlcNAc can also be incorporated into complex glycosylation pathways and in the production of other nucleotide sugars (Figure 1) (Wells et al., 2003a). The levels of the nucleotide sugar donor are regulated by amino acid, free fatty acid, nucleotide and glucose availability (Love & Hanover, 2005; Marshall et al., 1991; Wells & Hart, 2003; Wells et al., 2003a).
Figure 1.
The HBP and the O-GlcNAc Modification. The majority of glucose entering the cell is used in glycolysis, glycogen synthesis or the pentose phosphate pathway. However, a small portion is shunted into the HBP, whose end product is the nucleotide sugar donor UDP-GlcNAc. UDP-GlcNAc serves as a donor for several downstream events, including the synthesis of other nucleotide sugar donors, complex glycosylation events and the post-translational modification of nuclear and cytosolic proteins with O-GlcNAc. OGT is responsible for the enzymatic addition of this sugar moiety to the hydroxyl groups of serine and threonine residues, whereas OGA is the enzyme that removes the PTM. Altered flux through the HBP is one mechanism of attenuating O-GlcNAc cycling that influences numerous molecular events in the cell. Both GFAT and OGA inhibitors are highlighted in red and indicate the stage at which they function. (see colour version of this figure at www.informahealthcare.com/bmg).
First reported in 1984 (Torres & Hart), the addition of O-GlcNAc occurs on serine and threonine residues of nuclear and cytosolic proteins and is described as being analogous to phosphorylation. These modifications are both regulated by cycling enzymes in response to environmental stimuli and compete for similar amino acid residues. In fact, a dynamic interplay between the two PTMs has been described in several cases (Ande et al., 2009; Butkinaree et al., 2010). However, O-GlcNAc and phosphate can occur at adjacent and distal sites, suggesting additional regulatory roles for O-GlcNAcylation than just blocking phosphorylation. O-GlcNAc modified proteins regulate many cellular processes: cell cycle progression (Slawson et al., 2006), transcriptional control (Chou et al., 1995b; Kelly et al., 1993), signal transduction (Vosseller et al., 2002; Yang et al., 2008b), nutrient sensing (Parker et al., 2003; Wells et al., 2003a) stress responses (Zachara et al., 2004) and chromatin remodeling (Fujiki et al., 2009; Gambetta et al., 2009; Sakabe et al., 2010; Sinclair et al., 2009).
The O-GlcNAc cycling enzymes
Two genes in mammals encode the enzymes governing O-GlcNAc cycling: O-GlcNAc transferase (OGT) and β-N-acetylglucosaminidase (OGA), which add and remove the O-GlcNAc moiety respectively (Dong & Hart, 1994; Gao et al., 2001; Haltiwanger et al., 1992; Kreppel et al., 1997).
OGT, whose activity was initially characterized in 1992 (Haltiwanger et al., 1992), was cloned and partially characterized in the late 1990s (Kreppel & Hart, 1999; Kreppel et al., 1997; Lubas et al., 1997). Mammalian OGT knockouts are embryonic lethal, demonstrative of its importance in cell survival (Shafi et al., 2000). OGT has an N-terminal tetratricopeptide repeat (TPR) domain and a C-terminal catalytic domain (Kreppel & Hart, 1999; Kreppel et al., 1997). No clear consensus sequence has been identified for OGT substrate specificity, but several factors are proposed to regulate OGT activation. These include: protein–protein interactions mediated by the TPR region, localization in part by a phosphatidyl inositol phosphate (PIP)-binding domain, post-translational modifications and substrate availability (Whelan et al., 2008; Yang et al., 2008b). The gene encoding OGT can be alternatively spliced to produce three isoforms differing at their N-terminal TPR region (Hanover et al., 2003; Love et al., 2003).
OGA was cloned and partially characterized in the early 2000s and is found ubiquitously expressed in all tissues (Gao et al., 2001; Wells et al., 2002). OGA has a catalytic N-terminal O-GlcNAcase domain, and a C-terminal domain that has sequence similarity to histone acetyltransferase (HAT). Recently, work has convincingly demonstrated this enzyme lacks previously proposed HAT activity (Rao et al., 2013). In mammals, OGA is encoded as a single gene that can be alternatively spliced producing two isoforms and differ at their C-terminal ends (Toleman et al., 2004).
Methods for studying cellular regulation via O-GlcNAc
Manipulating HBP flux through glucose exposure, glucosamine (GlcN) addition or using the amidotransferase inhibitors 6-diazo-5-oxonorleucine (DON) or O-diazoacetyl-l-serine (Azaserine), can indirectly modulate O-GlcNAc levels (Wells et al., 2003a). More specific strategies modulating global O-GlcNAc levels can also be implemented to directly target the cycling enzymes. Overexpressing or knocking down OGA and OGT are commonly used genetic manipulation approaches, while specific OGA inhibitors can also be used to investigate O-GlcNAC-specific affects. O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) was the first established OGA inhibitor widely used in the field (Haltiwanger et al., 1998), but also affected the hexosaminadase enzyme family (Miller et al., 1993). Recently, several highly selective OGA inhibitors have been generated that exhibit greater specificity for N-acetylglucosaminidases compared to hexosaminidase A/B (Figure 1). These inhibitors include: GlcNAc-configured nagstatin derivative (GlcNAcstatin), 1,2-dideoxy-2′-methyl-α-d-glucopyranoso-[2,1-d]-D2′-thiazoline (NButGT) and Thiamet-G (Dorfmueller et al., 2006; Macauley et al., 2005; Yuzwa et al., 2008). Several OGT inhibitors are also documented in the literature (Gross et al., 2005), but have not been widely evaluated or used in the field to date.
Since its discovery, O-GlcNAc has been shown to modify thousands of proteins in numerous cellular pathways. However, recent work has begun to unravel the molecular importance of this PTM on specific sites of given proteins involved in diverse biological processes. The following sections will highlight this movement by presenting data published within the last several years, with an emphasis on epigenetics and several metabolically influenced diseases.
Epigenetic regulation by O-GlcNAc
Chromatin is a highly dynamic structure that critically regulates transcription (Gregory et al., 2001). Chromatin is composed of DNA and histones that are condensed to form nucleosomes (Lee & Young, 2000). This higher order chromatin structure regulates gene transcription and repression (Gregory et al., 2001; Lee & Young, 2000). Chromatin is composed of transcriptionally active euchromatin that is gene-rich and heterochromatin which is gene-poor and transcriptionally silent (Mahmoudi & Verrijzer, 2001). Nucleosomal rearrangement is crucial for the movement of the transcription machinery along the DNA (Lee & Young, 2000). Chromatin remodeling is a complex process involving several known PTMs like acetylation, methylation, ubiquitination and phosphorylation (Allfrey et al., 1964; Eberharter & Becker, 2002; Gregory et al., 2001).
The first studies implicating O-GlcNAc in epigenetic regulation were done in D. melanogaster. The findings identified elevated O-GlcNAc levels in transcriptionally repressed regions of polytene chromosomes and significantly lower levels in “puff” regions, indicative of active transcription (Gambetta et al., 2009; Kelly & Hart, 1989). RNA Polymerase II is O-GlcNAc modified (Kelly et al., 1993) and more recently OGT was shown to be a member of the preinitiation complex (Comer & Hart, 2001; Ranuncolo et al., 2012). Disruption of the activity of either OGT or OGA leads to transcriptional defects and impaired pre-initiation complex formation (Ranuncolo et al., 2012). Drosophila super sex combs (sxc) is a polycomb group (PcG) gene located in chromosome 2R that maps to the same region as OGT (Gambetta et al., 2009; Sinclair et al., 2009). PcGs form a multiprotein complex to orchestrate epigenetic regulation of target genes involved in developmental regulation, pluripotency and cancer (Pietersen & Van Lohuizen, 2008; Ringrose & Paro, 2007; Schuettengruber et al., 2007; Schwartz & Pirrotta, 2008). Mutations in sxc affect OGT protein expression and activity in vivo and both human and Drosophila OGT can rescue sxc mutations (Sinclair et al., 2009) convincingly establishing that OGT is in fact sxc. O-GlcNAc modification and PcG binding regions overlap at the polytene chromosomes (Sinclair et al., 2009). Sxc/OGT null mutants in Drosophila exhibit a loss of polycomb repression, providing further evidence for OGT involvement in gene silencing (Sinclair et al., 2009). The polycomb repressive complex 2 (PRC2) is also O-GlcNAc modified (Myers et al., 2011). In fact, PRC2 mutations in mouse embryonic stem cells (mESC) cause deregulated OGT and O-GlcNAcylation levels on proteins associated with the chromatin-remodeling complex (Myers et al., 2011).
O-GlcNAc and chromatin: transcriptional repression
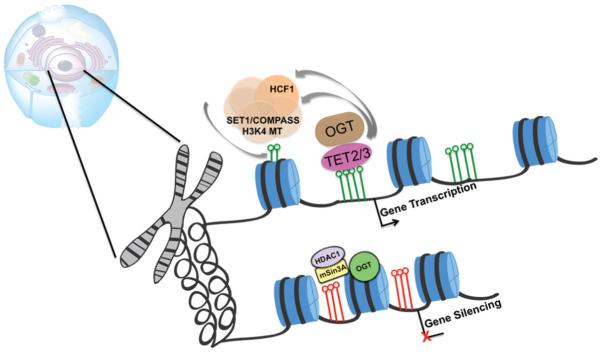
A breakthrough in identifying OGT in complex with mSin3A/HDAC1 revealed a potential role for OGT in gene silencing (Yang et al., 2002) (Figure 2). OGT and mSin3A act synergistically to repress basal and Sp1 mediated transcriptional activation (Yang et al., 2002). Moreover, estrogen target genes are hyperglycosylated in the absence of estrogen in Mcf-7 cells (Yang et al., 2002). mSin3A and HDAC1 are both known to be O-GlcNAc modified (Yang et al., 2002).
Figure 2.
associates with chromatin remodeling complexes. OGT associates with both transcriptional coactivator and corepressor complexes. OGT association with TET2/3 is necessary for the chromatin binding event of SETD1A methyl transferase. This facilitates the transcription of hematopoietic genes possibly in a HCF-1 dependent manner. OGT can also interact with mSin3A along with HDAC1 to functionally repress transcription including Sp1 activated genes. (see colour version of this figure at www.informahealthcare.com/bmg).
Many tissue-dependent differentially methylated regions (T-DMRs) have been identified in mammalian embryonic stem cells (ESC), where hyper- and hypomethylation play a role in silencing and activating loci respectively (Sato et al., 2010; Shiota, 2004; Yagi et al., 2008). In combination with histone modifications, these regions are vital in regulating gene activity at developmental stages in ESC (Armstrong, 2012; Ikegami et al., 2009). Investigation into ManNAc-stimulated hypocretin neuropeptide precursor (Hcrt) gene regulation revealed OGA and OGT are localized within the before mentioned T-DMRs (Hayakawa et al., 2013). ChIP experiments illustrate higher O-GlcNAc signal within the Hcrt promoter region (regions 1 and 2) during gene inactivity (Hayakawa et al., 2013). Enzymatic inhibition studies show a repressive role for O-GlcNAcylation in Hcrt expression. This is further strengthened by OGT association with repressive factors Sirt1 and Ezh2 at hypoacetylated T-DMR regions of non-neuronal differentiation cells (Hayakawa et al., 2013).
Histones 2A, 2B, 3 and 4 (H2A, H2B, H3, H4) are O-GlcNAc modified (Sakabe et al., 2010; Zhang et al., 2011) when assessed orthogonally by both click chemistry and immunoblotting methods (Sakabe et al., 2010). These findings are further verified in histone overexpression and O-GlcNAc immnunoblot studies using Hela cells (Sakabe et al., 2010). Click chemistry studies reveal the following O-GlcNAc modified histone sites: Thr101 on H2A, Ser36 on H2B and Ser47 on H4 (Sakabe et al., 2010). Alanine mutants of the three identified sites did not completely abrogate reactivity of the histones to O-GlcNAc specific antibodies (Sakabe et al., 2010) suggesting additional O-GlcNAc sites on each of the histones exist.
Glucosamine addition increases O-GlcNAc serine 10 (Ser10) of histone H3, subsequently decreasing the phosphorylation of the same residue (Fong et al., 2012; Zhang et al., 2011). Interestingly, when H3 Ser10 is O-GlcNAcylated, its neighboring residue lysine 9 (K9) presents with decreased acetylation (Zhang et al., 2011). Acetylation of H3K9 is a mark of active transcription (Allfrey et al., 1964; Fischle et al., 2003), which further validates H3 Ser10 O-GlcNAcylation as a repressive mark. Consistent with this, the transcriptional repression marks H3K9me3 and H3K27me3 are elevated upon increases in H3 O-GlcNAcylation, while the activation mark H3K4me3 decreases (Zhang et al., 2011). These data collectively describe the repressive role mediated by the O-GlcNAc modification of H3 Ser10.
O-GlcNAc and chromatin: transcriptional activation
Another study also identified O-GlcNAc sites on H2B and mapped three sites on this protein: Ser91, Ser112 and Ser123 of H2B (Fujiki et al., 2011). Alanine mutations of Ser112 significantly reduced O-GlcNAcylation by OGT in vitro (Fujiki et al., 2011). H2B modification at Ser112 is shown to be glucose dependent since 24-hour starvation results in its deglycosylation in Hela cells (Fujiki et al., 2011). Glucose replenishing restores the S112 O-GlcNAcylation gradually within a 24-hour period (Fujiki et al., 2011). This O-GlcNAc modification also influences H2B Lys120 monoubiquitination as highlighted by the replenishment of glucose facilitating this histone addition (Fujiki et al., 2011). This notion is validated considering OGT knockdown leads to diminished modification of Lys120 (Fujiki et al., 2011). HBP inhibitors attenuate the effect of glucose responsiveness as indicated by the loss of both Ser112 O-GlcNAcylation and Lys120 monoubiquitination (Fujiki et al., 2011). Further, Ser112Ala and Thr122Ala H2B mutations revealed the absence of K120 monoubiquitination even in the presence of extracellular glucose (Fujiki et al., 2011). However, mutating H2B Lys120Arg did not affect the O-GlcNAcylation at H2B Ser112 (Fujiki et al., 2011). This leads to the logical conclusion that Ser112 O-GlcNAcylation mediates Lys120 monoubiquitination of H2B. H2B monoubiquitination is an activation mark that has been previously described to be induced by glycolysis (Dong & Xu, 2004). H2B Ser112 O-GlcNAc is located within euchromatin of polytene chromosomes in fly (Fujiki, 2011) and co-localizes with H3K4me2, an activation mark rather than the H3K9me2/H3K27me3 repressive marks (Fujiki et al., 2011). Glycogen synthase kinase 3β (GSK3β) transcription was induced by Ser112-O-GlcNAcylated H2B, but totally ablated by OGT knockdown (Fujiki et al., 2011). These results suggest a potential role for Ser112-O-GlcNAc on H2B as a nutrient sensor to facilitate transcription of genes involved in gluconeogenesis. In pluripotent stem cells differentiating into orexin neurons, OGA is found to interact with the transcriptional activation machinery components p300 and CBP at the T-DMR of Hrct (Hayakawa et al., 2013). These events directly correlate with observed elevations in histone H3 and H4 acetylation marks during gene activation (Hayakawa et al., 2013).
Ten-eleven translocation (TET) proteins are Fe2+ and 2-oxoglutarate-dependent dioxygenases that oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) (Pastor et al., 2013; Tahiliani et al., 2009,). TET proteins mainly associate with CpG rich promoter regions (Ito et al., 2011; Williams et al., 2012; Wu & Zhang, 2011). Histone 3 lysine 4 trimethylation (H3K4me3), an activation mark, also marks CpG rich promoter regions (Balasubramanian et al., 2012). Interestingly, most Tet1-bound promoters are marked by H3K4me3 (Williams et al., 2012; Wu & Zhang, 2011). Mammals contain three TET proteins, namely TET1, TET2 and TET3. TET1 and TET2 colocalize with OGT in ESC, with TET1 being O-GlcNAc modified at residue Thr535 (Shi et al., 2013; Vella et al., 2013). TET1 in particular has been suggested to impart transcriptional regulation by interacting with chromatin remodeling and histone modification complexes Sin3a and NuRD (Downes et al., 2000). In addition, OGT and TET1 association in ESC appears to preferentially bind at unmethylated CpG-rich promoter regions in close proximity to the transcriptional start site (Vella et al., 2013). OGT siRNA-directed knockdown studies reduce Tet1 targeting and 5hmC enrichment on TET1 regulated genes (Shi et al., 2013; Vella et al., 2013).
Using affinity purification and MS techniques, OGT was found associated with TET2 and TET3 in vitro (Chen et al., 2013) (Figure 2). Moreover, in mESCs, TET2 interacts with OGT endogenously (Chen et al., 2013). The C-terminal catalytic double-strand beta-helix (DSBH) region of TET2 and TPRs 5 and 6 of OGT are essential for this interaction (Chen et al., 2013). OGT and TET2 interaction occurs at the chromatin with TET2 being necessary for OGT recruitment. This is verified by shRNA TET2 knockdown studies that totally ablate chromatin associated OGT levels (Chen et al., 2013). However, knockdown of OGT did not significantly alter TET2 retention at the chromatin (Chen et al., 2013; Fujiki et al., 2011). Both OGT and TET2 knockdowns impair histone O-GlcNAcylation with TET2 reduction dramatically reducing H2B Ser112 O-GlcNAc modification levels (Chen et al., 2013). TET2 knockout mice display impaired OGT activity and decreased global O-GlcNAcylation that parallel decreased H3K4me3 (Deplus et al., 2013). Genome-wide ChIP-Seq analysis provides insight on the distribution of OGT, TET2 and H2B Ser112 at transcription start sites (TSSs) with promoters that are H3K4me3 positive (Chen et al., 2013; Deplus et al., 2013). This study implicates the recruitment of OGT by TET2 to the chromatin to mediate transcriptional activation.
MS analysis and size-exclusion chromatography assays identify the existence of a larger complex consisting of OGT, TET1, TET2, mSin3A and host cell factor (HCF1) (Deplus et al., 2013; Shi et al., 2013; Vella et al., 2013). Interestingly, mSin3A and HDAC1 were shown to co-purify with OGT (Yang et al., 2002) and with TET1 (Ehrensberger & Svejstrup, 2012; Williams et al., 2012). OGT binding at H3K4me3-positive promoters directly corresponds with observed TET1 ChIP-Seq signal (Deplus et al., 2013; Vella et al., 2013). As previously described, OGT and the mSin3A/HDAC1 complex are involved in gene silencing in HepG2 cells as well as in in vitro studies (Yang et al., 2002) (Figure 2). HCF-1 is a known interacting and substrate partner of OGT (Capotosti et al., 2011; Ruan et al., 2012; Wysocka et al., 2003). OGT O-GlcNAcylates HCF-1 and is proposed to function as a protease to cleave HCF-1 (Capotosti et al., 2011). HCF-1 is also a component of the SET1/COMPASS H3K4 methyl transferase (MT) complex (Wysocka et al., 2003). OGT and TET2/3 have been identified in a complex with all members of the SET1/COMPASS H3K4 MT family including the methyl transferase SETD1A (Deplus et al., 2013). OGT and TET protein activities are required for the SETD1A-chromatin binding event facilitating transcriptional activation of hematopoietic genes (Deplus et al., 2013) (Figure 2)]. OGT inhibition reduces OGT interaction with HCF-1 (Capotosti et al., 2011; Deplus et al., 2013) and concomitantly decreases the association with SET1DA MT (Deplus et al., 2013). These data together suggest that HCF-1 interaction is required for the TET2/3-OGT mediated transcriptional activation by SET1/COMPASS H3K4 MT (Figure 2). A separate study highlights that OGT association with the histone lysine methyl transferase MLL5 is necessary to induce differentiation of promyelocytes by retinoic acid (RA) (Fujiki et al., 2009). OGT O-GlcNAc modifies MLL5 and activates its histone lysine methyl transferase (HKMT) activity to cause di-methylation of H3K4 (Fujiki et al., 2009). This causes RA stimulation leading to the expression of the differentiation promoting transcription factor C/EBPε (Fujiki et al., 2009). Given the role of OGT and O-GlcNAc in chronic lymphocytic leukemia (CLL) (discussed in the last section), further investigation could shed light on the role of TET2/3, OGT and MLL genes in leukemia.
Stem cells and development
Eukaryotic embryogenesis is a complex orchestration of molecular and environmental events working in concert at precise times. Glucose plays a vital role in determining many aspects of early development (Lucas, 1998; Waterland & Garza, 2002). Given the direct connection between glucose and the HBP, investigation into how O-GlcNAc impacts development has been widely studied.
OGT gene deletions in mESC provided the initial data suggesting O-GlcNAc plays an important role in development. Notably, complete knockout resulted in loss of embryonic stem cell viability and embryonic lethality due to incomplete embryogenesis (Shafi et al., 2000). Hyperglycemia was also shown to perturb blastocyst formation within the developing mouse through an HBP-directed mechanism (Pantaleon et al., 2010). O-GlcNAc appears to be the cause considering OGT inhibition prevented the hyperglycemia-induced complications observed during development (Pantaleon et al., 2010). Additional supporting evidence demonstrated mouse OGA knockouts were perinatally lethal (Yang et al., 2012). OGT and OGA targeted morpholino injection or enzyme over-expression studies results in stalled epiboly, preventing gastrulation and increasing embryonic death in zebrafish (Webster et al., 2009). Furthermore, disturbing the balance of O-GlcNAc during development in zebrafish significantly reduces body size and tissue disorganization in ectoderm, mesoderm and endoderm germ layers (Webster et al., 2009). These findings confirm the importance of precisely regulating OGT, OGA and O-GlcNAc during embryonic development and preempted further investigation into how this PTM influences developmental regulation of ESC and germ cell differentiation.
O-GlcNAc regulates ESC self-renewal
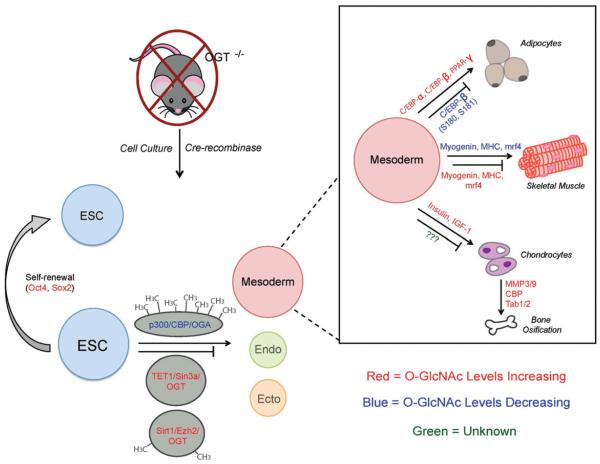
Self-renewal and pluripotency are hallmark characteristics of ESC and several studies have been conducted to determine how O-GlcNAc is involved in these processes (Figure 3). Integrin adhesion complexes are known to regulate embryonic development through the integrin β4 cytosolic domain and plectin interaction (Tarone et al., 2000). GlcN treated mESC contain decreased levels of integrin β4 mRNA and protein levels. Interestingly, these reductions disrupt the complex formation between integrin β4 and plectin necessary for proper development (Jeon et al., 2013; Margadant et al., 2010). Elevating O-GlcNAc levels through both GlcN flux and OGA inhibition increases mESC migration, while OGT inhibition blocks this action (Jeon et al., 2013). Several mESC proteins essential for self-renewal are O-GlcNAc modified, including Oct4, Sox2 and Zpf281 (Jang et al., 2012; Myers et al., 2011; Webster et al., 2009). Additionally, mSin3a is O-GlcNAc modified and is clearly demonstrated to be involved in epigenetic regulation during development (Myers et al., 2011; Yang et al., 2002). Elevating O-GlcNAc in mESC inhibits their self-renewal capacity and prevents somatic cell reprogramming into induced pluripotent stem cells (iPSC) (Jang et al., 2012). Oct4 and Sox2 are components of the core pluripotency network and part of somatic cell reprogramming cocktails to generate iPSC (Ng & Surani, 2011; Stadtfeld & Hochedlinger, 2010; Takahashi & Yamanaka, 2006). Both of these transcription factors are O-GlcNAc modified and Oct4 O-GlcNAcylation promotes mESC self-renewal and reprogramming through a transcriptionally regulated mechanism (Jang et al., 2012). In depth expression analysis reveals O-GlcNAc addition on Oct4 subsequently induces many pluripotency-related genes, including Klf2, Klf5, Nr5a2, Tbx3 and Tcl1 (Jang et al., 2012). This work establishes direct O-GlcNAc involvement in regulating key pluripotency and self-renewal proteins.
Figure 3.
O-GlcNAc levels regulate ESC characteristics and mesoderm differentiation. Complete OGT gene knockout is embryonic lethal, but studies in cell culture or the Cre-recombinase system enables O-GlcNAc investigation during differentiation and development. O-GlcNAc appears to influence ESC self-renewal that directly correlates with modulation of several embryonic transcription factors, including Oct4 and Sox2. The cycling enzymes OGT and OGA also interact with the chromatin remodeling and preinitiation complexes to control ESC pluripotency. Mesodermal cell fate is also regulated in response to O-GlcNAc levels, specifically affecting adipocyte, muscle, chondrocyte and bone differentiation. Blue font indicates reduced O-GlcNAc levels; red font indicates elevated O-GlcNAc levels; green font represents currently unknown O-GlcNAc affects. (see colour version of this figure at www.informahealthcare.com/bmg).
The previously discussed TET and T-DMRs are also shown to influence ESC fate determination through O-GlcNAc control (Figure 3). Increasing O-GlcNAc levels during development prevents the transition of ESC into germ cells provided OGT interacts with several epigenetic repressive members, including: TET1/2, mSin3a, Sirt1 and Ezh2 (Hayakawa et al., 2013; Shi et al., 2013; Vella et al., 2013). This is further supported by data demonstrating that OGA interacts with members of the transcriptional activation complex, p300 and CBP, at hypermethylated T-DMR region of Hrct (Hayakawa et al., 2013).
Studies on mouse embryonic fibroblasts (MEFs) demonstrate that O-GlcNAc plays a role in the cell cycle control (Dehennaut et al., 2007; Drougat et al., 2012; Fong et al., 2012; Sakabe & Hart, 2010; Tan et al., 2013; Zhang et al., 2011). As OGA null mice rarely reach maturity, MEFs can be isolated from mid-gestation embryos for investigation prior to glycosylation-linked lethality (Yang et al., 2012). In agreement with previous work (Slawson et al., 2005), O-GlcNAcylation fluctuates throughout the cell cycle stages, but constitutively increased O-GlcNAc levels in OGA null MEFs causes aberrant cell cycle progression (Yang et al., 2012). The observed loss of normal cell cycle control results in genomic instability as indicated by various abnormal nuclear morphologies that increases the number of senescent MEFs (Yang et al., 2012). Together these findings suggest that fluctuations in O-GlcNAc levels influence the self-renewal and pluripotent characteristics of ESC, but further investigation is needed to establish direct roles.
O-GlcNAc regulates differentiation into specialized cell-types
Upon stimulation by lineage-specific growth factors, multipotent stem cells differentiate into specialized cells during later development (Carpenter et al., 2003). Recent work implicates O-GlcNAc plays a major role in mesoderm germ cell differentiation to an even higher degree than in ESC pluripotency (Figure 3).
O-GlcNAc has long been associated with modulating many molecular aspects within adipose cells (Parker et al., 2003; Vosseller et al., 2002). It has since been identified as one of the main transcriptional regulatory modifications dictating adipocyte differentiation. Studies using the 3T3-L1-adipocyte cell line reveal protein O-GlcNAcylation increases during adipocyte differentiation (Hsieh et al., 2012; Ishihara et al., 2010). As expected, an increase in OGT and GFAT-1 protein levels, as well as GFAT-1 mRNA, directly correlate with observed O-GlcNAc elevations (Hsieh et al., 2012; Ishihara et al., 2010). OGT and GFAT inhibition decreases O-GlcNAc levels and prevents preadipocyte differentiation in 3T3-L1 cells (Hsieh et al., 2012).
Two basic leucine zipper transcription factors belonging to the CCAAT/enhancer-binding protein family (C/EBP) are implicated in O-GlcNAc-directed adipocyte differentiation. C/EBPα and C/EBPβ are critically important for controlling adipocyte differentiation (Christy et al., 1991; Mandrup & Lane, 1997; Yeh et al., 1995) and respond directly to changes in O-GlcNAc (Figure 3) (Hsieh et al., 2012; Ishihara et al., 2010; Li et al., 2009b; Maury et al., 2013). Elevating O-GlcNAc levels increases C/EBPα expression along with another adipose-related mesoderm marker, PPARg, during differentiation (Maury et al., 2013). Additionally, blocking glucose flux through the HBP in 3T3-L1 cells prevents lipid droplet formation during preadipocyte differentiation and correlates with decreased C/EBP α/β and PPARγ protein expression (Hsieh et al., 2012; Ishihara et al., 2010). A separate study looking at C/EBPb identified two amino acid residues as being O-GlcNAc modified: Ser180 and Ser181 (Li et al., 2009b). Interestingly, increasing O-GlcNAc occupancy at these sites in 3T3-L1 preadipocytes prevents subsequent phosphorylation at adjacent residues, decreases C/EBPβ DNA binding and transactivation and delays the adipocyte differentiation program (Li et al., 2009b). Considering these antagonistic roles for O-GlcNAc modification on C/EBPβ, further investigation is required to understand the molecular connection. However, it is clear that O-GlcNAcylation of C/EBPα and C/EBPβ directly influence adipocyte differentiation events.
While the primary focus on O-GlcNAc-mediated adipose differentiation has centered on C/EBPα and β, other factors involved in the developmental process have been identified. MS analysis confirms that vimentin, nucleoporin p62 and p98, Ewing sarcoma, long chain fatty acid-CoA ligase 1 and pyruvate carboxylase proteins are all more O-GlcNAc modified during preadipocyte differentiation (Ishihara et al., 2010). Along with C/EBP α and β, elevated O-GlcNAc increases the expression of the adiponectin, angiotensinogen, resistin and visfatin adipocytokines in 3T3-L1 cells to facilitate differentiation (Hsieh et al., 2012; Lim et al., 2008; Maury et al., 2013). While the precise mechanisms for O-GlcNAc regulation on these factors remains unknown, this PTM has been shown to be critical for adipocyte differentiation.
O-GlcNAcylation appears to be instrumental in spontaneously differentiating cardiac precursor cells as evident by O-GlcNAc reduction during embryoid body transition (Kim et al., 2009). This shift is likely due to a decrease in OGT protein levels during this developmental stage, which can be augmented by elevating HBP flux with GlcN addition and OGA inhibition to selectively increase O-GlcNAc (Kim et al., 2009). In a similar vein, work has been done to address whether changes in O-GlcNAc affect myoblast differentiation events. Myogenic stimulation queues activation of the skeletal myogenic program and the induction of multinucleated myotubes starting at day 1 and progressing thereafter (Berkes & Tapscott, 2005; Kitzmann & Fernandez, 2001). Protein observation during this time frame shows that O-GlcNAc levels in C2C12 myoblasts dramatically decrease between days 1 and 2 of myotubule formation, in parallel with increasing OGA and OGT mRNA and protein levels (Ogawa et al., 2012). OGA reduction using several pharmacological inhibitors or siRNAs perturbs myoblast differentiation from day 1 through day 5 as indicated by the persistence of mononucleated cells (Ogawa et al., 2012). Terminal differentiation of myoblasts is regulated by the activation of muscle-specific genes including: myogenin, myosin heavy chain (MHC) and muscle regulatory factor 4 (mrf4) (Berkes & Tapscott, 2005; Rhodes & Konieczny, 1989). OGA inhibition in C2C12 myoblasts significantly decreases the number of myogenin- and MHC-positive cells as well as myogenin, MHC and mrf4 gene expression, suggesting that O-GlcNAc reduction is critical during myogenesis (Figure 3) (Ogawa et al., 2012). Therefore, O-GlcNAc modulation is crucial for the temporal expression of genes during cardiac cell differentiation.
Although still in its infancy, new work demonstrates that O-GlcNAc may also be involved in chondrocyte differentiation and bone formation (Andres-Bergos et al., 2012; Nagel et al., 2013). Insulin and insulin-like growth factor-I (IGF-1) are strong stimulators of chondrogenesis and endochondral ossification (EO) during growth plate cartilage differentiation into bone (Hutchison et al., 2007; Kronenberg, 2003). During insulin-induced differentiation of ATCD5 pre-chondrogenic cells, O-GlcNAc levels are significantly increased and persist for the duration of development (Andres-Bergos et al., 2012). These results are also seen during ascorbic acid-induced ATCD5 differentiation, which is not directly related to the glucose metabolism pathway and insulin to suggest O-GlcNAc may independently regulate this transition (Andres-Bergos et al., 2012, Temu et al., 2010). OGA inhibition studies in the absence of insulin causes the activation of several pre-chondrogenic genes required for differentiation, indicating elevations in O-GlcNAc alone can regulate ATCD5 development (Andres-Bergos et al., 2012). This is further validated considering that reduction in HBP flux ablates insulin-stimulated differentiation and blocks the expression of these chondrogenic genes (Andres-Bergos et al., 2012). Additionally, matrix metalloproteinase (MMP) proteases 3 and 9, that are vital in ECM remodeling during chondrocyte differentiation (Brochhausen et al., 2009; Vu et al., 1998), are also upregulated during OGA inhibition to the same degree as with insulin stimulation (Andres-Bergos et al., 2012). OGA inhibition also influences several proteins that regulate CREB- and RUNX2-mediated gene expression during osteoblast differentiation, including CREB-binding protein (CBP) and TFGβ-activated kinase 1 and 2 (TAB1/TAB2) (Figure 3) (Kim et al., 2009; Nagel et al., 2013). As of now, the regulatory importance O-GlcNAc imparts in these proteins is unknown. In total, these findings demonstrate a clear role for O-GlcNAc in regulating the terminal differentiation of adipocytes, cardiac muscle, cartilage and bone.
The brain and central nervous system
The eukaryotic central nervous system (CNS) is an intricately intertwined signaling network controlling cognitive processing, emotional responsiveness and interpretive and integrative functions. The brain and spinal cord represent the main contributors to CNS function and enable whole system communication through synaptic stimulation (Moore, 1993; Tahayori & Koceja, 2012; Zhang et al., 2003). While only constituting a small portion of an organism’s mass, the CNS requires a significant amount of metabolic fuel, utilizing approximately 50% of the total glucose load (Fehm et al., 2006). Provided its well-documented dependency on glucose flux, it is logical to speculate that O-GlcNAc plays a major role in CNS regulation. To this end, proteomic analysis through a variety of mass spectrometry techniques identifies a large number of O-GlcNAc proteins within the CNS, some of which are pivotal in neuronal processes (Alfaro et al., 2012; Cole & Hart, 2001; Gao et al., 2001; Graham et al., 2011; Kang et al., 2013; Khidekel et al., 2007; Skorobogatko et al., 2011; Trinidad et al., 2012; Vosseller et al., 2002; Yuzwa et al., 2011). In fact, the presynaptic zone proteins Bassoon and Piccolo are two of the most heavily O-GlcNAc modified proteins ever observed (Trinidad et al., 2012). Recent studies have examined how O-GlcNAc contributes to synaptic signaling and have illustrated its involvement towards the establishment of Alzheimer’s disease as described below.
A neuroprotective role for O-GlcNAc in Alzheimer’s disease
Alzheimer’s disease is a neurodegenerative disorder that typically presents with aging. The hallmark phenotype includes: dementia, neurofibrillary tangles (NFTs), amyloid plaque accumulation, nerve cell degeneration and related brain physiological changes (Anderton, 1997; Carr et al., 1997). Considering the accelerated decline of glucose utilization in the Alzheimer’s disease brain (Heiss et al., 1991; McGeer et al., 1989, 1990; Minoshima et al., 1995, Smith et al., 1992), many groups have investigated the role O-GlcNAylation plays in disease progression.
One of the defining pathological features in Alzheimer’s is the oligomerization of the microtubule-associated protein tau, ultimately producing NFTs. This progression is controlled at the molecular level by hyperphosphorylation of tau, causing conformational rearrangements (Alonso et al., 2001; Weaver et al., 2000). Given the extensive crosstalk between protein phosphorylation and O-GlcNAcylation (Guo et al., 2012; Trinidad et al., 2012; Wang et al., 2010, 2012), tau O-GlcNAcylation has been investigated. Indeed, tau is shown to be O-GlcNAc modified at Thr123, Ser208, Ser333, Ser400 and Ser692, with Ser400 representing the primary functional site (Kang et al., 2013; Liu et al., 2004; Smet-Nocca et al., 2011; Wang et al., 2010; Yuzwa et al., 2011).
O-GlcNAc levels in the brain during Alzheimer’s progression appear to decrease as hyperphopsphorylation increases (Kang et al., 2013; Liu et al., 2012; Yuzwa et al., 2011). This may directly coincide with decreasing glucose metabolism observed in the aging brain (Kuhl et al., 1982; Petit-Taboue et al., 1998). Frontal cerebral cortex samples from deceased Alzheimer’s patients display significant reduction in global O-GlcNAc levels, but increased tau hyperphosphorylation as compared to wild-type controls (Liu et al., 2009). Immunofluorescent studies on human brain samples reveal a yin-yang relationship between tau O-GlcNacylation and phosphorylation (Liu et al., 2009). Non-hyperphosphorylated tau from patient brain samples are heavily O-GlcNAcylated compared to the hyperphosphorylated pool (Liu et al., 2009). This data suggests that the global decrease in O-GlcNAc may contribute to the hyperphosphorylated tau phenotype in Alzheimer’s diseased brains (Figure 4B). It also introduces OGA inhibition as a potential therapeutic target for disease treatment.
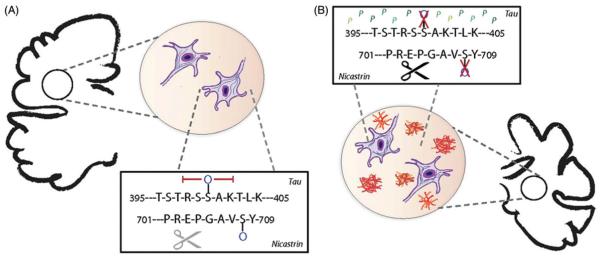
Figure 4.
O-GlcNAc protects against symptoms of neurodegeneration in the Alzheimer’s brain. (A) The microtubule-associated protein Tau can be O-GlcNAc modified at Ser400 and inhibit its subsequent hyperphosphorylation in Alzheimer’s brain samples and models. The nicastrin subunit of the γx secretase complex can also be O-GlcNAcylated at Ser708, preventing APP cleavage and aggregation observed during Alzheimer’s progression. (B) Reducing O-GlcNAc levels on both tau and nicastrin alleviates these protective affects, resulting in neurofibrillary tangles and amyloid β plaque accumulation. (see colour version of this figure at www.informahealthcare.com/bmg).
Manipulation of HBP flux and O-GlcNAc cycling enzymes directly influences Alzheimer’s disease. GFAT-1 inhibition in rat brains not only reduces the amount of O-GlcNAc, but also correlates with drastic elevation of tau phosphorylation to imply reducing glucose metabolism, and subsequently O-GlcNAc induces hyperphosphorylation of tau (Liu et al., 2012). Studies using mouse models mimicking tauopathy show that inhibiting OGA decreases phosphorylation of tau at several residues and protects against tau-driven neurodegeneration (Yuzwa et al., 2011). It also partially reduces the number of NFT-like structures in the brainstem, spinal cord, hypothalamus and cerebral cortex, while slowing tau aggregation and oligomerization (Figure 4A) (Yuzwa et al., 2011). Conversely, shOGT addition to HEK-293 cells transfected with human tau increases phosphorylation (Liu et al., 2012).
Another morphological feature of Alzheimer’s disease is the formation of amyloid plaques due to amyloid-β (Aβ) peptide accumulation. Plaque generation is caused by the sequential cleavage of amyloid precursor protein (APP) by β- and γ-secretase, respectively (Figure 4B) (Shoji et al., 1992). APP is recognized as the first plasma membrane protein identified to be O-GlcNAc modified (Griffith et al., 1995), but the functional role of this modification was not thoroughly investigated until recently. Experiments in mice suffering from Aβ aggregation-induced Alzheimer’s reveal that elevation in O-GlcNAc via OGA inhibition significantly reduces Aβ plaque load and decreases neuroinflammation in the brains of these animals (Kim et al., 2013). Active γ-secretase is a complex containing four protein subunits, including nicastrin (NCT) required for substrate recognition and binding (De Strooper, 2005, De Strooper et al., 1998). Mass spectrometry and mutational analysis confirms NCT is modified by O-GlcNAc at Ser708 and this PTM addition attenuates g-secretase activity and prevents APP cleavage (Figure 4A) (Kim et al., 2013).
The main proteolytic processing pathway for APP uses α- and γ-secretase to produce a secreted sAPPα fragment and prevents Aβ plaque aggregation (Araki et al., 1991; Mattson et al., 1993). Due to the observed neuroprotective properties of sAPPα (Goodman & Mattson, 1994) and the fact that APP is O-GlcNAc modified, investigation into a functional role for O-GlcNAylation in the non-amyloidogenic processing pathway has recently been elucidated. Cell culture experiments using human neuroblastoma cells show that elevations in O-GlcNAc levels via pharmacological inhibition of OGA increase the amount of sAPPα and prevents Aβ load (Jacobsen & Iverfeldt, 2011). Genetic and pharmacological manipulation studies targeting the O-GlcNAc cycling enzymes in SH-SY5Y human neuroblastoma cells confirm O-GlcNAcylation promotes sAPPα (Jacobsen & Iverfeldt, 2011).
Ubiquitin is a post-translational protein modification known to accumulate at Aβ plaques and NFTs in Alzheimer’s (Ii et al., 1997; Iqbal et al., 1998; Master et al., 1997). This PTM is crucial in regulating protein turnover via the proteasome (Hough & Rechsteiner, 1986; Hough et al., 1987) and is proposed to be dysfunctional in neurodegenerative diseases (Lam et al., 2000). Extensive research has established functional connections between O-GlcNAc, ubiquitination and the proteasome (Fujiki et al., 2011; Guinez et al., 2008; Klement et al., 2010; Liu et al., 2004; Ruan et al., 2013; Skorobogatko et al., 2011; Zaro et al., 2011; Zhang et al., 2007). Interestingly, mass spectrometry experiments identify an O-GlcNAc site on the 26S proteasome complex ubiquitin receptor subunit RPN13 (also known as ADRM1/ARM1). This protein recruits the deubiquinating enzyme UCH37 to the proteasome and serves as a ubiquitin receptor (Husnjak et al., 2008; Skorobogatko et al., 2011; Yao et al., 2006). Combined with the seemingly neuroprotective role O-GlcNAcylation plays in the brain, O-GlcNAc modification of RPN13 may decrease the ubiquitination status of Aβ and NFTs and diminish the Alzheimer disease phenotype. However, further investigation into this area is needed since a direct functional connection is yet to be established. These results collectively demonstrate that O-GlcNAc imparts neuroprotection in the aging brain and its decline exacerbates Alzheimer’s progression.
Synaptic signaling and memory
Cre-recombinase-expression experiments targeting OGT in both neonatal wild type and hemizygous female mice reveals significant changes in hypothalamic gene activity and the epigenetic microRNA environment (Howerton et al., 2013). Functional clustering analysis shows enrichment for genes involved in energy utilization, protein regulation and synapse formation to suggest that O-GlcNAc does more than protect against Alzheimer’s in the mammalian CNS (Howerton et al., 2013). Several independent studies reveal that O-GlcNAc appears to modulate synaptic communication at the signaling and trafficking stages, ultimately controlling long-term memory formation.
One of the more influential transcription factors determining the expression of genes in neuronal processes is cAMP-response element binding protein (CREB) (Kida et al., 2002; Lonze et al., 2002). It is long established that phosphorylation aids in regulating CREB activity within the nervous system, but is not the sole regulatory PTM (Chrivia et al., 1993; Conkright et al., 2003; Lonze & Ginty, 2002). CREB is now known to be O-GlcNAc modified at Ser40, whose induction increases in response to calcium- and kinase-dependent neuronal activation (Rexach et al., 2010, 2012). The major functionally relevant phosphorylation site of CREB is located at Ser133 (Sheng et al., 1991). Contrary to most instances, mutational studies demonstrate a cooperative role for O-GlcNAc and phosphorylation in mediating CREB activity (Rexach et al., 2012). Both OGA overexpression and Ser40Ala mutations illustrate that CREB glycosylation represses both basal transcription and activity-dependent CREB-induced gene expression in neurons (Rexach et al., 2012). In addition, obstructing Ser40 O-GlcNAc modification of CREB accelerates dendrite and axon elongation, while concurrently deregulating basal and activity-induced dendritic growth (Rexach et al., 2012).
Nerve cell communication in the CNS is a chemically regulated process requiring synaptic vesicle endocytosis. Clatherin-coated vesicles represent one specific type of trafficking molecule taking part in this process, promoting signal transmission following the removal of several inhibitory phosphorylation sites (Smith et al., 2008; Tan et al., 2003). AP180 is an important adapter protein mediating lipid and clatherin binding interaction during neurotransmitter release (Bao et al., 2005). Mass spectrometry reveals that AP180 can be O-GlcNAcylated at Thr310 and extensively phosphorylated at numerous residues in rodent brains (Graham et al., 2011; Wisniewski et al., 2010; Wu et al., 2003). Surprising results indicate that Thr310 of AP180 can be modified by a unique O-GlcNAc-phosphate moiety that is flanked by Ser306 and Ser313 phosphosites (Graham et al., 2011). Since both O-GlcNAc and phosphorylation events increase hydrophilicity and solubility, these adjacent PTMs on AP180 may hinder vesicle endocytosis by inhibiting protein–protein interactions (Graham et al., 2011). In contrast, these modifications may potentially serve as docking sites for specific substrate interaction (Graham et al., 2011). While enticing possibilities, neither has been confirmed experimentally to this point. This is not the first time O-GlcNAc sites have been found on synaptic vesicles involved in neurotransmitter signaling. Bassoon and Piccolo proteins vital for synapse assembly and vesicle docking have also been shown to be extensively O-GlcNAc modified, but impact on function has yet to be established (Trinidad et al., 2012)
As briefly mentioned, O-GlcNAc is suspected to contribute to nerve cell growth and elongation. Experiments in developing chicken forebrains show that O-GlcNAc localizes strongly in the cell bodies of axonal filopedia, lamellipodia protrusions and the growth cone (Francisco et al., 2009). Elevating O-GlcNAc by OGA inhibition increases axon branching events in neurons, while attenuating axonal filopodial numbers (Francisco et al., 2009). These results, together with the observation that elevating O-GlcNAc blocks forskolin-induced phosphorylation required for branching, suggest a repressive role for O-GlcNAc in axon branching and neuronal morphogenesis (Francisco et al., 2009). As nerve cell growth and plasticity are important in cognitive behavior, investigation into an O-GlcNAc-directed role in learning and memory is ongoing. Mek2, a kinase stimulating Erk 1/2 signaling via phosphorylation, is an important regulator in synaptic plasticity, learning and memory (Shalin et al., 2004). This protein can be O-GlcNAc modified (Ser396) as well as phosphorylated (Ser394) to trigger negative feedback inhibition and block the MEK pathway (Papin et al., 1995; Sharma et al., 2002; Skorobogatko et al., 2011; Xu et al., 1999). Reciprocity is likely to occur between these proximal sites on Mek2 to influence cognition through neuronal cell signaling control. Additionally, o-glycosylation of the previously discussed CREB protein appears to modulate long-term memory formation and consolidation (Rexach et al., 2012). In a somewhat similar study, the Drosophila PERIOD protein (dPER) is O-GlcNAcylated and temporally regulated in Schneider 2 cells (Kim et al., 2012). This protein interacts with several others to form a transcriptional feedback loop controlling circadian rhythms; the daily oscillations in behavioral and physiobiochemical processes (Dunlap, 1999; Hardin, 2011). OGT siRNA knockdown experiments dramatically shorten normal bimodal morning and evening behavior, while overexpressing OGT increased this behavioral period (Kim et al., 2012). Specifically, manipulation of OGT regulates dPER nuclear/cytoplasmic entry into pacemaker neurons to most likely account for the altered rhythms (Kim et al., 2012). Results strengthening this notion demonstrate that O-GlcNAc modification of dPER delays its phosphorylation-driven degradation, likely through the commonly observed reciprocal PTM relationship (Kim et al., 2012). While more work is needed to understand the specific functions for O-GlcNAc in the CNS, it is clear that this modification regulates synaptic signaling proteins in the circadian clock network and during memory formation.
O-GlcNAc in the heart: cardiac function and inflammatory signaling
O-GlcNAc has been implicated in pathogenesis and end-stage complications of type II diabetes for more than a decade (Akimoto et al., 2005; Buse, 2006; Issad et al., 2010; McClain et al., 2002; Vosseller et al., 2002). Because heart disease represents the largest group of diabetes-related problems, many studies have been aimed at identifying how O-GlcNAc impacts the molecular events leading to cardiac complications (Darley-Usmar et al., 2012; Fulop et al., 2007; McLarty et al., 2013; Zachara, 2012).
Post-injury cardiac protection by O-GlcNAc enrichment
Heart disease-related complications are responsible for the highest rate of annual deaths in the Western world (Prevention, 2011). Arterial blockage restricts blood flow from reaching tissues, starving them of oxygen and glucose required for normal cellular metabolism. This condition, also known as ischemia, is of major concern in the heart where myocardial damage attenuates physiological function. Cardiac injury is often exacerbated when normal blood supply returns to the site in an event called reperfusion. The rapid restoration of oxygen and nutrient supplies causes an inflammatory response and often leads to oxidative stress-induced tissue damage that can culminate in cellular apoptosis (Fliss & Gattinger, 1996; Gottlieb et al., 1994). Since O-GlcNAc levels are induced by stress and glucose flux, both of which occur during reperfusion, experimentalists have recently investigated whether this PTM may be involved in the process of ischemia-reperfusion injury.
Left ventricle myocardial biopsies from human patients displaying aortic stenosis have elevated O-GlcNAc levels compared to normal control samples (Lunde et al., 2012). Further analysis reveals that OGA and OGT protein levels are higher in these patients, coinciding with increased gene expression profiles for these cycling enzymes (Lunde et al., 2012). Rat models recapitulating the pathophysiology in the failing heart display similar results, suggesting O-GlcNAc signaling increases under cardiac stress (Lunde et al., 2012). Interestingly, manipulating O-GlcNAc levels in cardiomyocytes under basal conditions does not significantly impact heart function (Laczy et al., 2009). However, animals subjected to ischemia and reperfusion display considerable elevations in O-GlcNAc in damaged ventricle cells that can be augmented by increasing HBP flux with GlcN presupplementation (Champattanachai et al., 2008). Together these findings insinuate a strong correlation between elevated O-GlcNAcylation and cardiac complications, but does this synergism convey negative or positive effects within the heart?
Experiments investigating cardiac function in animals following ischemia/reperfusion show that OGA inhibition increases arterial and aortic vascular reactivity (Lima et al., 2009). Other studies inhibiting OGA demonstrate augmented cardiac contraction and relaxation, while significantly attenuating the appearance of arrhythmic activity during reperfusion (Laczy et al., 2009). Work using conditional OGT knockout mice (cmOGT) show that disrupting cardiomyocyte O-GlcNAc levels does not significantly influence cardiac function within the unstressed heart since there are no signs of increased hypertrophy, apoptosis or collagen accumulation compared to WT controls (Watson et al., 2010). However, cmOGT mice subjected to infarction exhibit worsening symptoms of heart failure, specifically: exaggerated left ventricular dilation in diastole, aggravated fractional shortening, impaired left ventricle contraction and relaxation and increased cases of pulmonary edema (Watson et al., 2010). Interestingly, there is no significant difference in myocyte hypertrophy and survival rate between cmOGT and WT mice post-infarction (Watson et al., 2010). However, noninfarcted myocardium in the hearts of cmOGT mice display greatly elevated levels of apoptosis and decreased expression of nutrient signaling molecules that together implies a veritable metabolic collapse when OGT is absent from the infarcted heart (Watson et al., 2010).
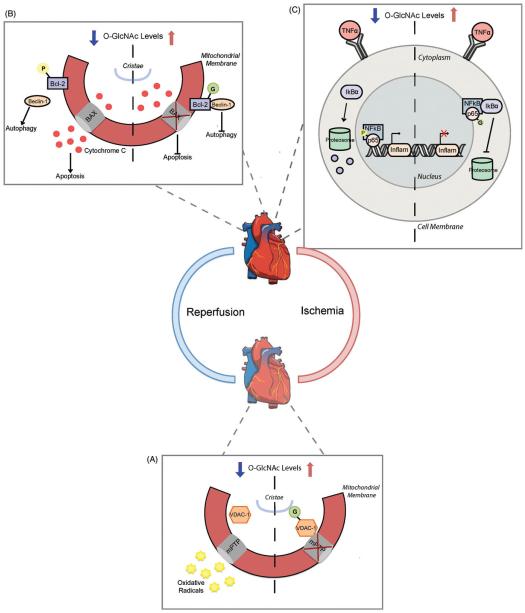
One of the major concerns of prolonged ischemia is irreversible myocyte infarction (Murry et al., 1986). A preventive measure to reduce tissue death is ischemic preconditioning, where periods of coronary artery occlusion are delicately interspersed with reperfusion events to establish an acute memory phase to prevent myocardial injury (Kersten et al., 1997). Various exogenous metabolites can trigger preconditioning, as can anesthetic treatment typically referred to as anesthetic preconditioning (APC) (Kersten et al., 1997; Tanaka et al., 2004). Mice subjected to APC through isoflurane supplementation express elevated O-GlcNAc levels within the heart compared to untreated controls (Hirose et al., 2011). APC mice display decreased myocardial infarction in the area at risk that can be reversed with OGT inhibitor pretreatment (Hirose et al., 2011). OGT inhibition combined with APC also significantly enhances myocyte viability following stimulated ischemia-reperfusion (Hirose et al., 2011). Isoflurane-initiated APC protects against ischemic injury at least in part by regulating mitochondrial ion flow through voltage-dependent anion channels (VDAC) (Hausenloy et al., 2002; Piriou et al., 2004). Previous studies reveal that O-GlcNAc modification of VDAC is essential for myocardial survival (Jones et al., 2008), but were never tested under ischemic conditions. APC treatment prevents the opening of the mitochondrial permeability transition pore in cardiac myocytes during ischemia, prohibiting the translocation of pro-apoptotic molecules (Bernardi et al., 1992; Crompton, 1999). Because VDAC is one of the structural components regulating pore opening and is O-GlcNAcylated, it is possible that this modification helps impart oxidative mitochondrial protection. Indeed, APC adult cardiac mitochondria displayed higher levels of O-GlcNAc modified VDAC compared to unconditioned controls, while OGT inhibition reverses this effect and abolishes APC oxidative protection (Figure 5A) (Hirose et al., 2011).
Figure 5.
Increased O-GlcNAcylation offers cardioprotection following ischemia-reperfusion injury. (A) Elevations in O-GlcNAc after vascular ischemia limit oxidative stress through a mitochondrial VDAC-1 mechanism. O-GlcNAc modification of VDAC-1 increases its interaction with the mitochondria permeability transition pore (mPTP) and prevents radical release. When VDAC-1 is unmodified, the mPTP can open and release harmful radical species into circulation. B Upon cardiac reperfusion the pro-autophagic protein Beclin-1 dissociates from its inhibitor Bcl-2 and stimulates constitutively active autophagy. Phosphorylation of Bcl-2 prevents its interaction with Bcl-2 associated X protein (BAX) in the mitochondrial membrane, causing cytochrome c release and apoptosis signal initiation. Bcl-2 O-GlcNAcylation during reperfusion promotes its interaction with Beclin-1 and BAX to inhibit downstream activation of autophagy and apoptosis pathways. (C) NFkB signaling is common following reperfusion in the heart. Decreasing O-GlcNAc promotes phosphorylation of the NFkB DNA binding subunit p65 and restricts IkBa protein inhibition. This enables p65 nuclear translocation where it can stimulate inflammatory gene activation. O-GlcNAc modified p65 subsequently blocks its phosphorylation to promote IkBa-mediated NFkB inhibition and prevents inflammatory gene activation. (see colour version of this figure at www.informahealthcare.com/bmg).
Multiple lines of research have established that O-GlcNAc offers cardioprotection in the heart, but there is also some evidence indicating a potential problematic role for the PTM. Arterial hypertension is a chronic elevation in blood pressure that significantly increases the heart’s workload (Chobanian et al., 2003). Rise in pressure can be caused by a number of events, including partial blood vessel occlusion, and if untreated can lead to myocardial infarction (Carretero & Oparil, 2000). Provided the degree of O-GlcNAc involvement after ischemia and reperfusion, it is reasonable that it may influence molecular aspects of hypertension. Deoxycorticosterone acetate (DOCA)-salt induced hypertension is a common mineralocorticoid model that elevates O-GlcNAc in treated rats compared to WT controls (Lima et al., 2009). DOCA-salt and OGA inhibited rats display decreased cardiac relaxation in response to acetylcholine and decreased phosphorylation of cardiovascular homeostatic proteins eNOS and Akt (Lima et al., 2009). Further experiments show that DOCA hypertension elevates O-GlcNAc-modified eNOS in the rat aorta, while decreasing levels of OGA, OGT and the HBP rate-limiting enzyme GFAT expression (Lima et al., 2009). Other work demonstrates that increasing O-GlcNAc via OGA inhibition reduces endothelial nitric oxide synthase activity to attenuate nitric oxide production (Federici et al., 2002) and appears to impair vasodilator activity in DOCA-salt models (Lima et al., 2009). Endothelin-1 (ET-1) is a peptide that induces vasoconstriction and has shown to be elevated in the vasculature of DOCA-salt hypertensive rats (Schiffrin, 2005). Interestingly, in hypertensive conditions ET-1 also activates transcription factors governing inflammation, oxidative stress and tissue damage (Carneiro et al., 2008; Shapira et al., 2003). Rat aortas incubated with ET-1 peptide display elevations in stimulated vasoconstriction in combination with increased vascular O-GlcNAcylation (Lima et al., 2010). OGT inhibition blocks this ET-1 induced effect on vascular activity, suggesting that O-GlcNAc in part mediates this ET-1 response (Lima et al., 2010). ETA receptor agonist supplementation diminishes vascular O-GlcNAc levels and augments vascular contractile function typically observed upon ET-1 stimulation (Lima et al., 2010). Together these results implicate O-GlcNAc as a possible culprit in cardiac dysfunction during salt-induced hypertension, although additional research is required to substantiate this claim. In all, these findings show that O-GlcNAc is essential for cardioprotection following ischemic and reperfusion injury, but additional studies are needed to determine its contribution during hypertension.
O-GlcNAc in cardiac inflammatory signaling
Hypertrophy and oxidative stress impinge on cardiovascular function by influencing the state of cellular inflammation. Acute vascular injury, as discussed previously, activates inflammatory signaling cascades to recruit primary immune system mediators as the initial protective response (Libby, 2001; Miller et al., 2004; Xing et al., 2004). Considering its vital role in responding to cellular stress, many studies have been aimed at determining the role of O-GlcNAc in cardiac inflammation and the purpose for this PTM within this process.
Phenylephrine (PE) stimulation is a commonly used model to recapitulate cardiac hypertrophy through activation of the neural factor of activated T-cells (NFAT) signaling cascade (Arany et al., 2006; Simpson, 1985). During hypertrophic events there is an observed increase in arterial natriuretic peptide (ANP) levels that appears to directly correlate with O-GlcNAc signaling. Not only does PE treatment elevate O-GlcNAcylation and OGT protein levels in neonatal rat cardiomyocytes, but also induces a higher expression of ANP mRNA (Facundo et al., 2012). Under conditions where HBP flux is blocked or OGA levels are elevated, both O-GlcNAc and ANP mRNA levels are significantly reduced in response to PE incubation (Facundo et al., 2012). Further studies indicate that O-GlcNAc reduction decreases ANP mRNA by blunting NFAT signaling and specifically prevents its nuclear translocation (Facundo et al., 2012). Previous work suggests that myocardial hypertrophy is at least partially caused by dysregulation of glucose uptake and utilization, wherein the insulin-dependent glucose transporter (GLUT1) is preferentially favored over its non-insulin dependent counterpart (GLUT4) (Montessuit & Thorburn, 1999). Strikingly, hypertrophic increases in O-GlcNAc directly correlate with a GLUT1 and GLUT4 expression imbalance, while OGA overexpression restores normal transporter proportions (Facundo et al., 2012). In contrast, cardiomyocytes from diabetic mice lack augmented ANP levels versus controls during PE supplementation, along with the reduction in other early markers of cardiac hypertrophy (Marsh et al., 2011). These findings may be in connection with O-GlcNAc signaling seeing in that GFAT inhibition in diabetic mice causes significantly elevated ANP expression and OGA inhibition completely blocks the observed increase in WT controls (Marsh et al., 2011). Although these results imply a possible protective role for O-GlcNAc in regards to hypertrophic cardiac signaling, it is important to consider the other metabolic irregularities at play in the diabetic phenotype that may be influencing this pathway.
Activation of the inflammatory signaling cascade in shown to impart arterial epithelial dysfunction through T lymphocyte-induced elevation in tumor necrosis factor (TNF) α (Kessler et al., 1997; Wimalasundera et al., 2003; Zemse et al., 2010). Overproduction of ROS through activated ROSenzymes, including inducible nitric oxide synthase (iNOS), is mediated by TNFα stimulation of the NFκB pathway (Busse & Mulsch, 1990; Goossens et al., 1995). Rat aortic rings treated with TNFα display impairment in depolarization-induced contractile responses that is reversed with GlcN or OGA inhibitor addition (Hilgers et al., 2012). Increasing O-GlcNAc also appears to drastically decrease TNFα-induced iNOS protein expression and the accumulation of free radical forming nitrotyrosine radicals often seen during oxidative stress (Hilgers et al., 2012). O-GlcNAc-induced iNOS attenuation is also observed in rats subjected to trauma-hemorrhage followed by full resuscitation and directly correlates with their significantly increased survival rate (Not et al., 2010). Several studies implicate O-GlcNAc involvement in regulating NFκB transduction (Golks et al., 2007; Ju et al., 2008; Zou et al., 2009), but more recent work provides a clear link in rat aortic smooth muscle cells. Phosphorylation of NFκB is essential in determining its transcriptional activity (Duran et al., 2003; Sakurai et al., 1999; Vermeulen et al., 2003; Zhong et al., 1997). Aortic smooth muscle cells incubated with GlcN or an OGA inhibitor limits inflammatory NFκB p65 DNA binding typically seen in TNFα stimulation (Xing et al., 2011). GlcN supplementation or OGA inhibition increases O-GlcNAc modification of NFκB p65 and prevents its concurrent nuclear phosphorylation at Ser536 (Figure 5C) (Xing et al., 2011). This reduction of phosphorylated p65 coincides directly with its enhanced interaction with the inhibitory complex protein IκBα and the reduction in TNFα triggered inflammatory signaling (Xing et al., 2011).
Genetically programmed cell death, or apoptosis, contributes to cell destruction following cardiac infarction and ischemia/reperfusion injury. OGT overexpression significantly reduces the ER stress response in cardiomyocytes subjected to hypoxia and reoxygenation and ultimately protects against unfolded protein response (UPR)-induced cell death (Ngoh et al., 2009). But until recently, little was known at a molecular level as to how increasing O-GlcNAc augments this cell survival. Autophagy is essential for cellular protection, but if constitutively activated can promote apoptosis (Maiuri et al., 2007). This process is extremely active in the injured cardiovascular system and its maladaptive control is thought to be primarily responsible for cell death in heart failure (Hamacher-Brady et al., 2007; Nakai et al., 2007). Two major interaction components in this system are Beclin-1 and Bcl-2, the pro- and anti-apoptosis promoting factors respectively (Pattingre et al., 2005). Dissociation of Bcl-2 from Beclin-1 induces autophagic events and is linked to pressure overload stress-induced cardiac hypertrophy (Zhu et al., 2006). Both interacting partners can be O-GlcNAcylated and phosphorylated to differentially control their interaction (Marsh et al., 2013). Interestingly, upon glucose starvation in the diabetic model pro-apoptotic protein Beclin-1 levels are reduced in cardiomyocytes to suggest a potential role for the HBP and O-GlcNAc (Marsh et al., 2013). Moreover, blocking HPB flux significantly increases the autophagic response in diabetic mice and OGA inhibition greatly reduces Beclin-1 expression (Marsh et al., 2013). Neonatal rat ventricular myocytes treated with GlcN and, to lesser extents OGA inhibition, display increased mitochondrial Bcl-2 that correlates with decreased post-ischemia and reperfusion cell injury during OGT over-expression (Champattanachai et al., 2008). Along with these findings, GlcN and OGT overexpression also prevent the loss of cytochrome c after cardiac damage, which serves as an apoptotic cell identifier when secreted from the mitochondria (Champattanachai et al., 2008). siRNA OGT-directed knockdown experiments verify these pharmacological findings by causing greatly reduced mitochondrial Bcl-2, exhibiting markedly higher cytochrome c secretion and disrupting mitochondrial membrane potential to promote higher cellular apoptosis after ischemia and reperfusion (Figure 5B) (Champattanachai et al., 2008). This set of studies clearly indicates the cardiac protection provided by O-GlcNAc occurs within cell signaling networks to prevent oxidative damage, apoptosis and uncontrolled autophagy.
O-GlcNAc regulates transcriptional activity in cancer
Pancreatic cancer
Nuclear factor kappa B (NF-κB) is a transcription factor known to play a role in various cellular processes like inflammation, cell survival, tumorigenesis and apoptosis (Ghosh & Karin, 2002; Karin & Greten, 2005). In its inactive state NF-κB is sequestered in the cytoplasm by binding to inhibitory kB (IκB). Following extracellular stimulation, IκB is phosphorylated by IκB kinase and subsequently ubiquitinated to facilitate proteosomal degradation (Karin & Ben-Neriah, 2000). The nuclear localization signal on NF-κB is uncovered in this state to allow for its nuclear translocation and facilitating transcription of downstream genes (Hayden & Ghosh, 2004). NF-κB is known to interact with OGT and contains several O-GlcNAc modification sites in lymphocytes with mutational analysis confirming T352 is required for NF-κB translocation and activation (Golks et al., 2007; Yang et al., 2008a) (Figure 6A). Hyperglycemia causes increased transcriptional activation of NF-κB due to nuclear translocation by decreased interactions between NF-κB and IκB in vascular smooth muscle cells (VSMCs) (Yang et al., 2008a). Interestingly, OGA overexpression under hyperglycemic conditions inhibits nuclear translocation of NF-κB while increasing O-GlcNAc with OGT overexpression is required for NF-κB activation in VSMCs (Yang et al., 2008a). OGT siRNA mediated knockdown in HEK293 cells display decreased mRNA levels of the NF-κB regulated genes IL-8 and BCL2A1 (Figure 6B). OGT overexpression in HEK293 cells increase transcription of these genes while conversely, OGA overexpression reduces their transcription suggesting OGT and O-GlcNAc cycling are required for the transcriptional activation of NF-κB (Allison et al., 2012) (Figure 6A and B). Attenuation of NF-κB signaling pathway can result in pancreatic ductal adenocarcinoma (PDAC) cell apoptosis (Liptay et al., 2003), while constitutive NF-κB signaling is a hallmark of several cancers including PDAC (Wang et al., 1999).
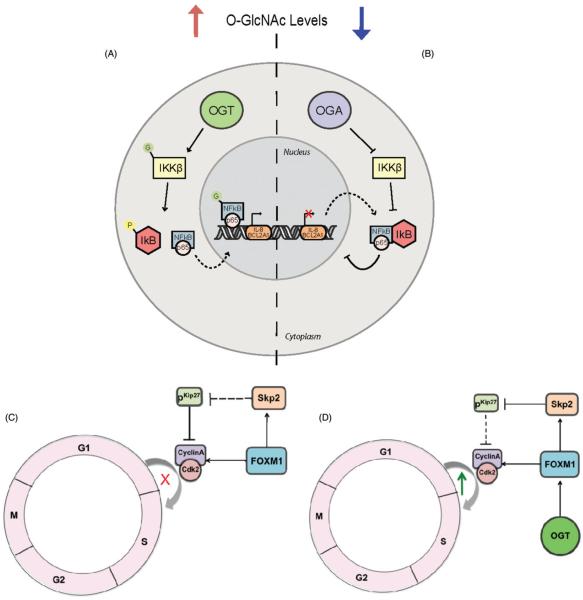
Figure 6.
OGT regulates transcription factors in the cancerous state. A IKKβ phosphorylates IκB facilitating its dissociation from NFκB. Elevating O-GlcNAc by overexpression of OGT or inhibition of OGA O-GlcNAcylates IKKβ and NFκB. NFκB that is O-GlcNAc modified can translocate to the nucleus. In cancer cells, there is an upregulation in this process allowing for increased gene transcription of NFκB targets. B Lowering O-GlcNAc levels by overexpressing OGA or using OGT inhibitors leads to deglycosylation of NFκB and its subsequent expulsion into the cytoplasm. Here it can stay sequestered with IκB, and affects NFκB downstream signaling. C In normal cells, G1/S transition is tightly regulated by pKip27 via inhibition of CyclinA/Cdk2. Skp2 negatively regulates pKip27 to allow for G1/S transition. D In cancer cells, upregulation of OGT levels cause an increase in FOXM1 and thereby Skp2 which inhibits pKip27. This simulates a constituitive G1/S transition that allows for proliferative capacity of the cells. (see colour version of this figure at www.informahealthcare.com/bmg).
O-GlcNAc and OGT levels are elevated in several different pancreatic cancer cell lines corresponding with decreased OGA levels (Ma et al., 2013). This observed increase in OGT and concomitant decrease in OGA is seen in other cancers, such as lung and colon (Mi et al., 2011). The observed hyper O-GlcNAcylation in many cancers like breast (Caldwell et al., 2010), pancreatic (Ma, 2013), prostate (Lynch et al., 2012), liver (Zhu et al., 2012), lung and colorectal (Mi et al., 2011; Yehezkel et al., 2012) may be attributed to the expression pattern of the cycling enzymes. Notably, UDP-GlcNAc levels are elevated in pancreatic cancer cell (Ma et al., 2013). OGT knockdown in PDAC cell line, MiaPaCa-2, led to an observed decrease in cell proliferation in both 2- and 3-dimensional cultures as well as colony formation (Ma et al., 2013). However, non-transformed human pancreatic epithelial cells (HPDE) did not display reduced cell proliferation when OGT was silenced to the same extent as PDAC cells (Ma et al., 2013). OGT inhibition (Gloster & Vocadlo, 2010) leads to reduced O-GlcNAcylation and inhibits both colony formation and cell proliferation (Ma et al., 2013). This is recapitulated in vivo by using OGT silenced orthotopic xenografts (Ma et al., 2013). Immunocompromised mice injected with OGT shRNA display smaller tumors in weight compared to scrambled shRNA (Ma et al., 2013). OGT shRNA mediated suppression of hyper O-GlcNAcylation induces caspase-3 and caspase-9 cleavage, indicative of apoptosis (Ma et al., 2013). Conversely, elevating O-GlcNAc levels by inhibiting OGA decreases caspase-3 cleavage and rescued cells from suspension-induced apoptosis (Ma et al., 2013). Collectively, these data establish a role for hyper O-GlcNAcylation in PDAC cell survival via inhibition of apoptosis. The p65 subunit of NF-κB and its kinase, IKKβ, are O-GlcNAc modified (Kawauchi et al., 2009) in PDAC cells (Ma et al., 2013). OGT knockdown studies in PDAC cells display reduced O-GlcNAcylation and IKKβ mediated phosphorylation at S536 of p65 that prevent its nuclear translocation and activation (Sakurai et al., 1999). Reduction in PDAC hyper O-GlcNAcylation decreases p65 nuclear localization and transcriptional activity (Ma et al., 2013), while also decreasing NF-κB targets Cyclin D1, Vimentin and Bcl-xL protein expression levels. Conversely, E-cadherin levels, normally inhibited by NF-κB, are increased in OGT knockdown PDAC cells (Ma et al., 2013). Furthermore, OGA inhibition mediated increase in O-GlcNAc lead to increased p65 O-GlcNAcylation (Ma et al., 2013). Additionally, anchorage-independent growth induced by p65 overexpression is ablated in OGT knockdown PDAC cells (Ma et al., 2013). These results show that increased O-GlcNAc levels seen in PDAC cells correspond to their increased proliferative capacity. This provides evidence to suggest that targeting OGT may be therapeutically useful to increase caspase-mediated apoptosis in these cells.
Breast cancer
Forkhead Box M1 (FOXM1) is a proliferation specific transcription factor controlling the cell cycle at the S phase, M phase, G1/S and G2/M phase (Wierstra & Alves, 2007). FOXM1 is shown to be upregulated in several cancers (Kalin et al., 2011) with some examples being breast and prostate cancers (Caldwell et al., 2010; Lynch et al., 2012). Furthermore, FOXM1 is clearly implicated in cell migration, invasion, angiogenesis, metastasis and inflammation (Kalin et al., 2011; Raychaudhuri & Park, 2011). Another protein of the Forkhead family, FOXO1 is a known O-GlcNAc modified protein (Housley et al., 2008). The functional impact of this modification is still unclear.
It is documented that OGT downregulation inhibits cell cycle progression (Lefebvre et al., 2005; Olivier-Van Stichelen et al., 2012; Sakabe & Hart, 2010; Slawson et al., 2005). Consistent with other studies (Lynch et al., 2012; Ma et al., 2013), OGT is required for in vivo tumorigenesis as evidenced by a four-fold reduction in tumor volumes in Nu/Nu mice injected with OGT shRNAs compared to scrambled control (Caldwell et al., 2010). FOXM1 protein expression is diminished in the breast cancer cell line MDA-MB-231 and oncogene over-expressing cell line MCF-10A-Erb2 when OGT is knocked down (Caldwell et al., 2010). Consistent with this data, targets of FOXM1 like Survivin, Nek2, PLK1 are also decreased in OGT knockdown in both cell lines (Caldwell et al., 2010). FOXM1 is a known transcriptional activator of Skp2 (Wang et al., 2005), which regulates the degradation of p27Kip1 during the G1/S transition (Chu et al., 2008) (Figure 6D). Interestingly, levels of p27Kip1 are increased in OGT knockdown in both MDA-MB-231 and MCF-10A-Erb2 cells (Caldwell et al., 2010). Furthermore, reduction in OGT causes accumulation of cells in G1 phase (Caldwell et al., 2010) (Figure 6C). Another target of FOXM1, matrix metalloproteinase 2 (MMP2) is down regulated in OGT knockdown MCF-10A-Erb2 cells. MMP2 is a major player in angiogenesis and metastasis (Jacob et al., 2013; Song et al., 2013) that is regulated by OGT levels through a possible mechanism via FOXM1. Inhibiting OGT pharmacologically decreases FOXM1 protein levels in MCF-10A-Erb2 cells, reducing their proliferation and invasion capacities in response to lower O-GlcNAc levels (Caldwell et al., 2010).
OGT knockdown studies also implicate O-GlcNAcylation in breast cancer metastasis via E-Cadherin/catenin complex (Gu et al., 2010). E-cadherin is pivotal for cell-cell adhesion, which is mediated by its interaction with β-catenin and p120 (Chen et al., 1999, Pokutta & Weis, 2007, Thoreson et al., 2000). OGT silencing in 4T1 breast cancer cells causes an elevation in E-Cadherin and β-catenin protein expression while p120 remains unaltered (Gu et al., 2010). In murine 4T1 cells which recapitulate human breast cancer phenotype, only p120 and β-catenin are O-GlcNAcylated (Gu et al., 2010) unlike E-Cadherin that is found O-GlcNAcylated in several other breast cancer cell lines (Zhu et al., 2001). Immunofluorescence detection portrays a significant increase in E-cadherin, β-catenin and p120 on the cell surface in OGT silenced cells while OGA inhibition displays lowered levels of E-cadherin, β-catenin and p120 at the cell surface (Gu et al., 2010). Interestingly, OGT and E-cadherin double knockdown of cells cannot inhibit cell migration as efficiently as OGT single knockdown in the 4T1 cells (Gu et al., 2010). O-GlcNAc modification of E-cadherin by endoplasmic stress-inducing agents block cell surface transport and cell adhesion capacity (Zhu et al., 2001). Given that loss of E-cadherin is associated with breast cancer transformation and metastases (Oka et al., 1993, Lu et al., 2012), this data suggests that OGT deregulates E-cadherin function in breast cancer cell line. Collectively, OGT is involved in breast cancer proliferation and metastases through its regulation of FOXM1 as well as E-cadherin.
Prostate cancer
Prostate carcinoma cell lines exhibit higher OGT mRNA, protein and O-GlcNAc levels compared to normal prostate cell that directly coincide with lower OGA protein levels (Lynch et al., 2012). Lentiviral knockdown of OGT in PC3-ML prostate carcinoma cell line leads to an 80% reduction in anchorage independent growth compared to PC3-ML control cells (Lynch et al., 2012). Both shOGT treatment and OGT inhibition display decrease in PC3-ML ability to grow in 3D culture and lower FOXM1 expression and elevated p27Kip1 expression (Lynch et al., 2012). FOXM1 is shown to play a role in angiogenesis by the regulation of VEGF in several cancers (Ahmad et al., 2010; Li et al., 2009a; Wang et al., 2007). Vascular endothelial growth factor (VEGF) mRNA is decreased by 50% in shOGT expressing PC3-ML cells and correlates with decreased VEGF mRNA by FOXM1 knockdown (Lynch et al., 2012). OGT regulates FOXM1 expression via protesosomal degradation and a non-degradable FOXM1 can rescue the angiogenic potential of shOGT expressing PC3-ML cells (Lynch et al., 2012). This suggests that OGT levels and its regulation of FOXM1 are crucial for the angiogenic potential of prostate cancer cells.
MMP2 and matrix metalloproteinase 9 (MM9) have been previously described to in prostate cancer metastasis (Sauer et al., 2004; Zhang et al., 2004). PC3-ML cells expressing shOGT have decreased ability to invade as observed by matrigel transwell assays (Lynch et al., 2012). Additionally, these cells have a significant reduction in their MMP2 and MMP9 mRNA and protein expression when compared to control PC3-ML cells (Lynch et al., 2012). Non-degradable FOXM1 mutant can restore MMP2 levels completely and MMP9 levels partially further reiterating the role of OGT mediated FOXM1 regulation of invasiveness in PC3-ML cells (Lynch et al., 2012). Moreover, PC3-ML cells expressing shOGT have reduced bone metastatic potential when introduced in immunocompromised mice, compared to control shRNA animals (Lynch et al., 2012) identifying OGT as a potential target for prostate cancer therapy.
OGT inhibition in LNCap, VCap and PC3 cancer cell lines causes loss of c-Myc protein expression (Itkonen et al., 2013). c-Myc, a proto-oncogene, is O-GlcNAcylated at T58 in its N-terminal transactivation domain (Chou et al., 1995a,b). c-Myc is a nuclear phosphoprotein containing a basic helix-loop-helix zipper domain that is a well-established transcriptional regulator involved in several cellular processes such as proliferation, differentiation and apoptosis (Eilers & Eisenman, 2008). O-GlcNAc modification of β-catenin in normal cells is higher than in cancer cell lines like LNCap (Sayat et al., 2008). O-GlcNAcylation negatively regulates the transcriptional activity of β-catenin through cytoplasmic sequestration, confirmed in OGA inhibition studies that decrease its nuclear accumulation and augments its cytoplasmic pool in DU-145 and LNCap prostate cancer cells (Sayat et al., 2008). The mechanism of dysregulating β-catenin O-GlcNAcylation and its nuclear localization is yet to be elucidated. This study highlights that O-GlcNAc levels can play a protective role against disease and antagonists of OGA can be exploited for prostate cancer therapy.
O-GlcNAc modulates metabolism in other cancers
Altered metabolism is a hallmark of cancer cells (Kroemer & Pouyssegur, 2008). Cancerous cells exhibit the “Warburg effect” whereby the cells display significantly increased glucose consumption and aerobic glycolysis (Dang & Semenza, 1999). Given that HBP is regulated by glucose flux and its end product is the substrate for OGT, the potential role of HBP, O-GlcNAc and OGT in cancers is being intensively studied.
CLL is characterized by the aberrant responses to micro-environment (Hammond et al., 2009). CLL patient samples display higher O-GlcNAc levels when immunoblotted with RL2 antibody in comparison to peripheral blood mononuclear cells (PBMCs) (Shi et al., 2010). Targets of OGT like p53, c-Myc, Akt and OGT itself are O-GlcNAcylated in CLL patients (Shi et al., 2010). Employing OGT inhibitor strategies, it is evident that Akt T308 phosphorylation is increased in CLL when O-GlcNAc is decreased (Shi et al., 2010). Conversely, elevation of O-GlcNAc levels by addition of uridine and GlcNAc attenuates Akt T308 phosphorylation and decreases its activity (Shi et al., 2010; Vosseller et al., 2002). Increasing O-GlcNAc levels in CLL patient cells impairs c-Jun N-terminal kinase (JNK) phosphorylation thereby affecting IκB phosphorylation (Shi et al., 2010). This defective phosphorylation of JNK is observed in normal B cells, as well as CLL when incubated overnight with uridine and glucosamine (Shi et al., 2010). Elevated O-GlcNAc levels affect JNK signaling to retard cell division and activation signals possibly describing the observed RL2 index of less severe CLL (Shi et al., 2010). Stage IV CLL patients have a lower RL2 index in comparison to a milder CLL phenotype suggesting that higher O-GlcNAc levels are indicative of indolent CLL phenotype (Shi et al., 2010). However, the mechanism leading to reduction in O-GlcNAcylation in the more aggressive CLL phenotypes is still unclear.
p53 is a tumor suppressor that is the target of many mutations in several cancers (Hamroun et al., 2006) and is stabilized by O-GlcNAc modification (Yang et al., 2006). p53 loss of function is associated with an increase in glycolysis (Bensaad & Vousden, 2007) via IKK-NF-κB pathway (Kawauchi et al., 2008). MCF-7 cells with p53 knockdown consume more glucose in comparison to control and exhibit elevated levels of O-GlcNAcylated IKKβ and activating phosphorylated IKKβ (Kawauchi et al., 2009). p53 deficient MEFs display higher O-GlcNAcylated IKKβ (Kawauchi et al., 2009). Additional studies confirm that p65-NF-κB is necessary for p53−/− mediated enhanced glycolysis (Kawauchi et al., 2008). Moreover, p65-NF-κB knockdown in p53−/− MEFs leads to decreased O-GlcNAcylated IKKβ and activating phosphorylation of IKKβ (Kawauchi et al., 2009). Transformed Tig-3 human primary fibroblasts also display increased glucose consumption as well as concomitant elevation of O-GlcNAcylated IKKβ and activation phosphorylation of IKKβ (Kawauchi et al., 2009). O-GlcNAc on S733 is important for enhanced glycolysis as mutating the serine to a glutamate or alanine both lead to lower glucose consumption (Kawauchi et al., 2009). TNFα stimulation of p53 deficient MEFs activates IKKβ and NF-κB in comparison to WT MEFs (Kawauchi et al., 2009). These data suggest that O-GlcNAcylation of IKKβ may mediate the constitutive NF-κB activation as seen in several cancers.
Increasing O-GlcNAc levels by over-expressing OGT in lung cancer cell line H1299 leads to decreased glucose consumption along with lower lactate and ATP levels (Yi et al., 2012). Elevated O-GlcNAc levels also lead to reduction in the activity of phosphofructokinase 1 (PFK1) activity (Yi et al., 2012), serving as a major player in and regulating the flux through glycolysis (Sola-Penna et al., 2010). PFK1 is O-GlcNAcylated in a variety of cell lines including LNCap, MDA-MB-231 and MCF-7 (Yi 2012). Under hypoxia and glucose deprivation, normally associated with tumorigenesis, PFK is O-GlcNAcylated in H1299 cells (Yi et al., 2012) at the residue S529 (Yi et al., 2012). S529 is the highly conserved residue on PFK1 that allows for allosteric regulation by fructose 2,6-bisphosphate (F-2,6 BP) (Ferreras et al., 2009). O-GlcNAcylation of S529 of PFK1 causes formation of low molecular weight complex while S529A is unperturbed and runs as a higher molecular weight complex (Yi et al., 2012). Overexpressing OGT in H1299 cells containing Flag-tagged knock in of WT PFK1 reduces lactate production and glycolysis, a key feature of cancer cell metabolism (Yi et al., 2012). No change in either glycolysis or lactate production is observable in S529A PFK knock in under OGT overexpression (Yi et al., 2012). Inhibiting flux through glycolysis can shift the levels of pentose phosphate pathway (PPP) (Yi et al., 2012). OGT overexpression increases PPP flux in WT PFK1 knock in demonstrating the deregulation of glycolysis (Yi et al., 2012). Consistent with PPP flux, NADPH and reduced glutathione (GSH) are increased in WT PFK1 knock in cells with OGT over-expression under hypoxia (Yi et al., 2012). S529A knock in cells demonstrate significantly lower levels of NADPH and GSH suggesting that blocking glycosylation may potentially restore glycolysis (Yi et al., 2012). Immunocompromised mice injected with WT PFK1 knock in cells with OGT overexpression display more tumorous growths while the S529A mice exhibit smaller tumors (Yi et al., 2012). PFK1 O-GlcNAcylation at S529 is required for enhanced tumor growth and this can be exploited for therapeutics against cancerous cells.
Concluding remarks
Extensive understanding into how O-GlcNAc influences biological systems has grown considerably in recent years. This dynamic and inducible nutrient sensor is a well-established regulator of metabolic- and stress-induced cellular activities. Unfortunately, direct functional connections have proven difficult due to technological limitations in combination with the field’s adolescence. More recent studies are beginning to substantiate previous claims that O-GlcNAc is essential in controlling molecular events. Epigenetics has exploded onto the scene as of late, providing an intricate model for environmental gene regulation. Although its biological introduction within this area was delayed compared to other PTMs, it is now clear that O-GlcNAcylation is a major part of the histone code. Various works demonstrate that histone proteins themselves can carry the O-GlcNAc moiety, while the cycling enzymes interact with numerous chromatin-associated complexes to affect nucleosome accessibility. Stem cell biology is extremely promising in terms of therapeutics, but details of the signaling pathways dictating cellular fates remain elusive. O-GlcNAc is now known to regulate ESC pluripotency and self-renewal, along with mesodermal differentiation into several cell types. Deregulated metabolism represents a common phenotype in many disease pathologies. Earlier works flirted with the notion that O-GlcNAc contributed substantial molecular regulation in these ailments, but were unable to show this decisively. Thanks to extensive investigation over the last several years, this PTM is definitively shown to influence the progression of multiple diseases, including: Alzheimer’s, diabetes, ischemic and reperfusion cardiac injury, hypertension and cancer. In all of these areas O-GlcNAc appears to exert its control at the cell cycle or transcriptional levels, further cementing it as a vital molecular component. While these new findings are exciting and encouraging, there is still much work to be done to validate these results and establish clear functional roles for site-specific O-GlcNAc modification on particular proteins. But science is a discipline that becomes more complicated with discovery and it appears that O-GlcNAc will only continue to beneficially confound our understanding for years to come.
Acknowledgements
We would like to thank members of the Wells laboratory for their insightful discussion. We would also like to apologize for any papers we failed to mention that were published in the last five years due to constraints of space.
Declaration of interest
This work was partially supported by U01CA128454 and P41RR018502 from NIH (LW co-investigator).
References
- Ahmad A, Wang Z, Kong D, et al. FoxM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast Cancer Res Treat. 2010;122:337–46. doi: 10.1007/s10549-009-0572-1. [DOI] [PubMed] [Google Scholar]
- Akimoto Y, Hart GW, Hirano H, Kawakami H. O-GlcNAc modification of nucleocytoplasmic proteins and diabetes. Med Mol Morphol. 2005;38:84–91. doi: 10.1007/s00795-004-0264-1. [DOI] [PubMed] [Google Scholar]
- Alfaro JF, Gong CX, Monroe ME, et al. Tandem mass spectrometry identifies many mouse brain O-GlcNAcylated proteins including EGF domain-specific O-GlcNAc transferase targets. Proc Natl Acad Sci USA. 2012;109:7280–5. doi: 10.1073/pnas.1200425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA. 1964;51:786–94. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DF, Wamsley JJ, Kumar M, et al. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-kappaB acetylation and transcription. Proc Natl Acad Sci USA. 2012;109:16888–93. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso AD, Zaidi T, Novak M, et al. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–8. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande SR, Moulik S, Mishra S. Interaction between O-GlcNAc modification and tyrosine phosphorylation of prohibitin: implication for a novel binary switch. PLoS One. 2009;4:e4586. doi: 10.1371/journal.pone.0004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton BH. Changes in the ageing brain in health and disease. Philos Trans R Soc Lond B Biol Sci. 1997;352:1781–92. doi: 10.1098/rstb.1997.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Bergos J, Tardio L, Larranaga-Vera A, et al. The increase in O-Linked N-acetylglucosamine protein modification stimulates chondrogenic differentiation both in vitro and in vivo. J Biol Chem. 2012;287:33615–28. doi: 10.1074/jbc.M112.354241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki W, Kitaguchi N, Tokushima Y, et al. Trophic effect of beta-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181:265–71. doi: 10.1016/s0006-291x(05)81412-3. [DOI] [PubMed] [Google Scholar]
- Arany Z, Novikov M, Chin S, et al. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. 2006;103:10086–91. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong L. Epigenetic control of embryonic stem cell differentiation. Stem Cell Rev. 2012;8:67–77. doi: 10.1007/s12015-011-9300-4. [DOI] [PubMed] [Google Scholar]
- Balasubramanian D, Akhtar-Zaidi B, Song L, et al. H3K4me3 inversely correlates with DNA methylation at a large class of non-CpG-island-containing start sites. Genome Med. 2012;4:47. doi: 10.1186/gm346. doi:10.1186/gm346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, Daniels RW, Macleod GT, et al. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J Neurophysiol. 2005;94:1888–903. doi: 10.1152/jn.00080.2005. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–91. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–95. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Vassanelli S, Veronese P, et al. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–9. [PubMed] [Google Scholar]
- Brochhausen C, Lehmann M, Zehbe R, et al. Tissue engineering of cartilage and bone: growth factors and signaling molecules. Orthopade. 2009;38:1053–62. doi: 10.1007/s00132-009-1496-5. [DOI] [PubMed] [Google Scholar]
- Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290:E1–8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Mulsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990;275:87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Butkinaree C, Park K, Hart GW. O-linked beta-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta. 2010;1800:96–106. doi: 10.1016/j.bbagen.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell SA, Jackson SR, Shahriari KS, et al. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–42. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- Capotosti F, Guernier S, Lammers F, et al. O-GlcNAc transferase catalyzes site-specific proteolysis of HCF-1. Cell. 2011;144:376–88. doi: 10.1016/j.cell.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Carneiro FS, Nunes KP, Giachini FR, et al. Activation of the ET-1/ETA pathway contributes to erectile dysfunction associated with mineralocorticoid hypertension. J Sex Med. 2008;5:2793–807. doi: 10.1111/j.1743-6109.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- Carr DB, Goate A, Phil D, Morris JC. Current concepts in the pathogenesis of Alzheimer’s disease. Am J Med. 1997;103:3S–10S. doi: 10.1016/s0002-9343(97)00262-3. [DOI] [PubMed] [Google Scholar]
- Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation. 2000;101:329–35. doi: 10.1161/01.cir.101.3.329. [DOI] [PubMed] [Google Scholar]
- Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol. 2008;294:C1509–20. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Chen Y, Bian C, et al. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature. 2013;493:561–4. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Stewart DB, Nelson WJ. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol. 1999;144:687–99. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chou TY, Dang CV, Hart GW. Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci USA. 1995a;92:4417–21. doi: 10.1073/pnas.92.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995b;270:18961–5. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- Christy RJ, Kaestner KH, Geiman DE, Lane MD. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA. 1991;88:2593–7. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–9. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Cole RN, Hart GW. Cytosolic O-glycosylation is abundant in nerve terminals. J Neurochem. 2001;79:1080–9. doi: 10.1046/j.1471-4159.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- Comer FI, Hart GW. Reciprocity between O-GlcNAc and O-phosphate on the carboxyl terminal domain of RNA polymerase II. Biochemistry. 2001;40:7845–52. doi: 10.1021/bi0027480. [DOI] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, et al. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413–23. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–49. [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- Darley-Usmar VM, Ball LE, Chatham JC. Protein O-linked beta-N-acetylglucosamine: a novel effector of cardiomyocyte metabolism and function. J Mol Cell Cardiol. 2012;52:538–49. doi: 10.1016/j.yjmcc.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B. Nicastrin: gatekeeper of the gamma-secretase complex. Cell. 2005;122:318–20. doi: 10.1016/j.cell.2005.07.021. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–90. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Dehennaut V, Lefebvre T, Sellier C, et al. O-linked N-acetylglucosaminyltransferase inhibition prevents G2/M transition in Xenopus laevis oocytes. J Biol Chem. 2007;282:12527–36. doi: 10.1074/jbc.M700444200. [DOI] [PubMed] [Google Scholar]
- Deplus R, Delatte B, Schwinn MK, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32:645–55. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem. 1994;269:19321–30. [PubMed] [Google Scholar]
- Dong L, Xu CW. Carbohydrates induce mono-ubiquitination of H2B in yeast. J Biol Chem. 2004;279:1577–80. doi: 10.1074/jbc.C300505200. [DOI] [PubMed] [Google Scholar]
- Dorfmueller HC, Borodkin VS, Schimpl M, et al. GlcNAcstatin: a picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-glcNAcylation levels. J Am Chem Soc. 2006;128:16484–5. doi: 10.1021/ja066743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes M, Ordentlich P, Kao HY, et al. Identification of a nuclear domain with deacetylase activity. Proc Natl Acad Sci USA. 2000;97:10330–5. doi: 10.1073/pnas.97.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drougat L, Olivier-Van Stichelen S, Mortuaire M, et al. Characterization of O-GlcNAc cycling and proteomic identification of differentially O-GlcNAcylated proteins during G1/S transition. Biochim Biophys Acta. 2012;1820:1839–48. doi: 10.1016/j.bbagen.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–90. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Duran A, Diaz-Meco MT, Moscat J. Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J. 2003;22:3910–8. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–9. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrensberger AH, Svejstrup JQ. Reprogramming chromatin. Crit Rev Biochem Mol Biol. 2012;47:464–82. doi: 10.3109/10409238.2012.697125. [DOI] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–66. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facundo HT, Brainard RE, Watson LJ, et al. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. Am J Physiol-Heart Circulat Physiol. 2012;302:H2122–30. doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici M, Menghini R, Mauriello A, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–72. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- Fehm HL, Kern W, Peters A. The selfish brain: competition for energy resources. Prog Brain Res. 2006;153:129–40. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- Ferreras C, Hernandez ED, Martinez-Costa OH, Aragon JJ. Subunit interactions and composition of the fructose 6-phosphate catalytic site and the fructose 2,6-bisphosphate allosteric site of mammalian phosphofructokinase. J Biol Chem. 2009;284:9124–31. doi: 10.1074/jbc.M807737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–83. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A, Janke J, Davies RE, Krebs HA. Chemistry of muscle contraction; contraction of muscle without fission of adenosine triphosphate or creatine phosphate. Nature. 1954;174:1081–3. doi: 10.1038/1741081a0. [DOI] [PubMed] [Google Scholar]
- Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–56. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- Fong JJ, Nguyen BL, Bridger R, et al. beta-N-Acetylglucosamine (O-GlcNAc) is a novel regulator of mitosis-specific phosphorylations on histone H3. J Biol Chem. 2012;287:12195–203. doi: 10.1074/jbc.M111.315804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco H, Kollins K, Varghis N, et al. O-GlcNAc post-translational modifications regulate the entry of neurons into an axon branching program. Develop Neurobiol. 2009;69:162–73. doi: 10.1002/dneu.20695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki R, Chikanishi T, Hashiba W, et al. GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature. 2009;459:455–9. doi: 10.1038/nature07954. [DOI] [PubMed] [Google Scholar]
- Fujiki R, Hashiba W, Sekine H, et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–60. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–97. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambetta MC, Oktaba K, Muller J. Essential role of the glycosyltransferase sxc/Ogt in polycomb repression. Science. 2009;325:93–6. doi: 10.1126/science.1169727. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wells L, Comer FI, et al. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–45. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gloster TM, Vocadlo DJ. Mechanism, structure, and inhibition of O-GlcNAc processing enzymes. Curr Signal Transduct Ther. 2010;5:74–91. doi: 10.2174/157436210790226537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golks A, Tran TT, Goetschy JF, Guerini D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 2007;26:4368–79. doi: 10.1038/sj.emboj.7601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- Goossens V, Grooten J, Devos K, Fiers W. Direct evidence for tumor necrosis factor-Induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–19. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Burleson KO, Kloner RA, et al. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–8. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ME, Thaysen-Andersen M, Bache N, et al. A novel post-translational modification in nerve terminals: O-linked N-acetylglucosamine phosphorylation. J Proteome Res. 2011;10:2725–33. doi: 10.1021/pr1011153. [DOI] [PubMed] [Google Scholar]
- Gregory PD, Wagner K, Horz W. Histone acetylation and chromatin remodeling. Exp Cell Res. 2001;265:195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- Griffith LS, Mathes M, Schmitz B. Beta-amyloid precursor protein is modified with O-linked N-acetylglucosamine. J Neurosci Res. 1995;41:270–8. doi: 10.1002/jnr.490410214. [DOI] [PubMed] [Google Scholar]
- Gross BJ, Kraybill BC, Walker S. Discovery of O-GlcNAc transferase inhibitors. J Am Chem Soc. 2005;127:14588–9. doi: 10.1021/ja0555217. [DOI] [PubMed] [Google Scholar]
- Gu Y, Mi W, Ge Y, et al. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010;70:6344–51. doi: 10.1158/0008-5472.CAN-09-1887. [DOI] [PubMed] [Google Scholar]
- Guinez C, Mir AM, Dehennaut V, et al. Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J. 2008;22:2901–11. doi: 10.1096/fj.07-102509. [DOI] [PubMed] [Google Scholar]
- Guo K, Gan L, Zhang S, et al. Translocation of HSP27 into liver cancer cell nucleus may be associated with phosphorylation and O-GlcNAc glycosylation. Oncol Rep. 2012;28:494–500. doi: 10.3892/or.2012.1844. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1992;267:9005–13. [PubMed] [Google Scholar]
- Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–17. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Logue SE, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differentiation. 2007;14:146–57. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- Hammond CM, Shi Y, White D, et al. The B-cell calcium sensor predicts progression of chronic lymphocytic leukemia. Leukemia. 2009;23:426–9. doi: 10.1038/leu.2008.351. [DOI] [PubMed] [Google Scholar]
- Hamroun D, Kato S, Ishioka C, et al. The UMD TP53 database and website: update and revisions. Hum Mutat. 2006;27:14–20. doi: 10.1002/humu.20269. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Yu S, Lubas WB, et al. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409:287–97. doi: 10.1016/s0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet. 2011;74:141–73. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–43. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Hirosawa M, Tabei Y, et al. Epigenetic switching by the metabolism-sensing factors in the generation of orexin neurons from mouse embryonic stem cells. J Biol Chem. 2013;288:17099–110. doi: 10.1074/jbc.M113.455899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Szelies B, Kessler J, Herholz K. Abnormalities of energy metabolism in Alzheimer’s disease studied with PET. Ann N Y Acad Sci. 1991;640:65–71. doi: 10.1111/j.1749-6632.1991.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Hilgers RH, Xing D, Gong K, et al. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am J Physiol Heart Circ Physiol. 2012;303:H513–22. doi: 10.1152/ajpheart.01175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Tsutsumi YM, Tsutsumi R, et al. Role of the O-linked beta-N-acetylglucosamine in the cardioprotection induced by isoflurane. Anesthesiology. 2011;115:955–62. doi: 10.1097/ALN.0b013e31822fcede. [DOI] [PubMed] [Google Scholar]
- Hough R, Pratt G, Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987;262:8303–13. [PubMed] [Google Scholar]
- Hough R, Rechsteiner M. Ubiquitin-lysozyme conjugates. Purification and susceptibility to proteolysis. J Biol Chem. 1986;261:2391–9. [PubMed] [Google Scholar]
- Housley MP, Rodgers JT, Udeshi ND, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–92. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Morgan CP, Fischer DB, Bale TL. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc Natl Acad Sci USA. 2013;110:5169–74. doi: 10.1073/pnas.1300065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TJ, Lin T, Hsieh PC, et al. Suppression of Glutamine:fructose-6-phosphate amidotransferase-1 inhibits adipogenesis in 3T3-L1 adipocytes. J Cell Physiol. 2012;227:108–15. doi: 10.1002/jcp.22707. [DOI] [PubMed] [Google Scholar]
- Husnjak K, Elsasser S, Zhang NX, et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–8. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison MR, Bassett MH, White PC. Insulin-like growth factor-I and fibroblast growth factor, but not growth hormone, affect growth plate chondrocyte proliferation. Endocrinology. 2007;148:3122–30. doi: 10.1210/en.2006-1264. [DOI] [PubMed] [Google Scholar]
- Ii K, Ito H, Tanaka K, Hirano A. Immunocytochemical co-localization of the proteasome in ubiquitinated structures in neurodegenerative diseases and the elderly. J Neuropathol Exp Neurol. 1997;56:125–31. doi: 10.1097/00005072-199702000-00002. [DOI] [PubMed] [Google Scholar]
- Ikegami K, Ohgane J, Tanaka S, et al. Interplay between DNA methylation, histone modification and chromatin remodeling in stem cells and during development. Int J Dev Biol. 2009;53:203–14. doi: 10.1387/ijdb.082741ki. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Alonso AD, Gong CX, et al. Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles. J Neural Transm Suppl. 1998;53:169–80. doi: 10.1007/978-3-7091-6467-9_15. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Takahashi I, Tsuchiya Y, et al. Characteristic increase in nucleocytoplasmic protein glycosylation by O-GlcNAc in 3T3-L1 adipocyte differentiation. Biochem Biophys Res Commun. 2010;398:489–94. doi: 10.1016/j.bbrc.2010.06.105. [DOI] [PubMed] [Google Scholar]
- Issad T, Masson E, Pagesy P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metab. 2010;36:423–35. doi: 10.1016/j.diabet.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Itkonen HM, Minner S, Guldvik IJ, et al. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73:5277–87. doi: 10.1158/0008-5472.CAN-13-0549. [DOI] [PubMed] [Google Scholar]
- Ito S, Shen L, Dai Q, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–3. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Jing J, Lee J, et al. Rab40b regulates MMP2 and MMP9 trafficking during invadopodia formation and breast cancer cell invasion. J Cell Sci. 2013;126:4647–58. doi: 10.1242/jcs.126573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen KT, Iverfeldt K. O-GlcNAcylation increases non-amyloidogenic processing of the amyloid-beta precursor protein (APP) Biochem Biophys Res Commun. 2011;404:882–6. doi: 10.1016/j.bbrc.2010.12.080. [DOI] [PubMed] [Google Scholar]
- Jang H, Kim TW, Yoon S, et al. O-GlcNAc regulates pluripotency and reprogramming by directly acting on core components of the pluripotency network. Cell Stem Cell. 2012;11:62–74. doi: 10.1016/j.stem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Jeon JH, Suh HN, Kim MO, Han HJ. Glucosamine-induced reduction of integrin beta4 and plectin complex stimulates migration and proliferation in mouse embryonic stem cells. Stem Cells Dev. 2013;22:2975–89. doi: 10.1089/scd.2013.0158. [DOI] [PubMed] [Google Scholar]
- Jones SP, Zachara NE, Ngoh GA, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–82. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- Ju Y, Hua J, Sakamoto K, et al. Modulation of TNF-alpha-induced endothelial cell activation by glucosamine, a naturally occurring amino monosaccharide. Int J Mol Med. 2008;22:809–15. [PubMed] [Google Scholar]
- Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle. 2011;10:396–405. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Kim C, Jeong H, et al. Synapsin-1 and tau reciprocal O-GlcNAcylation and phosphorylation sites in mouse brain synaptosomes. Exp Mol Med. 2013;45:e29. doi: 10.1038/emm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–18. doi: 10.1038/ncb1724. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. Loss of p53 enhances catalytic activity of IKKβeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci USA. 2009;106:3431–6. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WG, Dahmus ME, Hart GW. RNA polymerase II is a glycoprotein. Modification of the COOH-terminal domain by O-GlcNAc. J Biol Chem. 1993;268:10416–24. [PubMed] [Google Scholar]
- Kelly WG, Hart GW. Glycosylation of chromosomal proteins: localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 1989;57:243–51. doi: 10.1016/0092-8674(89)90962-8. [DOI] [PubMed] [Google Scholar]
- Kersten JR, Schmeling TJ, Pagel PS, et al. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–70. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- Kessler P, Bauersachs J, Busse R, Schini-Kerth VB. Inhibition of inducible nitric oxide synthase restores endothelium-dependent relaxations in proinflammatory mediator-induced blood vessels. Arterioscler Thromb Vasc Biol. 1997;17:1746–55. doi: 10.1161/01.atv.17.9.1746. [DOI] [PubMed] [Google Scholar]
- Khidekel N, Ficarro SB, Clark PM, et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nature Chemical Biology. 2007;3:339–48. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- Kida S, Josselyn SA, Pena De Ortiz S, et al. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5:348–55. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- Kim C, Nam DW, Park SY, et al. O-linked beta-N-acetylglucosaminidase inhibitor attenuates beta-amyloid plaque and rescues memory impairment. Neurobiol Aging. 2013;34:275–85. doi: 10.1016/j.neurobiolaging.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Kim EY, Jeong EH, Park S, et al. A role for O-GlcNAcylation in setting circadian clock speed. Genes Dev. 2012;26:490–502. doi: 10.1101/gad.182378.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Park SY, Choi YR, et al. Excessive O-GlcNAcylation of proteins suppresses spontaneous cardiogenesis in ES cells. FEBS Lett. 2009;583:2474–8. doi: 10.1016/j.febslet.2009.06.052. [DOI] [PubMed] [Google Scholar]
- Kitzmann M, Fernandez A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol Life Sci. 2001;58:571–9. doi: 10.1007/PL00000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement E, Lipinszki Z, Kupihar Z, et al. Enrichment of O-GlcNAc modified proteins by the periodate oxidation-hydrazide resin capture approach. J Proteome Res. 2010;9:2200–6. doi: 10.1021/pr900984h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Kornfeld R, Neufeld EF, O’brien PJ. The feedback control of sugar nucleotide biosynthesis in liver. Proc Natl Acad Sci USA. 1964;52:371–9. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs EG. Nobel Lecture. Protein phosphorylation and cellular regulation I. Biosci Rep. 1993;13:127–42. doi: 10.1007/BF01149958. [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–15. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Hart GW. Regulation of a cytosolic and nuclear O-GlcNAc transferase. Role of the tetratricopeptide repeats. J Biol Chem. 1999;274:32015–22. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Kuhl DE, Metter EJ, Riege WH, Phelps ME. Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F]fluorodeoxyglucose method. J Cereb Blood Flow Metab. 1982;2:163–71. doi: 10.1038/jcbfm.1982.15. [DOI] [PubMed] [Google Scholar]
- Laczy B, Marsh SA, Brocks CA, et al. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc dependent manner. FASEB J. 2009;23:H1715–27. doi: 10.1152/ajpheart.00337.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YA, Pickart CM, Alban A, et al. Inhibition of the ubiquitin-proteasome system in Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:9902–6. doi: 10.1073/pnas.170173897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Young RA. Transcription of eukaryotic protein-coding genes. Annu Rev Genet. 2000;34:77–137. doi: 10.1146/annurev.genet.34.1.77. [DOI] [PubMed] [Google Scholar]
- Lefebvre T, Guinez C, Dehennaut V, et al. Does O-GlcNAc play a role in neurodegenerative diseases? Expert Rev Proteomics. 2005;2:265–75. doi: 10.1586/14789450.2.2.265. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang N, Jia Z, et al. Critical role and regulation of transcription factor FoxM1 in human gastric cancer angiogenesis and progression. Cancer Res. 2009a;69:3501–9. doi: 10.1158/0008-5472.CAN-08-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Molina H, Huang H, et al. O-linked N-acetylglucosamine modification on CCAAT enhancer-binding protein beta: role during adipocyte differentiation. J Biol Chem. 2009b;284(1924):8–54. doi: 10.1074/jbc.M109.005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. Am J Cardiol. 2001;88:3J–6J. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- Lim JM, Sherling D, Teo CF, et al. Defining the regulated secreted proteome of rodent adipocytes upon the induction of insulin resistance. J Proteome Res. 2008;7:1251–63. doi: 10.1021/pr7006945. [DOI] [PubMed] [Google Scholar]
- Lima VV, Giachini FR, Carneiro FS, et al. O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1. Hypertension. 2010;55:180–8. doi: 10.1161/HYPERTENSIONAHA.109.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VV, Giachini FRC, Choi H, et al. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension. 2009;53:166–74. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptay S, Weber CK, Ludwig L, et al. Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105:735–46. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- Liu F, Shi J, Tanimukai H, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain. 2009;132:1820–32. doi: 10.1093/brain/awp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Paterson AJ, Zhang FX, et al. Accumulation of protein O-GlcNAc modification inhibits proteasomes in the brain and coincides with neuronal apoptosis in brain areas with high O-GlcNAc metabolism. J Neurochem. 2004;89:1044–55. doi: 10.1111/j.1471-4159.2004.02389.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li X, Yu Y, et al. Developmental regulation of protein O-GlcNAcylation, O-GlcNAc transferase, and O-GlcNAcase in mammalian brain. PLoS One. 2012;7:e43724. doi: 10.1371/journal.pone.0043724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–85. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- Love DC, Hanover JA. The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE. 2005;(2005):re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- Love DC, Kochan J, Cathey RL, et al. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci. 2003;116:647–54. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhou D, Jiang X, et al. Loss of E-cadherin in multidrug resistant breast cancer cell line MCF-7/Adr: possible implication in the enhanced invasive ability. Eur Rev Med Pharmacol Sci. 2012;16:1271–9. [PubMed] [Google Scholar]
- Lubas WA, Frank DW, Krause M, Hanover JA. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem. 1997;272:9316–24. doi: 10.1074/jbc.272.14.9316. [DOI] [PubMed] [Google Scholar]
- Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;128:401S–6S. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- Lunde IG, Aronsen JM, Kvaloy H, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiological Genomics. 2012;44:162–72. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- Lynch TP, Ferrer CM, Jackson SR, et al. Critical role of O-Linked beta-N-acetylglucosamine transferase in prostate cancer invasion, angiogenesis, and metastasis. J Biol Chem. 2012;287:11070–81. doi: 10.1074/jbc.M111.302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–30. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Whitworth GE, Debowski AW, et al. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem. 2005;280:25313–22. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- Mahmoudi T, Verrijzer CP. Chromatin silencing and activation by Polycomb and trithorax group proteins. Oncogene. 2001;20:3055–66. doi: 10.1038/sj.onc.1204330. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Mandrup S, Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272:5367–70. doi: 10.1074/jbc.272.9.5367. [DOI] [PubMed] [Google Scholar]
- Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–52. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- Marsh SA, Dell’italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40:819–28. doi: 10.1007/s00726-010-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SA, Powell PC, Dell’italia LJ, Chatham JC. Cardiac O-GlcNAcylation blunts autophagic signaling in the diabetic heart. Life Sci. 2013;92:648–56. doi: 10.1016/j.lfs.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–12. [PubMed] [Google Scholar]
- Master E, Chan SL, Ali-Khan Z. Ubiquitin (Ub) interacts non-covalently with Alzheimer amyloid precursor protein (betaPP): isolation of Ub-betaPP conjugates from brain extracts. Neuroreport. 1997;8:2781–6. doi: 10.1097/00001756-199708180-00026. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, et al. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–54. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- Maury JJ, Chan KK, Zheng L, et al. Excess of O-linked N-acetylglucosamine modifies human pluripotent stem cell differentiation. Stem Cell Res. 2013;11:926–37. doi: 10.1016/j.scr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Mcclain DA, Lubas WA, Cooksey RC, et al. Altered glycandependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA. 2002;99:10695–9. doi: 10.1073/pnas.152346899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgeer EG, Mcgeer PL, Akiyama H, Harrop R. Cortical glutaminase, beta-glucuronidase and glucose utilization in Alzheimer’s disease. Can J Neurol Sci. 1989;16:511–15. doi: 10.1017/s0317167100029851. [DOI] [PubMed] [Google Scholar]
- Mcgeer EG, Mcgeer PL, Harrop R, et al. Correlations of regional postmortem enzyme activities with premortem local glucose metabolic rates in Alzheimer’s disease. J Neurosci Res. 1990;27:612–19. doi: 10.1002/jnr.490270422. [DOI] [PubMed] [Google Scholar]
- McLarty JL, Marsh SA, Chatham JC. Post-translational protein modification by O-linked N-acetyl-glucosamine: its role in mediating the adverse effects of diabetes on the heart. Life Sci. 2013;92:621–7. doi: 10.1016/j.lfs.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Gu Y, Han C, et al. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011;1812:514–19. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Miller AP, Feng W, Xing D, et al. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–9. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Gong X, Shur BD. Sperm require beta-N-acetylglucosaminidase to penetrate through the egg zona pellucida. Development. 1993;118:1279–89. doi: 10.1242/dev.118.4.1279. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr. 1995;19:541–7. doi: 10.1097/00004728-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Montessuit C, Thorburn A. Transcriptional activation of the glucose transporter GLUT1 in ventricular cardiac myocytes by hypertrophic agonists. J Biol Chem. 1999;274:9006–12. doi: 10.1074/jbc.274.13.9006. [DOI] [PubMed] [Google Scholar]
- Moore RY. Principles of synaptic transmission. Ann N Y Acad Sci. 1993;695:1–9. doi: 10.1111/j.1749-6632.1993.tb23018.x. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Myers SA, Panning B, Burlingame AL. Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2011;108:9490–5. doi: 10.1073/pnas.1019289108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel AK, Schilling M, Comte-Walters S, et al. Identification of O-linked N-acetylglucosamine (O-GlcNAc)-modified osteoblast proteins by electron transfer dissociation tandem mass spectrometry reveals proteins critical for bone formation. Mol Cell Proteomics. 2013;12:945–55. doi: 10.1074/mcp.M112.026633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature Medicine. 2007;13:619–24. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Ng HH, Surani MA. The transcriptional and signalling networks of pluripotency. Nat Cell Biol. 2011;13:490–6. doi: 10.1038/ncb0511-490. [DOI] [PubMed] [Google Scholar]
- Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol. 2009;297:H1711–9. doi: 10.1152/ajpheart.00553.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Not LG, Brocks CA, Vamhidy L, et al. Increased O-linked beta-N-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit Care Med. 2010;38:562–71. doi: 10.1097/CCM.0b013e3181cb10b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Mizofuchi H, Kobayashi Y, et al. Terminal differentiation program of skeletal myogenesis is negatively regulated by O-GlcNAc glycosylation. Biochim Biophys Acta. 2012;1820:24–32. doi: 10.1016/j.bbagen.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Oka H, Shiozaki H, Kobayashi K, et al. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53:1696–701. [PubMed] [Google Scholar]
- Olivier-Van Stichelen S, Drougat L, Dehennaut V, et al. Serum-stimulated cell cycle entry promotes ncOGT synthesis required for cyclin D expression. Oncogenesis. 2012;1:e36. doi: 10.1038/oncsis.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleon M, Tan HY, Kafer GR, Kaye PL. Toxic effects of hyperglycemia are mediated by the hexosamine signaling pathway and o-linked glycosylation in early mouse embryos. Biol Reprod. 2010;82:751–8. doi: 10.1095/biolreprod.109.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papin C, Eychene A, Brunet A, et al. B-Raf protein isoforms interact with and phosphorylate Mek-1 on serine residues 218 and 222. Oncogene. 1995;10:1647–51. [PubMed] [Google Scholar]
- Parker GJ, Lund KC, Taylor RP, Mcclain DA. Insulin resistance of glycogen synthase mediated by O-linked N-acetylglucosamine. J Biol Chem. 2003;278:10022–7. doi: 10.1074/jbc.M207787200. [DOI] [PubMed] [Google Scholar]
- Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14:341–56. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu XP, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Petit-Taboue MC, Landeau B, Desson JF, et al. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–84. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- Pietersen AM, Van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol. 2008;20:201–7. doi: 10.1016/j.ceb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Piriou V, Chiari P, Gateau-Roesch O, et al. Desflurane-induced preconditioning alters calcium-induced mitochondrial permeability transition. Anesthesiology. 2004;100:581–8. doi: 10.1097/00000542-200403000-00018. [DOI] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–61. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Prevention CFDCA . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- Ranuncolo SM, Ghosh S, Hanover JA, et al. Evidence of the involvement of O-GlcNAc-modified human RNA polymerase II CTD in transcription in vitro and in vivo. J Biol Chem. 2012;287:23549–61. doi: 10.1074/jbc.M111.330910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao FV, Schüttelkopf AW, Dorfmueller HC, et al. Structure of a bacterial putative acetyltransferase defines the fold of the human O-GlcNAcase C-terminal domain. Open Biol. 2013;3:130021. doi: 10.1098/rsob.130021. doi: 10.1098/rsob.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71:4329–33. doi: 10.1158/0008-5472.CAN-11-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach JE, Clark PM, Mason DE, et al. Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nature Chem Biol. 2012;8:253–61. doi: 10.1038/nchembio.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach JE, Rogers CJ, Yu SH, et al. Quantification of O-glycosylation stoichiometry and dynamics using resolvable mass tags. Nature Chem Biol. 2010;6:645–51. doi: 10.1038/nchembio.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SJ, Konieczny SF. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–61. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R. Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development. 2007;134:223–32. doi: 10.1242/dev.02723. [DOI] [PubMed] [Google Scholar]
- Ruan HB, Han X, Li MD, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012;16:226–37. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan HB, Nie Y, Yang X. Regulation of protein degradation by O-GlcNAcylation: crosstalk with ubiquitination. Mol Cell Proteomics. 2013;12:3489–97. doi: 10.1074/mcp.R113.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe K, Hart GW. O-GlcNAc transferase regulates mitotic chromatin dynamics. J Biol Chem. 2010;285:34460–8. doi: 10.1074/jbc.M110.158170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakabe K, Wang Z, Hart GW. Beta-N-acetylglucosamine (O-GlcNAc) is part of the histone code. Proc Natl Acad Sci USA. 2010;107:19915–20. doi: 10.1073/pnas.1009023107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, et al. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–6. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Sato S, Yagi S, Arai Y, et al. Genome-wide DNA methylation profile of tissue-dependent and differentially methylated regions (T-DMRs) residing in mouse pluripotent stem cells. Genes Cells. 2010;15:607–18. doi: 10.1111/j.1365-2443.2010.01404.x. [DOI] [PubMed] [Google Scholar]
- Sauer CG, Kappeler A, Spath M, et al. Expression and activity of matrix metalloproteinases-2 and -9 in serum, core needle biopsies and tissue specimens of prostate cancer patients. Virchows Arch. 2004;444:518–26. doi: 10.1007/s00428-004-1016-2. [DOI] [PubMed] [Google Scholar]
- Sayat R, Leber B, Grubac V, et al. O-GlcNAc-glycosylation of beta-catenin regulates its nuclear localization and transcriptional activity. Exp Cell Res. 2008;314:2774–87. doi: 10.1016/j.yexcr.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, et al. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–45. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. Polycomb complexes and epigenetic states. Curr Opin Cell Biol. 2000;20:266–73. doi: 10.1016/j.ceb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Shafi R, Iyer SP, Ellies LG, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA. 2000;97:5735–9. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalin SC, Zirrgiebel U, Honsa KJ, et al. Neuronal MEK is important for normal fear conditioning in mice. J Neurosci Res. 2004;75:760–70. doi: 10.1002/jnr.20052. [DOI] [PubMed] [Google Scholar]
- Shapira M, Zhai RG, Dresbach T, et al. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–52. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- Sharma GD, Nguyen HT, Antonov AS, et al. Expression of atrial natriuretic peptide receptor-A antagonizes the mitogen-activated protein kinases (Erk2 and P38MAPK) in cultured human vascular smooth muscle cells. Mol Cell Biochem. 2002;233:165–73. doi: 10.1023/a:1015882302796. [DOI] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–30. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Shi FT, Kim H, Lu W, et al. Ten-eleven translocation 1 (tet1) is regulated by o-linked N-acetylglucosamine transferase (ogt) for target gene repression in mouse embryonic stem cells. J Biol Chem. 2013;288:20776–84. doi: 10.1074/jbc.M113.460386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tomic J, Wen F, et al. Aberrant O-GlcNAcylation characterizes chronic lymphocytic leukemia. Leukemia. 2010;24:1588–98. doi: 10.1038/leu.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota K. DNA methylation profiles of CpG islands for cellular differentiation and development in mammals. Cytogenet Genome Res. 2004;105:325–34. doi: 10.1159/000078205. [DOI] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–9. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Simpson P. Stimulation of hypertrophy of cultured neonatal rat-heart cells through an alpha-1-adrenergic receptor and induction of beating through an alpha-1-adrenergic and beta-1-adrenergic Receptor interaction – evidence for independent regulation of growth and beatin. Circulation Res. 1985;56:884–94. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Syrzycka M, Macauley MS, et al. Drosophila O-GlcNAc transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc) Proc Natl Acad Sci USA. 2009;106:13427–32. doi: 10.1073/pnas.0904638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorobogatko YV, Deuso J, Adolf-Bryfogle J, et al. Human Alzheimer’s disease synaptic O-GlcNAc site mapping and iTRAQ expression proteomics with ion trap mass spectrometry. Amino Acids. 2011;40:765–79. doi: 10.1007/s00726-010-0645-9. [DOI] [PubMed] [Google Scholar]
- Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- Slawson C, Zachara NE, Vosseller K, et al. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–56. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- Smet-Nocca C, Broncel M, Wieruszeski JM, et al. Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation. Mol Biosyst. 2011;7:1420–9. doi: 10.1039/c0mb00337a. [DOI] [PubMed] [Google Scholar]
- Smith GS, De Leon MJ, George AE, et al. Topography of cross-sectional and longitudinal glucose metabolic deficits in Alzheimer’s disease. Pathophysiologic implications. Arch Neurol. 1992;49:1142–50. doi: 10.1001/archneur.1992.00530350056020. [DOI] [PubMed] [Google Scholar]
- Smith SM, Renden R, Von Gersdorff H. Synaptic vesicle endocytosis: fast and slow modes of membrane retrieval. Trends Neurosci. 2008;31:559–68. doi: 10.1016/j.tins.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola-Penna M, Da Silva D, Coelho WS, et al. Regulation of mammalian muscle type 6-phosphofructo-1-kinase and its implication for the control of the metabolism. IUBMB Life. 2010;62:791–6. doi: 10.1002/iub.393. [DOI] [PubMed] [Google Scholar]
- Song C, Zhu S, Wu C, Kang J. HDAC10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J Biol Chem. 2013;288:28021–33. doi: 10.1074/jbc.M113.498758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–63. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahayori B, Koceja DM. Activity-dependent plasticity of spinal circuits in the developing and mature spinal cord. Neural Plast. 2012;2012:964843. doi: 10.1155/2012/964843. doi: 10.1155/2012/964843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tan EP, Caro S, Potnis A, et al. O-linked N-acetylglucosamine cycling regulates mitotic spindle organization. J Biol Chem. 2013;288:27085–99. doi: 10.1074/jbc.M113.470187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nature Cell Biol. 2003;5:701–10. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ludwig LM, Kersten JR, et al. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707–21. doi: 10.1097/00000542-200403000-00035. [DOI] [PubMed] [Google Scholar]
- Tarone G, Hirsch E, Brancaccio M, et al. Integrin function and regulation in development. Int J Dev Biol. 2000;44:725–31. [PubMed] [Google Scholar]
- Temu TM, Wu KY, Gruppuso PA, Phornphutkul C. The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells. Am J Physiol Endocrinol Metab. 2010;299:E325–34. doi: 10.1152/ajpendo.00145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson MA, Anastasiadis PZ, Daniel JM, et al. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleman C, Paterson AJ, Whisenhunt TR, Kudlow JE. Characterization of the histone acetyltransferase (HAT) domain of a bifunctional protein with activable O-GlcNAcase and HAT activities. J Biol Chem. 2004;279:53665–73. doi: 10.1074/jbc.M410406200. [DOI] [PubMed] [Google Scholar]
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–17. [PubMed] [Google Scholar]
- Trinidad JC, Barkan DT, Gulledge BF, et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Molecular Cellular Proteomics. 2012;11:215–29. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella P, Scelfo A, Jammula S, et al. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell. 2013;49:645–56. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, et al. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–24. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 2002;99:5313–18. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–22. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IC, Chen YJ, Hughes D, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25:10875–94. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Huang X, Sun DN, et al. Extensive crosstalk between O-GlcNAcylation and phosphorylation regulates akt signaling. PLoS One. 2012;7:e37427. doi: 10.1371/journal.pone.0037427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Abbruzzese JL, Evans DB, et al. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- Wang Z, Banerjee S, Kong D, et al. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- Wang Z, Udeshi ND, O’malley M, et al. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2010;9:153–60. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Garza C. Early postnatal nutrition determines adult pancreatic glucose-responsive insulin secretion and islet gene expression in rats. J Nutr. 2002;132:357–64. doi: 10.1093/jn/132.3.357. [DOI] [PubMed] [Google Scholar]
- Watson LJ, Facundo HT, Ngoh GA, et al. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA. 2010;107:17797–802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging. 2000;21:719–27. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- Webster DM, Teo CF, Sun Y, et al. O-GlcNAc modifications regulate cell survival and epiboly during zebrafish development. BMC Dev Biol. 2009;9:28. doi: 10.1186/1471-213X-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Gao Y, Mahoney JA, et al. Dynamic O-glycosylation of nuclear and cytosolic proteins: further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J Biol Chem. 2002;277:1755–61. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- Wells L, Hart GW. O-GlcNAc turns twenty: functional implications for post-translational modification of nuclear and cytosolic proteins with a sugar. FEBS Lett. 2003;546:154–8. doi: 10.1016/s0014-5793(03)00641-0. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–8. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci. 2003a;60:222–8. doi: 10.1007/s000180300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Whelan SA, Hart GW. O-GlcNAc: a regulatory post-translational modification. Biochem Biophys Res Commun. 2003b;302:435–41. doi: 10.1016/s0006-291x(03)00175-x. [DOI] [PubMed] [Google Scholar]
- Whelan SA, Lane MD, Hart GW. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J Biol Chem. 2008;283:21411–17. doi: 10.1074/jbc.M800677200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388:1257–74. doi: 10.1515/BC.2007.159. [DOI] [PubMed] [Google Scholar]
- Williams K, Christensen J, Helin K. DNA methylation: TET proteins-guardians of CpG islands? EMBO Rep. 2012;13:28–35. doi: 10.1038/embor.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalasundera R, Fexby S, Regan L, Hughes AD. Effect of tumour necrosis factor-alpha and interleukin 1 beta on endothelium-dependent relaxation in rat mesenteric resistance arteries in vitro. Br J Pharmacol. 2003;138:1285–94. doi: 10.1038/sj.bjp.0705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski JR, Nagaraj N, Zougman A, et al. Brain phosphoproteome obtained by a FASP-based method reveals plasma membrane protein topology. J Proteome Res. 2010;9:3280–9. doi: 10.1021/pr1002214. [DOI] [PubMed] [Google Scholar]
- Wu CC, Maccoss MJ, Howell KE, Yates JR. A method for the comprehensive proteomic analysis of membrane proteins. Nature Biotechnol. 2003;21:532–8. doi: 10.1038/nbt819. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436–52. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J, Myers MP, Laherty CD, et al. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Gong K, Feng W, et al. O-GlcNAc modification of NFkappaB p65 inhibits TNF-alpha-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS One. 2011;6:e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Miller A, Novak L, et al. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation. 2004;109:234–41. doi: 10.1161/01.CIR.0000105700.95607.49. [DOI] [PubMed] [Google Scholar]
- Xu R, Seger R, Pecht I. Cutting edge: extracellular signal-regulated kinase activates syk: a new potential feedback regulation of Fc epsilon receptor signaling. J Immunol. 1999;163:1110–14. [PubMed] [Google Scholar]
- Yagi S, Hirabayashi K, Sato S, et al. DNA methylation profile of tissue-dependent and differentially methylated regions (T-DMRs) in mouse promoter regions demonstrating tissue-specific gene expression. Genome Res. 2008;18:1969–78. doi: 10.1101/gr.074070.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Kim JE, Nam HW, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–83. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- Yang WH, Park SY, Nam HW, et al. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA. 2008a;105:17345–50. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Ongusaha PP, Miles PD, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008b;451:964–9. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- Yang YR, Song M, Lee H, et al. O-GlcNAcase is essential for embryonic development and maintenance of genomic stability. Aging Cell. 2012;11:439–48. doi: 10.1111/j.1474-9726.2012.00801.x. [DOI] [PubMed] [Google Scholar]
- Yao T, Song L, Xu W, et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol. 2006;8:994–1002. doi: 10.1038/ncb1460. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Cao Z, Classon M, Mcknight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–81. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- Yehezkel G, Cohen L, Kliger A, et al. O-linked beta-N-acetylglucosaminylation (O-GlcNAcylation) in primary and metastatic colorectal cancer clones and effect of N-acetyl-beta-D-glucosaminidase silencing on cell phenotype and transcriptome. J Biol Chem. 2012;287:28755–69. doi: 10.1074/jbc.M112.345546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W, Clark PM, Mason DE, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–80. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa SA, Macauley MS, Heinonen JE, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–90. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- Yuzwa SA, Yadav AK, Skorobogatko Y, et al. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids. 2011;40:857–68. doi: 10.1007/s00726-010-0705-1. [DOI] [PubMed] [Google Scholar]
- Zachara NE. The roles of O-linked beta-N-acetylglucosamine in cardiovascular physiology and disease. Am J Physiol Heart Circ Physiol. 2012;302:H1905–18. doi: 10.1152/ajpheart.00445.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, O’donnell N, Cheung WD, et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–42. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- Zaro BW, Yang YY, Hang HC, Pratt MR. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1. Proc Natl Acad Sci USA. 2011;108:8146–51. doi: 10.1073/pnas.1102458108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemse SM, Chiao CW, Hilgers RHP, Webb RC. Interleukin-10 inhibits the in vivo and in vitro adverse effects of TNF-alpha on the endothelium of murine aorta. Am J Physiol Heart Circulat Physiol. 2010;299:H1160–7. doi: 10.1152/ajpheart.00763.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FX, Hu Y, Huang P, et al. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem. 2007;282:22460–71. doi: 10.1074/jbc.M702439200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shi J, Feng J, et al. Type IV collagenase (matrix metalloproteinase-2 and -9) in prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:327–32. doi: 10.1038/sj.pcan.4500750. [DOI] [PubMed] [Google Scholar]
- Zhang LC, Zeng YM, Ting J, et al. The distributions and signaling directions of the cerebrospinal fluid contacting neurons in the parenchyma of a rat brain. Brain Res. 2003;989:1–8. doi: 10.1016/s0006-8993(03)03123-8. [DOI] [PubMed] [Google Scholar]
- Zhang S, Roche K, Nasheuer HP, Lowndes NF. Modification of histones by sugar beta-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated. J Biol Chem. 2011;286:37483–95. doi: 10.1074/jbc.M111.284885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Suyang H, Erdjument-Bromage H, et al. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–24. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- Zhu HX, Tannous P, Johnstone JL, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. Circulation. 2006;114:96–96. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zhou L, Yang Z, et al. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med Oncol. 2012;29:985–93. doi: 10.1007/s12032-011-9912-1. [DOI] [PubMed] [Google Scholar]
- Zhu W, Leber B, Andrews DW. Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J. 2001;20:5999–6007. doi: 10.1093/emboj/20.21.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou LY, Yang SL, Champattanachai V, et al. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-kappa B signaling. Am J Physiol Heart Circulat Physiol. 2009;296:H515–23. doi: 10.1152/ajpheart.01025.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]